Abstract
Background:
Dexmedetomidine can inhibit the perioperative stress response, which plays an important role in postoperative hypercoagulability. This study aimed to investigate whether dexmedetomidine could attenuate the activation of postoperative coagulation.
Methods:
Patients undergoing open radical gastrectomy under total intravenous anesthesia were randomly allocated to the control group (group Con) and the dexmedetomidine group (group Dex). Dexmedetomidine was intravenously infused at 0.5 μg/kg over 10 minutes before anesthesia induction and then infused at a rate of 0.5 μg/kg/h until peritoneal closure in group Dex, whereas saline was administered in group Con. Blood samples were collected for thrombelastograph (TEG) analysis [reaction time (R time), clot formation time (K time), and clot formation rate (α angle)] and laboratory coagulation testing before dexmedetomidine administration and at the end of surgery.
Results:
Coagulation was activated after radical gastrectomy, as indicated by TEG analysis and the increased concentrations of plasma fibrin (fibrinogen) degradation product (FDP) and thrombin-antithrombin complex (TAT). The R and K times were significantly prolonged and α angle was significantly decreased in group Dex compared with that in group Con at the end of surgery (P < .05). The concentrations of plasma TAT and FDP in group Dex were significantly lower than those in group Con at the end of surgery (P < .05 or .01).
Conclusion:
Adjunctive dexmedetomidine with general anesthesia attenuates the activation of coagulation following radical gastrectomy.
Keywords: coagulation, dexmedetomidine, radical gastrectomy, thromboelastograph
1. Introduction
The blood coagulation system is activated after surgery and helps reduce perioperative wound bleeding; however, blood coagulation also promotes postoperative thrombotic and thromboembolic complications.[1–4] Perioperative stress plays an important role in the development of a postoperative hypercoagulation state. Adopting appropriate anesthetic techniques to reduce the perioperative stress response can attenuate postoperative hypercoagulability. For example, compared with general anesthesia, intravertebral anesthesia has a beneficial effect on postoperative coagulation, which, in turn, reduces the incidence of postoperative thromboembolic events.[5,6] Dexmedetomidine is a highly selective α2-adrenergic agonist that can markedly reduce the perioperative stress response, but whether it can reduce postoperative coagulation system activation has not been reported.
Mahla et al[7] reported postoperative hypercoagulability following major abdominal surgery. Malignancies are associated with a high risk of thromboembolic complications, which is further increased by surgical trauma.[8,9] Therefore, the present study investigates the effect of adjunctive dexmedetomidine with general anesthesia on postoperative coagulation, with a focus on open radical gastrectomy.
2. Patients and methods
2.1. Patient selection
The study was approved by the medical ethics committee of the Affiliated People's Hospital of Jiangsu University and is registered at www.chictr.org (ChiCTR-IPR-17011152). After signing informed consent forms, 60 patients with ASA I or II, aged 51 to 70 years and weighing 53 to 75 kg who were scheduled for elective open radical gastrectomy were recruited. The exclusion criteria included body mass index ≥28 kg/m2, preoperative coagulation disorders and abnormal platelet count, past history of thromboembolic, hepatic, and renal dysfunction, any preoperative medication that interferes with coagulation, cardiovascular disease, and preoperative transfusion. Patients were randomly divided into 2 groups: the control group (group Con) and the dexmedetomidine group (group Dex); there were 30 patients in each group.
2.2. Study protocol
No preoperative medications were administered. Invasive radial artery blood pressure, nasopharyngeal temperature, bispectral index (BIS), electrocardiogram, and pulse oxygen saturation were monitored. A 16-G central venous catheter was inserted into the right internal jugular vein, and 8 mL/kg of lactated Ringer solution was infused before anesthesia induction. Normal saline without heparin was used to flush the arterial and central venous lines. In group Dex, 0.5 μg/kg dexmedetomidine (Hengrui Medicine Co., Ltd., Lianyungang, Jiangsu, China) was intravenously infused over 10 minutes before anesthesia induction followed by infusion at a rate of 0.5 μg/kg/h until peritoneal closure; volume-matched normal saline was administered in group Con. Effect-site target-controlled infusions of propofol and remifentanil were initiated for anesthesia induction. The initial effect-site concentrations of propofol were 1.5 and 3.0 μg/mL in group Dex and Con, respectively, and were gradually increased until BIS values reached 45 to 55. Then, effect-site target-controlled infusion of 3.0 ng/mL remifentanil was initiated. When the concentration of remifentanil reached the target value, 0.6 mg/kg rocuronium was administered to facilitate endotracheal intubation. The lungs were ventilated with a tidal volume of 8 mL/kg, and the breathing frequency was adjusted to maintain an end-tidal carbon dioxide partial pressure of 30 to 40 mm Hg. Target-controlled infusion of propfol and remifentanil was continued in the anesthesia maintenance phase. The concentrations of propofol and remifentanil were adjusted to maintain BIS values at 45 to 55 and mean arterial pressure at baseline values ± 20%. If the concentration of remifentanil was decreased to 2 ng/mL and mean arterial pressure was maintained below 80% of baseline values, phenylephrine was administered, whereas if the concentration of remifentanil was increased to 8 ng/mL and mean arterial pressure was maintained above 120% of baseline values, urapidil was administered. Patients with heart rates below 50 bpm and above 90 bpm were treated by atropine and esmolol, respectively. These treatments were repeated as necessary. Cisatracurium was infused at 1 μg/kg/min to maintain intraoperative muscle relaxation and discontinued before closing the abdomen. Propofol and remifentanil infusions were stopped before skin anastomosis. Afterwards, 3 μg/kg fentanyl and 0.3 mg of ramosetron were administered. Lactated Ringer solution was continuously infused at 8 mL/kg/h, and the volume of blood loss was supplemented with 3 times that volume of Ringer lactate solution during the operation. A heating blanket, warm intravenous fluids, and operative flushing fluids were applied to maintain normothermia.
After the operation, patients were transferred to the recovery room. Patients underwent routine reversal of neuromuscular blockade and were extubated after consciousness recovery. Lactated Ringer solution was infused at 2 mL/kg/h in the recovery room. Patient-controlled intravenous analgesia was used to maintain a visual analog scale (VAS) score ≤ 3.
Blood samples were collected from the central venous catheter before dexmedetomidine or placebo administration and at the end of surgery using the 2-syringe technique. Three 1.8-mL blood samples were drawn into vacutainer tubes containing 0.2 mL sodium citrate. The first blood sample was used for thrombelastograph (TEG) analysis (TEG 5000; Haemoscope Corporation, Niles, IL). TEG was used to monitor the clot development of whole blood by measurement of its viscoelastic changes associated with fibrin polymerization. TEG variables include reaction time (R time), clot formation time (K time), clot formation rate (α angle), and maximal amplitude of clot strength (MA) (Fig. 1). R time denotes the rate of initial fibrin formation and is related to the activity of clot factors. K time and α angle represent the rate of clot formation and are associated with the level of fibrin. MA is a reflection of the final clot strength and can be altered by the abnormalities of quantity and function of platelet. The shortening of R and k times and increase of α angle and MA indicate an enhancement of coagulation function. The second blood sample was used for laboratory tests, including detection of prothrombin time (PT), activated partial thromboplastin time (APTT), plasma fibrinogen concentration, and fibrin (fibrinogen) degradation product (FDP) concentration. The above coagulation assays were performed within 1 hour of collection. The third blood sample was centrifuged at 2300g for 15 minutes at 4°C, and the separated plasma was aliquoted and stored at –70°C. Samples were thawed within 1 month and prepared for the determination of thrombin-antithrombin complex (TAT) concentrations by ELISA. One 2-mL blood sample was drawn into vacutainer tubes containing ethylenediaminetetraacetate for blood counts.
Figure 1.
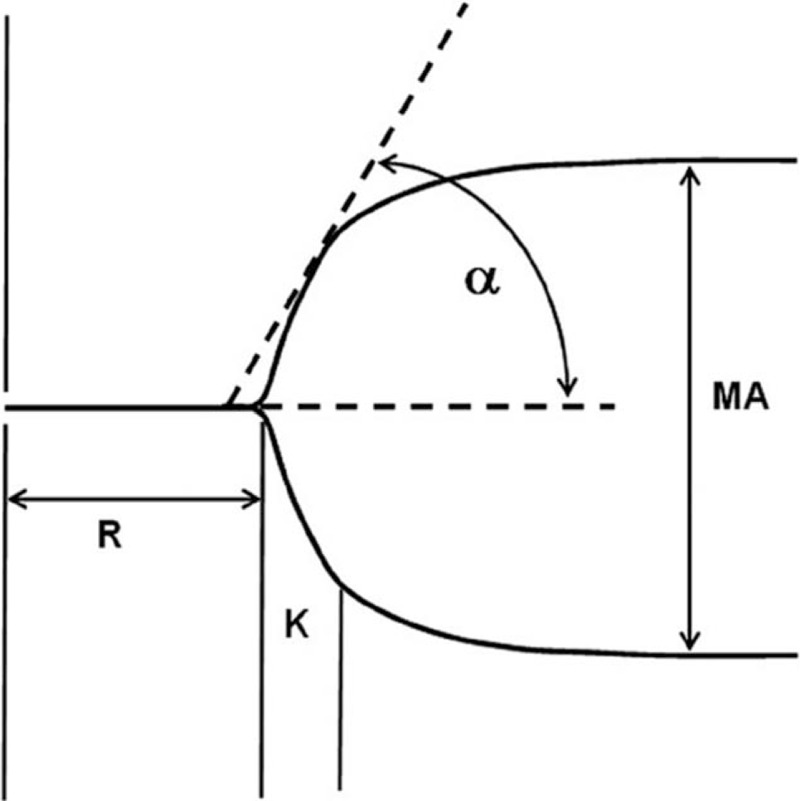
Thrombelastograph tracing. α = the slope of the tracing from the R to K value, K = time from R to reach a 20 mm of amplitude, MA = maximum amplitude, R = time from 0 to 2 mm of amplitude. Normal range of R time, K time, α angle, and MA: 5–10 min, 1–3 min, 53°–72°, and 50–70 mm.
The primary outcome of this study was the values for TEG variables and secondary outcomes included the values for standard coagulation indicators and the concentrations of plasma TAT and FDP.
The study designer used EXCEL random number generator to yield 60 random numbers, sorted them by size, and equally assigned patients into 2 groups. Anesthetist “A” enrolled study subjects in light of inclusion and exclusion criteria. Anesthetist “B” applied dexmedetomidine or placebo according to the grouping and adjusted the propofol concentration under BIS guidance. Anesthetist “C,” who was blinded to patient grouping, administered intraoperative anesthesia according to the study protocol.
2.3. Sample size and statistical analysis
The sample size was calculated based on the following assumptions: The primary endpoint was the R time; The preoperative R time of patients with gastric cancer was 5.61 ± 1.07 in the general surgical department of our hospital; On the basis of the findings of a previous study,[7] we predicted that the R time at the end of surgery would be reduced by 15% compared with preoperative baseline values, and On the basis of the study by Sharma and Philip,[5] we assumed that adjunctive dexmedetomidine with general anesthesia would prolong the R time by 20% at the end of surgery. A sample size of 29 subjects per group was required for a 2-tailed α of 0.05 and β of 0.10, assuming 10% loss to follow-up. We thus planned to enroll 60 subjects in this study.
Data were analyzed with SPSS 23.0 statistical package (SPSS Inc., Chicago, IL). Independent-sample t test was used for the comparison of demographic and intraoperative data between groups. The TEG, hematologic, and laboratory blood coagulation data were analyzed by Wilcoxon signed rank test within groups and Wilcoxon rank sum test between groups. Intergroup categorical data were compared with the Chi-square test or Fisher exact test. A P value of less than .05 was considered to be statistically significant (Tables 1 and 2).
Table 1.
Demographic and intraoperative data.
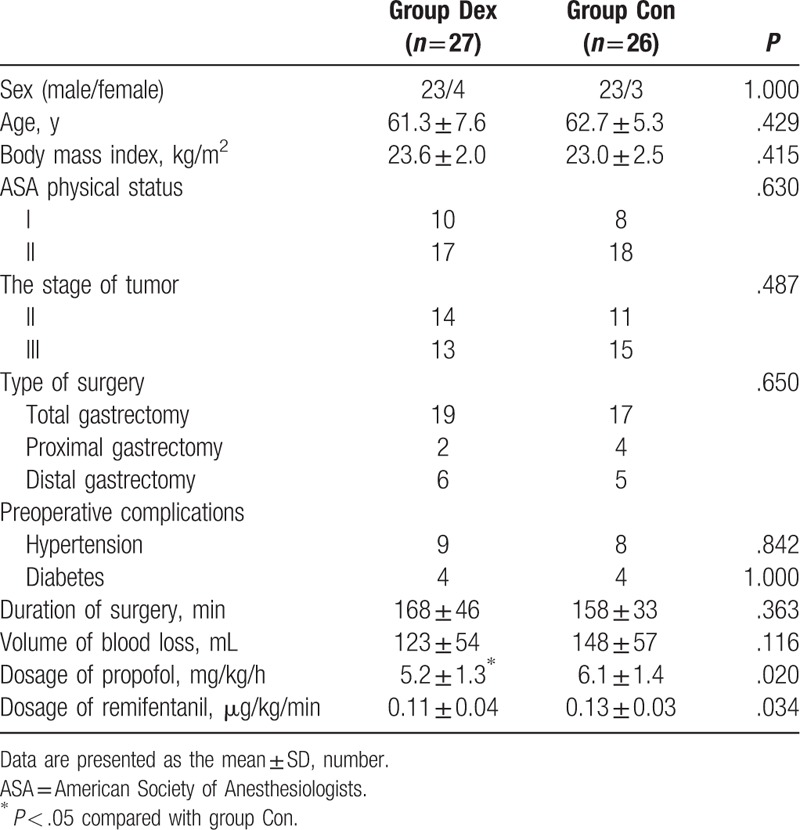
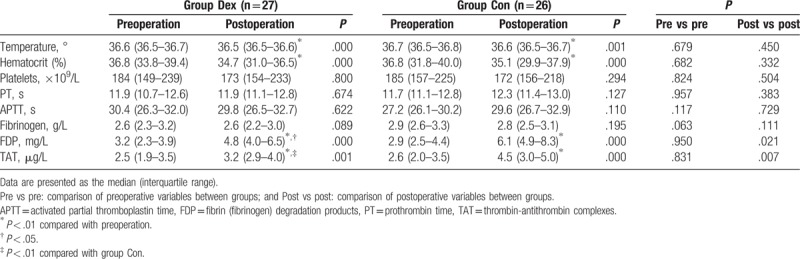
Table 2.
Hematologic and laboratory blood coagulation data.
3. Results
Two and three patients in group Dex and group Con, respectively, were excluded from the study because the tumor invaded into surrounding tissue and the surgeon failed to carry out radical gastrectomy. One patient in each group was excluded from the study because of intraoperative transfusion. Thus, the data of 27 and 26 patients, respectively, in group Dex and group Con were included in the statistical analysis (see flow diagram).
There were no differences in gender, age, body mass index, the type of operation, the duration of operation, the total blood loss in operation, preoperative complications, and tumor staging between groups. The consumptions of both propofol and remifentanil were significantly lower in group Dex than group Con (P < .05).
The body temperature and hematocrit in the postoperative period were significantly lower than those in the preoperative period in both groups (P < .01). The platelet counts, PT, APTT, and plasma fibrinogen concentration were comparable between the preoperative and postoperative period in both groups. In both groups, the concentrations of plasma FDP and TAT were significantly increased in the postoperative period when compared with the preoperative period (P < .01). In the preoperative period, the levels of FDP and TAT were similar between the groups. In the postoperative period, the concentrations of plasma FDP and TAT in group Dex were lower than those in group Con (P < .05 or .01).
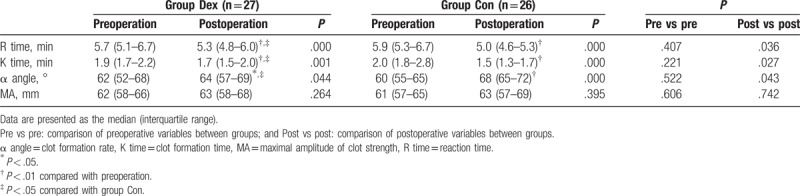
In both groups, the R and K times and α angle were significantly reduced in the postoperative period when compared with the preoperative period (P < .05 or .01), and there was no significant difference in the MA value between the preoperative and postoperative periods. In the preoperative period, the values for all TEG variables were similar between groups, whereas in the postoperative period, the R and K times were prolonged and α angle was decreased in group Dex compared with that in group Con (P < .05); however, there was no significant difference in MA value between groups (Table 3).
Table 3.
Thromboelastographic variables.
4. Discussion
A number of factors, including the type of surgery, anesthetic regimens, massive blood loss, transfusion, hypothermia, and the use of artificial colloids, can affect perioperative coagulation.[5,10–12] To avoid the influence of the above factors on coagulation in the present study, we limited our observation to one type of operation, applied a series of warming measures, used only crystalloid solutions, and excluded patients who experienced more bleeding and received transfusions. Our study demonstrated that the postoperative coagulation system was activated in patients undergoing radical gastrectomy and that intraoperative adjunctive dexmedetomidine administration had a beneficial effect on postoperative coagulation.
TEG analyses survey the mechanical properties of the coagulation process from blood clot formation to lysis and reflect the interaction among clotting factors, platelets, and the fibrinolytic system, whereas the routine laboratory coagulation profile monitors only a portion of the coagulation cascade. Numerous studies have documented that TEG analysis was more sensitive in identifying postoperative hypercoagulability than standard coagulation tests.[13,14] Concurrently, in our study, TEG analysis indicated the activation of postoperative coagulation with reduced R and K times and increased α angle, whereas PT and APTT tests showed no changes after radical gastrectomy. Moreover, our results suggested that adding dexmedetomidine to general anesthesia might alleviate postoperative activation of the coagulation system, which was reflected by a reduction in the postoperative shortening of R and K times and increase of α angle.
Antithrombin-thrombin complex formation reflects activation of the coagulation system and the generation of thrombin. FDP is a fibrin (fibrinogen) degradation product and indicates that blood clots have been formed and degraded by fibrinolysis. In accordance with previous studies,[15,16] we also observed significant postoperative increases in plasma TAT and FDP concentrations. However, the levels of postoperative plasma TAT and FDP after the application of dexmedetomidine with general anesthesia were lower than those in the absence of dexmedetomidine. Therefore, dexmedetomidine might reduce the postoperative increase in coagulation response and intravascular clot formation at a subclinical level.
Perioperative stress response plays an important role in postoperative hypercoagulability, which promotes thrombotic and thromboembolic complications. The release of stress hormones intraoperatively leads to an increase in blood coagulability.[17,18] Dexmedetomidine is a potent sympatholytic agent that can reduce central sympathetic outflow and intraoperatively attenuate neuroendocrine responses to surgical stress.[19] Cytokine release following surgical trauma can also activate the blood coagulation system and induce a hypercoagulable state.[20,21] Recent studies have shown that dexmedetomidine has an anti-inflammatory effect. Dong et al[22] found that dexmedetomidine could effectively reduce the release of cytokines in patients who underwent radical gastrectomy. The study by Li et al[23] also showed that adjunctive dexmedetomidine in general anesthesia could notably reduce the concentration of plasma catecholamines and cytokines to a degree that was similar to the combination of general and epidural anesthesia. Therefore, the inhibitory effect of dexmedetomidine on postoperative coagulation activation might be attributed to a reduced intraoperative stress response and an anti-inflammatory effect from dexmedetomidine.
Dexmedetomidine directly stimulates the α2-adrenoceptor on the surface of the platelet and activates the platelet[24]; however, dexmedetomidine also reduces tonic levels of sympathetic outflow and decreases catecholamine release, which indirectly inhibit platelet function. Martins et al[25] showed that the pro- and antiplatelet effects from the direct and indirect mechanisms resulted in mild hypocoagulation, as shown by TEG analysis with increased R time and decreased coagulation index, which supported our hypothesis that dexmedetomidine could attenuate postoperative hypercoagulability via its sympatholytic effect.
Anesthetics themselves can affect blood coagulation; for example, propofol has a suppressive effect on platelet aggregation and reduces coagulability.[26,27] Dexmedetomidine can reduce the intraoperative requirement for propofol, which may have an adverse effect on coagulation. However, our results showed a beneficial effect of dexmedetomidine on coagulation, which further supported that dexmedetomidine can reduce the activation of postoperative coagulation.
The prevention of postoperative thromboembolism involves multiple measures, including administration of low molecular heparin and early ambulation.[28] It may be difficult to reduce the incidence of thromboembolism in the early postoperative period by intraoperatively administered adjunctive dexmedetomidine alone. Therefore, we did not use Doppler or ultrasound to examine leg veins. Nevertheless, our study provided the basis for attenuating postoperative hypercoagulability by optimizing anesthetics.
Our results contradict the findings of Ganter et al,[29] who reported that clonidine had no effect on postoperative coagulation. However, all patients were perioperatively administered low-molecular-weight heparin to prevent postoperative thrombosis in that study. The effect of clonidine on postoperative coagulation may be difficult to isolate. Similarly, regional anesthesia attenuated the activation of postoperative coagulation in patients without heparin prophylaxis but did not have a beneficial effect on patients receiving perioperatively administered anticoagulant therapy.[30]
After major abdominal surgery, hypercoagulability can persist for at least 1 week.[1,7] However, our observation was terminated only after operation, which was a limitation of the study. However, dexmedetomidine is short-acting and the effect of dexmedetomidine gradually weakens after discontinuation. Furthermore, there are many uncontrolled postoperative variables, especially the use of hemostatic drugs, which can affect coagulation. Therefore, we chose to evaluate the effect of dexmedetomidine on postoperative coagulation function only at the end of surgery.
In summary, the application of dexmedetomidine in radical gastrectomy can attenuate the activation of coagulation and ameliorate hypercoagulability at the end of surgery.
Author contributions
Data curation: Da-Peng Zhang.
Funding acquisition: Dong-Hua Shao.
Investigation: Zheng Chen, Dong-Hua Shao, Zu-min Mao, Xiao-Dong Ma, Da-Peng Zhang.
Project administration: Lei-lei Shi.
Supervision: Dong-Hua Shao.
Writing – original draft: Zheng Chen.
Writing – review & editing: Zheng Chen.
Footnotes
Abbreviations: α angle = clot formation rate, APTT = activated partial thromboplastin time, BIS = bispectral index, FDP = fibrin (fibrinogen) degradation product, K time = clot formation time, MA = maximal amplitude of clot strength, PT = prothrombin time, R time = reaction time, TAT = thrombin-antithrombin complex, TEG = thrombelastograph.
There is no external funding and no competing interests declared.
The authors of this work have nothing to disclose.
References
- [1].Lison S, Weiss G, Spannagl M, et al. Postoperative changes in procoagulant factors after major surgery. Blood Coagul Fibrinolysis 2011;22:190–6. [DOI] [PubMed] [Google Scholar]
- [2].Bezeaud A, Denninger MH, Dondero F, et al. Hypercoagulability after partial liver resection. Thromb Haemost 2007;98:1252–6. [PubMed] [Google Scholar]
- [3].Caprini JA, Arcelus JI, Laubach M, et al. Postoperative hypercoagulability and deep-vein thrombosis after laparoscopic cholecystectomy. Surg Endosc 1995;9:304–9. [DOI] [PubMed] [Google Scholar]
- [4].Saleh J, El-Othmani MM, Saleh KJ. Deep vein thrombosis and pulmonary embolism considerations in orthopedic surgery. Orthop Clin North Am 2017;48:127–35. [DOI] [PubMed] [Google Scholar]
- [5].Sharma SK, Philip J. The effect of anesthetic techniques on blood coagulability in parturients as measured by thromboelastography. Anesth Analg 1997;85:82–6. [DOI] [PubMed] [Google Scholar]
- [6].Hollmann MW, Wieczorek KS, Smart M, et al. Epidural anesthesia prevents hypercoagulation in patients undergoing major orthopedic surgery. Reg Anesth Pain Med 2001;26:215–22. [DOI] [PubMed] [Google Scholar]
- [7].Mahla E, Lang T, Vicenzi MN, et al. Thromboelastography for monitoring prolonged hypercoagulability after major abdominal surgery. Anesth Analg 2001;92:572–7. [DOI] [PubMed] [Google Scholar]
- [8].Eichinger S. Cancer associated thrombosis: risk factors and outcomes. Thromb Res 2016;suppl 1:S12–7. [DOI] [PubMed] [Google Scholar]
- [9].Angchaisuksiri P. Cancer-associated thrombosis in Asia. Thromb J 2016;14(suppl 1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tsiminikakis N, Chouillard E, Tsigris C, et al. Fibrinolytic and coagulation pathways after laparoscopic and open surgery: a prospective randomized trial. Surg Endosc 2009;23:2762–9. [DOI] [PubMed] [Google Scholar]
- [11].Topçu I, Civi M, Oztürk T, et al. Evaluation of hemostatic changes using n thromboelastography after crystalloid or colloid fluid administration during major orthopedic surgery. Braz J Med Biol Res 2012;45:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gong P, Zhang MY, Zhao H, et al. Effect of mild hypothermia on the coagulation-fibrinolysis system and physiological anticoagulants after cardiopulmonary resuscitation in a porcine model. PLoS One 2013;8:e67476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Goobie SM, Soriano SG, Zurakowski D, et al. Hemostatic changes in pediatric neurosurgical patients as evaluated by thrombelastograph. Anesth Analg 2001;93:887–92. [DOI] [PubMed] [Google Scholar]
- [14].Arcelus JI, Traverso CI, Caprini JA. Thromboelastography for the assessment of hypercoagulability during general surgery. Semin Thromb Hemost 1995;21 suppl 4:21–6. [DOI] [PubMed] [Google Scholar]
- [15].Monaco M, Di Tommaso L, Stassano P, et al. Impact of blood coagulation and fibrinolytic system changes on early and mid term clinical outcome in patients undergoing stent endografting surgery. Interact Cardiovasc Thorac Surg 2006;5:724–8. [DOI] [PubMed] [Google Scholar]
- [16].Schietroma M, Carlei F, Mownah A, et al. Changes in the blood coagulation, fibrinolysis,;1; and cytokine profile during laparoscopic and open cholecystectomy. Surg Endosc 2004;18:1090–6. [DOI] [PubMed] [Google Scholar]
- [17].Liuboshevskiĭ PA, Artamonova NI, Ovechkin AM. Haemostasis disturbances as the component of the surgical stress-response and possibilities of their correction. Anesteziol Reanimatol 2012;44–8. [PubMed] [Google Scholar]
- [18].Rosenfeld BA, Faraday N, Campbell D, et al. Hemostatic effects of stress hormone infusion. Anesthesiology 1994;81:1116–26. [DOI] [PubMed] [Google Scholar]
- [19].Wang XW, Cao JB, Lv BS, et al. Effect of perioperative dexmedetomidine on the endocrine modulators of stress response: a meta-analysis. Clin Exp Pharmacol Physiol 2015;42:828–36. [DOI] [PubMed] [Google Scholar]
- [20].Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation 2004;109:2698–704. [DOI] [PubMed] [Google Scholar]
- [21].Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res 2016;118:1392–408. [DOI] [PubMed] [Google Scholar]
- [22].Dong W, Chen MH, Yang YH, et al. The effect of dexmedetomidine on expressions of inflammatory factors in patients with radical resection of gastric cancer. Eur Rev Med Pharmacol Sci 2017;21:3510–5. [PubMed] [Google Scholar]
- [23].Li Y, Wang B, Zhang LL, et al. Dexmedetomidine combined with general anesthesia provides similar intraoperative stress response reduction when compared with a combined general and epidural anesthetic technique. Anesth Analg 2016;122:1202–10. [DOI] [PubMed] [Google Scholar]
- [24].Kawamoto S, Hirakata H, Sugita N, et al. Bidirectional effects of dexmedetomidine on human platelet functions in vitro. Eur J Pharmacol 2015;766:122–8. [DOI] [PubMed] [Google Scholar]
- [25].Martins CR, Tardelli MA, Amaral JL. Effects of dexmedetomidine on blood coagulation evaluated by thromboelastography. Rev Bras Anestesiol 2003;53:705–19. [DOI] [PubMed] [Google Scholar]
- [26].Aoki H, Mizobe T, Nozuchi S, et al. In vivo and in vitro studies of the inhibitory effect of propofol on human platelet aggregation. Anesthesiology 1998;88:362–70. [DOI] [PubMed] [Google Scholar]
- [27].Fourcade O, Simon MF, Litt L, et al. Propofol inhibits human platelet aggregation induced by proinflammatory lipid mediators. Anesth Analg 2004;99:393–8. [DOI] [PubMed] [Google Scholar]
- [28].Davis JD. Prevention diagnosis and treatment of venous thromboembolic complications of gynecologic surgery. Am J Obstet Gynecol 2001;184:759–75. [DOI] [PubMed] [Google Scholar]
- [29].Ganter MT, Hofer CK, Spahn DR, et al. The effect of clonidine on perioperative blood coagulation. J Clin Anesth 2005;17:456–62. [DOI] [PubMed] [Google Scholar]
- [30].Brueckner S, Reinke U, Roth-Isigkeit A, et al. Comparsion of general and spinal anesthesia and their influence on hemostatic markers in patients undergoing total hip arthroplasty. J Clin Anesth 2003;15:433–40. [DOI] [PubMed] [Google Scholar]


