Abstract
Rationale:
Charcot arthropathy, also known as Neuropathic arthropathy (NA), is an unusual chronic degenerative disease. To date, there exists a paucity of research on NA caused by syringomyelia.
Patients concerns:
A 52-year-old non-diabetic female presented with progressive swelling, pain and limited movement in her left shoulder joint combined with asthenia of her left upper extremity for three months.
Diagnoses:
Neuropathic arthropathy of the shoulder associated with the cervicothoracic syrinx and basilar impressions was diagnosed.
Interventions:
The treatment is directed to its potential cause to cease its progression. A posterior fossa decompression (PFD) was conducted for this patient.
Outcomes:
Postoperatively, the patient's symptoms were relieved and the size of syrinx was reduced at the 6-month follow-up.
Lessons:
Taken together with 34 previous reports identified from a PubMed search, an analysis of 35 cases of Charcot arthropathy was conducted. Surgical decompression is an effective treatment, but the optimal treatment remains controversial. Thus, the aim of this literature review was to remind us to diagnose the potential cause as early as possible and we should spare no efforts on the exploration of etiology and adjuvant therapy for this disease.
Keywords: basilar impression, Charcot arthropathy, syringomyelia
1. Introduction
Charcot arthropathy, also called neuropathic arthropathy (NA) or Charcot joint, is an unusual chronic degenerative disease that can affect the sensory nerve associated with involved joints, which is secondary to diabetes, syringomyelia, traumatic spinal cord injury, leprosies, congenital pain insensitivity, and chronic alcoholic intoxication. Syringomyelia is the main cause in shoulder Charcot arthropathy, which is found in approximately 6% of cases of syringomyelia.[1] In syringomyelia, the cavity in the spinal cord leads to the progressive destruction of the shoulder joint caused by spinal cord compression and abnormal never conduction.[2] Recently, efforts to cure the disease are directed towards treating syringomyelia to cease the progression. To date, several reports have described NA caused by Chiari malformation (CM) with syringomyelia, but nobody has reported the syringomyelia caused by basilar impression. Therefore, we recruited a patient with Charcot arthropathy of the shoulder caused by basilar impressions (BI) with syringomyelia, which remained undiagnosed for 3 months and reviewed 34 previously documented cases to gain a better understanding of Charcot arthropathy, including clinical manifestations, radiographic features, and surgical strategies.
2. Case report
A 52-year-old nondiabetic female presented with progressive swelling, pain, and limited movement in her left shoulder joint combined with asthenia of her left upper extremity for 3 months. Accordingly, she was hospitalized for diagnosis and treatment. The patient denied experiencing neck pain, steeping on cotton, unstable walking and muscle atrophy. A review of her personal and family medical history was unremarkable. In addition, it was noted that she was not taking any medication.
A physical examination revealed that lateral flexion and extension of the cervical spine was slightly painful and limited at the end of movement. Her left shoulder abduction was recorded as 50°, flexion 90°, internal rotation 30°, and external rotation 40°, all of which were recorded as painful for the patient in all directions. There was swelling in the left shoulder joint, and the left upper extremity was swollen compared with the right. Interestingly, Spurling's test was negative. A magnetic resonance image (MRI) of the left shoulder showed left shoulder dislocation, downward displaced humeral head, and irregular glenoid shape. The left shoulder joint cavity contained corpus liberum and effusion (Fig. 1).
Figure 1.
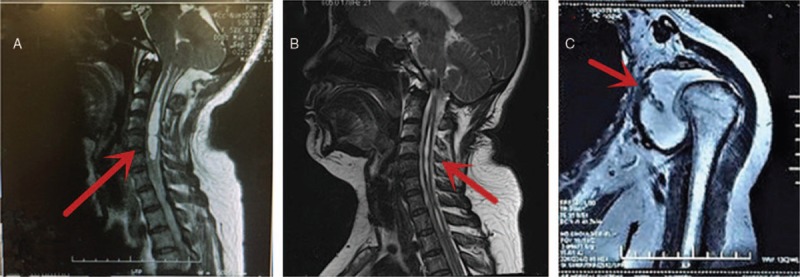
(A) A sagittal T2-weighted magnetic resonance imaging scan of the cervical spine demonstrates a syrinx extending from the C2 vertebral body down to the T2 vertebral body; (B) 6-month follow-up after foramen magnum decompression shows the decrease in size of syrinx. (C) Magnetic resonance imaging of the left shoulder joint showing the shoulder joint disclosed and the glenoid losing its normal shape. A large amount of effusion can be seen in the joint cavity (arrow).
On neurological examination, left and right upper extremities were observed to have a muscle strength of grades 4 and 5 according to the muscle strength testing scale, respectively. Tendon reflexes were hypoactive in the patient's left upper extremity. Moreover, the sensation of pain and temperature in the left hand, upper extremities, and shoulder were decreased. Sensation of deep pain was normal, and joint position sense was preserved in bilateral upper extremities. Pathologic reflex was absent. Complete blood count, biochemical analysis, C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, and tumor markers were normal.
A MRI) of the cervical spine revealed a syringohydromyelia cavity extending from C2 to T4 vertebral levels (Fig. 1). This odontoid tip is above the Chamberlain's line (a line traced from the posterior margin of the hard palate to the dorsal margin of the foramen magnum) in 6.0 mm (Fig. 2), which the diagnosis of basilar impressions is clear.
Figure 2.
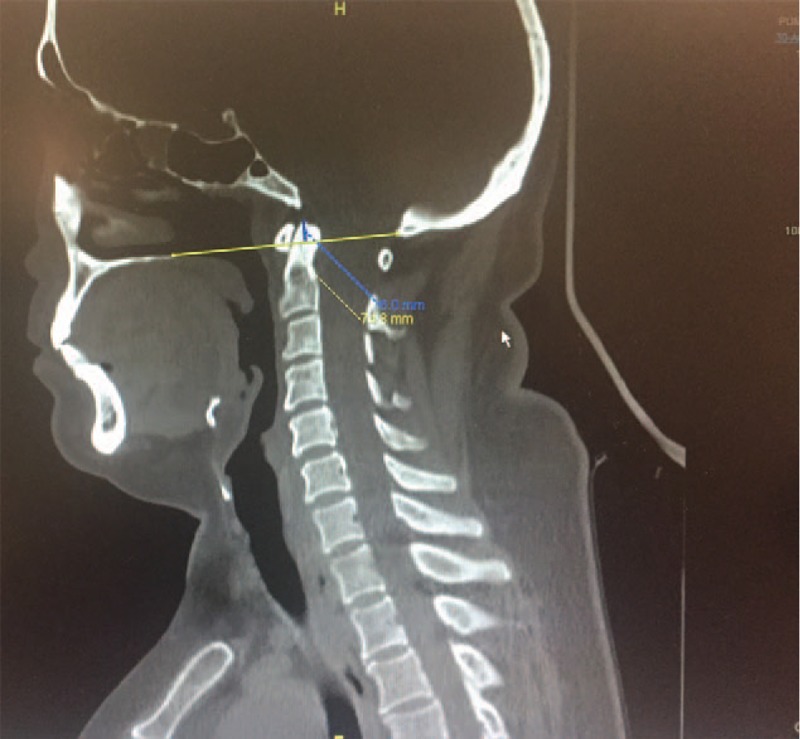
Computed tomography scans show that odontoid tip is above the Chamberlain's line in 6.0 mm.
In the light of the clinical, laboratory, and imaging findings, the case was diagnosed as the NA of the shoulder associated with syringomyelia and BI.
Accordingly, a surgical treatment plan was formulated to solve the patients’ disease. The patient was not observed to have atlanto-occipital dislocation (AOD). As such, a posterior fossa decompression (PFD) was conducted. The patient was administered general anesthesia and placed in a prone position. Their skin, subcutaneous tissues, and occipital were dissected with a midline incision extending from the occipital protuberance to the C2 spinous process. The inferior part of the occipital bone and the posterior lamina of C1 were removed to achieve a bony decompression (approximately 3 cm × 2 cm). Next, a thick Atlas pillow fascia, compressed the dura and was then removed. Therefore, the dura mater of the AOD restored the pulsation. Finally, the outer layers were sutured step-by-step to achieve anatomical reduction.
After operation, the pain of left shoulder was relieved, but the other symptoms have not improved. At the 6-month follow-up, the asthenia of her left upper extremity was also improved and the patient felt clinically well. The physical examination revealed that her left shoulder abduction was recorded as 90°, flexion 90°, internal rotation 50°, and external rotation 50°, all of which were improved postoperatively. MRI at this time showed adequate foramen magnum decompression and reduction of the size of syrinx (Fig. 2).
3. Literature review
The literature search was performed with the PubMed search engine of the National Library of Medicine of the National Institutes of Health (http://www.ncbi.nlm.nih.gov/pubmed), using the following keywords: “Charcot arthropathy;” “Neuropathic arthropathy;” “Charcot joint;” “Chiari malformation;” “Syringomyelia;” “Basilar impressions.” The search was restricted to English-language publications without date limitations. Any case reports involving Charcot arthropathy due to syringomyelia caused by CM or BI were included. No mention of the clear etiology can be excluded.
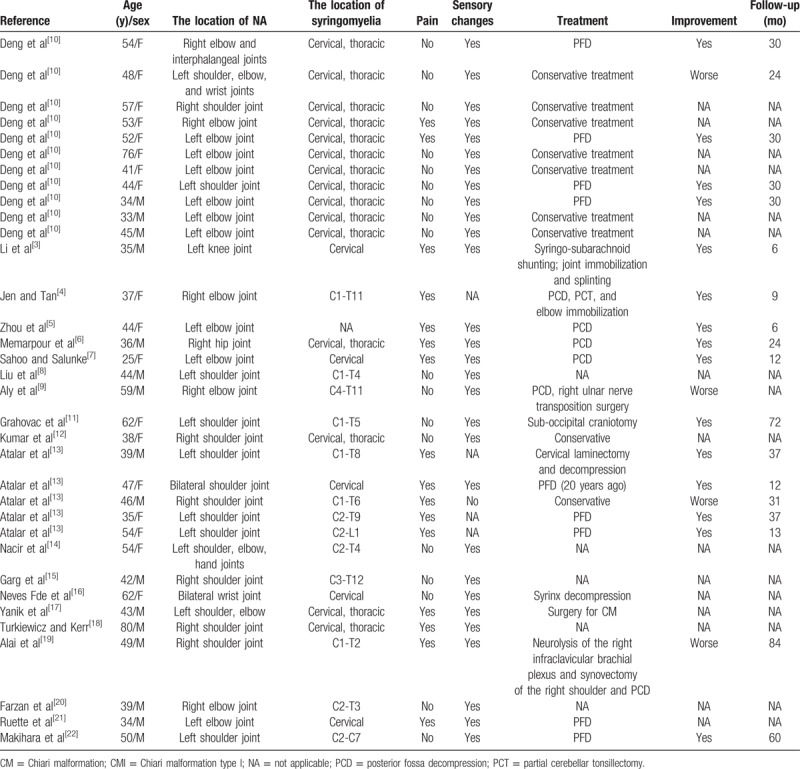
In our updated review, totally 34 patients with NA caused by CM with syringomyelia were found, and the mean age of these patients was 45.21 years (range 25–80 years) with a female predominance (M/F = 0.89). We did not find the relevant literature about Charcot arthropathy caused by basilar impression with syringomyelia. Clinical data for all 33 patients are shown in Table 1.[3–22] A total of 17 (50.0%) cases presented NA involving the shoulder, 17 (50.0%) cases involving the elbow, 2 (5.88%) cases involving the wrist and interphalangeal joints, and 1 (2.94%) case involving the hip and the knee joint. Moreover, we found that 2 (5.88%) cases involved bilateral joints and 4 (11.76%) cases involved more than one joint. Besides, the distribution of syringomyelia was mainly in cervical and thoracic. In addition, 19 (55.88%) cases underwent surgery, out of which 15 cases had improvement during the follow-up. A total of 9 (26.47%) cases were treated with conservative treatment, the symptoms of which aggravated in 2 patients.
Table 1.
Summary of clinical data in 34 patients with neuropathic arthropathy caused by CM with syringomyelia published in English.
4. Discussion
NA is defined as a gradual undermining of the joint or joints, which is commonly associated with sensory deprivation and temperature changes demonstrated in weight-bearing joints including the ankle, knee, and hip.[23] NA occurs most commonly in patients with diabetic neuropathies which affects the foot and ankle joints frequently.[24] Upper extremity involvement is common in syringomyelia. There are few case reports of the wrist and interphalangeal joints involvement.[10]
Charcot arthropathy of the shoulder, which often manifested destruction of the proximal aspect of the humerus and the glenoid, generally progresses slowly, but it can make rapid progress in a few months or even weeks. However, the pathogenesis of this disease is debatable. Two main theories exist: neurotraumatic and neurovascular theories. The neurovascular theory emphasizes that normal neurovascular reflexes at the joints will destroy because of sensory deprivation and the results in hyperemia and activation of osteoclasts leading to bone resorption.[17] However, the neurotraumatic theory is that somatic muscular reflexes normally prevent joints from exceeding specific safe limits. Specifically, ranges of movement are degraded by account of absent or decreased sensations, which can cause in repeated subclinical trauma and joint destruction.[17,25] Nevertheless, the 2 theories are still defective as to why the syrinx refers to both halves of the cord but present unilaterally.
NA has a variety of clinical manifestations, including painful and painless, joint instability, swelling, and dysfunction. Pain does not always exit due to neuropathy, but joint welling and limited activity occurs frequently. In general, joint symptoms can emerge earlier than neurological symptoms. Some clinicians regard NA as an exclusion diagnosis. Therefore, other general diseases must be excluded including diabetes, infection, congenital pain insensitivity, and inflammatory arthropathy. X-rays show severe joint deterioration and significant bone loss, both of which may be akin to suppurative arthritis or tumors. However, a biopsy may discard these diagnoses. Physical examination revealed joint relaxation, effusion, and decreased muscle strength.
On examination, sensory and temperature changes were revealed. All in all, our case's symptoms were in line with the above description.
Syringomyelia is a chronic, progressive, and degenerative disorder of the spinal cord, which exists a fluid cavity inside the spinal cord. The etiology of the disease can be congenital, often occurring due to obstruction of the foramen Magnum, trauma, infection, degeneration, vascular problem, or tumor.[26] Syringomyelia is most common in the spine of the neck, and it can also diffuse throughout the central canal of the spinal cord. In general, the upper limb begins to lose pain and temperature, while retaining tactile and proprioceptive sensations referred to as “dissociative anesthesia.” The clinical manifestation of syringomyelia is highly dependent on its position. As with our patient, whose left hand, upper extremities, and shoulder incurred loss of pain and temperature, a syringohydromyelic cavity extending from C2 to T4 vertebral levels was observed. Meyer et al reported that NA as having developed in 25% of patients with syringomyelia.[14] In addition, syringomyelia is commonly associated with CM type I,[26] which are characterized by the herniation of the cerebellar tonsils by the foramen magnum into the cervical spinal canal.
Interestingly, Paliwal et al[27] reported Charcot knee secondary to lumbar spinal cord syringomyelia due to spinal anesthesia. In addition, Deng et al[10] reported Charcot elbow caused by syringomyelia due to tethered spinal cord. However, as demonstrated in our patient, the syringomyelia is caused by congenital disease—BI.
BI is an evolutionary abnormality of the occipital bone and upper cervical spine that can lead to abnormally high vertebral column prolapse into the skull base, where incidence in the general population is about 1%.[28] Most reports describe CM with syringomyelia, which develops in approximately 75% to 85% of patients with CM type I.[2] However, this case is complicating with BI. They all can lead to Syringomyelia. Although CM and BI have much in common, they are not described as having the same pathological process.
Once the Charcot joint was observed, it was most likely in late stage and hard for therapy. The treatment is usually conservative, which is included controlling pain, physical therapy to enhance range of motion, and nonsteroid anti-inflammatory drugs given to decrease synovial inflammation.[2] Despite this, however, alendronate treatment has produced excellent results for NA caused by diabetes mellitus.[29] However, total replacement arthroplasty for the Charcot joints has been disappointing because of a high risk of recurrence and infection.
The goal of treatment for patients with NA are early diagnosis, maintenance of joint and extremity function, and treatment of underlying disease, which it means immediately go to neurosurgery for syrinx evaluation.[30] Our patients can be accounted for by the complex interactions of syringomyelia complicating with BI. The progression of joint destruction was halted by neurosurgical decompression. Accordingly, surgery of PFD for syringomyelia was performed on our patient. After surgery, the patient's symptoms were improved. Our review found that 19 patients underwent neurosurgical decompression and 15 of them showed neurologic improvement without joint deterioration. These data indicated early neurosurgical intervention can stabilize the joint degenerative process. Besides operative treatment, we should also give conservative methods, including restricted weight bearing, immobilization, passive stretching, physical therapy and nonsteroidal anti-inflammatory medication. Due to the scarcity of data regarding conservative treatment, further research is necessary. Therefore, an early diagnosis of Charcot shoulder would inevitably contribute to treating our patient at the earliest stage possible.
5. Conclusion
In the present study, the differential diagnosis of NA of the joints should be considered in patients suffering from swelling, pain, and a loss in range of joint motion, and neurological examination should be conducted carefully. What's more, a cranium or cervical MRI should be made to exclude syringomyelia, where the effective treatment is PFD. Accordingly, thorough medical history and timely diagnosis are critical to manage patients successfully. Therefore, we should spare no efforts on the exploration of etiology and adjuvant therapy for this disease.
5.1. Patient consent statement
The patient has consented to submission of this case report to the journal.
Author contributions
Conceptualization: Xin Wang, Jun Gao, Yongning Li, Tianyu Wang, Zhimin Li.
Data curation: Xin Wang, Jun Gao, Tianyu Wang, Zhimin Li.
Formal analysis: Jun Gao.
Methodology: Xin Wang, Tianyu Wang.
Supervision: Yongning Li, Jun Gao.
Visualization: Yongning Li.
Writing – original draft: Xin Wang.
Writing – review & editing: Yongning Li.
Footnotes
Abbreviations: NA = neuropathic arthropathy, AOD = atlanto-occipital dislocation, BI = basilar impressions, CM = Chiari malformation, MRI = magnetic resonance image, PFD = posterior fossa decompression.
Ethical statement: Ethics committee approval is not included as it is commonly accepted that case reports do not require such approval. Our work did not use patients’ data that would allow identifying them, thus no ethical approval is required.
Funding/support: This research did not receive any specific grant from funding agencies in the public or commercial.
The authors have no conflicts of interest to disclose.
References
- [1].Hatzis N, Kaar TK, Wirth MA, et al. Neuropathic arthropathy of the shoulder. J Bone Joint Surg Am 1998;80:1314–9. [DOI] [PubMed] [Google Scholar]
- [2].Jones J, Wolf S. Neuropathic shoulder arthropathy (Charcot joint) associated with syringomyelia. Neurology 1998;50:825–7. [DOI] [PubMed] [Google Scholar]
- [3].Li G, Ding Y, Zhang C, et al. Syringomyelia with left knee Charcot arthropathy: a case report. Br J Neurosurg 2018;1–2. [DOI] [PubMed] [Google Scholar]
- [4].Jen CL, Tan JC. Neuropathic arthropathy of the elbow treated with double-plate arthrodesis and resection site bone graft. Shoulder & elbow 2016;8:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhou Y, Zhu L, Lin YX, et al. Charcot elbow joint as the initial symptom in Chiari malformation with syringomyelia. Chin Med J 2015;128:3381–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Memarpour R, Tashtoush B, Issac L, et al. Syringomyelia with Chiari I malformation presenting as hip Charcot arthropathy: a case report and literature review. Case Rep Neurol Med 2015;2015:487931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sahoo SK, Salunke P. Charcot arthropathy of the elbow joint as a presenting feature of Chiari malformation with syringomyelia. Br J Neurosurg 2014;28:811–2. [DOI] [PubMed] [Google Scholar]
- [8].Liu H, Wang Y, Yang Z, et al. A case report of Charcot arthropathy caused by syringomyelia and Chiari malformation complicated with scoliosis. BMC Res Notes 2014;7:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aly AR, Rajasekaran S, Obaid H, et al. Bilateral ulnar neuropathy at the elbow secondary to neuropathic arthropathy associated with syringomyelia. PM R 2013;5:533–8. [DOI] [PubMed] [Google Scholar]
- [10].Deng X, Wu L, Yang C, et al. Neuropathic arthropathy caused by syringomyelia. J Neurosurg Spine 2013;18:303–9. [DOI] [PubMed] [Google Scholar]
- [11].Grahovac G, Vilendecic M, Srdoc D. Charcot shoulder caused by Chiari type I malformation with syringomyelia with six-year follow-up. Wien Klin Wochenschr 2011;123:512–4. [DOI] [PubMed] [Google Scholar]
- [12].Kumar S, Sharma V, Kumar S, et al. Imaging findings in Chiari I malformation with syringomyelia in a case of Charcot shoulder. J Clin Imaging Sci 2011;1:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Atalar AC, Sungur M, Demirhan M, et al. Neuropathic arthropathy of the shoulder associated with syringomyelia: a report of six cases. Acta Orthop Traumatol Turc 2010;44:328–36. [DOI] [PubMed] [Google Scholar]
- [14].Nacir B, Arslan Cebeci S, Cetinkaya E, et al. Neuropathic arthropathy progressing with multiple joint involvement in the upper extremity due to syringomyelia and type I Arnold-Chiari malformation. Rheumatol Int 2010;30:979–83. [DOI] [PubMed] [Google Scholar]
- [15].Garg RK, Kar AM. Charcot shoulder in syringomyelia. Intern Med J 2008;38:868–9. [DOI] [PubMed] [Google Scholar]
- [16].Neves Fde S, Goncalves DP, Goncalves CR. Syringomyelia, neuropathic arthropathy and rheumatoid arthritis as diagnostic dilemmas in two different cases: confounding factor and true coexistence. Clin Rheumatol 2007;26:98–100. [DOI] [PubMed] [Google Scholar]
- [17].Yanik B, Tuncer S, Seckin B. Neuropathic arthropathy caused by Arnold-Chiari malformation with syringomyelia. Rheumatol Int 2004;24:238–41. [DOI] [PubMed] [Google Scholar]
- [18].Turkiewicz AM, Kerr G. Clinical images: syrinx-induced Charcot shoulder. Arthritis Rheum 2004;50:2380. [DOI] [PubMed] [Google Scholar]
- [19].Alai A, Reddy CG, Amrami KK, et al. Charcot arthropathy of the shoulder associated with typical and atypical findings. Clin Anat 2013;26:1017–23. [DOI] [PubMed] [Google Scholar]
- [20].Farzan M, Eraghi AS, Mardookhpour S, et al. Neuropathic arthropathy of the elbow: two case reports. Am J Orthop 2012;41:E39–42. [PubMed] [Google Scholar]
- [21].Ruette P, Stuyck J, Debeer P. Neuropathic arthropathy of the shoulder and elbow associated with syringomyelia: a report of 3 cases. Acta Orthop Belg 2007;73:525–9. [PubMed] [Google Scholar]
- [22].Makihara T, Onishi S, Wadano Y, et al. Regrowth of the deteriorated glenoid in advanced Charcot shoulder after suboccipital decompression for syringomyelia: a case report. J Shoulder Elbow Surg 2015;24:e223–228. [DOI] [PubMed] [Google Scholar]
- [23].Allman RM, Brower AC, Kotlyarov EB. Neuropathic bone and joint disease. Radiol Clin North Am 1988;26:1373–81. [PubMed] [Google Scholar]
- [24].Brower AC, Allman RM. Pathogenesis of the neurotrophic joint: neurotraumatic vs. neurovascular. Radiology 1981;139:349–54. [DOI] [PubMed] [Google Scholar]
- [25].Johnson JT. Neuropathic fractures and joint injuries. Pathogenesis and rationale of prevention and treatment. J Bone Joint Surg Am 1967;49:1–30. [PubMed] [Google Scholar]
- [26].Fernandez AA, Guerrero AI, Martinez MI, et al. Malformations of the craniocervical junction (Chiari type I and syringomyelia: classification, diagnosis and treatment). BMC Musculoskelet Disord 2009;10(suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Paliwal VK, Singh P, Rahi SK, et al. Charcot knee secondary to lumbar spinal cord syringomyelia: complication of spinal anesthesia. J Clin Rheumatol 2012;18:207–8. [DOI] [PubMed] [Google Scholar]
- [28].Burwood RJ, Watt I. Assimilation of the atlas and basilar impression: a review of 1,500 skull and cervical spine radiographs. Clin Radiol 1974;25:327–33. [DOI] [PubMed] [Google Scholar]
- [29].Pitocco D, Ruotolo V, Caputo S, et al. Six-month treatment with alendronate in acute Charcot neuroarthropathy: a randomized controlled trial. Diabetes Care 2005;28:1214–5. [DOI] [PubMed] [Google Scholar]
- [30].Snoddy MC, Lee DH, Kuhn JE. Charcot shoulder and elbow: a review of the literature and update on treatment. J Shoulder Elbow Surg 2017;26:544–52. [DOI] [PubMed] [Google Scholar]


