Abstract
Ogilvie's syndrome, also known as acute colonic pseudo-obstruction, refers to pathologic dilation of the colon without underlying mechanical obstruction, occurring primarily in patients with serious comorbidities. Diagnosis of Ogilvie's syndrome is based on clinical and radiologic grounds, and can be treated conservatively or with interventions such as acetylcholinesterase inhibitors (such as neostigmine), decompressive procedures including colonoscopy, and even surgery. Based on our clinical experience we hypothesized that conservative management yields similar, if not superior, results to interventional management. Therefore, we retrospectively examined all patients over the age of 18 with Ogilvie's syndrome who presented to the Medical University of South Carolina (MUSC). The diagnosis of Ogilvie's syndrome was confirmed by clinical criteria, including imaging evidence of colonic dilation ≥9 cm. Patients were divided and analyzed in 2 groups based on management: conservative (observation, rectal tube, nasogastric tube, fluid resuscitation, and correction of electrolytes) and interventional (neostigmine, colonoscopy, and surgery). Use of narcotics in relation to maximal bowel size was also analyzed. Over the 11-year study period (2005–2015), 37 patients with Ogilvie's syndrome were identified. The average age was 67 years and the average maximal bowel diameter was 12.5 cm. Overall, 19 patients (51%) were managed conservatively and 18 (49%) underwent interventional management. There was no significant difference in bowel dilation (12.0 cm vs 13.0 cm; P = .21), comorbidities (based on the Charlson Comorbidity Index (CCI), 3.2 vs 3.4; P = .74), or narcotic use (P = .79) between the conservative and interventional management groups, respectively. Of the 18 patients undergoing interventional management, 11 (61%) had Ogilvie's-syndrome-related complications compared to 4 (21%) of the 19 patients in the conservative management group (P < .01). There was no difference in overall length of stay in the 2 groups. Two patients, one in each group, died from complications unrelated to their Ogilvie's syndrome. We conclude that Ogilvie's syndrome, although uncommon, and typically associated with severe underlying disease, is currently associated with a low inpatient mortality. While interventional management is often alluded to in the literature, we found no evidence that aggressive measures lead to improved outcomes.
Keywords: anti-cholinesterase, colonic pseudo-obstruction, colonoscopy, decompression, mortality, nasogastric tube, neostigmine, prognosis, rectal tube
1. Introduction
Acute colonic pseudo-obstruction, also known as Ogilvie's syndrome, was first described in 1948 and refers to massive dilation of the colon without underlying mechanical obstruction or other organic cause.[1] The pathophysiologic basis of Ogilvie's syndrome remains unclear but is believed to be due to a functional disturbance in the enteric nervous, leading to an “adynamic colon,” massive dilation, and perforation.[2] Ogilvie's syndrome is typically found in hospitalized patients, who most often have severe comorbid conditions, such as severe musculoskeletal abnormalities, trauma, surgery, or sepsis, and is associated with increased morbidity and mortality.[1,3] Other conditions that appear to increase the risk of Ogilvie's syndrome include electrolyte imbalances, medications (ie, narcotics, anticholinergics), and debilitation.[4,5]
Clinical manifestations of Ogilvie's syndrome vary, and include abdominal distention and pain (80%), nausea with vomiting (60%), and obstipation (60%).[3] The diagnosis depends on exclusion of structural and known causes of colonic dilation, as well as clinical and radiologic evidence.[6–9] Ogilvie's syndrome usually involves the cecum and right colon, but can involve any part or all of the colon.[10] Typically, diameters >14 cm are believed to be associated with a high risk of perforation.[4,5] Perforation and intestinal ischemia are the most serious complications and are the major reason that intervention is often attempted.[2]
Many different approaches have been used to manage Ogilvie's syndrome. It may be managed by addressing underlying conditions (ie, discontinuation of narcotics, correction of electrolyte abnormalities[3]) and/or decompressing the gastrointestinal tract via nasogastric (NG) tube and/or rectal tube insertion. Additionally, neostigmine has gained popularity due to a small randomized study suggesting its benefit.[11] Decompressive colonoscopy, although not established in randomized clinical trials, is also often used, as it can provide immediate colonic decompression.[12,13]
Despite having been first described nearly 70 years ago, literature focused on the management of Ogilvie's syndrome remains sparse and consists primarily of single case reports and expert opinion. In this study, we have hypothesized that conservative management, with treatment of underlying conditions, discontinuation of drugs that alter bowel motility, and correction of underlying electrolyte abnormalities is highly effective and is rarely associated with progression to bowel perforation. Therefore, we have investigated the prevalence, etiology, management, and outcomes of Ogilvie's syndrome among patients at our institution.
2. Methods
2.1. Study population
All patients over the age of 18 with Ogilvie's syndrome (pseudo-obstruction of the colon ICD-9: 560.82) between January 2005 and December 2015 at the Medical University of South Carolina (MUSC), a tertiary care and academic medical center in Charleston, SC, were included in this retrospective cohort. A diagnosis of Ogilvie's syndrome required radiographic evidence of colonic dilatation >9 cm, the historically accepted diameter at which treatment to avoid complications was warranted.[4] The data collected included demographics, medical and surgical history, reason for admission, admitting service, interventions or procedures during the admission, and discharge status. Patients missing historical, clinical, laboratory, or treatment data, or with Ogilvie's syndrome discovered incidentally during surgery were excluded (Fig. 1).
Figure 1.
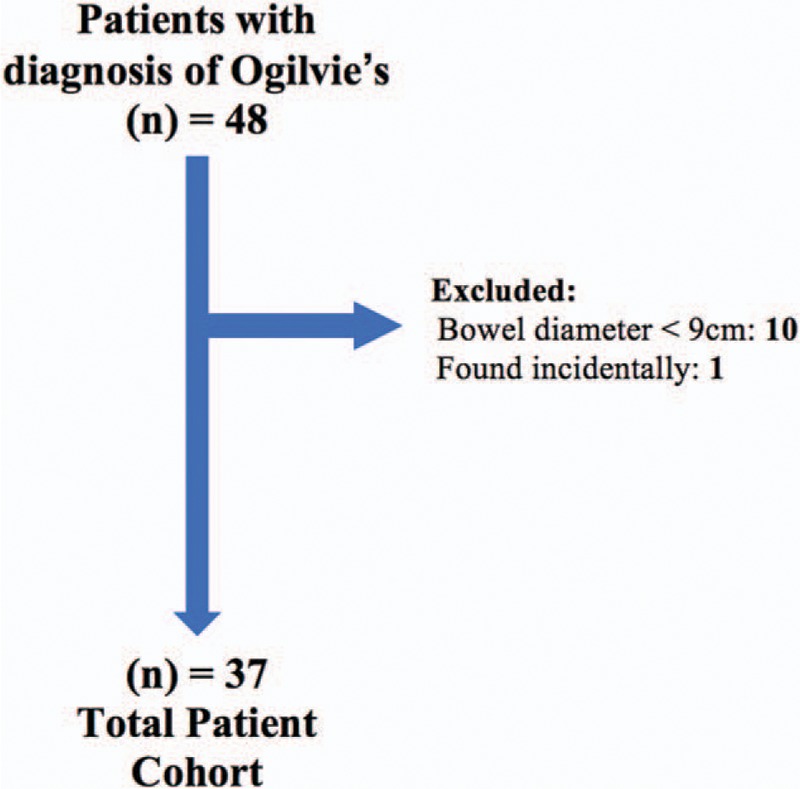
Potential patients from 2005 to 2015 at the Medical University of South Carolina were screened using the ICD-9 code: 560.82. Forty-eight patients with Ogilvie's syndrome were identified. Ten patients were excluded because they had a maximal bowel diameter <9 cm and 1 patient was excluded due to incidental discovery of colonic obstruction during unrelated laparotomy.
Patients were divided into 2 groups: those receiving conservative management and those receiving interventional management. Conservative management was defined as placement of a NG tube and/or rectal tube to aid in gastrointestinal tract decompression, correction of serum electrolytes, withdrawal of narcotics or other predisposing medications, and administration of bowel prep. Interventional management included administration of neostigmine, decompressive colonoscopy and/or sigmoidoscopy, placement of a gastrostomy tube with wall suction, and surgical interventions such as colostomy or colectomy.
2.2. Data and statistical analysis
Primary endpoints were inpatient mortality and time to resolution of obstruction. Secondary endpoints included clinical complications associated with management of Ogilvie's syndrome, defined as ischemia or perforation of the colonic wall, primary failure of treatment or recurrence of Ogilvie's syndrome during the same inpatient admission, or severe bradycardia leading to clinical symptoms. Resolution was defined as normal imaging (by abdominal radiographic series or computed tomography) or relief of abdominal distention on physical examination with return of bowel movements. The Charlson comorbidity index (CCI) was used to assess the severity of underlying comorbidities, as previously reported.[14]
Statistical analyses were performed using Statistical Analysis System (SAS) versions 9.4 (SAS Institute, Cary, NC) and GraphPad Prism version 6.04 for Mac (GraphPad software, La Jolla, CA). Student's t test was used for continuous variables and was reported as means with corresponding standard deviations. Fisher exact test was used for categorical variables and was reported as percentages. The correlation between narcotic use and dose over 48 hours, and maximal bowel diameter was assessed using Spearman analysis. All narcotics were calculated to the equianalgesic dose of oral oxycodone using the Johns Hopkins Opioid Conversion Program Calculator.[15,16] Results are reported as percentages, odds ratios with corresponding 95% confidence intervals (CIs), and mean with standard deviations, where appropriate. Statistical significance was set at P < .05 and all reported P-values were 2-tailed.
2.3. Study approval and data use agreement
The Institutional Review Board at the Medical University of South Carolina (MUSC) approved the research protocol prior to initiation of this research. A data use agreement was in place with the Clinical Data Warehouse at MUSC for the identification and use of inpatient data. The study met all guidelines for good clinical practice.[17]
3. Results
From January 2005 to December 2015, 48 patients diagnosed as having Ogilvie's syndrome were identified as outlined in Section 2 (Fig. 1), with 38 patients having colonic dilatation >9 cm. One patient was excluded after colonic dilatation was discovered during an unrelated laparotomy for complicated pancreatitis, leaving a cohort of 37 patients with symptomatic Ogilvie's syndrome. Women represented 27% of the cohort, the average age was 67 years, and the average maximal bowel diameter was 12.5 cm (Table 1). Nineteen patients (51%) were managed conservatively and 18 (49%) had some form of interventional management (Table 1). Age, race, and clinical features were similar among the 2 groups. Women were more likely to receive conservative management compared to males (P = .008).
Table 1.
Patient cohort characteristics.
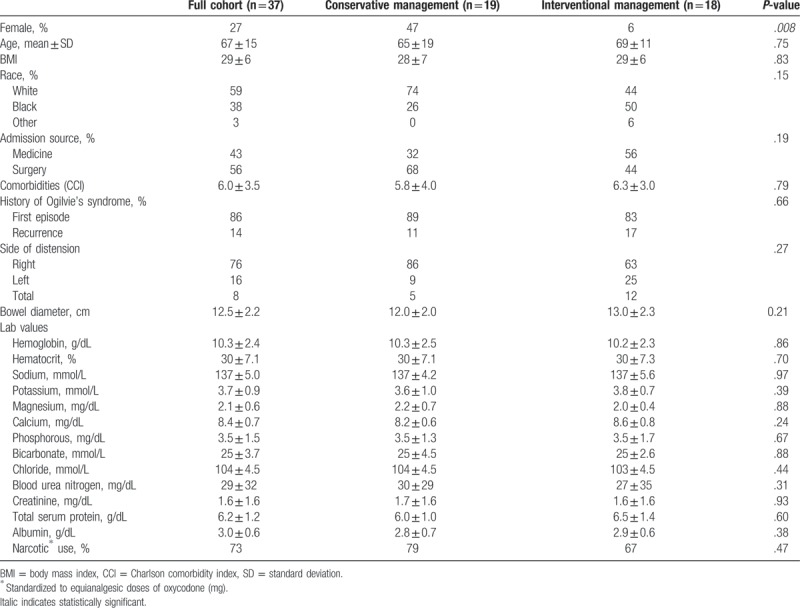
The average maximal bowel dilation size was 12.0 cm in the conservative management group and 13.0 cm in the interventional management group (P = .21)). Of note, comorbidities were similar in both groups of patients as evidenced by similar CCI scores (Table 1). The majority of patients in both groups were admitted with their first episode of Ogilvie's syndrome, although several patients had recurrent disease (17% vs 11%; 95% CI; P = .66) (Table 1). While more patients in the conservative management group were admitted through a surgical service (68% vs 44%), this was not significantly different among the groups (95% CI; P = .191).
Overall, the most common location of dilation was the cecum and/or right hemicolon (76%) (Table 1). A greater number of patients in the interventional management group had a left-sided or total colonic dilatation, compared to the conservative management group (Table 1). All left-sided and total colonic dilatations occurred in males. As expected, primary neurologic and orthopedic disorders were common in patients in the cohort (Table 2). Stroke, primary and metastatic brain tumors, and subdural hemorrhages were responsible for the neurologic disorders, while hip replacements, spinal surgeries, and fractures after motor vehicle accidents characterized orthopedic disorders in the cohort (Table 2).
Table 2.
Underlying clinical conditions among inpatients developing Ogilvie's syndrome (n = 37).

Electrolyte levels at onset of Ogilvie's syndrome were similar in the conservative and intervention management groups (Table 1). Hypocalcemia (serum Ca2+ < 8.4 mg/dL) was present in 62% of patients at onset of Ogilvie's syndrome, with hypokalemia (serum K+ < 3.5 mg/dL) and hypophosphatemia (serum PO43- < 2.4 mg/dL) present in 35% (Table 3). Serum calcium levels at onset were found to correlate with maximal bowel diameter (P = .01, R2 = 0.17) (Fig. 2), though no correlation was identified with the other electrolytes. We also investigated the influence of narcotics as a predisposing factor in this cohort of patients. Narcotic doses were converted to equianalgesic doses of oral oxycodone (mg) as in Section 2 (Methods); a trend between maximal bowel size and the 48-hour narcotic dose immediately preceding the index diagnosis of Ogilvie's syndrome was noted (Fig. 3).
Table 3.
Electrolyte abnormalities at onset of Ogilvie's syndrome (n = 37).
Figure 2.
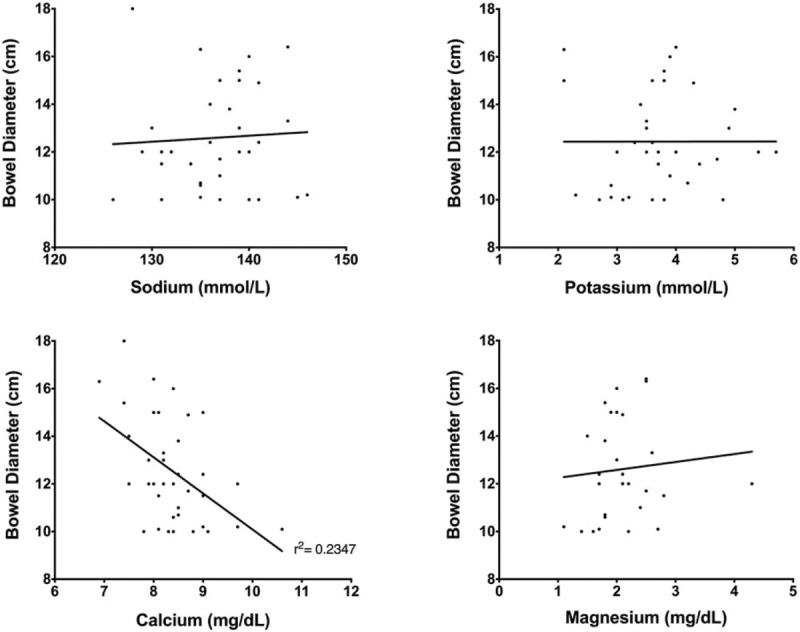
Maximal bowel diameter (cm) and serum electrolytes. Correlation analyses were used to investigate the relationship between maximal bowel diameter (cm) and serum electrolytes at onset (defined as the date of radiologic diagnosis of Ogilvie's syndrome). The correlation between the 2 variables was determined using Spearman correlation coefficient for non-normal data. A correlation was found only between serum calcium levels and bowel size (r2 = 0.2347).
Figure 3.
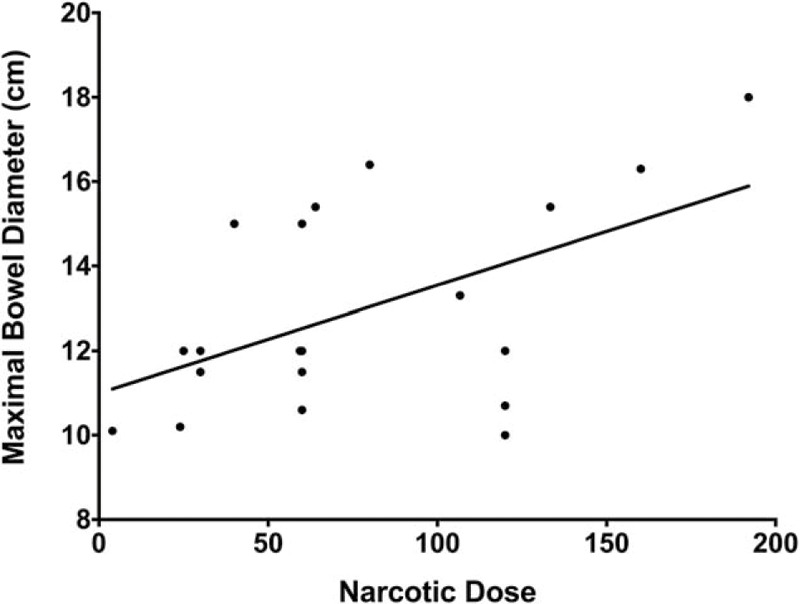
Relationship between narcotics and colon bowel diameter. Spearman correlation coefficient was determined for the 20 patients who received narcotics before and during the onset of Ogilvie's syndrome to assess a relationship between 48-hour dose of narcotics and maximal bowel size (cm). All narcotics doses were standardized to equianalgesic doses of oxycodone (mg), as described in Methods.
The overall time to resolution, defined as radiographic evidence of decreased bowel diameter or clinical evidence of decreased abdominal distension and/or resolution of pain or obstipation, was 5 days, with no difference in either group (Table 4). Overall inpatient length of stay (LOS) was often prolonged, consistent with substantial underlying disease in the entire cohort of patients. One patient in each group died (inpatient mortality 5.4% and 5.6% in the conservative and interventional management groups, respectively) from complications unrelated to their Ogilvie's syndrome. The rate of resolution also did not differ between the 2 groups (Fig. 4A). Additionally, the rate of resolution did not differ with the different interventional management options including neostigmine, colonoscopy, and surgery (Fig. 4B).
Table 4.
Outcomes.
Figure 4.
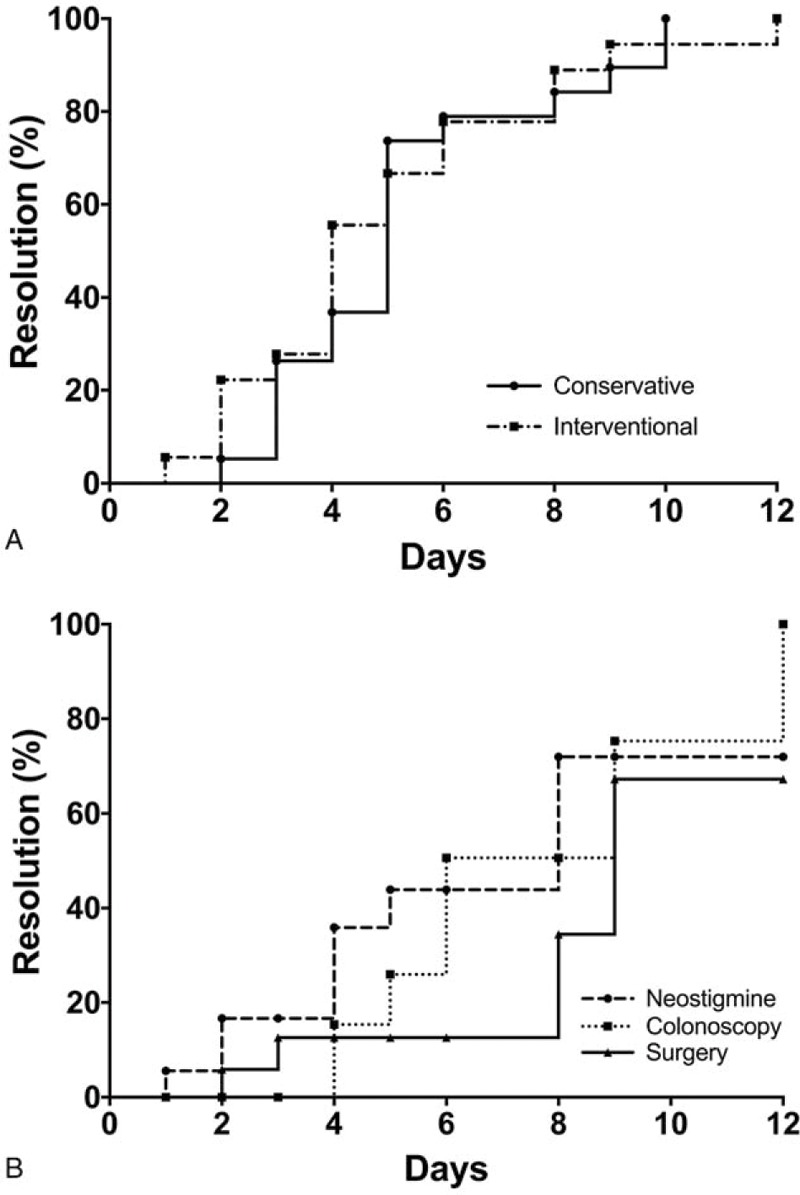
Outcome of patients with Ogilvie's syndrome. Resolution of bowel dilation was defined as decrease in bowel diameter to <9 cm radiologically or clinical resolution of abdominal distension. (A) A comparison of the 19 patients treated conservatively and those who were treated with interventional methods (neostigmine, colonoscopy, surgery). (B) The response to the different interventional methods including neostigmine, colonoscopy, and surgery.
Overall, 15 patients (41%) experienced an Ogilvie-syndrome-related complication, as defined, with recurrence of Ogilvie's syndrome being the most common (24%) (Table 4). Patients in the interventional management group were more likely to experience complications (61% vs 21% in the conservatively managed group; P = .01); bradycardia after administration of neostigmine was the most common complication experienced in this group (17%) and 1 patient (6%) developed colonic ischemia after an initial colonoscopy, requiring urgent colectomy (Table 4). We used multiple regression analysis to identify independent risk factors for developing an Ogilvie's-syndrome-related complication, as defined earlier. Age, CCI, gender, narcotic use, maximal bowel diameter, and management approach were assessed. The risk of a complication (as defined) was reduced when conservative management was used (OR: 0.03, 95% CI 0.002–0.512; P = .02).
4. Discussion
Here, we have identified a large cohort of patients with Ogilvie's syndrome and have demonstrated that conservative treatment appears to be safe and effective. Interventional treatments for Ogilvie's syndrome such as acetylcholinesterase inhibitors (ie, neostigmine) and decompressive colonoscopy have been recommended by experts and become popular.[18,19] In this study, we report that although these more traditional interventional treatments are commonly used, they do not appear to be any more effective than conservative maneuvers.
Patients with Ogilvie's syndrome typically have serious underlying comorbid conditions, as was the case in our study. Over half of our patients had serious underlying neurologic or orthopedic disease; this is critically important to recognize, as these illnesses are associated with decreased mobility, which in turn appears to be a major predisposing factor in the development of Ogilvie's syndrome. Additionally, many patients had electrolyte abnormalities, notably hypocalcemia (62%), which may alter gut motility, predisposing to pseudo-obstruction.[4,12,20] In combination with decreased ambulation and movement associated with chronic illness, such metabolic disturbances are critical to identify and correct, and their correction likely contributed to the success of conservative treatment in this study.
While the exact pathogenesis of Ogilvie's syndrome is not understood, the fact that it is present in the setting of chronic illness, electrolyte abnormalities, and often narcotic use implies that management of these underlying contributory metabolic or pharmacologic factors is critical. The results of our study suggest that the correction of such inciting factors should be the foundation of management. Although we cannot exclude potential bias (ie, that “sicker” patients received interventional management), we doubt this to be the case since at baseline there was no difference in age, CCI, admission source, or baseline electrolytes between the 2 groups. Additionally, there was no difference in overall LOS, time to resolution, or rate of recovery in the different treatment groups.
We found that patients who had conservative management were less likely to experience an Ogilvie-syndrome-related complication than those having interventional management. Complications included bradycardia secondary to neostigmine, recurrence of Ogilvie's syndrome, progression of distension, and colonic ischemia requiring surgery. While neostigmine was used in 67% of patients in the interventional management group, its use did not appear to lead to superior results to conservative management. Indeed, although neostigmine has been found in small randomized clinical trials to be effective for treatment of Ogilvie's syndrome, those same trials reported numerous adverse effects ranging from increased abdominal pain, severe cholinergic symptoms, as well as severe bradycardia, and syncope.[21] Furthermore, patients with Ogilvie's syndrome receiving neostigmine require monitoring on cardiac telemetry and immediate bedside access to atropine, a setting often difficult to achieve outside of an intensive care unit[18,21]); increasing the hospital costs incurred to the patient. Conservative management, however, is practical, safe, and efficacious.
We recognize strengths and limitations of this study. Perhaps the greatest strength is that this is a large cohort of patients with an uncommon disease. Additionally, we were able to obtain detailed clinical, laboratory, and outcome data on all patients. We recognized the retrospective nature of the study, which could lead to potential cases having been missed due to coding errors. Further, given the retrospective nature of the study, it is possible that there was bias as to which patients had conservative or interventional management (ie, sicker patients with more advanced disease had more aggressive interventional management). While this is certainly possible, we think that it is highly unlikely since the patients were evenly matched for comorbidity and bowel diameter. Additionally, this was a single center study, and thus our experience may not be generalizable to other settings. For example, our practitioners may have pursed interventional management early, which could have biased our results toward either a more favorable outcome for interventional therapy or less favorable interpretation of outcomes for the conservative group. We tend to discount the latter possibility, however, as the outcomes in the conservative group were similar to those in the interventional group.
In summary, despite the fact that Ogilvie's syndrome is a relatively uncommon condition, it is important to recognize early and manage appropriately, particularly in already chronically ill patients. We report here that many patients will respond to conservative management. In fact, from a quality and safety standpoint, the data suggest that at least for certain patients, conservative management may be preferable. Given our findings, clinicians should be highly motivated to implement basic acts of supportive care when managing Ogilvie's syndrome, including correcting electrolyte abnormalities, weaning narcotics, providing a NG or rectal tube for decompression, and encouraging ambulation when possible.
Author contributions
Magda Haj: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
Mona Haj: analysis and interpretation of data; statistical analyses; critical revision of the manuscript for important intellectual content
Don C. Rockey: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; supervisory activities
Conceptualization: Maggie Haj, Mona Haj, Don Rockey.
Data curation: Maggie Haj, Mona Haj.
Formal analysis: Maggie Haj, Mona Haj.
Funding acquisition: Don Rockey.
Investigation: Maggie Haj.
Methodology: Maggie Haj, Mona Haj.
Project administration: Mona Haj, Don Rockey.
Resources: Don Rockey.
Supervision: Mona Haj, Don Rockey.
Writing – original draft: Maggie Haj, Mona Haj, Don Rockey.
Writing – review & editing: Maggie Haj, Mona Haj, Don Rockey.
Footnotes
Abbreviations: CCI = Charlson comorbidity index, LOS = length of stay, MUSC = Medical University of South Carolina, NG = nasogastric.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Kaiser AM. Ogilvie transition to colonic perforation. Am J Surg 2010;200:e15–6. [DOI] [PubMed] [Google Scholar]
- [2].Ross SW, Oommen B, Wormer BA, et al. Acute colonic pseudo-obstruction: defining the epidemiology, treatment, and adverse outcomes of Ogilvie's syndrome. Am Surg 2016;82:102–11. [DOI] [PubMed] [Google Scholar]
- [3].Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie's syndrome). An analysis of 400 cases. Dis Colon Rectum 1986;29:203–10. [DOI] [PubMed] [Google Scholar]
- [4].Maloney N, Vargas HD. Acute intestinal pseudo-obstruction (Ogilvie's syndrome). Clin Colon Rectal Surg 2005;18:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Saunders MD, Kimmey MB. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther 2005;22:917–25. [DOI] [PubMed] [Google Scholar]
- [6].Kahi CJ, Rex DK. Bowel obstruction and pseudo-obstruction. Gastroenterol Clin North Am 2003;32:1229–47. [DOI] [PubMed] [Google Scholar]
- [7].Sheikh RA, Yasmeen S, Pauly MP, et al. Pseudomembranous colitis without diarrhea presenting clinically as acute intestinal pseudo-obstruction. J Gastroenterol 2001;36:629–32. [DOI] [PubMed] [Google Scholar]
- [8].Choi JS, Lim JS, Kim H, et al. Colonic pseudoobstruction: CT findings. AJR Am J Roentgenol 2008;190:1521–6. [DOI] [PubMed] [Google Scholar]
- [9].Godfrey EM, AddleyLee HC, Shaw AS. The use of computed tomography in the detection and characterisation of large bowel obstruction. N Z Med J 2009;122:57–73. [PubMed] [Google Scholar]
- [10].Jacob SE, Lee SH, Hill J. The demise of the instant/unprepared contrast enema in large bowel obstruction. Colorectal Dis 2008;10:729–31. [DOI] [PubMed] [Google Scholar]
- [11].De Giorgio R, Barbara G, Stanghellini V, et al. Review article: the pharmacological treatment of acute colonic pseudo-obstruction. Aliment Pharmacol Ther 2001;15:1717–27. [DOI] [PubMed] [Google Scholar]
- [12].Jetmore AB, Timmcke AE, Gathright JB, Jr, et al. Ogilvie's syndrome: colonoscopic decompression and analysis of predisposing factors. Dis Colon Rectum 1992;35:1135–42. [DOI] [PubMed] [Google Scholar]
- [13].Geller A, Petersen BT, Gostout CJ. Endoscopic decompression for acute colonic pseudo-obstruction. Gastrointest Endosc 1996;44:144–50. [DOI] [PubMed] [Google Scholar]
- [14].D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med 1993;32:382–7. [PubMed] [Google Scholar]
- [15].Hopkins TSKCCCaJ. The Hopkins Opioid Program. Available at: http://www.hopweb.org/index.cfm?cfid=4634198&cftoken=46515837. Accessed August 1, 2016. [Google Scholar]
- [16].Shaheen PE, Walsh D, Lasheen W, et al. Opioid equianalgesic tables: are they all equally dangerous? J Pain Symptom Manage 2009;38:409–17. [DOI] [PubMed] [Google Scholar]
- [17].World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- [18].Nicholson D. Neostigmine for acute colonic pseudo-obstruction. N Engl J Med 1999;341:1623. [DOI] [PubMed] [Google Scholar]
- [19].Jain A, Vargas HD. Advances and challenges in the management of acute colonic pseudo-obstruction (ogilvie syndrome). Clin Colon Rectal Surg 2012;25:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vazquez-Sandoval A, Ghamande S, Surani S. Critically ill patients and gut motility: are we addressing it? World J Gastrointest Pharmacol Ther 2017;8:174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med 1999;341:137–41. [DOI] [PubMed] [Google Scholar]


