Abstract
Introduction:
Tumor-infiltrating lymphocytes (TILs) have been shown to be of prognostic significance in patients with gastric cancer. This study aims to investigate the association between density of TILs and prognoses of patients with gastric cancer.
Methods:
The relative studies of tumor-infiltrating lymphocytes in tumor tissue from patients with gastric cancer were systematically searched from PubMed and Embase until October 31, 2017. The pooled hazard ratios (HRs) and their 95% confidence intervals (95%CI) for overall survival (OS) were estimated.
Results:
Twenty-nine studies involving 4,942 patients were included into analyses. Subset of TILs included CD8+, CD3+, CD4+, and FOXP3+ T cell density. Results from meta-analyses revealed that high density of intratumoral CD8+ T cells (HR = 0.77, 95% CI 0.63–0.95) and CD3+ (HR = 0.62, 95% CI 0.49–0.77) were associated with significantly higher OS than those with low density in patients with gastric cancer. Moreover, a larger number of general TILs density also suggested a favorable prognosis (HR 0.75, 95% CI 0.67–0.84). However, patients with high density of intratumoral FOXP3+ T or CD4+ T cells were not statistically associated with higher or lower OS than those with low density (HR 1.41, 95% CI 0.97–2.05; HR = 0.86, 95% CI 0.47–1.57). Sample size and follow-up period seemed to influence study outcomes.
Conclusion:
The present study revealed that high density of intratumoral CD8+ and CD3+ T cells were associated with better OS in patients with gastric cancer.
Keywords: gastric cancer, meta-analysis, overall survival, tumor infiltrating lymphocytes
1. Introduction
Gastric cancer is the most common type of gastrointestinal malignancies in the world.[1] Surgical resection remains the primary curative treatment for gastric cancer. However, less than 30% of patients eligible for curative resection because the majority of gastric cancer cases present in advanced stage due to late onset and nonspecific symptoms.[2] In recent decade, although treatment of gastric cancer has been significantly improved, the prognoses of patients represented by liver metastases is extremely poor, with a 3-year overall survival (OS) lower than 40% even after hepatectomy,[2] or less than 10% after chemotherapy.[3] Therefore, exploring tumor markers to predict patients’ prognoses has important clinical value to prolong their survival time.
Previous studies revealed that immune markers are associated with OS for patients with gastric cancer.[4,5] Tumor-infiltrating lymphocytes (TILs) are the major type of infiltrating immune cells.[4,5] The density of TILs is considered a manifestation of the host immune response against tumor cells. Nowadays, the association between TILs and patients’ clinical outcomes have been investigated in non-small cell lung cancer,[6] esophageal squamous cell carcinoma,[7] hepatocellular carcinoma,[8] and breast cancers,[9] and so on. Moreover, lots of studies have investigated the prognostic impact of TILs on patients with gastric cancer, but their results were inconsistent. Therefore, this systematic review comprehensively investigated the prognostic effect of TILs for patients with gastric cancer.
2. Materials and methods
Two independent authors performed a systematic review (PCY and DL) according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[10] The included studies were evaluated as a cohort study performed in accordance with the Newcastle-Ottawa Quality Assessment Scale (NOS) for quality assessment.[11] This tool was chosen because of the unavailability of randomized controlled trials and large heterogeneity between studies. The NOS scale includes the following: selection; comparability; and outcome. Full marks according to NOS are represented by 9 points; scores of 0 to 4 indicate low-quality research, and scores of 5 to 9 indicate high-quality research.[11]
3. Literature search
We searched the literature for studies published in Embase, PubMed, Web of Science, and the Cochrane Library through 31 December 2017 reporting the prognostic role of TILs or its subsets among patients with gastric cancer.
Search keywords included “immune cells” OR “tumor infiltrating lymphocytes” AND “gastric cancer” OR “stomach neoplasm” AND “overall survival” OR “prognostic” OR “prognosis.” The literature search was restricted to English-language publications. Besides, all the reference lists of identified articles were also reviewed in order to find out potential studies. When the effective data included in the literature were not reported or when data published in different studies overlapped, we contacted the author to confirm the appropriate data. Two authors were responsible for searching the comprehensive database and evaluating availability independently (PCY and DL).
4. Eligibility criteria
Literatures that were eligible for inclusion in this meta-analysis should meet the following criteria: patients were diagnosed with gastric cancer; studies investigated the role of general TILs or T lymphocyte subsets (including CD3+, CD8+, CD4+, and FoxP3+ lymphocytes) on patients with gastric cancer; the TILs measurement must be detected in situ of tumor tissue by application of HE or immunohistochemistry; the lymphocyte infiltration site should be within the tumor tissue, such as tumor parenchyma, tumor stroma; adequate data (hazard ratio [HR] and its associated 95% confidence interval [95% CI]) were provided for further analysis. When more than one studies were published based on the same population, only the latest information and the most complete study were used.
5. Data extraction and quality assessment
Two authors (PCY and DL) independently extracted data from all eligible studies. Uncertainties were resolved through discussion or the third author (SZ). The following information was extracted from all included studies: name of first author, country, recruitment period, sample size, lymph node metastasis, tumor stage, TIL detection method, cut-off for overexpression, TILs subsets and distribution site, follow-up period, and outcome measures. HR and 95% CI for OS were extracted directly from the enrolled studies.
6. Statistical analysis
HR combination analysis was performed under the assumption of clinical homogeneity. The 95% CI represents the statistical effect. The heterogeneity of each study was analyzed by the χ2 test. With P < .1 as the significance level, heterogeneity was expressed as an I2 value. When heterogeneity was present, a random effects model (DerSimonian–Laird method) was used; otherwise, a fixed effects model (Mantel–Haenszel test) was employed.
An HR >1.0 was considered to indicate a poor OS for patients with low TILs or its subsets infiltration group; an HR < 1.0 in the high TILs or its subsets infiltration group was associated with good OS. A lack of overlap of the HR (CI) with 1 suggested that the results of visual interpretation had statistical value for OS prediction. All P-values were calculated with 2-sided tests.
Effect-quantity pooling, heterogeneity testing, sensitivity analysis, and bias testing were analyzed using the meta-package in R (ver.3.2.3; a language and environment for statistical computing; https://www.R-project.org/).
7. Results
7.1. Results of literature search and study characteristics
A total of 656 studies were identified using our search criteria (Fig. 1), of which 445 were rejected and 211 were retained for abstract review. On the basis of the abstract, 145 studies were excluded and 66 retained and read in full. The main reason of exclusion was because these studies were not investigated the role of general TILs or T lymphocyte subsets (including CD3+, CD8+, CD4+, and FoxP3+ lymphocytes) on patients with gastric cancer. In the end, 29 studies involving 3020 patients were included in the systematic review (Table 1).[12–40] The flow diagram for study selection was shown in Fig. 1. Though 2 studies were based on the same population, different variables were investigated. Therefore, all these 2 studies were included into this meta-analysis.[22,33]
Figure 1.
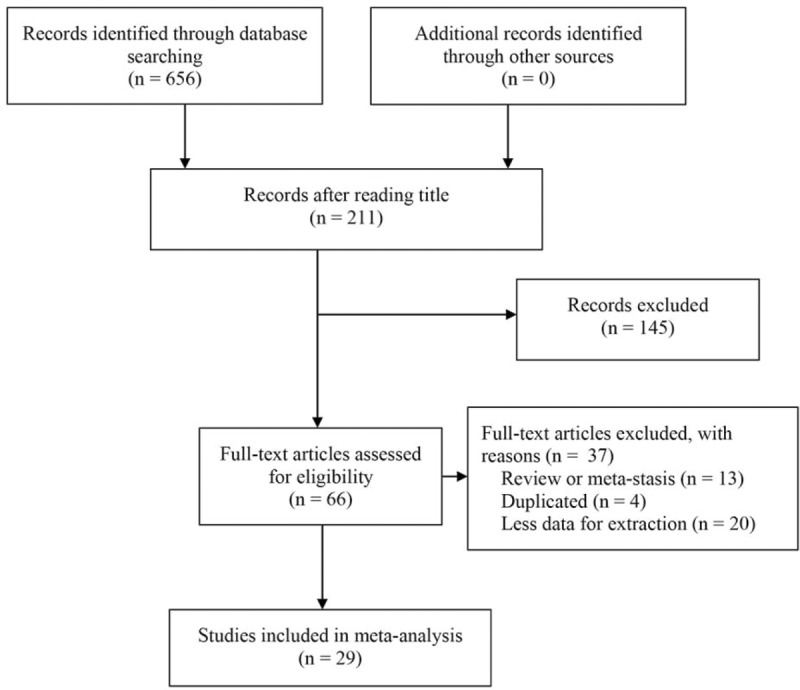
PRISMA flow diagram.
Table 1.
Clinicpathological characteristics of the included studies.
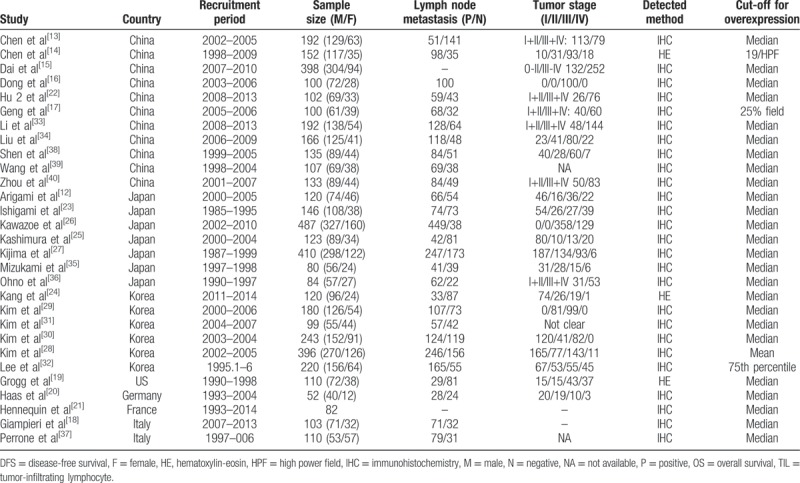
Of the 29 studies, 11 came from China,[13–17,22,33–34,38–40] 7 from Japan,[12,23,25–27,35,36] 6 from Korea,[24,28–32] and the other 5 from the United States,[19] Germany,[20] and Italy,[18,37] respectively. Three studies used hematoxylin-eosin staining[14,19,24] while the other 26 studies used immunohistochemistry staining for detection of general or specific TILs subsets of the tumor tissue.[12,13,15–18,20–23,25–40]
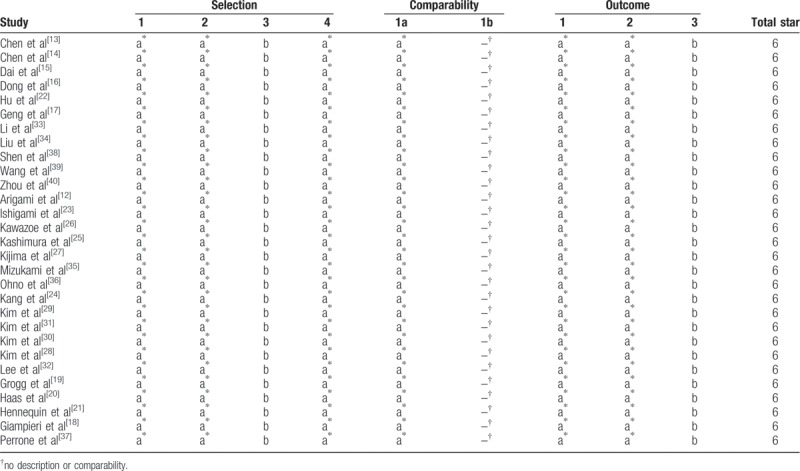
The quality of the 29 eligible studies was assessed in strict accordance with the NOS. The scores were 6 points, suggesting that the methodological quality level of each eligible study was sufficiently high (Table 2).
Table 2.
Newcastle–Ottawa score quality assessment scale for cohort studies.
7.2. Pooled analysis
Six studies reported the prognostic value of general TILs,[14,15,18,19,23,24] 9 studies about CD8+ T lymphocytes,[13,16,20,25,27,31–33,36] 14 studies about FOXP3+ T lymphocytes,[17,20–22,26,28,29,31,34–35,37–40] 2 about CD4+ T lymphocytes,[29,33] and 5 about CD3+ T lymphocytes.[12,20,28,30,32] Association between general TILs or its subsets and patients’ prognoses in each study was described in Table 3.
Table 3.
Associated between tumor-infiltrating lymphocytes and patients’ prognoses in each study.
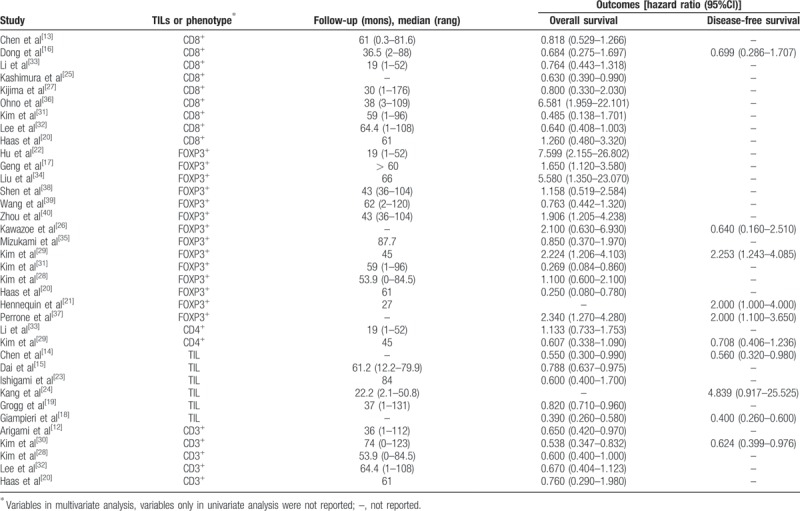
Pooled analysis found that high infiltration of general TILs was associated with statistically higher OS than those with low infiltration (HR 0.75, 95% CI 0.67–0.84; Fig. 2). Moreover, high infiltration of CD8+ T lymphocytes (HR = 0.77, 95% CI 0.63–0.95; Fig. 3) and CD3+ T lymphocytes (HR 0.62, 95% CI 0.49–0.77; Fig. 4) were also associated with statistically higher OS than those with low infiltration. However, gastric cancer patients with high infiltration of FOXP3+ T lymphocytes (HR 1.41, 95% CI 0.97–2.05; Fig. 5) or CD4+ T lymphocytes (HR = 0.86, 95% CI 0.47–1.57; Fig. 6) were only associated with slightly higher OS than those with low infiltration.
Figure 2.
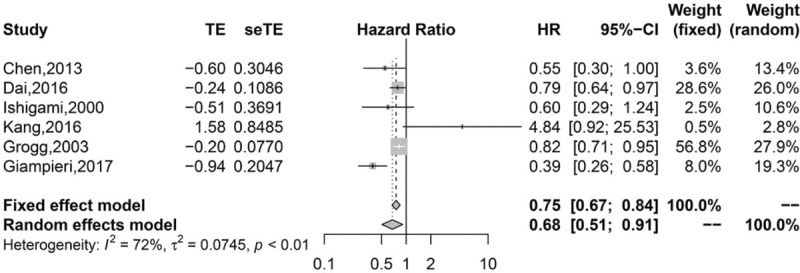
Pooled results for general TILs in patients with gastric cancer.
Figure 3.
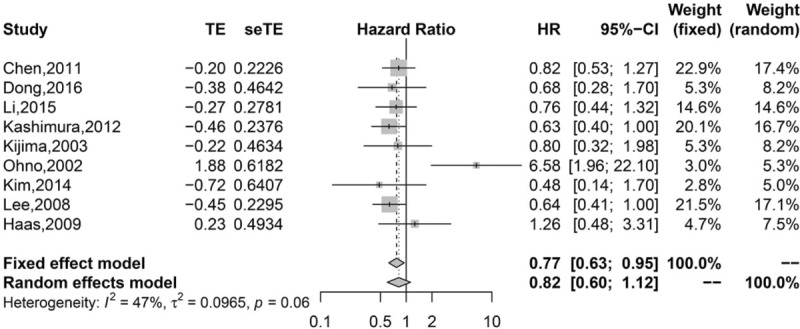
Pooled results for CD8+ TILs in patients with gastric cancer.
Figure 4.
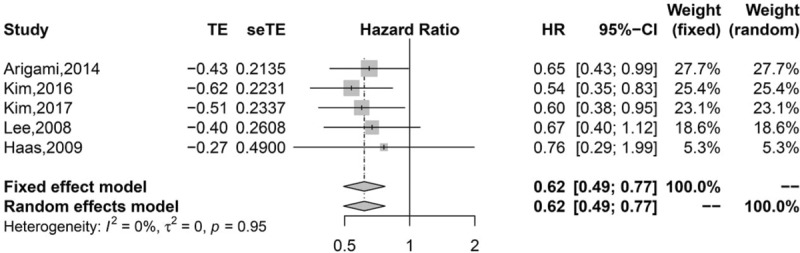
Pooled results for CD3+ TILs in patients with gastric cancer.
Figure 5.
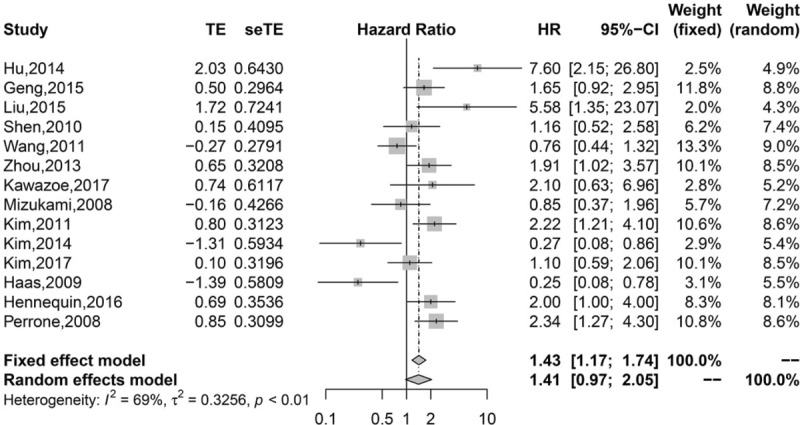
Pooled results for FOXP3+ TILs in patients with gastric cancer.
Figure 6.
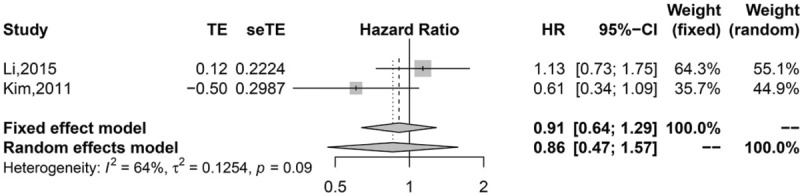
Pooled results for CD4+ TILs in patients with gastric cancer.
8. Discussion
The microenvironment contributes to patients’ survival and growth of cancer cells. The gastric tumor microenvironment is frequently filled with a wide range of immune cells, which have been reported to impact on cancer development, progression, and cancer-related immune reactions, emerged as the hotspot of cancer research.
Subgroup analyses based on subset of TILs was performed because of the bidirectional role of TILs in tumor-associated immune responses. Our study confirmed the importance of intratumoral TILs and its subsets as a prognostic factor, which is in agreement with previous studies.[41–44] Our meta-analysis identified 29 studies that evaluated the prognostic significance of different TIL subsets. It provides evidence that high densities of intratumor general TILs, CD8+, or CD3+ TILs alone are indicative of improved survival, but the presence of FOXP3+ and CD4+ TILs alone are not significantly associated with the prognosis.
Each subset of TILs plays its own roles in the development and progression of gastric cancer. CD8+ TILs is the surface antigens of cytotoxic T lymphocytes. And it is the main effective cells in the anti-tumor immune response. In our study, we found CD8+ TILs were associated with better OS. However, 2 included studies were not in accordance with our drawn conclusions,[20,36] which may be due to sample size bias. However, CD4+ lymphocytes are composed of T helper and regulatory cells. These cells can secret diverse cytokines. Therefore, the roles of CD4+ T cells are complicated by their heterogeneity trait. In the present study, we did not found the significant prognostic value of CD4+ TILs.
CD3 is a common surface antigen of T cells. We found gastric cancer patients with high infiltration of CD3+ TILs had significantly higher OS than those with low infiltration. This finding is consistent with that the infiltration of CD3+ TILs significantly correlated with tumor stage, lymph node metastasis, and depth of tumor invasion.[12,32] Higher infiltration of CD3+ TILs is also significantly correlates with higher OS in other cancers.[45,46] Therefore, as an immunological predictor of tumor stage and disease outcome in cancer patients, the infiltration of CD3+ TILs may decreases during tumor progression.[12] Considering the close relationship between the infiltration of CD3+ TILs and patients’ prognoses, immunohistochemical analysis of CD3+ TIL infiltration in endoscopic resected specimens might help to identify tumor stage after endoscopic resection. In addition, measuring CD3+ TIL infiltration in resected specimens may be helpful to identify the induction of adjuvant chemotherapy in patients with gastric cancer.
The prognostic value of intratumoral FOXP3+ TILs infiltration in gastric cancer is still in debate. Some studies showed that FOXP3+ TILs infiltration was associated with decreased overall survival in gastric cancer[17,22,34,40] while other studies failed to uncover such an association.[26,28,35,38,39] A previous meta-analysis found tumor-infiltrating FOXP3+ TILs were a factor for a poor prognosis for hepatocellular carcinoma and gastric cancer, but a good prognosis for colorector cancer.[47] Our study included 14 studies investigating the relationship between FOXP3+ T lymphocytes and the prognoses of patients with gastric cancer. We did not found a positive or negative association. These discrepancies may be partly attributed to differences on the method for specimen processing, the difference on the selection of FOXP3+ TILs markers in each study, the ethnics of population, and the histology of gastric cancer patients.
This study has some limitations. First, cut-off for overexpression of TILs is different in some studies. Second, median follow-up in some included studies may be too short to observe long term prognoses. Third, the method of immunohistochemical technique may be discrepency in tissue fixation, antibodies used for T cell detection. For example, 3 studies[14,19,24] with hematoxylin-eosin staining. It may be difficult to distinguish the biomarkers of TIL only via hematoxylin-eosin staining. And fourth, the heterogeneity of some meta-analysis is still remarkable.
9. Conclusion
This meta-analysis suggests that TILs were prognostic markers for OS in gastric cancer patients. In addition, a high density of introtumoral CD8+ and CD3+ lymphocyte indicated good prognosis in gastric cancer, while CD4+ and FOXP3+ lymphocytes demonstrated no obvious effect on survival outcomes. However, due to the limitations of this study, TILs can’t fully explain its impact on prognosis. Therefore, further studies should focus on high-quality prospective studies, including a comprehensive clinical pathology, evaluation of information, follow-up strategy, and standardized cut-off values, similar treatment strategies, multivariate analyses of clinicopathological variables of the patients, which will make the study more standardized. Besides, quantitative studies of TILs alone are far from explaining the complex effects of tumor microenvironment, which requiring more rigorous design, greater sample size, more standardized survival analyzes, and longer follow-up studies to produce more credible statistics.
Author contributions
Data curation: Peng-Cheng Yu.
Data acquisition: Peng-Cheng Yu, Di Long, Cheng-Cheng Liao.
Data analysis: Peng-Cheng Yu, Di Long, Cheng-Cheng Liao.
Data interpretation: Peng-Cheng Yu, Di Long, Cheng-Cheng Liao.
Study Design: Sen Zhang.
Supervision: Sen Zhang.
Writing – original draft: Sen Zhang, Peng-Cheng Yu, Cheng-Cheng Liao, Di Long.
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, NOS = Newcastle–Ottawa Quality Assessment Scale, OS = overall survival, TILs = tumor-infiltrating lymphocytes.
This research was funded by the National Natural Science Foundation of China (81160235), the Graduate Course Construction Project of Guangxi Medical University (YJSA2017014), and the Self-Raised Scientific Research Fund of the Ministry of Health of Guangxi Province (Z2016176).
The authors declare no conflicts of interest.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Liao YY, Peng NF, Long D, et al. Hepatectomy for liver metastases from gastric cancer: a systematic review. BMC Surg 2017;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Song HJ, Srivastava A, Lee J, et al. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology 2010;139:84–92. e82. [DOI] [PubMed] [Google Scholar]
- [6].Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 2008;14:5220–7. [DOI] [PubMed] [Google Scholar]
- [7].Cho Y, Miyamoto M, Kato K, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res 2003;63:1555–9. [PubMed] [Google Scholar]
- [8].Zhong JH, Xiang X, Qin HG, et al. Expression and prognostic significance of CD4+/CD8+ T lymphocytes in patients with hepatocellular carcinoma. Chin J Oncol Prev Treat 2017;9:311–6. [Google Scholar]
- [9].Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32:2959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [11].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [12].Arigami T, Uenosono Y, Ishigami S, et al. Decreased density of CD3+ tumor-infiltrating lymphocytes during gastric cancer progression. J Gastroenterol Hepatol 2014;29:1435–41. [DOI] [PubMed] [Google Scholar]
- [13].Chen JG, Xia JC, Liang XT, et al. Intratumoral expression of IL-17 and its prognostic role in gastric adenocarcinoma patients. Int J Biol Sci 2011;7:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen LJ, Zheng X, Shen YP, et al. Higher numbers of T-bet(+) intratumoral lymphoid cells correlate with better survival in gastric cancer. Cancer Immunol Immunother 2013;62:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dai C, Geng R, Wang C, et al. Concordance of immune checkpoints within tumor immune contexture and their prognostic significance in gastric cancer. Mol Oncol 2016;10:1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dong J, Li J, Liu S, et al. Prognostic potential of an immune score based on the density of CD8+ T cells, CD20+ B cells, and CD33+/p-STAT1+ double-positive cells and HMGB1 expression within cancer nests in stage IIIA gastric cancer patients. Chin J Cancer Res 2016;28:543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Geng Y, Wang H, Lu C, et al. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance. Int J Clin Oncol 2015;20:273–81. [DOI] [PubMed] [Google Scholar]
- [18].Giampieri R, Maccaroni E, Mandolesi A, et al. Mismatch repair deficiency may affect clinical outcome through immune response activation in metastatic gastric cancer patients receiving first-line chemotherapy. Gastric Cancer 2017;20:156–63. [DOI] [PubMed] [Google Scholar]
- [19].Grogg KL, Lohse CM, Pankratz VS, et al. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol 2003;16:641–51. [DOI] [PubMed] [Google Scholar]
- [20].Haas M, Dimmler A, Hohenberger W, et al. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol 2009;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hennequin A, Derangere V, Boidot R, et al. Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients. Oncoimmunology 2016;5:e1054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hu M, Li K, Maskey N, et al. Decreased intratumoral Foxp3 Tregs and increased dendritic cell density by neoadjuvant chemotherapy associated with favorable prognosis in advanced gastric cancer. Int J Clin Exp Pathol 2014;7:4685–94. [PMC free article] [PubMed] [Google Scholar]
- [23].Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 2000;88:577–83. [PubMed] [Google Scholar]
- [24].Kang BW, Seo AN, Yoon S, et al. Prognostic value of tumor-infiltrating lymphocytes in Epstein-Barr virus-associated gastric cancer. Ann Oncol 2016;27:494–501. [DOI] [PubMed] [Google Scholar]
- [25].Kashimura S, Saze Z, Terashima M, et al. CD83(+) dendritic cells and Foxp3(+) regulatory T cells in primary lesions and regional lymph nodes are inversely correlated with prognosis of gastric cancer. Gastric Cancer 2012;15:144–53. [DOI] [PubMed] [Google Scholar]
- [26].Kawazoe A, Kuwata T, Kuboki Y, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer 2017;20:407–15. [DOI] [PubMed] [Google Scholar]
- [27].Kijima Y, Ishigami S, Hokita S, et al. The comparison of the prognosis between Epstein-Barr virus (EBV)-positive gastric carcinomas and EBV-negative ones. Cancer Lett 2003;200:33–40. [DOI] [PubMed] [Google Scholar]
- [28].Kim EK, Yoon SO, Jung WY, et al. Implications of NOVA1 suppression within the microenvironment of gastric cancer: association with immune cell dysregulation. Gastric Cancer 2017;20:438–47. [DOI] [PubMed] [Google Scholar]
- [29].Kim HI, Kim H, Cho HW, et al. The ratio of intra-tumoral regulatory T cells (Foxp3+)/helper T cells (CD4+) is a prognostic factor and associated with recurrence pattern in gastric cardia cancer. J Surg Oncol 2011;104:728–33. [DOI] [PubMed] [Google Scholar]
- [30].Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 2016;19:42–52. [DOI] [PubMed] [Google Scholar]
- [31].Kim KJ, Lee KS, Cho HJ, et al. Prognostic implications of tumor-infiltrating FoxP3+ regulatory T cells and CD8+ cytotoxic T cells in microsatellite-unstable gastric cancers. Hum Pathol 2014;45:285–93. [DOI] [PubMed] [Google Scholar]
- [32].Lee HE, Chae SW, Lee YJ, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer 2008;99:1704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li K, Zhu ZP, Luo J, et al. Impact of chemokine receptor CXCR3 on tumor-infiltrating lymphocyte recruitment associated with favorable prognosis in advanced gastric cancer. Int J Clin Exp Pathol 2015;8:14725–32. [PMC free article] [PubMed] [Google Scholar]
- [34].Liu K, Yang K, Wu B, et al. Tumor-infiltrating immune cells are associated with prognosis of gastric cancer. Medicine (Baltimore) 2015;94:e1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mizukami Y, Kono K, Kawaguchi Y, et al. Localisation pattern of Foxp3+ regulatory T cells is associated with clinical behaviour in gastric cancer. Br J Cancer 2008;98:148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ohno S, Tachibana M, Fujii T, et al. Role of stromal collagen in immunomodulation and prognosis of advanced gastric carcinoma. Int J Cancer 2002;97:770–4. [DOI] [PubMed] [Google Scholar]
- [37].Perrone G, Ruffini PA, Catalano V, et al. Intratumoural FOXP3-positive regulatory T cells are associated with adverse prognosis in radically resected gastric cancer. Eur J Cancer 2008;44:1875–82. [DOI] [PubMed] [Google Scholar]
- [38].Shen Z, Zhou S, Wang Y, et al. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol 2010;136:1585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang B, Xu D, Yu X, et al. Association of intra-tumoral infiltrating macrophages and regulatory T cells is an independent prognostic factor in gastric cancer after radical resection. Ann Surg Oncol 2011;18:2585–93. [DOI] [PubMed] [Google Scholar]
- [40].Zhou S, Shen Z, Wang Y, et al. CCR7 expression and intratumoral FOXP3+ regulatory T cells are correlated with overall survival and lymph node metastasis in gastric cancer. PLoS One 2013;8:e74430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mao Y, Qu Q, Chen X, et al. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. PLoS One 2016;11:e0152500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yu X, Zhang Z, Wang Z, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol 2016;18:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zheng X, Song X, Shao Y, et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis. Oncotarget 2017;8:57386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Laghi L, Bianchi P, Miranda E, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol 2009;10:877–84. [DOI] [PubMed] [Google Scholar]
- [46].Gabrielson A, Wu Y, Wang H, et al. Intratumoral CD3 and CD8 T-cell densities associated with relapse-free survival in HCC. Cancer Immunol Res 2016;4:419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Huang Y, Liao H, Zhang Y, et al. Prognostic value of tumor-infiltrating FoxP3+ T cells in gastrointestinal cancers: a meta analysis. PLoS One 2014;9:e94376. [DOI] [PMC free article] [PubMed] [Google Scholar]


