Abstract
The seroprevalence of hepatitis B virus (HBV) and its impact on pregnancy outcomes of women from Shaanxi Province (China) was assessed. Risk factors for mother-to-child transmission (MTCT) were evaluated based on HBV-related seroprevalence data.
Viral markers and biochemical parameters were assessed in HBsAg-positive mothers and their infants out of 13,451 cases recruited. A pretested and structured questionnaire was used to test the general HBV knowledge. Descriptive statistics and logistic regression analysis were done to reveal possible risk factors for MTCT.
The overall prevalence of HBsAg in pregnant women was 7.07% (951/13,451), and a rate as high as 9.40% was observed. Among the HBsAg-positive pregnant women, 30.49% (290/951) were HBeAg-positive, 22.08% (210/951) had HBV DNA levels >106 IU/mL and only 16.19% with a high risk of MTCT (34/210) had received antiviral treatment. The overall MTCT rate was 5.21%. Noteworthy, the risk ratio and 95% confidence interval (95% CI) of MTCT in HBeAg-negative mothers with HBV DNA levels >2 × 103 IU/mL and HBsAg >104 IU/mL was 26.062 (2.633–258.024), which was significantly higher than that of HBeAg-positive mothers with HBV DNA level >106 IU/mL. Moreover, the awareness and knowledge about HBV transmission, risk factors, and intervention for MTCT were generally lacking among HBsAg-positive mothers.
As a higher HBsAg seroprevalence and a higher MTCT rate among HBeAg-negative mothers with lower HBV DNA level was observed, our study emphasizes different interventional criteria for HBeAg-positive and HBeAg-negative mothers. Extensive health education, routine screening, and immunization against HBV during pregnancy are highly warranted to minimize the possibility of perinatal transmission.
Keywords: epidemiology, hVB DNA, mother-to-child transmission, pregnant women
1. Introduction
Chronic hepatitis B virus (HBV) infection is the major cause of end-stage liver diseases in China, among which 30% to 50% owes to mother-to-child transmission (MTCT),[1] and are associated with increased risk of morbidity and mortality later in life.[2,3] Despite the successful implementation of hepatitis B vaccine and hepatitis B immunoglobulin (HBIG) at birth, about 10% of infants born to HBV-infected mothers still suffer from chronic HBV infection.[4] Moreover, 50 million new cases continue to be diagnosed annually, and MTCT is responsible for the highest proportion.[5]
In China, HBV infection is one of the infectious diseases with the highest morbidity, causing both end-stage liver diseases with high mortality and a substantial economic burden for the patients, their families, and the society.[6] Routine antenatal hepatitis B surface antigen (HBsAg) screening for pregnant women and combined immunoprophylaxis (HBIG and hepatitis B vaccine] for neonates born to HBsAg-positive mothers have reduced the rate of HBV infection in infants. Nevertheless, there is still approximately 5% to 10% of infants born to HBsAg-positive mothers suffering from HBV infection in their early life,[7,8] of which as much as 90% develops chronic hepatitis infection. Moreover, 25% to 40% of patients with chronic HBV infection eventually develop severe complications such as cirrhosis and hepatocellular carcinoma.[9] Considering the large population and high prevalence of HBV infection in China, the absolute number of HBV-infected women is important. HBV infection and its related complications will continue to remain a major public health concern in China.
Vertical transmission of HBV infection may adversely affect the pregnancy and newborn, leading to spontaneous abortion, premature delivery, growth retardation, and low birth weight.[10] HBsAg is the surface antigen of HBV, while hepatitis B e antigen (HBeAg) is an envelope antigen. Presence of HBsAg in the blood indicates the presence of intact virus, its association with viral replication, and cccDNA have been confirmed widely. HBeAg does not necessarily indicate the presence of intact virus, but denotes higher infectivity.[11,12] Hepatitis B status of pregnant women detected through serum HBsAg screening is essential for the effective management of the disease and prevention of MTCT, as it confers 20% risk of transmission to infants without any intervention such as immunoprophylaxis. In fact, this risk adds up to 90% if the mother is also positive for HBeAg.[13–16]
Several factors affect the prevalence of perinatal HBV transmission and are related to MTCT, such as the levels of maternal HBV DNA, HBeAg status, and neonatal immune deficiency.[10] Some published studies have identified maternal HBV DNA levels corresponding to viral load as the most relevant factor, and the antiviral treatment in pregnant women is decided based on this single threshold factor.[3] MTCT, including intrafamilial horizontal transmission and vertical transmission, is still the main transmission route of HBV infection in China.[17] It is believed that MTCT accounts for clustering of infection in families with unfavorable prognoses.[18] It has been reported that the overall prevalence of HBsAg in childbearing women is 6.61% in China,[19] and the HBsAg-positive rate of pregnant women is about 5% in eastern regions of China.[20] Nevertheless, there is scarce report about the HBV infection rate and infection status of pregnant women in northwestern regions of China. As most of the regions in this area are underdeveloped, improvement of general awareness among pregnant women and primary health care doctors regarding the risk factors for MTCT, mode of transmission, and indicators of higher transmissibility rate of HBV infection and implementation of additional health care government policies will help eliminate HBV infection.
Currently, we carried out this study to assess the seroepidemiology and awareness of information about HBV infection among pregnant women in Northwest China, as well as risk factors for HBV MTCT. The results should provide the latest and updated data for the effective implementation of better prevention strategies and control programs for HBV in this area.
2. Methods
2.1. Study area and population
A cross-sectional study was conducted at 7 county-level hospitals, 9 general hospitals, and 2 mother and child health centers in Shaanxi Province, a northwestern region of China. From November 2010 to December 2015, pregnant women over 18 years of age who delivered at these 18 hospitals were enrolled. Women with prior vaccination, those who were unwilling to give consent for the blood test, or those who gave responses to questionnaire that were deemed invalid, incomplete, or defective were exempted from the study. The study complied with all ethical standards for research and was approved by the ethics committee of First Affiliated Hospital of Xi’an Jiaotong University, protocol number XJTU-2010–127. All participants in this study gave consent for participation.
2.2. Sample and data collection
Venous blood (5 mL) was collected aseptically and centrifuged to separate the serum. Serum samples were then stored at -20°C until assayed. Serum HBsAg was tested in all the pregnant women before delivery. Other HBV serum markers (anti-HBs, HBeAg, and anti-HBc), HBV DNA level, and liver function tests for liver enzymes were tested among HBsAg-positive pregnant women. Medication status of the mothers was recorded.
After childbirth, data in case report forms (CRFs) were collected from the enrolled women's medical records and via interviews with the mothers. The content of the CRFs included the basic characteristics, delivery situation and infant-feeding patterns of the mothers, and the birth date, gender, and development of the infants. Semi-structured questionnaires that had been pretested were used to obtain sociodemographic characteristics and risk factors for HBV infection.
2.3. Study design
All the neonates born to HBsAg-positive mothers received standard combined immunoprophylaxis (HBIG 200 IU; Hualan Biological Engineering Inc., Henan, China) and the first dose of the hepatitis B vaccine (Shenzhen Kangtai Biological Products Co., Ltd. Guangdong, China) at different injection sites within 12 hours postpartum (the other 2 doses of vaccine are given at 1 and 6 months of age, respectively). Some of those infants were followed to the age of 12 months. Peripheral venous serum was stored at -80°C for HBV markers and HBV DNA detection. Infants with persistent HBV DNA or HBsAg-positive results from birth to the age of 7 months were diagnosed as having HBV intrauterine infection.[21–24]
2.4. Laboratory analyses
HBV serum markers (HBsAg, anti-HBs, HBeAg, and anti-HBc) were quantified using the Abbott ARCHITECT assays (Abbott Laboratories, Chicago, IL). The HBV DNA levels in infants were measured using the Roche COBAS AmpliPrep/COBAS TaqMan HBV test (lower limit of the test range: 12 IU/mL; Roche Molecular Systems. Inc, NJ). The viral titer of the mothers was detected with a real-time quantitative fluorescence PCR (detection range: 1.0 × 102–1.0 × 109 IU/mL; Sun Yat-sen University, Da An Gene Co., Ltd. Guangzhou, China). Liver function tests were performed with an Olympus automated bioanalyzer (Olympus AU5400; Olympus kabushikikaisha, Tokyo, Japan).
2.5. Questionnaire assessment of HBV knowledge
Survey questions were developed by leading physicians from the Department of Infectious Diseases and the Department of Gynecology and Obstetrics, the First Affiliated Hospital of Xi’an Jiaotong University, which is a Chinese grade III hospital with the highest ability to provide medical care and medical education, and to perform medical research. Sociodemographic information was collected from all the participants using a standard questionnaire that was pretested. All participants gave written informed consent before enrollment and the test results were communicated for proper management and care of the study participants. The questionnaire was designed to capture the basic information about HBsAg-positive mothers (age, location, and vocation), knowledge about HBV transmission (routes of transmission, risk factors for MTCT), and prevention (measures, efficacy, and safety). All interviewers received training and certification in interviewing techniques and were familiar with the medical terminology used in the questionnaires. Participants were randomly selected from HBsAg-positive mothers who were requested to complete paper-based survey.
2.6. Quality control
To make sure that the questionnaire was appropriate and understandable, it was pretested on 30 pregnant women at health centers other than the actual study sites. The collected data were checked daily for consistency and accuracy. All questionnaires were reviewed to eliminate the incomplete responses. Responses that were deemed invalid or defective were not included in the analyses. No statistical tests were pre-performed. The data are presented as percentages and described. Standardized procedures were strictly followed during blood sample collection, storage, and analytical process. Positive and negative controls were run alongside of the test subjects.
2.7. Statistical analysis
EpiData 3.0 was used to establish the clinical database. Data were double-checked for transcription errors and were then analyzed statistically with SPSS 13.0 (SPSS, Inc., Chicago, IL). Descriptive analysis (calculations of averages, frequencies, proportions, or rates) was conducted. The Chi-squared for R × C table test was used to compare more than 2 groups. The t test was used to assess whether the means of 2 groups were significantly different from each other. Univariate and multivariate logistic regression analyses were used to determine odds ratios (ORs), adjusted ORs, and their 95% confidence intervals (95% CIs) to different variables. P < .05 was considered statistically significant.
3. Results
3.1. The prevalence of HBV infection among pregnant women
Among the subjects, 7.07% (951/13,451) were seropositive for HBsAg, which was consistent with the Chinese prevalence rate of HBsAg.[19] It is noteworthy that the HBsAg prevalence rate in pregnant women in the southern region of Shaanxi province, close to a mountain area, was 9.40% (311/3309), which was significantly higher than the overall rate (9.40% vs 7.07%; P < .001). More importantly, among the 951 HBsAg-positive women, 36.17% (344/951) did not receive regular prenatal examination. The demographics and characteristics of these HBsAg-positive mothers are summarized in Table 1.
Table 1.
Demographics and characteristics of HBsAg-positive pregnant women.
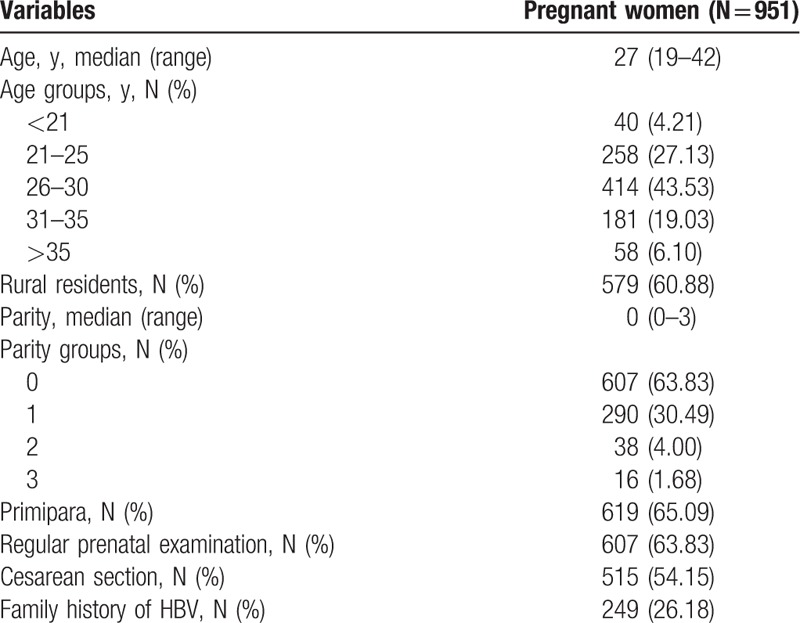
3.2. The virological and biochemical characteristics of HBsAg-positive pregnant women
As summarized in Table 2, among the HBsAg-positive mothers, 30.49% (290/951) were HBeAg-positive, and 22.08% (210/951) pregnant women had HBV DNA levels >1×106 IU/mL and 32.60% (310/951) had HBV DNA levels >2×103 IU/mL, while 73.40% (698/951) had HBsAg titer >1000 IU/mL and 34.07% (324/951) had HBsAg titer >10,000 IU/mL. Of these high-risk mothers, only 16.19% (34/210) received antiviral treatment during pregnancy; those without antiviral intervention notified that the main reason for omission was due to no recommendation from their obstetrician. At delivery, 13.35% (127/951) showed ALT levels elevation (median, 77 U/L; range, 740–41 U/L). Three of these HBsAg-positive mothers suffered from fulminant liver disease, 2 cases were improved after treatment, and another one had to terminate pregnancy at 35th week of gestation because of serious abnormal liver function.
Table 2.
Virological and biochemical characteristics of HBsAg-positive pregnant women.

3.3. Pregnancy complications
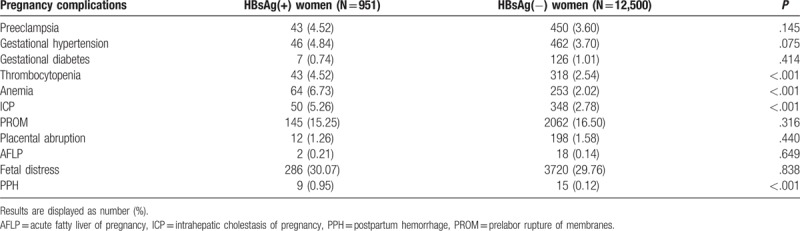
The complications that occurred in those HBsAg-positive pregnant women are summarized in Table 3. With further analysis, we noticed that the incidence of intrahepatic cholestasis of pregnancy (ICP), thrombocytopenia, postpartumhemorrhage, and anemia was significantly higher in HBsAg-positive pregnant women (P < .001, Table 3) than pregnant women free of HBV infection.
Table 3.
Pregnancy complications with respect to HBsAg status.
3.4. Risk factors associated with mother-to-child HBV transmission
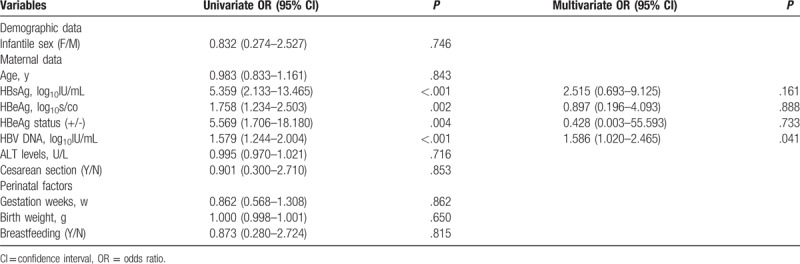
In this study, 499 infants born to HBsAg-positive mothers were followed to 12 months of age, and 26 of them (5.21%) were diagnosed as having HBV infection showing seropositivity for HBsAg or HBV DNA at 7 and 12 months. Possible factors related to HBV infection of infants are presented in Table 4. The results of univariate analysis showed that maternal HBsAg titer, HBeAg titer, HBeAg-positive, and HBV DNA levels were associated with HBV infection in infants (P < .001, P = .002, P = .004, P < .001, respectively). With further multivariate regression analysis, only maternal HBV DNA levels were related to HBV infection of infant (P = .041).
Table 4.
Factors associated with intrauterine infection among HBsAg-positive pregnant women.
As shown in Fig. 1A, when maternal HBV DNA levels were stratified, the incidence of infantile HBV infection increased with maternal HBV DNA levels (13.33% vs 5.80% vs 0%; P < .001). The overall rate of intrauterine HBV infection was 5.21%, while it increased to 13.33%, 13.53%, and 21.28% with HBV DNA >6 log10IU/mL, HBsAg >4 log10IU/mL, or HBeAg>3 log10s/co, respectively.
Figure 1.
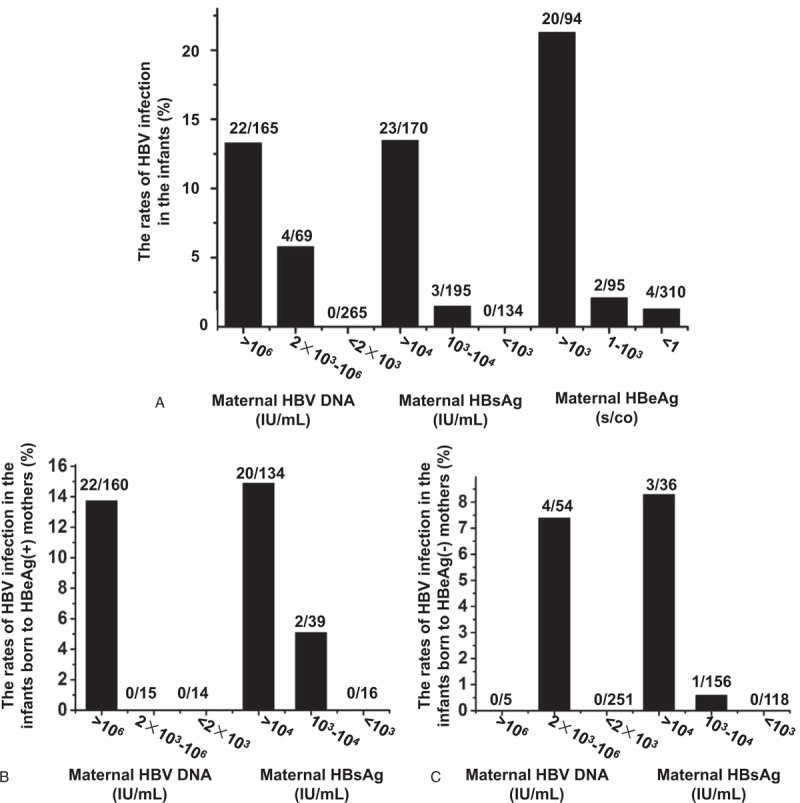
Differential rates of HBV infection in infants related to varying concentrations of maternal HBV DNA, HBsAg, and HBeAg levels. (A) The rates of HBV infection in infants due to varying levels of maternal HBV DNA, HBsAg, and HBeAg. (B) The rates of HBV infection in infants born to HBeAg-positive mothers with varying levels of maternal HBV DNA and HBsAg. (C) The rates of HBV infection in infants born to HBeAg-negative mothers with varying levels of maternal HBV DNA and HBsAg.
It was noteworthy that 4 of these vertically transmitted infants (4/26) were born to HBeAg-negative mothers, compared with the 22 other infants born to HBeAg-positive mothers. Furthermore, the HBV DNA levels of the 4 HBeAg-negative mothers were significantly lower than HBeAg-positive mothers (4.35 ± 0.61 vs 7.65 ± 1.02; P = .005). For the 189 HBeAg-positive mothers, the risk ratio and 95% CI of infantile HBV infection in the mothers with HBV DNA levels >106 IU/mL was 1.210 (1.129–1.297), while for the 310 HBeAg-negative mothers, the risk ratio and 95% CI in mothers with HBV DNA level >2×103 IU/mL was 6.652 (5.097–8.683), which was much higher than that of those HBeAg-positive mothers with HBV DNA level >106 IU/mL. Moreover, a significantly higher titer of HBsAg was observed in HBeAg-negative mothers compared with HBeAg-positive mothers with MTCT (4.13 ± 0.74 vs 3.08 ± 1.03 log10IU/mL, Z = −2.106, P = .035). With further analysis, a risk ratio and 95% CI of MTCT as high as 26.062 (2.633–258.024) was displayed in HBeAg-negative mothers with HBV DNA level >2 × 103 IU/mL and HBsAg >104 IU/mL.
3.5. Awareness and knowledge of HBV transmission and prevention among HBsAg-positive pregnant women
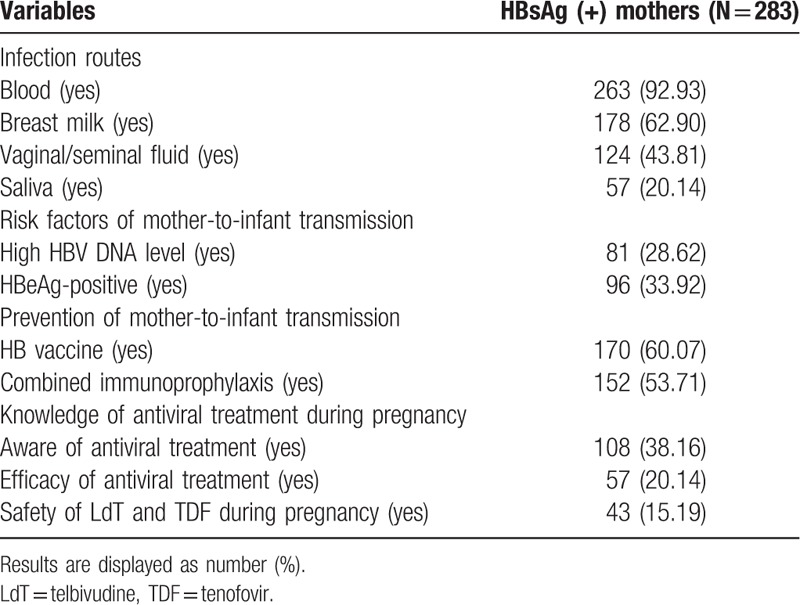
Questionnaire of knowledge about HBV transmission, risk factors for MTCT, and intervention for MTCT was randomly distributed to 300 HBsAg-positive pregnant women. None of these interviewees declined participation but 12 did not return their questionnaire and 5 did not complete the questionnaires. A total of 283 questionnaires with complete data were analyzed; 263 (92.93%) respondents were aware of blood as a medium of HBV transmission. However, knowledge of other body fluids as source of infection varies among HBsAg-positive mothers. Regarding the knowledge about risk factors of HBV MTCT, 82 women (28.62%) were aware of high HBV DNA levels as a risk factor and 152 (53.71%) HBsAg-positive mothers knew that combined immunoprophylaxis administration could prevent their infants from HBV infection. Compared with the awareness of combined immunoprophylaxis, the knowledge of efficacy of antiviral treatment during pregnancy to prevent MTCT was poorer (20.14% vs 53.71%; P < .001), and only 15.19% mothers had in-depth knowledge about safety of telbivudine and tenofovir administration during pregnancy. Compared with data from other parts of China and other countries,[25–27] the awareness and knowledge of HBV MTCT of the mothers included in the present study were deficient. The results suggest that there is much room for improvement regarding the knowledge of vertical transmission of HBV.
4. Discussion
A 5-year hospital-based cross-sectional study was carried out and included 13,451 pregnant women from 18 hospitals in Shaanxi Province in Northwestern China. This study aimed at identifying the prevalence and risk factors for HBV infection among pregnant women and to assess the awareness and knowledge about the MTCT of HBV infection among this population. A HBsAg prevalence rate of 7.07% was observed in this study, which was much higher than the common population in China.[28] Compared with survey results from Shenyang (Northeastern China),[20] Guizhou (Southwestern China),[29] and Hainan (Southern China),[30] the prevalence of HBV in this study (Northwestern China) was substantively high, which may be attributed to the relatively low gross domestic product and education level in this closed hinterland. Furthermore, the deficiency of awareness and knowledge about periodic reviewing and prevention during pregnancy indicates the necessity of strengthening the cognizance among primary health care doctors.
With the wide implementation of hepatitis B vaccination in the past 20 years, the HBsAg carrier rate in the Chinese population has significantly decreased.[19] Combined immunoprophylaxis to newborns of HBsAg-positive mothers largely aims to eliminate HBV infection through antenatal transmission. Considering the imbalance of economic development and education levels among different areas in China, we envisioned the significance to update and assess the HBsAg-positive situation in Shaanxi Province, China, a large rural province in Northwestern China with a population of 37 million. We further anticipated that these recent findings might help enlighten the importance to formulate new policy and guidelines for the prevention of HBV infection.
The majority of the CHB pregnant women did not display severe complications during pregnancy. Nevertheless, compared with pregnant women without HBV infection, the incidence of ICP (5.26%) and postpartum hemorrhage (0.95%) were significantly higher in HBsAg-positive pregnancies. In a previous study conducted by Tse et al,[31] a high risk of antepartum hemorrhage had been identified to be associated with HBV infection, which may be related to the chronic inflammatory state in this population. The close association of ICP with HBV has also been explored in other observations,[32] and the inflammation caused by chronic HBV infection was responsible for this. Thrombocytopenia and anemia are the common complications in patients with liver stiffness. In our study, the incidence of thrombocytopenia (4.52%) and anemia (6.73%) was also substantively higher in HBsAg-positive mothers, which may be attributed to the inhibitory effect of HBV on the maturation of the bone marrow, another identified factor responsible for thrombocytopenia and anemia. For the safety of CHB pregnant women and their fetus, assessing and monitoring liver function before and during pregnancy is essential.
In this current investigation, we noticed that among the HBsAg-positive mothers, about one-third was HBeAg-positive and 22.08% pregnancies had high viremia (>106 IU/mL), which is consistent with a previous study.[33] Surprisingly, our data revealed that only 16.19% of these mothers with high viral load were managed with antiviral intervention during pregnancy, indicating the importance of strengthening the training for primary care doctors about management and therapeutic intervention of CHB in pregnant women. In accordance with previous studies,[34,35] vaginal delivery and breastfeeding do not contribute to HBV infection in infants receiving combined immunoprophylaxis. Obviously, high HBV DNA levels indicating viral load and HBeAg positivity reflecting the infectivity status are the 2 independent risk factors for MTCT.[36] Currently, maternal HBsAg titer, HBeAg status, and HBV DNA levels are 3 closely linked parameters of prime importance and are considered relevant with vertical transmission. Even though, the overall MTCT rate was 5.21%, a much substantially higher risk was demonstrated in mothers with HBV DNA >6 log10 IU/mL, HBsAg >4 log10 IU/mL, or HBeAg >1000 s/co (13.33%, 13.53%, and 21.28%, respectively), which is alarming and points toward a dire need of immediate attention and proper action by the responsible authorities. Nevertheless, the exact molecular mechanisms responsible for these observations require additional studies.
It is recommended by all major liver society guidelines that antiviral therapy should be administrated to pregnant women with high viremia during the third trimester.[37–39] On the contrary, a previous study of 869 infant–mother pairs showed that the MTCT rate of HBV infection due to failure of immunoprophylaxyin HBeAg-positive mothers with HBV DNA levels ≥6 log10 copies/mL was 3.1%.[40] Another investigation showed the rate of intrauterine transmission at maternal viral load >7 log10 copies/mL was 6.6%.[33] Accordingly, these data suggest that about 90% of these mothers may not require antiviral therapy during pregnancy, and therefore, it is necessary to figure out the exact high-risk population with large sample randomized controlled studies. Of note, in the present study, 4 infants born to HBeAg-negative mothers got intrauterine HBV infection. With further analysis, we noticed that the risk ratio of intrauterine transmission for HBeAg-negative mothers with HBV DNA level >2 × 103 IU/mL was substantially high [OR = 6.652 (5.097–8.683)], even higher than the HBeAg-positive mothers with HBV DNA level >106 IU/mL. Surprisingly, HBeAg-negative mothers with HBV DNA levels >2 × 103 IU/mL and HBsAg >104 IU/mL displayed a significantly higher risk of MTCT [risk ratio = 26.062 (2.633–258.024)]. In this case, antiviral therapy is recommended for this special population (HBeAg-negative and HBsAg >104 IU/mL) even with low HBV DNA levels to prevent MTCT, which is not consistent with recommendation by the major liver society guidelines, in which antiviral therapy is recommendation to initial in the third trimester in pregnant women with high viremia (>2 × 105 IU/mL). The discrepancy of immune state and viral mutation in HBeAg-negative mothers may be response for the higher MTCT, which needs further study to clarify the molecular mechanisms. Consequently, we think that different interventional strategies should be followed for CHB pregnant women with HBeAg-negative and HBeAg-positive; for HBeAg (+) pregnant women, antiviral therapy is recommended for those with HBV DNA >2 × 105 IU/mL to prevent MTCT, while for the HBeAg (-) mothers, antiviral therapy is recommended for those with HBV DNA >2 × 103 IU/mL and HBsAg >104 IU/mL. Moreover, the exact timing of the therapeutic intervention during pregnancy needs to be determined through further clinical investigations.
This cross-sectional study design could not differentiate the data based on the fluctuating serological marker profile of chronic hepatitis infection at different stages of the disease. Another major limitation of this study is the failure to distinguish the true inactive carriers from active HBeAg-negative individuals, which could be possible by performing varying levels of ALT in blood. Although we did not include the awareness rate of HBsAg-negative pregnant women as controls, we compared our results with other studies on the awareness rate of knowledge about maternal-infantile HBV infection, so as to draw the conclusion of a low awareness rate of knowledge about HBV in our subjects. The awareness rate of vertical transmission knowledge among the Chinese population varies from 40% to 91% in different regions,[26,27] of which Shaanxi is an area of low awareness. Compared with foreign countries, the awareness rate in our country is also low.[26] Nevertheless, according to the data we obtained, we could not get a cut-off score for the lack of awareness.
The prevalence rate of HBsAg-positivity in pregnant women from Shaanxi is still high, the awareness and knowledge of the disease are deficient, and further epidemiological training and education should be implemented in primary level doctors and pregnant women. The precise interventional and monitoring strategies for HBeAg-negative and HBeAg-positive mothers need to clarify with an elaborate comprehensive multicenter study including large-scale subjects (Table 5).
Table 5.
Knowledge of HBV transmission and prevention among HBsAg-positive mothers.
Acknowledgment
We are very grateful to the pregnant women and their children participating in our survey.
Author contributions
Data curation: Tianyan Chen, Jing Wang, Hongtao Qiu, Taotao Yan, Yuan Yang, Yingli He, Yingren Zhao.
Formal analysis: Furong Cao.
Investigation: Hongtao Qiu, Qiang Yu, Caijing Qi, Naijuan Yao, Jinfeng Liu.
Methodology: Tianyan Chen, Hongtao Qiu, Qiang Yu, Taotao Yan, Furong Cao, Zhen Tian, Yuan Yang, Yingren Zhao, Jinfeng Liu.
Project administration: Taotao Yan, Caijing Qi, Furong Cao, Dandan Guo, Jinfeng Liu.
Resources: Tianyan Chen, Jinfeng Liu.
Validation: Tianyan Chen.
Writing – original draft: Tianyan Chen, Jing Wang, Dandan Guo, Jinfeng Liu.
Writing – review & editing: Tianyan Chen, Jing Wang, Zhen Tian, Naijuan Yao, Yuan Yang, Yingli He, Jinfeng Liu.
Footnotes
Abbreviations: CRFs = case report forms, HBeAg = hepatitis B e antigen, HBIG = hepatitis B immunoglobulin, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, ICP = intrahepatic cholestasis of pregnancy, MTCT = mother-to-child transmission.
TC and JW contributed equally to this work.
Funding/support: This study was funded by the grants obtained from the National Science and Technology Projects on Major Infectious Diseases of China (13th Five Year, China) (Project No. 2017ZX10202202-002-006) and the National Natural Sciences foundation of China (Project No. 81670537 and 81770594).
All authors declare that they have no any conflict of interests.
References
- [1].Wang Y, Zhou H, Zhang L, et al. Prevalence of chronic hepatitis B and status of HBV care among rural women who planned to conceive in China. Sci Rep 2017;7:12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shepard CW, Simard EP, Finelli L, et al. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006;28:112–25. [DOI] [PubMed] [Google Scholar]
- [3].Emechebe GO, Emodi IJ, Ikefuna AN, et al. Hepatitis B virus infection in Nigeria. A review. Niger Med J 2009;50:18–22. [Google Scholar]
- [4].Patton H, Tran TT. Management of hepatitis B during pregnancy. Nat Rev Gastroenterol Hepatol 2014;11:402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fujiko M, Chalid MT, Turyadi, et al. Chronic hepatitis B in pregnant women: is hepatitis B surface antigen quantification useful for viral load prediction? Int J Infect Dis 2015;41:83–9. [DOI] [PubMed] [Google Scholar]
- [6].Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology 2014;60:2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shao ZJ, Zhang L, Xu JQ, et al. Mother-to-infant transmission of hepatitis B virus: a Chinese experience. J Med Virol 2011;83:791–5. [DOI] [PubMed] [Google Scholar]
- [8].Marion SA, Tomm Pastore M, Pi DW, et al. Long-term follow-up of hepatitis B vaccine in infants of carrier mothers. Am J Epidemiol 1994;140:734–46. [DOI] [PubMed] [Google Scholar]
- [9].McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009;49:S45–55. [DOI] [PubMed] [Google Scholar]
- [10].Willey JM, Sherwood LM, Woolverton CJ, et al. Human diseases caused by viruses and prions, Direct Contact Diseases: Viral hepatitides Prescott's Microbiology. International Edition. 8th ed.2011;New York: The McGraw Hill Companies, 919–921. [Google Scholar]
- [11].Chen DS, Lai MY, Lee SC, et al. Serum HBsAg, HBeAg, anti-HBe, and hepatitis B viral DNA in asymptomatic carriers in Taiwan. J Med Virol 1986;19:87–94. [DOI] [PubMed] [Google Scholar]
- [12].Gowans EJ. Relationship between HBeAg and HBV DNA in patients with acute and persistent hepatitis B infection. Med J Aust 1986;145:439–41. [DOI] [PubMed] [Google Scholar]
- [13].Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006;55:1–33. quiz CE1-4. [PubMed] [Google Scholar]
- [14].Xu ZY, Liu CB, Francis DP, et al. Prevention of perinatal acquisition of hepatitis B virus carriage using vaccine: preliminary report of a randomized, double-blind placebo-controlled and comparative trial. Pediatrics 1985;76:713–8. [PubMed] [Google Scholar]
- [15].Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983;2:1099–102. [DOI] [PubMed] [Google Scholar]
- [16].Yi P, Chen R, Huang Y, et al. Management of mother-to-child transmission of hepatitis B virus: propositions and challenges. J Clin Virol 2016;77:32–9. [DOI] [PubMed] [Google Scholar]
- [17].Chow WC, Chong R, Guan R, et al. Ministry of Health clinical practice guidelines: chronic hepatitis B infection. Singapore Med J 2011;52:307–11. quiz 312-313. [PubMed] [Google Scholar]
- [18].Yang Y, Du D, Jin L, et al. A molecular epidemiology study investigating familial clustering of hepatitis B virus infection in families with unfavorable prognoses in Northwest China. J Med Virol 2017;89:1427–34. [DOI] [PubMed] [Google Scholar]
- [19].Liang X, Bi S, Yang W, et al. Epidemiological serosurvey of hepatitis B in China: declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009;27:6550–7. [DOI] [PubMed] [Google Scholar]
- [20].Ding Y, Sheng Q, Ma L, et al. Chronic HBV infection among pregnant women and their infants in Shenyang, China. Virol J 2013;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jiang HX, Han GR, Wang CM, et al. [Abstract efficacy of combined vaccine for the prevention of HBV transmission in highly viremic HBeAg+ mothers and the HBV markers’ dynamic change of babies in follow-up]. Zhonghua Gan Zang Bing Za Zhi 2011;19:818–22. [DOI] [PubMed] [Google Scholar]
- [22].Yi W, Li MH, Hu YH, et al. [Predictive study of HBsAg in different stages of neonatal venous blood on failure of blocking HBV mother to infant transmission]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2011;25:338–41. [PubMed] [Google Scholar]
- [23].Liu Y, Timani K, Mantel C, et al. TIP110/p110nrb/SART3/p110 regulation of hematopoiesis through CMYC. Blood 2011;117:5643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sun KX, Li J, Zhu FC, et al. A predictive value of quantitative HBsAg for serum HBV DNA level among HBeAg-positive pregnant women. Vaccine 2012;30:5335–40. [DOI] [PubMed] [Google Scholar]
- [25].Dun-Dery F, Adokiya MN, Walana W, et al. Assessing the knowledge of expectant mothers on mother-to-child transmission of viral hepatitis B in Upper West region of Ghana. BMC Infect Dis 2017;17:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Han Z, Yin Y, Zhang Y, et al. Knowledge of and attitudes towards hepatitis B and its transmission from mother to child among pregnant women in Guangdong Province, China. PLoS One 2017;12:e0178671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chan OK, Lao TT, Suen SS, et al. Deficient knowledge on hepatitis B infection in pregnant women and prevalence of hepatitis B surface antigen carriage in an endemic area: a review. Hepat Res Treat 2012;2012:317451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu J, Zhang S, Wang Q, et al. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21-49 years in rural China: a population-based, cross-sectional study. Lancet Infect Dis 2016;16:80–6. [DOI] [PubMed] [Google Scholar]
- [29].Huang Y, Li L, Sun X, et al. Screening of pregnant women for hepatitis B virus surface antigen (HBsAg) and subsequent management, Qiandongnan prefecture, Guizhou, China, 2010. Vaccine 2013;31(suppl 9):J62–5. [DOI] [PubMed] [Google Scholar]
- [30].Zhang Y, Fang WM, Fan LC, et al. Hepatitis B surface antigen prevalence among 12 393 rural women of childbearing age in Hainan Province, China: a cross-sectional study. Virol J 2013;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tse KY, Ho LF, Lao T. The impact of maternal HBsAg carrier status on pregnancy outcomes: a case-control study. J Hepatol 2005;43:771–5. [DOI] [PubMed] [Google Scholar]
- [32].Hu Y, Ding YL, Yu L. The impact of intrahepatic cholestasis of pregnancy with hepatitis B virus infection on perinatal outcomes. Ther Clin Risk Manag 2014;10:381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wen WH, Chang MH, Zhao LL, et al. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol 2013;59:24–30. [DOI] [PubMed] [Google Scholar]
- [34].Wang JS, Zhu QR, Wang XH. Breastfeeding does not pose any additional risk of immunoprophylaxis failure on infants of HBV carrier mothers. Int J Clin Pract 2003;57:100–2. [PubMed] [Google Scholar]
- [35].Wang J, Zhu Q, Zhang X. Effect of delivery mode on maternal-infant transmission of hepatitis B virus by immunoprophylaxis. Chin Med J (Engl) 2002;115:1510–2. [PubMed] [Google Scholar]
- [36].Wen WH, Chen HL, Ni YH, et al. Secular trend of the viral genotype distribution in children with chronic hepatitis B virus infection after universal infant immunization. Hepatology 2011;53:429–36. [DOI] [PubMed] [Google Scholar]
- [37].Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661–2. [DOI] [PubMed] [Google Scholar]
- [38].Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008;2:263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012;57:167–85. [DOI] [PubMed] [Google Scholar]
- [40].Zou H, Chen Y, Duan Z, et al. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat 2012;19:e18–25. [DOI] [PubMed] [Google Scholar]


