Supplemental Digital Content is available in the text
Keywords: network meta-analysis, primary nephrotic syndrome, tripterygium wilfordii Hook F
Abstract
Background:
The present study aims to comprehensively determine the efficacy of different therapy regimens based on Tripterygium wilfordii Hook F (TwHF) for patients with primary nephrotic syndrome (PNS) using network meta-analysis method.
Methods:
Seven electronic databases were searched to identify randomized controlled trials (RCTs) that compared the differences between different therapy regimens based on TwHF for patients with PNS. The risk of bias in included RCTs was evaluated according to the Cochrane Handbook version 5.2.0. Network meta-analysis was performed to compare different regimens. Primary outcomes were complete remission rate and total remission rate. The secondary outcomes were hr urinary protein excretion, serum albumin, serum creatinine, and urea nitrogen. Data analysis was performed using R software.
Results:
A total of 40 studies involving 2846 patients with PNS were included. Compared with prednisone, the improvement in total remission rate and complete remission rate was associated with TwHF alone (odds ratio [OR] = 4.80, 95% credible intervals [CrI]: 2.20–10.00; OR = 6.30, 95% CrI: 2.90–13.00, respectively), TwHF+prednisone (OR = 2.10, 95% CrI: 1.30–3.50; OR = 2.40, 95% CrI: 1.50–3.80, respectively), TwHF+CPA (OR = 12.00, 95% CrI: 1.10–150.00; OR = 16.00, 95% CrI: 1.60–170.00, respectively), and TwHF+Cyclosporine A (OR = 28.00, 95% CrI: 3.20–250.00; OR = 35.00, 95% CrI: 4.50–270.00, respectively). Compared with TwHF alone, TwHF+prednisone showed less benefit in improving total remission rate and complete remission rate (OR = 0.44, 95% CrI: 0.21–0.91; OR = 0.38, 95% CrI: 0.19–0.77, respectively). TwHF alone, TwHF+prednisone could significantly reduce hr urinary protein excretion (MD = −0.69, 95% CrI: −1.30 to −0.14; MD = −1.00, 95% CrI: −1.90 to −0.14, respectively) and increase serum albumin (MD = 5.90, 95% CrI: 2.50–9.30; MD = 3.40, 95% CrI: 1.30–5.50, respectively) when compared to prednisone alone. TwHF alone showed significant reduction in serum creatinine when compared to CPA (MD = −19.00, 95% CrI: −37.00 to −0.56).
Conclusions:
TwHF alone, the addition TwHF to prednisone showed more benefit in improving total and complete remission rate, hr urinary protein excretion, serum albumin, and serum creatinine.
1. Introduction
Primary nephrotic syndrome (PNS) is an etiology unknown and a relatively rare kidney disease.[1] It is estimated that the incidence is 3/100,000 annually in adults.[2] However, the acute complications caused by PNS are not neglected, which include infection, acute kidney injury, and thromboembolism.[1] Glucocorticoid (GC) and cyclophosphamide (CPA) are the main therapy option for PNS,[3] whereas a considerable part of patients are becoming dependence or resistance to GC, and even cause some toxic effects.[4]. Previous studies have shown that CPA pulse therapy could improve short-term remission; however, some patients still relapsed or could not obtain remission.[5,6]
Tripterygium wilfordii Hook F (TwHF), a traditional Chinese herbal medicine, is a kind of vine-like plant which grows in Southeast China, has been used as an immunosuppressive agent for patients with PNS in China >20 years.[1,3] The ingredient of TwHF contains the bioactive compounds possessing immunosuppressive agents.[3] Combined TwHF with GC has been considered as a beneficial regimen in improving the remission of PNS and preventing the relapse.[7,8] Song et al's study showed that TwHF plus CPA could reduce hr urinary protein excretion and increased serum albumin.[9] Several systematic reviews and meta-analyses have investigated the impact of TwHF, CPA, or prednisone in patients with PNS[1,10,11]. However, it has been difficult to determine the superiority among treatment agents using pairwise meta-analysis and randomized controlled trial.[12] Network meta-analysis, an increasingly popular statistical method, allows to estimate the relative efficacy between different interventions of interest and to rank the interventions even though head-to-head comparisons are lacking.[12]
The present study aims to comprehensively compare the differences between all alternative regimens based on TwHF in improving patient outcomes for PNS using Bayesian network meta-analysis.
2. Methods
Ethics approval and patient consent are not required because this study is a meta-analysis based on the published original studies.
2.1. Information source
We systematically researched Cochrane Library, EMBASE, PubMed, CNKI (Chinese National Knowledge Infrastructure), CBM (Chinese Biological Medical Database), and WanFang databases from their inception to May 2017. We also tracked the references of relevant systematic reviews, meta-analyses, and included articles to identify additional studies. The search terms were combined as follows: (“lei gong teng” OR “leigong teng” OR Common Threewingnut Root Extract OR Glucosidorum Tripterygll Totorum OR leigongtengduogan OR Tripterygium Wilfordii OR tripterygium OR triptolide OR Tripterygium Glycosides) AND (random∗) AND (Nephrotic syndrome [MeSh] OR Nephrotic syndrome OR Nephropathy OR NS).
2.2. Inclusion criteria
Studies met all of the following criteria were included: (1) patients were diagnosed with PNS or refractory PNS; (2) randomized controlled trials (RCTs); (3) treatment regimens based on TwHF; (4) primary outcomes were complete remission and total remission. The secondary outcomes were hr urinary protein excretion, serum albumin, serum creatinine, and urea nitrogen. (5) There were no limitations on year of publication and publication status.
2.3. Study selection
We used ENDNOTE X7 literature management software to manage literature search records. According to a prior eligibility criteria, 2 reviewers independently screened the title and abstract of all the retrieved records. Full-texts of any potentially eligible were downloaded for further screening. Any conflict was resolved by discussion.
2.4. Data items
Microsoft Excel 2010 (Microsoft Corp, Redmond, WA, www.microsoft.com) was used to create a data abstraction form for data collection. One reviewer extracted the following information: first author, year of publication, health status, journal of publication, kidney function, sample, mean age, intervention arms, intervention duration, producer of intervention, and dosage of intervention, and outcomes), and another reviewer checked out them.
2.5. Risk of bias of individual studies
We assessed risk of bias in included RCTs according to the Cochrane Handbook version 5.2.0 that included random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias and detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other resources of bias.[13] We answered these domains as low, high, or unclear risk of bias by 2 dependent reviewers, and conflict was resolved by a third reviewer.
2.6. Data analysis
We conducted a Bayesian network meta-analysis using gemtc package version 0.8.1 of R-3.4.0 software.[14] Four Markov chains ran simultaneously. For each chain we set 5000 simulations as the ‘burn-in’ period. Then posterior summaries were based on 50, 000 subsequent simulations. The model convergence was estimated using Brooks-Gelman-Rubin plots method.[15]
Heterogeneity across head-to-head trials was assessed using I2 statistics. The values of 25%, 50%, and 75% for the I2 were considered as an indication of low, moderate, and high statistical heterogeneity, respectively. Pooled odds ratio (OR) with 95% credible intervals (CrIs) was calculated for dichotomous data. Mean difference (MD) or standard mean difference (SMD) for continuous data. In addition, rank probability was also calculated, which indicated the probability for each treatment to be best, second best, and so on. We assessed statistical inconsistency between direct and indirect evidence at the paired comparison level using node splitting method. A post hoc subgroup analysis was conducted to explore the differences based on the type of PNS (refractory PNS and PNS) for primary outcomes.
3. Results
3.1. Literature selection
A total of 386 records were identified initially. Of them, 158 records were duplicates. Upon further assessment, 17 records were excluded because they were animal studies. 152 citations were excluded during screening of titles and abstracts. After reviewing full-texts, 18 articles were further excluded. Finally, 40 studies with 2846 patients met our inclusion criteria.[16–56] The flow graph of literature selection is presented in Figure 1.
Figure 1.

The flow graph of literature selection.
3.2. Characteristics of included studies
The characteristics of included articles are shown in Table 1. Included RCTs were published between 1998 and 2016. A total of 11 intervention regimens were included in this study (Fig. 2): TwHF, CPA, prednisone, CPA+prednisone, TwHF+prednisone, leflunomide+TwHF, leflunomide+prednisone, Cyclosporine A (CSA)+TwHF, CSA+prednisone, TwHF+CPA, leflunomide+TwHF+prednisone. A total of 19 studies involving 1260 patients received interventions of TwHF and TwHF+prednisone. However, only one study was identified for the following interventions: leflunomide+TwHF, leflunomide+prednisone, CSA+TwHF, CSA+prednisone, and leflunomide+TwHF+prednisone. The risk of bias of included studies was high. Most of studies did not report the methods of random sequence generation, allocation concealment, blinding, and incomplete outcome (Fig. 3).
Table 1.
Characteristic of included studies.

Figure 2.
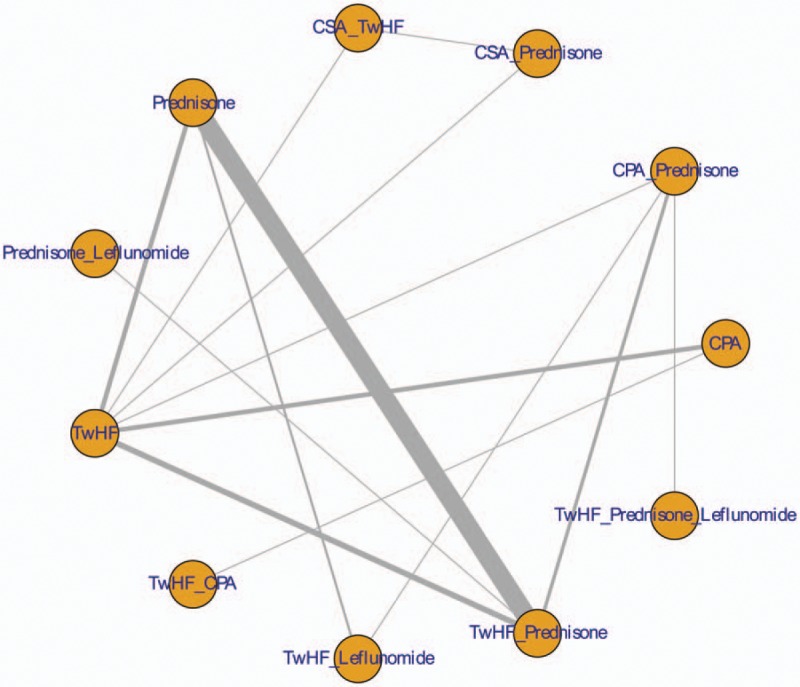
Network plot.
Figure 3.

Results of risk of bias.
3.3. Network meta-analysis
Compared with prednisone, the improvement in total remission rate and complete remission rate were associated with TwHF alone (OR = 4.80, 95% CrI: 2.20–10.00; OR = 6.30, 95% CrI: 2.90–13.00, respectively), TwHF+prednisone (OR = 2.10, 95% CrI: 1.30–3.50; OR = 2.40, 95% CrI: 1.50–3.80, respectively), TwHF+CPA (OR = 12.00, 95% CrI: 1.10–150.00; OR = 16.00, 95% CrI: 1.60–170.00, respectively), and TwHF+CSA (OR = 28.00, 95% CrI: 3.20–250.00; OR = 35.00, 95% CrI: 4.50–270.00, respectively). Compared with TwHF alone, TwHF+prednisone showed less benefit in improving total remission rate and complete remission rate (OR = 0.44, 95% CrI: 0.21–0.91; OR = 0.38, 95% CrI: 0.19–0.77, respectively). The differences between other comparisons were minimal (Table 2). For continuous outcomes, TwHF alone, TwHF+prednisone could significantly reduce hr urinary protein excretion (MD = −0.69, 95% CrI: −1.30 to −0.14; MD = −1.00, 95% CrI: −1.90 to −0.14, respectively) and increase serum albumin (MD = 5.90, 95% CrI: 2.50–9.30; MD = 3.40, 95% CrI: 1.30–5.50, respectively) when compared with prednisone (Table 3). TwHF alone showed significant reduction in serum creatinine compared with CPA (MD = −19.00, 95% CrI: −37.00 to −0.56) (Table 4). The detailed results of network meta-analysis and direct meta-analysis are summarized in Appendix 1 and Appendix 2.
Table 2.
Results of network meta-analysis for total remission and complete remission.

Table 3.
Results of network meta-analysis for hr urinary protein excretion and serum albumin.

Table 4.
Results of network meta-analysis for urea nitrogen and serum creatinine.

3.4. Ranking results
We did not plan to present the results of ranking probability because the number of included studies and the sample size was small, that would be possible to mislead evidence users.
3.5. Inconsistency between direct and indirect comparisons
We used node-splitting model to assess inconsistency between direct and indirect comparisons, the results showed that there were no inconsistency between all comparison groups (all P > 0.05). Results of node-splitting analysis are provided in Appendix 3.
3.6. Subgroup analysis
We conducted a post hoc subgroup analysis to explore the differences between refractory PNS and PNS. The results of subgroup analysis showed that there were no statistically significant differences between patients with refractory PNS and PNS (Appendix S4).
4. Discussion
The main characteristics of nephrotic syndrome include heavy proteinuria, edema, hypoalbuminemia, and hypercholesterolemia. In the past few years, the incidence of PNS was gradually increasing. Pulse therapy with GC is considered as an effective therapeutic method. However, the toxic effects from GC regimens and high recurrence rate have been often neglected. Chinese herbal medicines, such as TwHF, gained growing attention and interest, and may be proved to be one of viable treatment options for PNS patients.[57] Tripdiolide and triptolide were regarded as the major active components of TwHF.[58] Mechanism study indicated that triptolide could inhibit effectively B7, CD40 h, and C3 expression in vitro and further to inhibit the immunoregulatory and proinflammatory capacity of TNF-α that activate human renal proximal tubular epithelial cells.[59]
This systematic review aims to provide a comprehensive evidence to compare the efficacy of TwHF in the treatment of PNS. The results combining direct with indirect evidence, in terms of total remission, complete remission, hr urinary protein excretion, serum albumin, serum creatinine, and urea nitrogen, proved that TwHF appeared to provide more benefit for PNS patients, although other regimens also showed slightly differences in improving the outcomes of patients. Chen et al assessed the efficacy of TwHF using traditional meta-analysis methods, resulted that TwHF significantly increased complete remission; however, the effect of TwHF on urinary protein excretion and serum albumin had no significant difference.[1] In the present study, we combined direct with indirect evidence to inform the effect of TwHF. Similar to Chen et al's study, we found that TwHF significantly improved total remission rate and complete remission rate. However, we also found that the addition TwHF to prednisone, CPA, and CSA could significantly improve the total remission rate and complete remission rate compared to prednisone alone. In addition, TwHF alone and TwHF+prednisone could significantly reduce hr urinary protein excretion and increase serum albumin compared to prednisone. Furthermore, we added the evidence of comparing TwHF alone to TwHF+prednisone, which indicated TwHF alone showed more benefit than TwHF+prednisone.
In this meta-analysis, we mainly focused on the efficacy of different therapy regimens based on TwHF. The reporting of included studies on adverse events has been inconsistent; we could not synthesis the data of adverse events. According to the results, TwHF alone and the addition TwHF to prednisone showed more benefit than other regimens in improving total and complete remission rate, hr urinary protein excretion, serum albumin, and serum creatinine for patients with PNS; however, some adverse events related to these regimens were reported in individual studies, including leukopenia (TwHF:1.15%[22,32,51]; TwHF+prednisone: 0.45%[17,26,27]), slightly impaired liver function (TwHF:3.00%[32,52,56]; TwHF+prednisone: 1.49%[16–18,20,27]), gastrointestinal reaction (TwHF:3.93%[27,32,45,51,56]; TwHF+prednisone: 2.24%[17,18,22,23,26,38,44]), cushing syndrome (TwHF:0%; TwHF+prednisone: 0.60%[18,20]), and complicated with bacterial infection (TwHF:0%; TwHF+prednisone: 0.60%[18,20]).
Ranking of interventions is one of the most appealing elements of network meta-analysis, which could indicate the probability for each treatment to be best, second, and so on. Methodological study has showed that treatment rankings derived from network meta-analyses have a substantial degree of imprecision, especially when the sample size was small.[60] In the present study, we included extremely small number of studies and small sample sizes for most of comparisons, for example, only 2 studies involving 47 patients assessed the efficacy of CSA+TwHF, and one study involving 33 patients assessed leflunomide+TwHF. We considered that the present study existed the serious uncertainty of rankings. To avoid mislead evidence users, we did not plan to present the results of ranking.
There was no network meta-analysis to examine relative efficacy between different regimens based on TwHF until now. Our study firstly combined direct with indirect evidence to compare relatively efficacy of TwHF, CPA, prednisone, and their combination regimens. We performed a comprehensive search of the literature including Cochrane Library, EMBASE, and PubMed to identify all potential studies. However, all studies were conducted in China and published in Chinese. The reporting of included studies was poor, which led to the most of answers to risk of bias were unclear. Although the benefit of TwHF was observed, more high quality studies were warned. Second, because of the inconsistent reporting about adverse effects of TwHF, we could not perform a network meta-analysis to compare the differences of adverse events in different treatment regimens, which indicated that future studies should concern the relevant adverse events of the use of TwHF. In addition, although a detailed subgroup analysis on the type of nephropathy was useful in clinical practice as a kind of precise evidence, because of the limitation of reporting of included studies, we only conducted a subgroup analysis to compare the differences between refractory PNS and primary PNS.
In conclusions, both direct and indirect evidence indicated that TwHF alone, the addition TwHF to prednisone, CPA, and CSA might be better to improve total remission rate, complete remission rate, hr urinary protein excretion, serum albumin, and serum creatinine. However, studies included paid less attention to the adverse effects from the use of TwHF. More RCTs with large sample size and high quality are warned to confirm the important role of TwHF and traditional Chinese medicine.
Author contributions
WXB and DEL planned and designed the research; XGZ and MRL provided methodological support/advice; WXB, DEL, and XGZ tested the feasibility of the study; WXB, DEL, XGZ, and DRL extract data; WXB and WXB performed the statistical analysis; WXB wrote the manuscript; all authors approved the final version of the manuscript.
Conceptualization: Xin-bin Wang, En-lai Dai.
Data curation: Xin-bin Wang, En-lai Dai, Rui-ling Ma.
Formal analysis: Xin-bin Wang.
Investigation: Xin-bin Wang, Guo-zhong Xue, Rui-ling Ma.
Methodology: Xin-bin Wang, En-lai Dai.
Software: Xin-bin Wang, Guo-zhong Xue.
Supervision: En-lai Dai.
Writing – original draft: Xin-bin Wang.
Writing – review and editing: Xin-bin Wang, En-lai Dai, Guo-zhong Xue, Rui-ling Ma.
Supplementary Material
Footnotes
Abbreviations: CBM = Chinese Biological Medical Database, CNKI = Chinese National Knowledge Infrastructure, CPA = cyclophosphamide, CrIs = credible intervals, CSA = Cyclosporine A, GC = glucocorticoid, MD = mean difference, OR = odds ratio, PNS = primary nephrotic syndrome, RCTs = randomized controlled trials, SMD = standard mean difference, TwHF = Tripterygium wilfordii Hook F.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Chen Y, Gong Z, Chen X, et al. Tripterygium wilfordii Hook F (a traditional Chinese medicine) for primary nephrotic syndrome. Cochrane Database Syst Rev 2013;11:CD008568. [DOI] [PubMed] [Google Scholar]
- [2].Hull RP, Goldsmith DJ. Nephrotic syndrome in adults. BMJ 2008;336:1185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xu G, Tu W, Jiang D, et al. Tripterygium wilfordii Hook F treatment for idiopathic refractory nephrotic syndrome in adults: a meta-analysis. Nephron Clin Pract 2009;111:c223–8. [DOI] [PubMed] [Google Scholar]
- [4].Xiong F, Shao DN. Meta-analysis on Tripterygium wilfordii Hook F in the treatment of nephrotic syndrome. J Clin Nephrol 2011;11:1671–2390. [Google Scholar]
- [5].Valeri A, Radhakrishnan J, Estes D, et al. Intravenous Pulse cyclophosphamide treatment of Sever lupus nephritis: a prospective five-year study. Clin Nephrol 1994;42:71. [PubMed] [Google Scholar]
- [6].Peng XM. Treatment of refractory nephrotic syndrome with large dosage of thalidomide intravenous shock therapy. Chin J Nephrol 1996;03:64. [Google Scholar]
- [7].Wang YL. Clinical observation on the treatment of nephrotic syndrome with integrated traditional Chinese and Western Medicine. Central Plains Med J 2006;33:64–5. Chinese. [Google Scholar]
- [8].Wang WF, Chen XH. Treatment of 36 cases of primary nephrotic syndrome with integrated traditional Chinese and Western Medicine. JIANGXI JOURNAL OF TRADITIONAL CHINESE MEDICINE 1993;1:44–5. [Google Scholar]
- [9].Song HX, Wang ZF, Chen XT. Clinical study of cyclophpsphamide and yripterygium xwilfordii in treating nephrotic syndrome. J Linyi Med Coll 1998;20:109–12. [Google Scholar]
- [10].Luo L, Lei ZJ. Meta-analysis on clinical therapeutic effects and safety of Tripterygium Glycosides on Nephrotic Syndrome Compared with Cytoxan. Haixia Yixue 2016;7:82–5. [Google Scholar]
- [11].Hu LH, Wang CQ. Evaluation of efficacy and safety of Tripterygium wilfordii combined with prednisone in the treatment of adult primary nephrotic syndrome. Chin J Integr Trad Western Nephrol 2016;17:50–3. [Google Scholar]
- [12].Bafeta A, Trinquart L, Seror R, et al. Reporting of results from network meta-analyses: methodological systematic review. BMJ 2014;23:g1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0, Cochrane. 2017. Available from: www.training.cochrane.org/handbook. Accessed December 6, 2016. [Google Scholar]
- [14].Van Valkenhoef G, Kuiper J. gemtc: Network meta-analysis using Bayesian methods. 2014. Available from: http://cran. rproject. org/web/packages/gemtcpdf. Accessed December 6, Dec 2016. [Google Scholar]
- [15].Wu HY, Huang JW, Lin HJ, et al. Comparative effectiveness of renin-angiotensin system blockers and other antihypertensive drugs in patients with diabetes: systematic review and bayesian network meta-analysis. BMJ 2013;347:f6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shen LR, Zhang LJ, Wang SJ. To observe the clinical efficacy of cyclophosphamide combined with methylprednisolone in the treatment of refractory nephrotic syndrome. Chin J Med Guide 2012;14:163–4. Chinese. [Google Scholar]
- [17].Su ZY. Clinical observation of 40 cases of senile primary nephrotic syndrome treated with Tripterygium wilfordii combined with prednisone. Chin Foreign Med Res 2014;13:43–4. [Google Scholar]
- [18].Bao Y. Clinical study of Tripterygium wilfordii combined with small dose prednisone in the treatment of senile primary nephrotic syndrome. Asia-Pacific Trad Med 2013;9:172–3. [Google Scholar]
- [19].Xiao YC. Clinical observation of 56 cases of primary nephrotic syndrome treated with Tripterygium Wilfordii Hook. Appl J Gen Pract 2007;5:312–3. [Google Scholar]
- [20].Du JL. The short-term efficacy of Tripterygium Wilfordii in the treatment of primary nephrotic syndrome. Health Weekly 2016;6:65–7. Chinese. [Google Scholar]
- [21].Xu YZ, Huang ZQ, Tang R. Clinical observation of Tripterygium wilfordii combined with prednisone in the treatment of senile nephrotic syndrome. Chin J Primary Med Pharm 2009;16:781–2. [Google Scholar]
- [22].Gong JH. Clinical observation of Tripterygium wilfordii combined with low-dose prednisone in the treatment of senile primary nephrotic syndrome. Chin Foreign Med Res 2014;21:11–2. Chinese. [Google Scholar]
- [23].Jiang YL. Clinical observation of Tripterygium wilfordii combined with low-dose prednisone in the treatment of senile primary nephrotic syndrome. Strait Pharm J 2013;25:76–7. [Google Scholar]
- [24].Chen WW. Efficacy of Tripterygium wilfordii combined with moderate dose prednisone in the treatment of primary nephrotic syndrome in the elderly. World J Integr Trad Western Med 2013;8:1031–3. [Google Scholar]
- [25].Guo peng. Comparison of Tripterygium wilfordii glycosides and cyclophosphamide in the treatment of nephrotic syndrome. Everybody Health 2016;2:143–143. [Google Scholar]
- [26].Zhou ZZ. Clinical observation of small dose prednisone combined with Tripterygium Wilfordii in the treatment of senile primary nephrotic syndrome. Mod Diagn Treat 2015;26:4860–1. [Google Scholar]
- [27].Wang HT. Efficacy of conventional dose of Tripterygium wilfordii combined with low-dose prednisone in the treatment of primary nephrotic syndrome in the elderly. Contemp Med Forum 2014;10:190–1. [Google Scholar]
- [28].Guan ZX, Chen JH. Effect of medium dose prednisone combined with Tripterygium wilfordii on primary nephrotic syndrome in the elderly. J Clin Exp Med 2012;11:345–6. [Google Scholar]
- [29].Li XH. Effect of tripterygium glycosides on primary nephrotic syndrome in middle-aged and elderly patients. Chin J Clin Healthcare 2015;5:514–6. [Google Scholar]
- [30].Liu JP. Observation on the effect of multi Ying and supplementary treatment of primary nephrotic syndrome in middle and old aged patients. Mod Med 2016;22:135–6. Chinese. [Google Scholar]
- [31].Tan W. The clinical effect of Tripterygium Duoer tablets combined with drug treatment of nephrotic syndrome was observed in 30 cases. Guide China Med 2014;12:140–1. [Google Scholar]
- [32].Song B. Comparison between the therapeutic effects of cyclophamide and tripterygium wilfrdii hooks on children's refractory nephrotic syndrome. Chin Pract Med 2008;14:7–8. [Google Scholar]
- [33].Jiang GR. The clinical efficacy of leflunomide combined with tripterygium glycosides and low-dose prednisone in the treatment of refractory nephrotic syndrome in middle and elderly patient. Chin JN Clin Med 2014;6:519–21. [Google Scholar]
- [34].Cui XX, Wang XF, Fan JX. Clinical observation of leflunomide combined with Tripterygium Wilfordii in the treatment of refractory nephrotic syndrome. Med Innov China 2011;8:38–9. [Google Scholar]
- [35].Liu ZH. Leflunomide combined with Tripterginum Wilfordii polyglycoside for refractory nephrotic syndrome. Prog Mod Biomed 2011;11:738–41. Chinese. [Google Scholar]
- [36].Wang HW, Chen JH. A comparative study of Tripterygium Wilfordii and prednisone in the treatment of senile nephrotic syndrome. J Youjiang Med Coll Nationalities 2000;22:883–4. [Google Scholar]
- [37].Ma P. Effect of Tripterygium wilfordii combined with small dose of prednisone on nephrotic syndrome. Clin Focus 1991;05:210–1. [Google Scholar]
- [38].Du X. Tripterygium wilfordii combined with methylprednisolone curative effect observation on the treatment of nephrotic syndrome in the elderly. Jilin Med J 2012;33:283–4. [Google Scholar]
- [39].Liu ZH, Yuan F, Wang YF. Treatment of 30 cases of refractory nephrotic syndrome with Tripterygium Wilfordii and prednisone. Med Innov China 2010;7:1–2. [Google Scholar]
- [40].Zhou B. Efficacy of Tripterygium wilfordii combined with glucocorticoid in the treatment of refractory nephrotic syndrome. J Trad Chin Med Univ of Hunan 2016;36:62. [Google Scholar]
- [41].Niu HJ, Wang X, Chen XY, et al. Effect of Tripterygium wilfordii combined with glucocorticoid in the treatment of nephrotic syndrome. Anhui Med J 2015;3:310–2. Chinese. [Google Scholar]
- [42].Liu DJ, Liu HH, Wei G. Tripterygium wilfordii combined with medium dose methylprednisolone curative effect observation on the treatment of nephrotic syndrome in the elderly. Chin J Geriatr Care 2010;08:51–2. Chinese. [Google Scholar]
- [43].Zhang XJ, Zhou SJ. Comparison of the effects of Tripterygium Wilfordii and leflunomide in the treatment of refractory nephrotic syndrome. Jilin Med J 2015;9:1766–1766. [Google Scholar]
- [44].Fan DY, Xu CG. Efficacy of Tripterygium wilfordii combined with glucocorticoid in the treatment of refractory nephrotic syndrome. Chin Arch Trad Chin Med 2014;32:958–60. [Google Scholar]
- [45].Luo ZM. Comparative study of tripterygium glycosides and cyclophosphamide in the treatment of nephrotic syndrome. Med People 2014;5:65. [Google Scholar]
- [46].Wu B. Clinical efficacy of Tripterygium Wilfordii and glucocorticoid in the treatment of refractory nephrotic syndrome. J Qiqihar Univ Med 2015;36:3326–7. [Google Scholar]
- [47].Piao CM. Clinical observation of treatment of refractory nephrotic syndrome with Tripterygium wilfordii combined with glucocortico. Chin J Mod Drug Appl 2015;9:135–6. Chinese. [Google Scholar]
- [48].Huo J, Su M. Clinical study of Tripterygium wilfordii combined with glucocorticoid in treatment of refractory nephrotic syndrome. Shenzhen J Integr Trad ChinWestern Med 2015;25:46–7. [Google Scholar]
- [49].Wang XX. Effect analysis of prednisone combined with Tripterygium Glycosides in the treatment of refractory nephrotic syndrome. China Pract Med 2016;11:136–7. [Google Scholar]
- [50].Wang MH, Qin M. Efficacy of Tripterygium wilfordii combined with glucocorticoids in the treatment of refractory nephrotic syndrome. Contemp Med Forum 2014;12:129–129. [Google Scholar]
- [51].Chen ZM, Chen ZJ. Clinical analysis of 36 cases of refractory nephrotic syndrome treated with large dose of Tripterygium wilfordii glycosides. Chin Gen Pract 2003;6:948. [Google Scholar]
- [52].Han JH, Mou ZL, Wang JP. Cyclosporine A combined with Tripterygium wilfordii multi 2 treatment of refractory nephrotic syndrome. Clin Focus 1999;18:841–2. [Google Scholar]
- [53].Song HX, Wang ZF, Chen XT. Clinical study on the treatment of nephrotic syndrome with cyclic adenosine phosphate combined with Tripterygium wilfordii. Linyi Med Special Newspaper 1998;20:109–12. [Google Scholar]
- [54].Zhao DG, Zhao X. Tripterygium wilfordii combined with prednisone and chronic glomerular disease in 64 cases. Shanxi J TCM 1999;15:26–7. Chinese. [Google Scholar]
- [55].Ada, Su JH, Wang ZC. A clinical randomized controlled trial of Tripterygium Wilfordii in the treatment of 18 cases of primary IgA nephropathy syndrome. Contemp Med 2008;06:12–3. [Google Scholar]
- [56].Zhao L. A comparative study of Tripterygium Wilfordii and cyclophosphamide in the primary nephrotic syndrome. Med J 2009;31:2160–1. [Google Scholar]
- [57].Patavino T, Brady DM. Natural medicine and nutritional therapy as an alternative treatment in systemic lupus erythematosus. Altern Med Rev 2001;6:460–71. [PubMed] [Google Scholar]
- [58].Tao X, Cai JJ, Lipsky PE. The identity of immunosuppressive components of the ethyl acetate extract and chloroform methanol extract (T2) of Tripterygium wilfordii Hook. F. J Pharm Exp Ther 1995;272:1305–12. [PubMed] [Google Scholar]
- [59].Hong Y, Zhou W, Li K, et al. Triptolide is a potent suppressant of C3, CD40 and B7 h expression in activated human proximal tubular epithelial cells. Kidney Int 2002;62:1291–300. [DOI] [PubMed] [Google Scholar]
- [60].Trinquart L, Attiche N, Bafeta A, et al. Uncertainty in treatment rankings: reanalysis of network meta-analyses of randomized trials. Ann Intern Med 2016;164:666–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


