Abstract
Thalamic pain is a severe pain that is often unresponsive to medical therapy. Repetitive transcranial magnetic stimulation (rTMS) entirely non-invasively modulates neuronal plasticity to produce therapeutic benefit. Since the rTMS stimulation parameters varied, it is difficult to determine which specific parameters are best for clinical use. The aim of this study was to evaluate the analgesic lasting effect of 10-Hz rTMS over the motor cortex (M1) for 10 consecutive days to treat thalamic pain.
Patients were treated with daily 10-Hz rTMS sessions for 1000 pulses applied over the M1 for 10 consecutive days. Pain severity and mood were assessed at baseline, immediately after, 2 weeks, 4 weeks, 6 weeks, 8 weeks after rTMS. Pain severity was measured by the visual analogue scale (VAS) and the percentage of pain relief on VAS score was calculated between baseline and final examination. Mood was monitored using the Hamilton Anxiety Scale (HAMA) and Hamilton Depression Scale (HAMD).
Seven patients with thalamic pain were enrolled. VAS score was significantly decreased after rTMS. Mean VAS scores were 7 at baseline and decreased to 5.6 at 2 weeks after rTMS and then decreased to 3.9 at 8 weeks after rTMS. The analgesic effect of rTMS can last up to 8 weeks. The percentage of pain relief ranges from 25.0% to 66.7% at the 8th week. Four patients (3 moderate pain and 1 severe pain) achieved satisfactory relief (pain relief ≥40–69%).
Although this was an open-label study without a control group, our findings show that 10 Hz rTMS over the M1 for 10 consecutive days can produce satisfactory or partial antalgic effect on patients with thalamic pain.
Keywords: motor cortex, thalamic pain, transcranial magnetic stimulation
1. Introduction
Thalamic pain, one of the central pain, is a severe pain producing an intolerable sensation described as burning. It impairs not only the daily activities of patients but also the quality of life. Alleviation of the pain is highly desirable for patients. But the severe pain is often unresponsive to medical therapy. Medications are often ineffective or cause various adverse effects, especially in elderly patients. Surgical intervention such as cortex electrical stimulation and deep brain stimulation (DBS) are also used for patients who cannot be treated with these drugs, although some patients cannot undergo such procedures. High costs and rates of complication have limited invasive neurostimulation use. So, better approaches are needed.
The success of epidural cortex electrical stimulation inspired consideration of even less invasive stimulation methods. Repetitive transcranial magnetic stimulation (rTMS) is entirely non-invasive and triggers neuronal plasticity to produce long-lasting therapeutic benefit. rTMS already has US and European approval for treating refractory depression. But there is lesser evidence of efficacy of rTMS in treatment of chronic central pain and the results vary.[1–4] So many questions remain, concerning indications, target cortex, stimulation frequency, predictive factors, and further technical matters in rTMS therapy.
Motor cortex stimulation (MCS) was proposed by Tsubokawa in 1991 for the treatment of post-stroke thalamic pain.[5] The results reported in the literature are quite good. Several clinical trials and a recent meta-analysis have reported 64% to 70% of neuropathic pain or thalamic pain patients achieved excellent pain relief by epidural MCS.[6–9] So primary motor cortex (M1) has been proven to be a potential targets for neuropathic pain therapy.
We hypothesized that high-frequency rTMS over M1 is effective for pain relief in patients with thalamic pain. In this study, we assessed the antalgic effect and the lasting duration of 10 Hz rTMS over M1.
2. Methods
2.1. Patient selection
From 2011 to 2013, 7 patients with thalamic pain were enrolled at the Capital Medical University Xuanwu Hospital. Clinical data of each patient are summarized in Table 1. Of the patients, 5 were men and 2 were women. The mean patient age was 53.57 ± 7.16 years and the average duration of pain was 3.7 ± 3.69 years.
Table 1.
Clinical characteristics of patients.
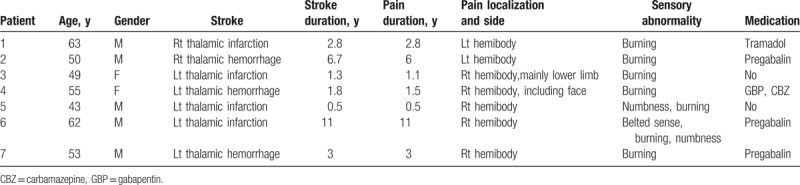
Inclusion criteria were according to the following: age between 18 and 80; presence of unilateral or lateralized neuropathic pain due to thalamic lesion or disease; magnetic resonance imaging done within 1 year of the first visit showing a lesion that involves the posterior thalamic region or a lesion in the dorsal or ventral vicinity of the thalamus; chronic pain for >6 months; failure to respond adequately to medication or physical therapy; pain level >5° by the visual analogue scale (VAS); participants maintain their current medications during the study period, and written informed consent.
Patients presenting any of the following conditions were excluded: pregnancy; malignant disease; history of epileptic seizures; unstable heart disease or presence of cardiac pacemaker; heart, renal, or hepatic failure; psychotic disorder; contraindication for TMS, or patient unable and/or unwilling to cooperate with study procedures or to comply with the required follow-up visits.
2.2. Defining the motor cortex and resting motor threshold (RMT)
The optimal scalp location of motor cortex was determined using a TMS system (Magstim Super Rapid stimulator, Magstim Ltd, UK) and a 70-mm figure-of-eight coil. Motor responses were recorded using a standard EMG machine (Viking, Nicolet, Madison, WI) and surface electrodes were placed over the contralateral first dorsal interosseus muscle with the belly-tendon method. The handle of the coil was oriented at 45° posterior to the midline and the stimulator was moved over the scalp in 1-cm increments. The motor cortex site was at which single pulse TMS evoked a contralateral motor evoked potential of maximal amplitude in a hand muscle. Once the cortical site was identified, single-pulse TMS was delivered to the location and the RMT was defined as the lowest stimulus intensity that produced 5 responses with peak-to-peak amplitude of at least 50 mV in 10 consecutive trials.[10]
2.3. rTMS intervention
Patients received 10 daily sessions of rTMS over the motor cortex of the affected hemisphere corresponding to the painful hand. rTMS included 10 consecutive trains of 10 seconds duration and inter-trains interval of 60 seconds. Stimulations were applied at 10 Hz and 90% RMT. A total of 1000 pulses were delivered (Fig. 1). The rTMS protocol used in the present study is in accordance with safety guidelines for rTMS application.[11]
Figure 1.
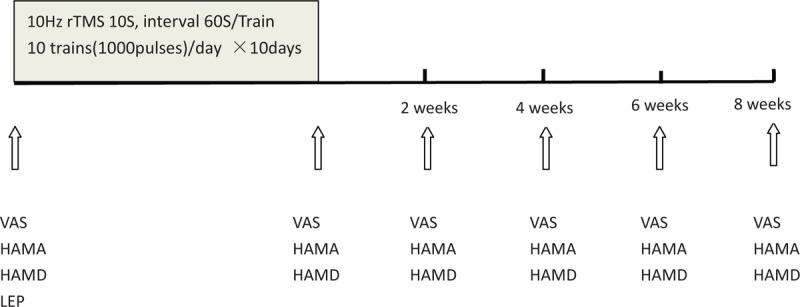
Experimental design: all patients received 10 daily sessions of 10 Hz rTMS (1000 pulses) over the motor cortex of the affected hemisphere. Pain intensity was measured by the VAS before, immediately after, and 2 weeks, 4 weeks, 6 weeks, 8 weeks after rTMS. Mood was assessed before, immediately after, and 2 weeks, 4 weeks, 6 weeks, 8 weeks after rTMS using HAMA and HAMD. HAMA = the Hamilton Anxiety Scale, HAMD = Hamilton Depression Scale, LEP = laser evoked potentials, rTMS = repetitive transcranial magnetic stimulation, VAS = visual analogue scale.
2.4. Laser evoked potentials recording
Laser stimulation was delivered by a CO2 laser stimulator (Beijing Electronic and Technology Company, China). Spot diameter was 0.1 mm, pulse duration 10 ms, and interstimulus intervals 6 seconds. The stimulus intensity was set at 15 ± 20% above the pain threshold obtained in the healthy side. Laser stimuli were applied to the dorsum of both hands (radial nerve territory). The output energy was kept below 700 mJ to avoid skin damage.
Electroencephalographic (EEG) registrations were made from 5 electrodes (Fz, Cz, Pz, T3, and T4) according to the international 10–20 system, using ISA1008 EP recording device (Micromed, Italian). The recordings of Fz, Cz, and Pz were referenced to linked bilateral earlobes (A1 + A2) and T3/T4 referenced to Fz .The impedance was maintained below 5 Ω. Brain signals were sampled at 1024 Hz, and bandpass filtered at 0.1 to 30 Hz. Evoked potentials were averaged over blocks of 30 ± 10 stimulus repetitions.
Room temperature was 22 to 23 °C and skin temperature was always above 30 °C. The subjects were instructed to keep their eyes open, to focus on a fixed point on the wall, and to avoid blinking.
2.5. Clinical evaluation
The pain level and characteristics of each patient were assessed by a multidisciplinary group. Sensory function was checked with standard clinical methods. A blunted safety pin may be used to test pain (which is often referred to as pinprick sensation). Light touch may be tested with a wisp of cotton. A tube of warm or cool water may be used to test temperature sensation. Vibration sensation is assessed by apply a vibrating 128 Hztuning fork to a bony structure such as the ankle or knuckle. Pain intensity was measured by the VAS before and after rTMS, and for 8 weeks following the rTMS course (Fig. 1). Mood was measured before and after rTMS using the Hamilton Anxiety Scale (HAMA) and Hamilton Depression Scale (HAMD) (Fig. 1).
2.6. Analysis of data
The scores of VAS, HAMA, and HAMD were assessed before, immediately after, 2 weeks, 4 weeks, 6 weeks, 8 weeks after rTMS. The percentage of pain relief on VAS score was calculated between preoperative baseline and final examination by the following equation:
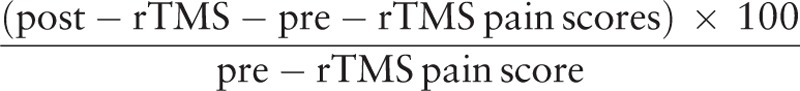 |
Individual analgesic effects of rTMS were also classified into 3 categories[12]:
good—reduction of pain score by ≥70%
satisfactory—reduction of pain score by ≥40% to 69%
poor—reduction of pain score by <40%
3. Results
3.1. Pain relief
Table 2 summarized outcomes after rTMS treatment and 8 weeks later. In all patients, the VAS score was significantly decreased after rTMS. Their mean VAS scores were 7 at initial assessment and decreased to 5.6 at 2 weeks after rTMS and then decreased to 3.9 at 8 weeks after rTMS. The VAS scores of the patients decreased gradually during 2 to 8 weeks’ following-up after rTMS compared with baseline. So the analgesic effect of rTMS can last up to 8 weeks. Among the 7 patients analyzed, the percentage of pain relief range from 25.0% to 66.7%. Four patients (3 moderate pain and 1 severe pain) achieved satisfactory relief (pain relief ≥40–69%).
Table 2.
Response to rTMS among patients with thalamic pain.
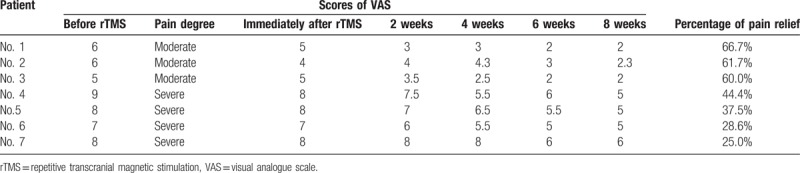
Figure 2 shows the distribution of pain relief in the patients with moderate pain and severe pain after rTMS. It is clear that the effect is greater in the patients with moderate pain than others with severe pain.
Figure 2.
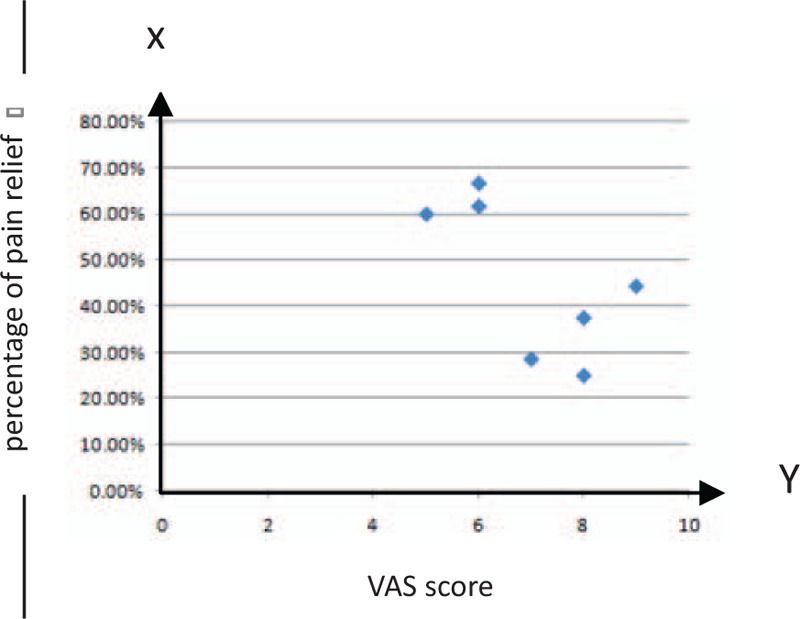
Patient distribution according to percentage of pain relief at the end of the follow-up period, corresponding to the patients with moderate pain and severe pain.
Pain relief at final examination did not correlate with age, sex, etiology, pain duration, but with the severity of anxiety or depression before rTMS treatment. Figure 3 showed that pain relief after rTMS was significantly low in the patients with severe anxiety or depression.
Figure 3.
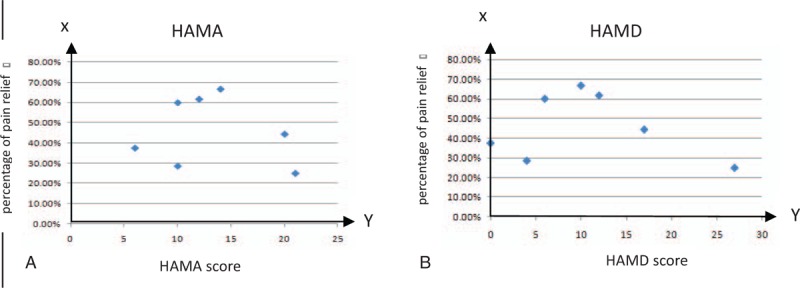
Patient distribution according to percentage of pain relief at the end of the follow-up period, corresponding to the score of HAMA (A) or HAMD (B) before rTMS. HAMA = the Hamilton Anxiety Scale, HAMD = Hamilton Depression Scale, rTMS = repetitive transcranial magnetic stimulation.
3.2. Laser evoked potentials amplitudes
In clinical studies, only the late Laser evoked potentials (LEP) components are routinely evaluated (Fig. 4). These components are maximal at the vertex Cz lead. The LEP N2 occurs at about 240 ms and the P2 at about 380 ms.[13] It is a negative–positive complex comprising the so-called N2 and P2, measured individually from baseline (or both together peak-to-peak). Among 7 patients, LEP recording were not performed in 2 patients. The number 2 patient felt too painful to cooperate the examination. The number 5 patient was afraid of skin burns and rejected the examination. The results of LEP amplitudes in 5 patients are given in Table 3. LEP were found to be abnormal in 5 patients with thalamic pain that led to altered pain sensitivity. The larger LEP amplitudes were, the higher the percentage of pain relief were. LEP examination may thus also be useful in patients with thalamic lesions.
Figure 4.
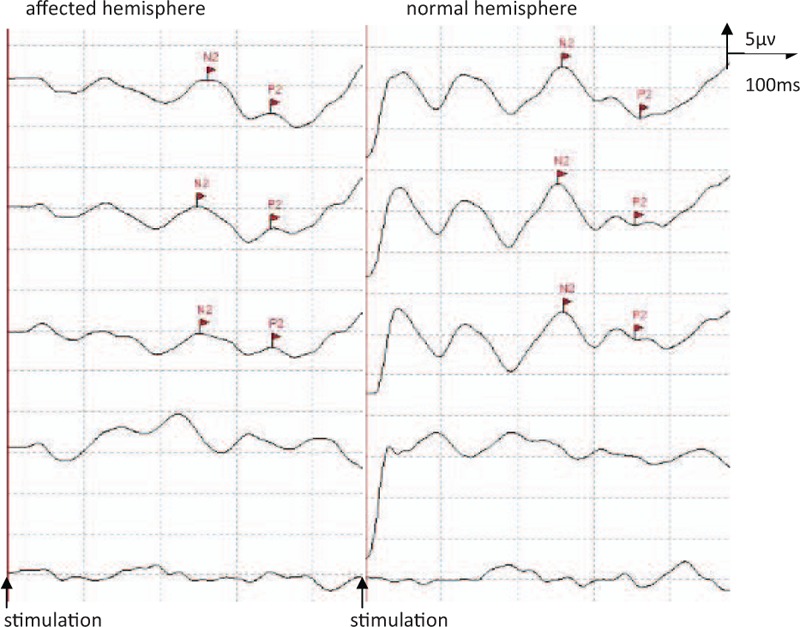
The late LEP components of affected and normal hemisphere in patient No. 6. LEP = laser evoked potentials.
Table 3.
LEP amplitudes among patients with different pain degree.

4. Discussion
This study was an open-label study without a control group. The study showed that high-frequency rTMS applied over the M1 for 10 consecutive days can produce satisfactory or partial antalgic effect (66.7%–25.0%) on patients with thalamic pain. This effect can persist for 8 weeks after the completion of the rTMS intervention.
Selection of target cortex is a crucial step for the efficacy of rTMS therapy. Larger series of epidural MCS continued to report significant pain relief for chronic neuropathic pain,[9,14–16] which sparked development of non-invasive technologies. TMS is a non-invasive method that enables the stimulation of specific cortical areas by an electric current induced by a coil placed on the scalp. The technique most frequently studied in chronic pain by predicting the efficacy of MCS.[17] So M1 may be a potential targets for rTMS therapy of neuropathic pain. Several studies report that high-frequency rTMS of motor cortex reduces chronic pain.[18–20] Their results came most from different chronic pain conditions, such as fibromyalgia, spinal cord injury, and mixed neuropathic pain. Only a few of study recruited homogeneous populations of patients. Thalamic pain was included in the concept of central poststroke pain (CPSP), which is commonly refractory to current pharmacologic treatments. The incidence of poststroke thalamic pain is about 10% within the first year,[21] which is a significant population that is worthy of study.
Migita et al[22] first reported the patient with central pain obtained 30% pain relief after rTMS over M1. A number of following studies have showed the therapeutic potential of rTMS in patients with CPSP,[23–27] but the results vary. Kobayashi et al[23] reported that the rTMS (10 trains of 10-second 5 Hz-rTMS) of M1, when maintained once a week, was effective in 61.1% of the patients with CPSP at the 12th week. In the 6 patients who continued rTMS for 1 year, the pain relief effects also were sustained. Five sessions of open-label M1 rTMS consisting of 2000 stimuli/10 Hz each session provided analgesia effects on CPSP.[25] Of the 14 patients, 6 patients reported pain reductions between 18% and 26%. Ohn et al[27] also demonstrated that daily 10-Hz rTMS sessions for 1000 pulses applied over the M1 for 5 consecutive days can produce a partial antalgic effect on poststroke central pain. Of the 22 participants, the VAS score of 14 participants was significantly decreased after rTMS, which showed an antalgic effect of rTMS within CPSP. This effect can persist for 2 weeks after the completion of the rTMS intervention. de Oliveira et al[24] performed a placebo controlled double-blind study to assess the analgesic effect of 10 repeated sessions of M1/the dorsolateral prefrontal cortex (DLPFC)rTMS in CPSP. But an interim analysis showed a consistent lack of analgesic effect, and the study was terminated. The conclusion was that rTMS of the M1/DLPFC was not effective in relieving CPSP.
Since the rTMS stimulation parameters varied among different studies such as frequency, intensity of RMT, and total number of pulses, it is difficult to determine which specific parameters are best for clinical use. In order to find a better rTMS stimulation parameters for patients with thalamic pain, we proposed a daily 10-Hz rTMS sessions for 1000 pulses applied over the M1 for 10 consecutive days to treat thalamic pain. The study produced satisfactory antalgic effect (66.7%–25.0%) on patients with thalamic pain. The VAS score of all patients was significantly decreased after rTMS and the analgesic effect of rTMS can last up to 8 weeks after the completion of the rTMS intervention. Furthermore, the analgesic effect was more prominent in patients with moderate pain, less anxiety or depression, and higher LEP amplitudes.
The mechanisms underlying the analgesic effect of rTMS applied over the M1 remain unclear. Hosomi et al[26] evaluated the alterations of cortical excitability in M1 in CPSP patients with high-frequency rTMS in M1. Intracortical facilitation (ICF) in the responders (≥30% pain reduction after rTMS) was lower at baseline and significantly increased after rTMS, which suggested that the mechanism of action of rTMS might be related to restoration of abnormal cortical excitability in CPSP. Some studies reported that a preserved thalamocortical connectivity or sensory neural network was important to the effect of rTMS on pain modulation.[27–29] Goto et al[28] found that there were greater antalgic effects of rTMS in patients with preserved superior thalamocortical tract (TCT). Ohn et al[27] confirmed further that the antinociceptive effect of rTMS was greater when the TCT was more preserved. So it was believed that such modulation of rTMS over M1 may spread to other areas of the brain via a distributed pain network of corticosubcortical and corticocortical connections.
LEPs were used to evaluate a pain rating scale and to test the integrity of the nociceptive system. Treede et al[30] reported that the amplitude of LEPs may also be increased when heat/pain sensitivity is pathologically increased. Several changes in the LEPs are shown that fibromyalgia patients tend to have lower pain thresholds and higher N2 amplitudes.[31] So the amplitudes of LEPs correlate with the subjective perception of the intensity of pain. In this study, the magnitude of LEPs was used to assess the index of the subjective pain experience accurately and it is also helpful to identify psychogenic pain. LEP were found to be abnormal in 5 patients with thalamic pain that led to altered pain sensitivity in this study. The larger LEP amplitudes were, the higher the percentage of pain relief were. But this potential indication has not been investigated well enough.
In conclusion, high-frequency rTMS applied over the M1 for 10 consecutive days can provide long-term pain relief in patients with thalamic pain without causing severe adverse effects. Since the results were based on an open-label study with a limited number of the patients, further studies will be necessary before any robust conclusions can be drawn about the effects of rTMS therapy and optimal patient selection.
Author contributions
Data curation: Wenjuan Li.
Investigation: Jiaxiang Ni.
Supervision: Yuping Wang.
Writing – original draft: HUA LIN.
Footnotes
Abbreviations: CPSP = central poststroke pain, DBS = deep brain stimulation, DLPFC = the dorsolateral prefrontal cortex, EEG = electroencephalographic, HAMA = Hamilton Anxiety Scale, HAMD = Hamilton Depression Scale, ICF = intracortical facilitation, LEP = laser evoked potentials, M1 = motor cortex, MCS = motor cortex stimulation, RMT = resting motor threshold, rTMS = repetitive transcranial magnetic stimulation, TCT = thalamocortical tract, VAS = visual analogue scale.
The authors of this work have no conflicts of interest to disclose.
References
- [1].Lefaucheur JP, Drouot X, Menard-Lefaucheur I, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry 2004;75:612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andre-Obadia N, Peyron R, Mertens P, et al. Transcranial magnetic stimulation for pain control: double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin Neurophysiol 2006;117:1536–44. [DOI] [PubMed] [Google Scholar]
- [3].Lefaucheur JP, Drouot X, Nguyen JP. Interventional neurophysiology for pain control: duration of pain relief following repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol Clin 2001;31:247–52. [DOI] [PubMed] [Google Scholar]
- [4].Yoo WK, Kim YH, Doh WS, et al. Dissociable modulating effect of repetitive transcranial magnetic stimulation on sensory and pain perception. Neuroreport 2006;17:141–4. [DOI] [PubMed] [Google Scholar]
- [5].Tsubokawa T, Katayama Y, Yamamoto T, et al. Treatment of thalamic pain by chronic motor cortex stimulation. Pacing Clin Electrophysiol 1991;14:131–4. [DOI] [PubMed] [Google Scholar]
- [6].Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg 1993;78:393–401. [DOI] [PubMed] [Google Scholar]
- [7].Nuti C, Peyron R, Garcia-Larrea L, et al. Motor cortex stimulation for refractory neuropathic pain: four year outcome and predictors of efficacy. Pain 2005;118:43–52. [DOI] [PubMed] [Google Scholar]
- [8].Sokal P, Harat M, Zieliński P, et al. Motor cortex stimulation in patients with chronic central pain. Adv Clin Exp Med 2015;24:289–96. [DOI] [PubMed] [Google Scholar]
- [9].Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology 2008;70:2329–37. [DOI] [PubMed] [Google Scholar]
- [10].Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application: report of an IFCN committee. Electroencephalogr Clin Neurophysiol 1994;91:79–92. [DOI] [PubMed] [Google Scholar]
- [11].Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120:2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Khedr EM, Kotb H, Kamel NF, et al. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry 2005;76:833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hüllemann P, von der Brelie C, Manthey G, et al. Reduced laser-evoked potential habituation detects abnormal central pain processing in painful radiculopathy patients. Eur J Pain 2017;21:918–26. [DOI] [PubMed] [Google Scholar]
- [14].Walter J, Fagundes-Pereyra WJ, Teixeira MJ, et al. Motor cortex electric stimulation for the treatment of neuropathic pain. Arq Neuropsiquiatr 2010;68:923–9. [DOI] [PubMed] [Google Scholar]
- [15].Ostergard T, Munyon C, Miller JP. Motor cortex stimulation for chronic pain. Neurosurg Clin N Am 2014;25:693–8. [DOI] [PubMed] [Google Scholar]
- [16].Im SH, Ha SW, Kim DR, et al. Long-term results of motor cortex stimulation in the treatment of chronic intractable neuropathic pain. Stereotact Funct Neurosurg 2015;93:212–8. [DOI] [PubMed] [Google Scholar]
- [17].Saitoh Y, Hirayama A, Kishima H, et al. Reduction of intractable deafferentation pain due to spinal cord or peripheral lesion by high-frequency repetitive transcranial magnetic stimulation of the primary motor cortex. J Neurosurg 2007;107:555–9. [DOI] [PubMed] [Google Scholar]
- [18].Galhardoni R, Correia GS, Araujo H, et al. Repetitive transcranial magnetic stimulation in chronic pain: a review of the literature. Arch Phys Med Rehabil 2015;96:S156–72. [DOI] [PubMed] [Google Scholar]
- [19].Mhalla A, Baudic S, Ciampi de Andrade D, et al. Long-term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. Pain 2011;152:1478–85. [DOI] [PubMed] [Google Scholar]
- [20].Treister R, Lang M, Klein MM, et al. Non-invasive transcranial magnetic stimulation (TMS) of the motor cortex for neuropathic pain—at the tipping point? Rambam Maimonides Med J 2013;4:e0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Andersen G, Vestergaard K, Ingeman-Nielsen M, et al. Incidence of central post-stroke pain. Pain 1995;61:187–93. [DOI] [PubMed] [Google Scholar]
- [22].Migita K, Uozumi T, Arita K, et al. Transcranial magnetic coil stimulation of motor cortex in patients with central pain. Neurosurgery 1995;36:1037–9. [DOI] [PubMed] [Google Scholar]
- [23].Kobayashi M, Fujimaki T, Mihara B, et al. Repetitive transcranial magnetic stimulation once a week induces sustainable long-term relief of central poststroke pain. Neuromodulation 2015;18:249–54. [DOI] [PubMed] [Google Scholar]
- [24].de Oliveira RA, de Andrade DC, Mendonça M, et al. Repetitive transcranial magnetic stimulation of the left premotor/dorsolateral prefrontal cortex does not have analgesic effect on central poststroke pain. J Pain 2014;15:1271–81. [DOI] [PubMed] [Google Scholar]
- [25].Hasan M, Whiteley J, Bresnahan R, et al. Somatosensory change and pain relief induced by repetitive transcranial magnetic stimulation in patients with central poststroke pain. Neuromodulation 2014;17:731–6. [DOI] [PubMed] [Google Scholar]
- [26].Hosomi K, Kishima H, Oshino S, et al. Cortical excitability changes after high-frequency repetitive transcranial magnetic stimulation for central poststroke pain. Pain 2013;154:1352–7. [DOI] [PubMed] [Google Scholar]
- [27].Ohn SH, Chang WH, Park CH, et al. Neural correlates of the antinociceptive effects of repetitive transcranial magnetic stimulation on central pain after stroke. Neurorehabil Neural Repair 2012;26:344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Goto T, Saitoh Y, Hashimoto N, et al. Diffusion tensor fiber tracking in patients with central poststroke pain; correlation with efficacy of repetitive transcranial magnetic stimulation. Pain 2008;140:509–18. [DOI] [PubMed] [Google Scholar]
- [29].Drouot X, Nguyen JP, Peschanski M, et al. The antalgic efficacy of chronic motor cortex stimulation is related to sensory changes in the painful zone. Brain 2002;125:1660–4. [DOI] [PubMed] [Google Scholar]
- [30].Treede RD, Lorenz J, Kunze K. Bromm B, Desmedt JE, et al. Assessment of nociceptive pathways with laser-evoked potentials in normal subjects and patients. Pain and the Brain. Advances in Pain Research and Therapy, Vol. 22. New York: Raven Press; 1995. 377–92. [Google Scholar]
- [31].Plazier M, Ost J, Snijders E, et al. Laser-evoked potentials in fibromyalgia: the influence of greater occipital nerve stimulation on cerebral pain processing. Neuromodulation 2015;18:376–83. [DOI] [PubMed] [Google Scholar]


