Abstract
Background:
Many studies have indicated that leptin is correlated with breast cancer occurrence and tumor behavior. However, this issue remains controversial. Therefore, we conducted an updated meta-analysis to investigate the role of leptin in breast cancer.
Methods:
We performed a systematic literature search and identified relevant papers up to 1 September 2017. Standardized mean differences (SMDs) with 95% confidence intervals (CIs) were used to evaluate effect sizes.
Results:
Thirty-five eligible studies were included in the current meta-analysis. Serum leptin levels were related to breast cancer risk as demonstrated by calculations of the overall SMD = 0.46 (95% CI = 0.31-0.60, I2 = 93.5%). A subgroup analysis of BMI identified an association between breast cancer and serum leptin levels in patients who are overweight and obese (overweight: SMD = 0.35, 95% CI = 0.13–0.57, I2 = 88.1%; obesity: SMD = 1.38, 95% CI = 0.64–2.12, I2 = 89.6%). Additionally, menopausal status subgroup analysis revealed a significant association in postmenopausal women (SMD = 0.26, 95% CI = 0.12–0.40, I2 = 77.9%). Furthermore, we identified a significant association between breast cancer and serum leptin levels in Chinese women (SMD = 0.61, 95% CI = 0.44–0.79, I2 = 40.6%).
Conclusion:
The results of this meta-analysis suggested that leptin could be a potential biomarker for breast cancer risk in women, especially overweight/obese or postmenopausal women. Therefore, it may be useful for identifying subjects with a high risk for breast cancer who may benefit from preventive treatments.
Keywords: breast cancer, leptin, meta-analysis, risk
1. Introduction
Breast cancer is common cancer and the second leading cause of cancer-related death in women worldwide.[1] Multiple factors, including genetic, environmental, and hormonal factors, are related to breast cancer. Obesity is a well-recognized risk factor for breast cancer.[2,3] Data in the literature shows that in obesity adipocytes induces chronic inflammation, which increases the local and systemic levels of cytokines such as tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6).[4] The adipokines secretion profile alters the condition of obesity, modulates the immune response, and in particular affects the production and activation of neutrophil, such as leptin, which regulates the total neutrophil count and has a granulocyte-stimulating effect.[5]
Leptin, a 16-kDa protein, is a product of the obese (Ob) gene and is secreted into the bloodstream, where it circulates in both bound and free forms.[6] The potential pro-tumoral effects of leptin are facilitated by its mitogen actions. Leptin has been shown to exert neoplastic effects in breast cancer by acting directly on tumor growth, migration and invasion signaling pathways or by decreasing tissue sensitivity to insulin or regulating inflammatory responses and tumor angiogenesis.[7,8] Leptin is a pleiotropic molecule that influences energy balance, appetite control, angiogenesis, reproduction, the immune response, and bone development. Leptin also affects the proliferation of different cell types, including the cells of the breast.[9,10] The results of several studies strongly support the hypothesis that leptin activity is correlated with breast cancer occurrence and tumor behavior. A few studies noted no associations between these parameters, a finding attributed to small patient samples and the presence of concomitant pathologies.[11–14] To better clarify this issue, Niu et al[15] conducted a meta-analysis and confirmed that leptin is positively associated with breast cancer. They suggested that serum leptin levels vary by population and increase from low to high in the following order: healthy people < patients with benign breast diseases < patients with breast cancer < patients with positive lymph node metastasis.
However, we noted that the results of recent studies were controversial. Rodrigo et al[16] did not find any relationships between serum leptin levels and sporadic breast cancer in post-menopausal women. Moreover, Georgia et al[17] found that leptin levels in healthy women are slightly higher than those in premenopausal women with breast cancer. Besides, the association between leptin and cancer risk was weak in the study by Touvier et al.[18] Moreover, no updated meta-analysis and systematic reviews have been performed recently. Therefore, we conducted this meta-analysis to verify whether serum leptin plays a vital role in breast cancer pathogenesis.
2. Materials and methods
2.1. Search strategy
The present meta-analysis followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.[19] All analyses were based on previously published studies. Thus no ethical approval and patient consent are required. We performed a systematic combined computerized, manual literature search and identified relevant scientific articles published up to September 1, 2017. Published reports were identified through searches of PubMed (Medline), Embase, Chinese Wanfang Data, and Chinese National Knowledge Infrastructure databases using the following MeSH terms and phrases: (leptin) AND (breast neoplasms) OR (breast neoplasm) OR (breast tumors) OR (breast tumor) OR (cancer, breast[MeSH terms]) OR (breast cancer) OR (carcinoma, human mammary[MeSH terms]) OR human mammary neoplasms) OR (human mammary neoplasms) OR (human mammary carcinoma) OR (mammary neoplasm, human[MeSH terms]). No language, year, or publication status restrictions were imposed. We also performed manual searches of the references cited in the retrieved articles and earlier reviews on the topic.
2.2. Inclusion and exclusion criteria
Case-control and cohort studies published as original articles, and reports containing relative risk estimates or raw data about serum leptin levels were eligible for inclusion in the study. We assessed the relevance of the corresponding abstracts and retrieved full copies of the manuscripts to determine whether they met our primary inclusion criteria. We ultimately selected only those studies:[1] reported sufficient information for estimating standardized mean differences (SMDs) or odds ratios (ORs) with 95% confidence intervals (CIs)[2]; were independent and did not duplicate previously published results[3]; included patients who were newly diagnosed and had histopathologically confirmed breast cancer and had not been treated via surgery, chemotherapy, or radiotherapy[4]; and indicated that all blood specimens were collected early in the morning after a period of fasting and before the initiation of any therapeutic approach. In cases in which multiple publications pertained to the same study population, the study with the largest number of case subjects was used. Also, data from articles with more detailed reporting were used for sub-analyses. Breast cancer diagnoses were classified using the International Classification of Diseases for Oncology (Third Edition codes C50.5–C50.9).
The following studies were excluded from the analysis[1]: studies reporting recurrence risk estimates of breast cancer among patients[2]; studies reporting mortality risk estimates of breast cancer[3]; and studies with patients who had a prior malignancy or coexisting severe medical conditions. Review articles not reporting original data were also excluded from the study, although they were checked for useful references.
2.3. Data extraction
Two researchers independently extracted detailed information and data using a pre-designed data extraction form and assessed the quality of the individual studies. In the event of disagreement, a third researcher would assess the relevant articles further. Data regarding the following parameters were extracted from each study: the first author's name, the year of publication, countries, ethnicities, sample sizes, age of the participants, cancer types, cancer stages, body mass indices, serum leptin levels (means and standard differences), treatment status, test methods, leptin levels among controls, and sources of controls (hospitals or populations). Moreover, data pertaining to serum leptin levels among women of different menopausal statuses were also retrieved.
2.4. Quality assessment
The Newcastle–Ottawa scale was modified to capture information pertaining to the following relevant quality characteristics of the included reports: sampling representativeness, sample sizes, exposure definitions, famine severity assessments, confounding adjustments, outcome assessments and statistical methods. The total score ranged from 0 to 9 (studies with scores of 0–3, 4–6, and 7–9 were considered low-, moderate-, and high-quality studies, respectively).
2.5. Statistical analysis
The command metan in Stata 14.0 (StataCorp LLC, College Station, TX) was used to combine the results of the included studies to perform the meta-analysis. SMDs and 95% CIs were calculated for each study, based on the sample size and the mean (and standard deviation [SD]) serum leptin levels in the case and control groups. We used the I2 statistic to investigate whether heterogeneity was present among the studies.[20] The pooled effect size (SMD) was evaluated using a random-effects model if the heterogeneity was considered statistically significant (I2 >50% and P < .10); otherwise, a fixed-effects model was used. We calculated the heterogeneity with the χ2 based Q-test and the I2 statistics test.
To identify the sources of heterogeneity, we using meta-regression and sensitivity analysis to evaluate between-study heterogeneity by assessing the influence of different study features, such as quality, sample size, year of publication, test method, BMI, and menopausal status.[21] To examine the impacts of effect modifiers, we conducted subgroup analyses based on information from the primary studies. Publication bias was assessed by Begg and Egger tests (α= 0.05).[22,23]
3. Results
3.1. Search results
We identified 1482 records and screened 109 full texts for relevance based on the titles and abstracts. Thirty-five studies met our inclusion criteria. The excluded studies and the reasons for their exclusion are presented in detail in Figure 1. A total of 781 studies remained after exclusion of duplicated studies, and 452 articles were removed after the title and abstract screening because they were not relevant to our study aims. Fifty-nine items were excluded after full-text testing because they did not pertain to the relationship between breast cancer (n = 30) and serum leptin levels (n = 29). Five articles were excluded because they did not have sufficient information regarding baseline patient characteristics. Eight articles were excluded because they were meta-analyses. Two studies were excluded because they were 2 of multiple publications from the same study population, and the study with the largest number of case subjects was used. One study was composed of various ethnicities, which we stratified into different groups for subgroup analysis of ethnicity. All articles were in English and were published between 2000 and February 2017.
Figure 1.
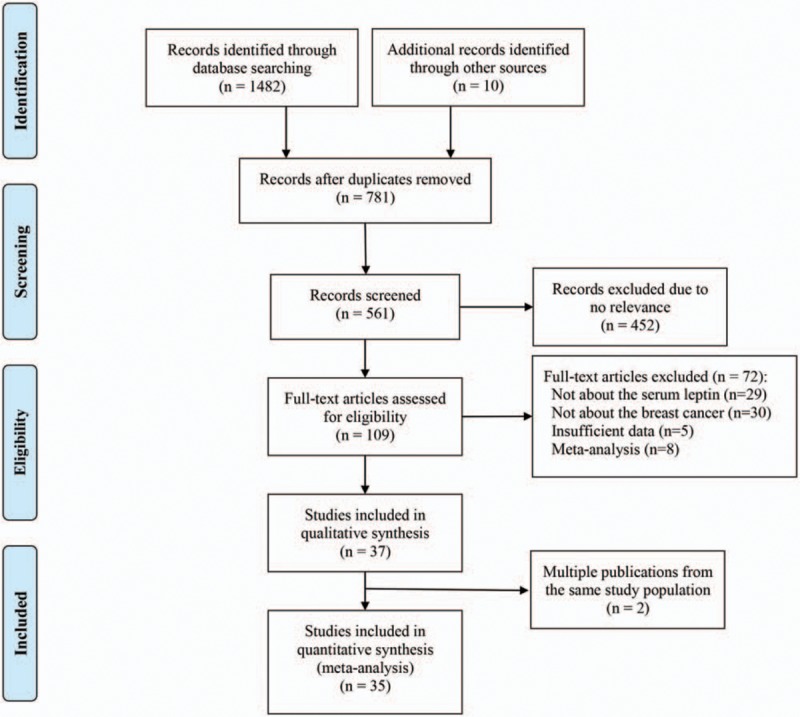
Flow diagram of the study selection process.
Tables 1 and 2 present the descriptive characteristics of the eligible studies. Among the studies included in the analysis, 13 were performed in Asia. Five of those thirteen were conducted in China (2 in Taiwan and 3 in inland China; 2 articles pertained to the same study population, and the study with the smaller number of case subjects was used for supplemental information).[6,24–27] Three studies were conducted in Turkey,[28–30] 2 in the Kingdom of Saudi Arabia,[11,31] 1 in Korea,[32] 1 in Sri Lanka,[16] and 1 in Iran.[33] Twenty-one studies involved Caucasian patients. Seven of these 21 studies involved patients living in the United States,[14,34–39] 5 in Greece,[17,40–43] 2 in Mexico,[44,45] 2 in France,[18,46] 1 in Portugal,[8] 1 in Germany,[47] 1 in Norway,[48] 1 in Australia,[49] and 1 in Italy.[50] Two studies were conducted among Africans, 1 study was conducted in Egypt,[13] and 1 United States study included persons of African descent.[39] Among the 35 studies, 2 included only premenopausal women,[36,38] and 4 contained only postmenopausal women.[34,35,40,41]
Table 1.
Characteristics of studies included in the meta-analysis.
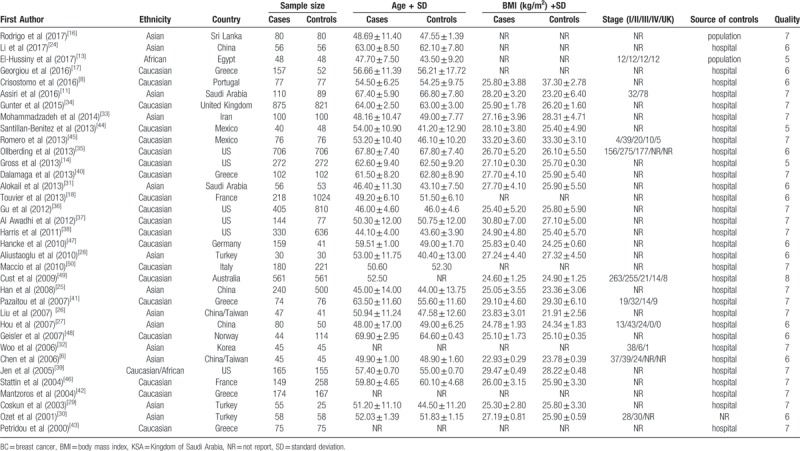
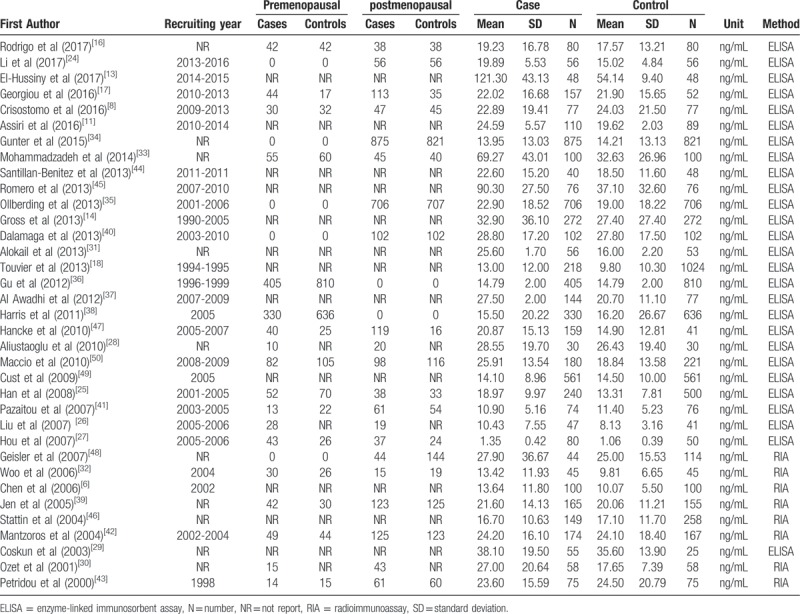
Table 2.
The levels of serum leptin in each primary study.
3.2. Pooling of studies and subgroup analysis
All 35 eligible studies were included in the meta-analysis, and the results of the overall and subgroup analyses of the studies providing data on the relationship between serum leptin levels and breast cancer are presented in Table 3. Serum leptin levels were related to breast cancer risk as demonstrated by calculations of the overall SMD = 0.46 (95% CI = 0.31–0.60). However, significant and non-ignorable heterogeneity (I2 = 93.5%) was present. Therefore, we performed subgroup analyses of specific variables to determine if they were sources of the heterogeneity.
Table 3.
The pooled results of the serum leptin levels in breast cancer patients compared with control groups.
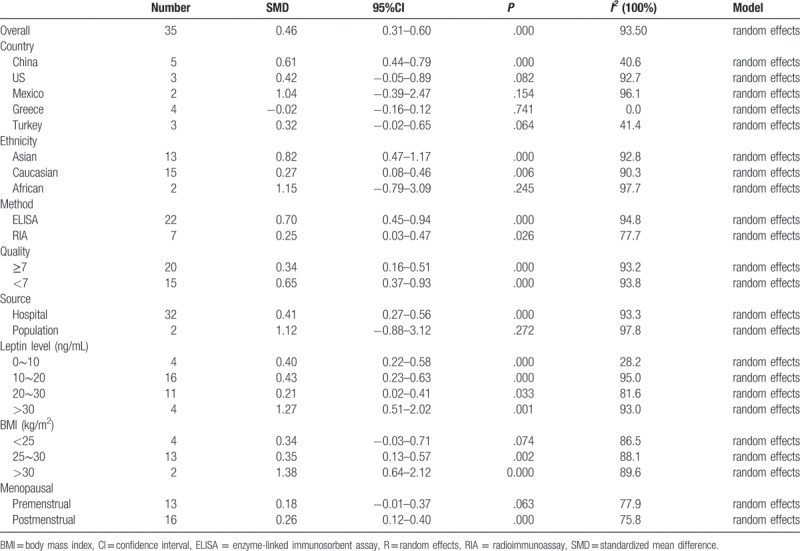
We conducted subgroup analyses of several factors that may modify the association between serum leptin levels and breast cancer, including country, ethnicity, BMI, test methods, quality, leptin levels among controls, sources of controls, and menopausal status. First, we performed a subgroup analysis in which the studies were organized into groups according to their countries. Five studies reported estimates of the significance of the relationship between serum leptin levels and breast cancer in China, 3 in the United States, 2 in Mexico, 4 in Greece, and 3 in Turkey. The pooled SMD showed weak statistical significance in Chinese people (SMD = 0.61, 95% CI = 0.44–0.79, I2 = 40.6%). In addition, we found no association between serum leptin levels and breast cancer in the United States (SMD = 0.42, 95% CI = –0.05 to 0.89, I2 = 92.7%), Mexico (SMD = 1.04, 95% CI = –0.39 to 2.47, I2 = 96.1%), Greece (SMD = –0.02, 95% CI = –0.16 to 0.12, I2 = 0.0%), or Turkey (SMD = 0.32, 95% CI = –0.02 to 0.65, I2 = 41.4%) (Fig. 2). We then analyzed the effect of ethnicity on the relationship between serum leptin levels and breast cancer. We found an association between the two variables in Asian (SMD = 0.82, 95% CI = 0.47–1.17, I2 = 92.8%) and Caucasian patients (SMD = 0.27, 95% CI = 0.08–0.46, I2 = 90.3%), but not in African patients (SMD = 1.15, 95% CI = –0.79 to 3.09, I2 = 97.7%). The subgroup analysis of test methods showed that both enzyme-linked immunosorbent assay (ELISA) (SMD = 0.70, 95% CI = 0.45–0.94, I2 = 94.8%) and radioimmunoassay (RIA) (SMD = 0.25, 95% CI = 0.03–0.47, I2 = 77.7%) had significant effects on the relationship between serum leptin levels and breast cancer. Additionally, the subgroup analysis of the quality of the included studies revealed that scores greater than or equal to 7 (SMD = 0.34, 95% CI = 0.16–0.51, I2 = 93.2%) and scores below 7 (SMD = 0.65, 95% CI = 0.37–0.93, I2 = 93.8%) had significant effects. The subgroup analysis of leptin levels among controls showed that all of the 4 subgroups (leptin levels from 0–10ng/mL: SMD = 0.40, 95% CI = 0.22–0.58, I2 = 28.2%; 10–20 ng/mL: SMD = 0.43, 95% CI = 0.23–0.83, I2 = 95.0%; 20–30 ng/mL: SMD = 0.21, 95% CI = 0.02–0.41, I2 = 81.6%; and >30 ng/mL: SMD = 1.27, 95% CI = 0.51–2.02, I2 = 93.0%) had significant effect on the relationship between serum leptin levels and breast cancer. Moreover, the effect of sources of controls on the relationship between serum leptin levels and breast cancer revealed an association in hospital groups (SMD = 0.41, 95% CI = 0.27–0.56, I2 = 93.3%) but not in population groups (SMD = 1.12, 95% CI = –0.88 to 3.12, I2 = 97.8%). Interestingly, analysis of the SMD of BMI showed that when the BMI was below 25 (SMD = 0.34, 95% CI = –0.03 to 0.71, I2 = 86.5%), there was no difference in the leptin-breast cancer relationship between the case and control groups; however, we noted significant differences in the relationship between the case and control groups when the BMI was between 25 and 30 (SMD = 0.35, 95% CI = 0.13–0.57, I2 = 88.1%) and when the BMI exceeded 30 (SMD = 1.38, 95% CI = 0.64–2.12, I2 = 89.6%) (Fig. 3). Finally, we assessed menopausal status and found no significant association among premenopausal women (SMD = 0.18, 95% CI = –0.01 to 0.37, I2 = 77.9%); however, a significant association was found between serum leptin levels and breast cancer in postmenopausal women (SMD = 0.26, 95% CI = 0.12–0.40, I2 = 75.8%) (Fig. 4).
Figure 2.
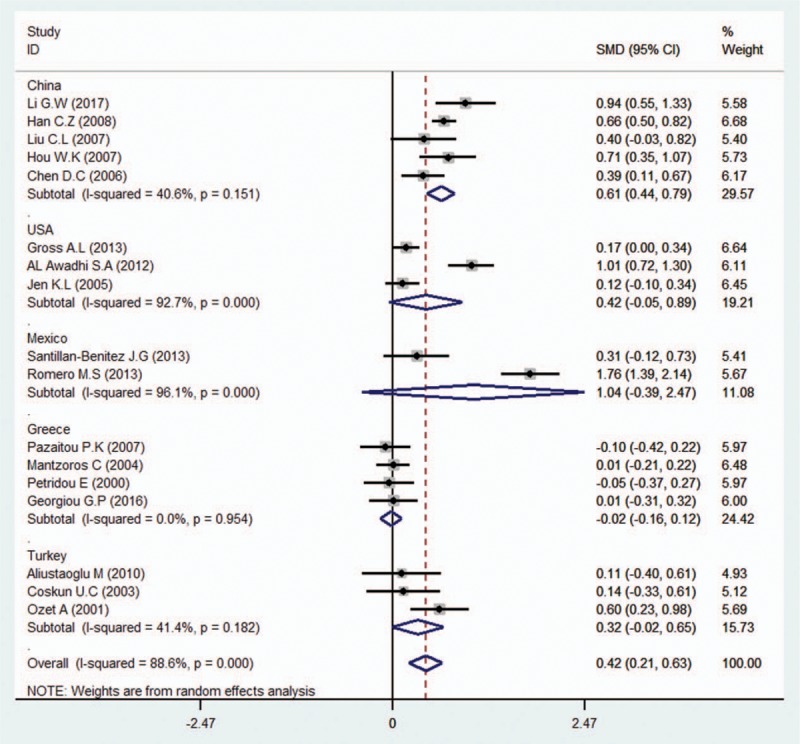
Subgroup analysis of the association between serum leptin levels and breast cancer in different countries. Zero is not included in this confidence interval, which indicates that the relationship between leptin levels and breast cancer is different between the case and control groups. The positive SMD value suggests that the mean circulating leptin level in the case group is higher than that in the control group in Asian and Caucasians, while the negative SMD value indicates that the mean leptin level in the case group is lower than that in the control group in Africans.
Figure 3.
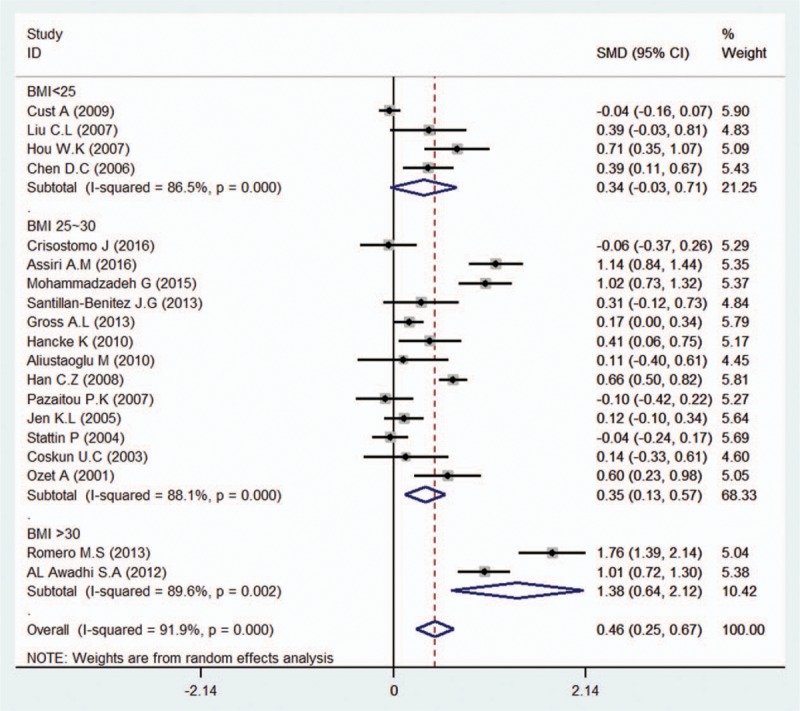
Subgroup analysis of the association between serum leptin levels and breast cancer in different BMI groups. Zero is not included in this confidence interval, which indicates a difference between the case and control groups. The negative SMD value suggests that the mean of the case groups is are lower than that of the control groups for the BMI below 25 group, while the positive SMD value suggests that the mean of serum leptin level in the case groups is higher than that in the control groups in both the BMI between 25 to 30 and the BMI exceeding 30 groups.
Figure 4.
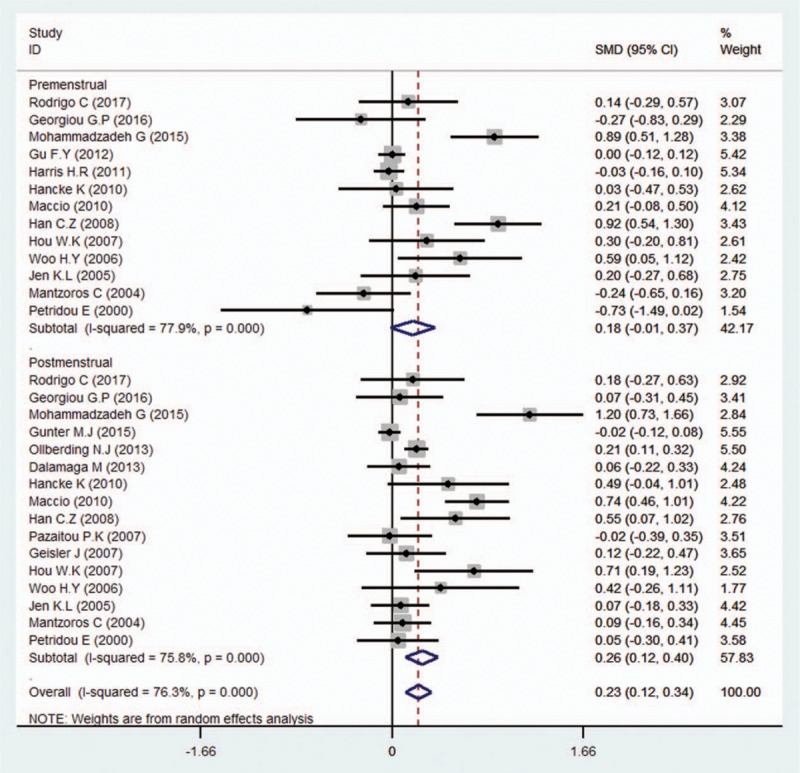
Subgroup analysis of the association between serum leptin levels and breast cancer in different menopausal status groups. Zero is not included in this confidence interval, which indicates a difference between the case and control groups. A negative SMD value suggests that the mean of the case groups is lower than that of the control groups in the premenstrual group, while the positive SMD value suggests that the mean serum leptin level in the case groups is higher than that in the control groups in the postmenstrual group.
3.3. Sensitivity and meta-regression analysis
We conducted sensitivity analysis by sequentially excluding studies from the meta-analysis to further investigate the possible sources of the heterogeneity among the studies. The sensitivity analysis results suggested that our meta-analysis was stable (Fig. 5). We also performed multivariate meta-regression to evaluate the influence of several factors that may modify the association between serum leptin levels and breast cancer, including quality, sample size, year of publication, test method, BMI and menopausal status (the adjusted P values are .982, .230, 1.000, .700, .844, and .978, respectively). The results showed that these confounding factors did not substantially affect the heterogeneity. No publication bias was found by either Begg (P = .163) (Fig. 6) or Egger test (P = .093).
Figure 5.
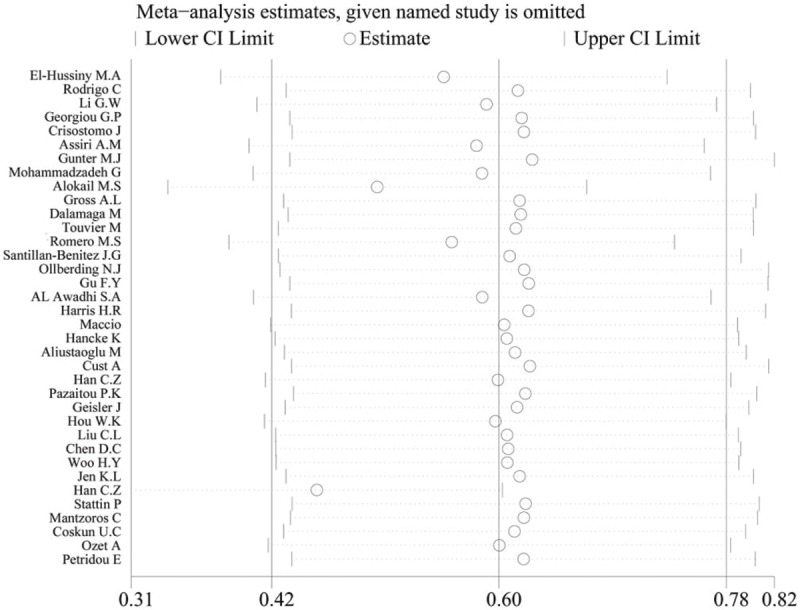
The result of the sensitivity analysis on the association between circulating leptin levels and breast cancer.
Figure 6.
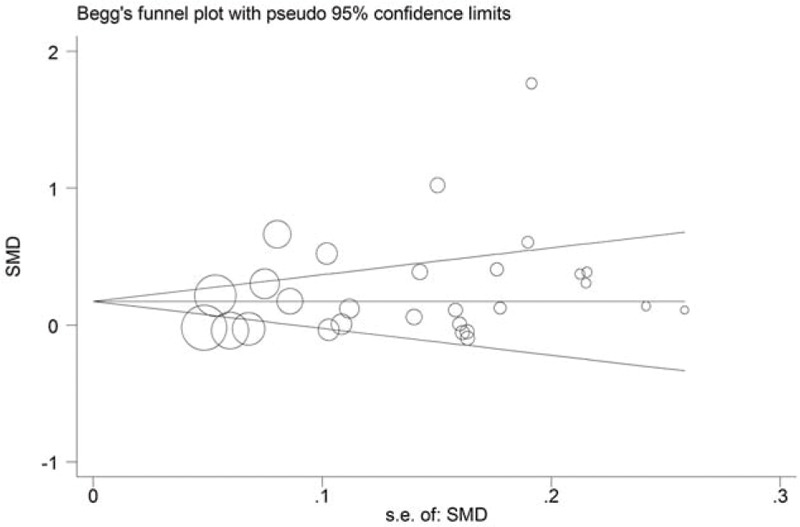
Funnel plot for the effect of publication bias on the data regarding the association between circulating leptin levels and breast cancer. Each circle represents a separate study pertaining to the indicated association. The circles in the funnel plot imply that an asymmetrical distribution was not present, suggesting that no publication biases were present.
4. Discussion
The results of this meta-analysis were consistent with those of previous studies indicating that leptin plays a potential role in breast cancer progression. However, we noted non-ignorable heterogeneity among studies included in the meta-analysis (I2 = 93.5%). We used a random-effects model to account for the heterogeneity; however, the model also increased the probability of a type-I error. To investigate the causes of the heterogeneity, we identified several characteristics, such as ethnicity, country, BMI, quality, measurement method, control source, breast cancer stage, cancer type, and menopausal status, as factors that may have added variability to the results.
To identify the source of the heterogeneity, we first conducted a multivariate meta-regression and sensitivity analysis by sequentially excluding each study. We did not observe any differences in heterogeneity among all the analyzed confounding factors, including ethnicity, quality, sample size, publication year, measurement method, BMI, and menopausal status. The other potential factors were not included in the meta-regression because we did not have sufficient information about them. The results of the sensitivity analysis indicated that the reliability of the meta-analysis was good. We found that none of the confounding factors introduced significant variability; however, we performed subgroup analysis to investigate the non-ignorable heterogeneity further. We used a random-effects model to account for the between-study variability.
Increasing amounts of evidence in the literature support the idea that an association exists between breast cancer risk and leptin.
In a previous meta-analysis, Niu et al[15] presented the combined-effect (d) values for serum leptin levels in different population groups. They showed that leptin levels vary among different groups and increase from low to high in the following order: healthy people < patients with benign breast disease < patients with breast cancer < patients with positive lymph node metastasis. They divided the 23 studies included in their analysis into eight groups. The quantity of literature was limited in some of the groups. The fail-safe number suggests that their results were unstable. Moreover, the authors investigated whether menopausal status and the sources of the patients and controls affected their results; however, they did not examine whether any additional confounders influenced the results of their investigation. Thus, the effects of several other potential confounding factors required evaluation. Furthermore, the authors included 11 (47.8%) Chinese studies in the analysis. These studies accounted for almost 50% of the studies included in the report; thus, the results may be applicable only to Chinese patients. We contained higher numbers of case-control studies (number = 35) and events (cases vs. controls = 6086 vs 7158) and similar numbers of studies involving patients of specific ethnicities (14 studies included Asians, 20 studies involved Caucasians and 2 studies involved Africans) in our analysis. We excluded 2 studies (Han et al[51] and Dalamaga et al[52]) that may have involved the same study population. We used the data from the study with the larger number of subjects and included the data from the survey with the smaller number of items as supplemental information when necessary. Moreover, our analysis included only patients who were newly diagnosed and had histopathologically confirmed breast cancer that had not been treated via surgery, chemotherapy, or radiotherapy. Thus, prior treatments were unlikely to have affected our results. Therefore, we may be able to improve the statistical power of the analysis.
Some studies have suggested that BMI, ethnicity, and family history are closely related to breast cancer.[6,14,39,45,49] Besides, Crisostomo et al[8] showed that leptin is positively correlated with BMI in obese patients with breast cancer. In our analysis, we first evaluated the correlation between serum leptin levels and breast cancer risk and, found that serum leptin levels are significantly positively correlated with breast cancer risk. In our subgroup analysis in which BMI stratified patients, we found that serum leptin levels were not associated with breast cancer in healthy normal-weight individuals or normal-weight individuals with breast cancer. Interestingly, we noted a strong association between breast cancer risk and higher leptin levels in patients who are overweight or obese, a finding consistent with those of previous studies by Romero et al[45] and Cust et al.[49]
Many authors have evaluated whether the association between leptin and breast cancer varies according to menopausal status. Interestingly, the relationship between breast cancer risk and leptin levels has been shown to be menopause status specific in several studies.[33,35,39,41,47] However, leptin levels have been shown to be inversely related to menopausal status in other studies.[8,11,13,16–18,24,31,34,40,44] Based on the results of our meta-analysis, we agree that leptin levels are associated with breast cancer among postmenopausal women but not among premenopausal women.
We also performed several subgroup analyses to assess whether several factors can modify the association between leptin levels and breast cancer risk. In the report in which the included studies were organized into groups by country, serum leptin levels were only weakly associated with breast cancer in Chinese people and were, not significantly associated with breast cancer in individuals from the United States, Mexico, Greece, or Turkey, possibly because the number of the studies evaluating patients from these countries were small. The analysis of the effect of ethnicity showed that serum leptin levels were significantly associated with breast cancer risk in Asians and Caucasians, this association was not identified in Africans because only a small number of studies evaluated African patients. The analysis of leptin levels among controls revealed that serum leptin levels were significantly associated with breast cancer in all groups. In the analysis of sources of controls, we found that the SMDs in hospital groups were consistent with the overall SMD; however, the SMDs in population groups showed that serum leptin levels were not significantly associated with breast cancer, possibly because the studies evaluating patients from population groups were a few (2 articles). Moreover, the test method analysis showed that ELISA and RIA significantly affected the relationship between serum leptin levels and breast cancer. Finally, the subgroup analysis in which the included studies were stratified according to their quality showed no differences in the leptin-breast relationship between the studies with scores ≥7 and those with scores <7. The results indicate that this factor did not affect the association between breast cancer risk and leptin levels.
There were several limitations to our meta-analysis. First, we were unable to perform additional subgroup analyses to investigate the effects of other factors, such as cancer stage, specific cancer classifications, and age of menarche, because the included studies lacked sufficient information regarding these factors. Second, we included only studies published in English in the analysis so that other biases may have affected the results of the present study. Third, the inclusion rates of hospital and population groups in the studies were 94.29% (33 articles) and 5.71% (2 items), respectively. The number of studies including population groups was small, so the power of this subgroup analysis was weak, and further research is required. Fourth, the participation rate of controls is an essential factor for assessing selecting bias. However, we found out only 1 study[40] had reported the number of the control group who agreed to take part in the concluded studies compared to the numbers who were asked to take part. Only 4[26,28,40,50] of 35 studies stated that the controls were sources from volunteers, and 31 remained reported the controls were randomly selected from routine physical examination and were strictly matched for age. We were constrained by the lack of more detailed information to go further analyzation, and extensive sample size studies are needed to be conducted for eliminating the confounding factor of participation rates of controls. Despite these limitations, we minimized the possibility that a bias would affect our results by creating a detailed protocol and by carefully selecting and analyzing the data.
In summary, the current meta-analysis suggests that leptin may have a potential role as a biomarker for breast cancer risk, especially in overweight/obese and postmenopausal women. Thus, it may be helpful in identifying subjects at high risk for breast cancer who may benefit from preventive treatments. However, some information, namely, information regarding specific cancer types and stages, was insufficient in the primary studies. In the future, the corresponding researchers will conduct additional prospective or longitudinal studies focusing on the above information to corroborate the association between leptin levels and breast cancer risk.
Acknowledgments
The authors would like to acknowledge all the authors of the original studies included in this meta-analysis. We would also like to thank American Journal Experts (AJE) for its linguistic assistance during the preparation of this article.
Author contributions
Conceptualization: Hui Pan, Li Wang.
Data curation: Hui Pan, Lin-Li Deng.
Formal analysis: Hui Pan, Lin-Li Deng, Jiang-Hui Luo.
Methodology: Jia-Qi Cui, Li Wang.
Project administration: Hui Pan, Li Wang.
Resources: Jia-Qi Cui.
Software: Lin Shi, Yi-Chun Yang, Jiang-Hui Luo, Dan Qin.
Validation: Lin Shi, Dan Qin.
Writing – original draft: Hui Pan.
Writing – review & editing: Lin-Li Deng, Lin Shi.
Footnotes
Abbreviations: BMI = body mass index, CIs = confidence intervals, ELISA = enzyme-linked immunosorbent assay, IL-6 = interleukin 6; kDa, kilodalton, KSA = Kingdom of Saudi Arabia, MOOSE = Meta-analysis Of Observational Studies in Epidemiology, Ob = obese, ORs = odds ratios, RIA = radioimmunoassay, SD = standard deviation, SMDs = standardized mean differences, TNF-α = tumour necrosis factor α.
The authors declares no conflicts of interest.
References
- [1].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [2].van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 2000;152:514–27. [DOI] [PubMed] [Google Scholar]
- [3].Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States). Cancer Causes Control 2002;13:741–51. [DOI] [PubMed] [Google Scholar]
- [4].Huh JY, Park YJ, Ham M, et al. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells 2014;37:365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maurizi G, Della Guardia L, Maurizi A, et al. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J Cell Physiol 2018;233:88–97. [DOI] [PubMed] [Google Scholar]
- [6].Chen DC, Chung YF, Yeh YT, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett 2006;237:109–14. [DOI] [PubMed] [Google Scholar]
- [7].Moon HS, Dalamaga M, Kim SY, et al. Leptin's role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev 2013;34:377–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Crisostomo J, Matafome P, Santos-Silva D, et al. Hyperresistinemia and metabolic dysregulation: a risky crosstalk in obese breast cancer. Endocrine 2016;53:433–42. [DOI] [PubMed] [Google Scholar]
- [9].Ando S, Barone I, Giordano C, et al. The multifaceted mechanism of leptin signaling within tumor microenvironment in driving breast cancer growth and progression. Front Oncol 2014;4:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jarde T, Perrier S, Vasson MP, et al. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer 2011;47:33–43. [DOI] [PubMed] [Google Scholar]
- [11].Assiri AM, Kamel HF. Evaluation of diagnostic and predictive value of serum adipokines: leptin, resistin, and visfatin in postmenopausal breast cancer. Obes Res Clin Pract 2016;10:442–53. [DOI] [PubMed] [Google Scholar]
- [12].Han C, Zhang HT, Du L, et al. Serum levels of leptin, insulin, and lipids in relation to breast cancer in china. Endocrine 2005;26:19–24. [DOI] [PubMed] [Google Scholar]
- [13].El-Hussiny MA, Atwa MA, Rashad WE, et al. Leptin receptor Q223R polymorphism in Egyptian female patients with breast cancer. Contemp Oncol (Pozn) 2017;21:42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gross AL, Newschaffer CJ, Hoffman-Bolton J, et al. Adipocytokines, inflammation, and breast cancer risk in postmenopausal women: a prospective study. Cancer Epidemiol Biomarkers Prev 2013;22:1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Niu J, Jiang L, Guo W, et al. The association between leptin level and breast cancer: a meta-analysis. PloS One 2013;8:e67349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rodrigo C, Tennekoon KH, Karunanayake EH, et al. Circulating leptin, soluble leptin receptor, free leptin index, visfatin and selected leptin and leptin receptor gene polymorphisms in sporadic breast cancer. Endocr J 2017;64:393–401. [DOI] [PubMed] [Google Scholar]
- [17].Georgiou GP, Provatopoulou X, Kalogera E, et al. Serum resistin is inversely related to breast cancer risk in premenopausal women. Breast 2016;29:163–9. [DOI] [PubMed] [Google Scholar]
- [18].Touvier M, Fezeu L, Ahluwalia N, et al. Association between prediagnostic biomarkers of inflammation and endothelial function and cancer risk: a nested case-control study. Am J Epidemiol 2013;177:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [20].Higgins JPTS. Quantifing heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [21].Harbord R, Higgins J. METAREG: Stata module to perform meta-analysis regression. Statistical Software Components 2009; 8. [Google Scholar]
- [22].Chaimani A, Mavridis D, Salanti G. A hands-on practical tutorial on performing meta-analysis with Stata. Evid Based Ment Health 2014;17:111–6. [DOI] [PubMed] [Google Scholar]
- [23].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li G, Xu Z, Zhuang A, et al. Magnetic resonance spectroscopy-detected change in marrow adiposity is strongly correlated to postmenopausal breast cancer risk. Clin Breast Cancer 2017;17:239–44. [DOI] [PubMed] [Google Scholar]
- [25].Han CZ, Du LL, Jing JX, et al. Associations among lipids, leptin, and leptin receptor gene Gin223Arg polymorphisms and breast cancer in China. Biol Trace Elem Res 2008;126:38–48. [DOI] [PubMed] [Google Scholar]
- [26].Liu CL, Chang YC, Cheng SP, et al. The roles of serum leptin concentration and polymorphism in leptin receptor gene at codon 109 in breast cancer. Oncology 2007;72:75–81. [DOI] [PubMed] [Google Scholar]
- [27].Hou WK, Xu YX, Yu T, et al. Adipocytokines and breast cancer risk. Chin Med J (Engl) 2007;120:1592–6. [PubMed] [Google Scholar]
- [28].Aliustaoglu M, Bilici A, Gumus M, et al. Preoperative serum leptin levels in patients with breast cancer. Med Oncol 2010;27:388–91. [DOI] [PubMed] [Google Scholar]
- [29].Coskun U, Gunel N, Toruner FB, et al. Serum leptin, prolactin and vascular endothelial growth factor (VEGF) levels in patients with breast cancer. Neoplasma 2003;50:41–6. [PubMed] [Google Scholar]
- [30].Ozet A, Arpaci F, Yilmaz MI, et al. Effects of tamoxifen on the serum leptin level in patients with breast cancer. Jpn J Clin Oncol 2001;31:424–7. [DOI] [PubMed] [Google Scholar]
- [31].Alokail MS, Al-Daghri N, Abdulkareem A, et al. Metabolic syndrome biomarkers and early breast cancer in Saudi women: evidence for the presence of a systemic stress response and/or a pre-existing metabolic syndrome-related neoplasia risk? BMC Cancer 2013;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Woo HY, Park H, Ki CS, et al. Relationships among serum leptin, leptin receptor gene polymorphisms, and breast cancer in Korea. Cancer Lett 2006;237:137–42. [DOI] [PubMed] [Google Scholar]
- [33].Mohammadzadeh G, Ghaffari MA, Bafandeh A, et al. The relationship between -2548 G/A leptin gene polymorphism and risk of breast cancer and serum leptin levels in Ahvazian women. Iran J Cancer Prev 2015;8:100–8. [PMC free article] [PubMed] [Google Scholar]
- [34].Gunter MJ, Wang T, Cushman M, et al. Circulating adipokines and inflammatory markers and postmenopausal breast cancer risk. J Natl Cancer Inst 2015;107:djv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ollberding NJ, Kim Y, Shvetsov YB, et al. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer Prev Res (Phila) 2013;6:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gu F, Kraft P, Rice M, et al. Leptin and leptin receptor genes in relation to premenopausal breast cancer incidence and grade in Caucasian women. Breast Cancer Res Treat 2012;131:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Al Awadhi SA, Al Khaldi RM, Al Rammah T, et al. Associations of adipokines & insulin resistance with sex steroids in patients with breast cancer. Indian J Med Res 2012;135:500–5. [PMC free article] [PubMed] [Google Scholar]
- [38].Harris HR, Tworoger SS, Hankinson SE, et al. Plasma leptin levels and risk of breast cancer in premenopausal women. Cancer Prev Res (Phila) 2011;4:1449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jen KL, Buison A, Darga L, et al. The relationship between blood leptin level and bone density is specific to ethnicity and menopausal status. J Lab Clin Med 2005;146:18–24. [DOI] [PubMed] [Google Scholar]
- [40].Dalamaga M, Karmaniolas K, Papadavid E, et al. Hyperresistinemia is associated with postmenopausal breast cancer. Menopause 2013;20:845–51. [DOI] [PubMed] [Google Scholar]
- [41].Pazaitou-Panayiotou K, Kelesidis T, Kelesidis I, et al. Growth hormone-binding protein is directly and IGFBP-3 is inversely associated with risk of female breast cancer. Eur J Endocrinol 2007;156:187–94. [DOI] [PubMed] [Google Scholar]
- [42].Mantzoros C, Petridou E, Dessypris N, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab 2004;89:1102–7. [DOI] [PubMed] [Google Scholar]
- [43].Petridou E, Papadiamantis Y, Markopoulos C, et al. Leptin and insulin growth factor I in relation to breast cancer (Greece). Cancer Causes Control 2000;11:383–8. [DOI] [PubMed] [Google Scholar]
- [44].Santillan-Benitez JG, Mendieta-Zeron H, Gomez-Olivan LM, et al. The tetrad BMI, leptin, leptin/adiponectin (L/A) ratio and CA 15-3 are reliable biomarkers of breast cancer. J Clin Lab Anal 2013;27:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Romero-Figueroa Mdel S, Garduno-Garcia Jde J, Duarte-Mote J, et al. Insulin and leptin levels in obese patients with and without breast cancer. Clin Breast Cancer 2013;13:482–5. [DOI] [PubMed] [Google Scholar]
- [46].Stattin P, Soderberg S, Biessy C, et al. Plasma leptin and breast cancer risk: a prospective study in northern Sweden. Breast Cancer Res Treat 2004;86:191–6. [DOI] [PubMed] [Google Scholar]
- [47].Hancke K, Grubeck D, Hauser N, et al. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res Treat 2010;119:367–77. [DOI] [PubMed] [Google Scholar]
- [48].Geisler J, Haynes B, Ekse D, et al. Total body aromatization in postmenopausal breast cancer patients is strongly correlated to plasma leptin levels. J Steroid Biochem Mol Biol 2007;104:27–34. [DOI] [PubMed] [Google Scholar]
- [49].Cust AE, Stocks T, Lukanova A, et al. The influence of overweight and insulin resistance on breast cancer risk and tumour stage at diagnosis: a prospective study. Breast Cancer Res Treat 2009;113:567–76. [DOI] [PubMed] [Google Scholar]
- [50].Maccio A, Madeddu C, Gramignano G, et al. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: preliminary results and therapeutic implications. J Mol Med (Berl) 2010;88:677–86. [DOI] [PubMed] [Google Scholar]
- [51].Han CZ, Shi J, Du LL, et al. Association among lipids, leptin and leptin receptor polymorphisms with risk of breast cancer. Zhonghua Liu Xing Bing Xue Za Zhi 2007;28:136–40. [PubMed] [Google Scholar]
- [52].Dalamaga M, Karmaniolas K, Papadavid E, et al. Elevated serum visfatin/nicotinamide phosphoribosyl-transferase levels are associated with risk of postmenopausal breast cancer independently from adiponectin, leptin, and anthropometric and metabolic parameters. Menopause 2011;18:1198–204. [DOI] [PubMed] [Google Scholar]


