Abstract
Background:
The relationship between male pattern baldness and incidence of prostate cancer remains inconclusive. Hence, we performed the present meta-analysis based on all eligible cohort and case–control studies.
Methods:
A comprehensive literature search was performed in October 2017 based on PubMed and Web of Science databases. Pooled relative risk (RR) and its 95% confidence interval (95% CI) was calculated with a DerSimonian and Laird random-effects model.
Results:
A total of 15 studies were included in this meta-analysis. Overall, no statistically significant association between baldness (any pattern) and prostate cancer risk was identified (RR: 1.03, 95% CI 0.96–1.11). There was obvious heterogeneity across included studies (P < .078 for heterogeneity, I2 = 36.4%). When subgroup analysis by types of baldness, a statistically significant association was observed for vertex baldness (RR 1.24, 95% CI 1.05–1.46) but not for other types of baldness.
Conclusion:
Individuals with vertex baldness may have an increased risk of prostate cancer. Given the obvious heterogeneity and null results in overall analysis and most of subgroup analyses, further large well-designed prospective cohort studies are warranted to confirm our preliminary findings.
Keywords: male pattern baldness, meta-analysis, prostate cancer, risk
1. Introduction
Prostate cancer is the second-most commonly diagnosed cancer among men worldwide, with a total of 1,111,700 new cases and 307,500 deaths estimated in 2012.[1] With the wide utilization of the prostate-specific antigen (PSA) testing, a growing number of prostate cancer cases at local or regional stages have been diagnosed.[2] The only well-established risk factors for prostate cancer are age, race/ethnicity, and family history of prostate cancer.[3] Hence, an additional study to improve our understanding of the etiology of prostate cancer is warranted.
Recently, several researchers have explored whether male pattern baldness (or androgenic alopecia) is a potential indicator for future prostate cancer incidence, as male pattern baldness seems to share pathologic mechanisms with prostate cancer, such as advancing age, hereditability, and endogenous hormones.[4,5] As male pattern baldness generally occurs decades earlier than prostate cancer, baldness may serve as a potential noninvasive screening indicator for prostate cancer if a clear association between male pattern baldness and prostate cancer could be established. However, findings from previous epidemiologic studies on the relationship between male pattern baldness and prostate cancer risk are inconclusive due to the different study designs, limited sample size, various baldness measurements and classification, and time window (age of baldness onset). Al Edwan et al,[6] Zeigler-Johnson et al,[7] and Giles et al[8] reported a significant positive association of baldness with prostate cancer risk, while many other epidemiological studies failed to confirm this association.
Given the conflicting findings in literature as discussed above, we performed the present systematic review and meta-analysis to summarize evidence on the association of male pattern baldness with risk of prostate cancer.
2. Materials and methods
2.1. Literature search
A comprehensive literature search was performed in October 2017 based on PubMed and Web of Science databases with the following search algorithm: (“baldness” or “alopecia” or “hair loss”) and (“prostate cancer” or “prostate neoplasm” or “prostatic carcinoma”). In addition, the reference lists of retrieved articles and related reviews were checked to identify any potential relevant studies. No language or publication date restrictions were adopted. The present systematic review and meta-analysis was designed, performed, and reported in accordance with the standards of quality for reporting a systematic review and meta-analysis.[9]
2.2. Selection criteria
Articles included in this study had to meet all the following criteria: the exposure of interest was male pattern baldness; the outcome of interest was risk of prostate cancer; study design was cohort, nested case–control, or case-control; and the risk estimates with their corresponding 95% CIs were reported or sufficient data were provided to calculate them. If multiple articles used the same study population, the article with the largest sample size was included in the present meta-analysis.
2.3. Quality assessment
Newcastle–Ottawa Scale (NOS) was introduced to evaluate the quality of included studied by two independent authors (HH and BX). NOS is a 9-star system, which includes 3 dimensions: selection (4 items), comparability (1 item), and exposure/outcome (3 items) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Each item represents 1 point, except for comparability (2 points). A study with ≥ 7 points was considered as a high-quality study.
2.4. Data extraction
Data were extracted independently by the same 2 authors (HH and BX) and any discrepancies were resolved by discussion. The following information from each included study were recorded: first author's surname, the country where the study was conducted, study design, publication year, age of study population, sample size and number of cases, types of baldness, fully adjusted risk estimates with their corresponding 95% confidence intervals (95% CIs), and matched or adjusted variables in the study design or statistical analysis.
2.5. Statistical methods
Considering that prostate cancer is a rare disease, the odds ratio (OR) was assumed approximately the same as relative risk (RR), and the RR was adopted as the study outcome. Pooled RR and its 95% CI was calculated with a DerSimonian and Laird random-effects model,[10] which takes account for both within- and between-study variability. For studies that reported separate effect size estimates for different types of baldness, we combined these risk estimates within each study, weighted by inverse of the variance.[11] Subgroup analyses were performed based on geographical region, types of baldness, age of baldness onset, study design, and stage of prostate cancer.
Heterogeneity across included studies was assessed with the Cochran Q test (the level of significance was set at 0.1).[12] The I2 score was also used to determine the degree of heterogeneity (I2 < 25%, no obvious heterogeneity; I2 = 25–50%, moderate heterogeneity; I2 > 50%, large or extreme heterogeneity).[12] Finally, Galbraith plot [13] was introduced to identify the studies that contributed to the heterogeneity and meta-analysis was performed again after removing these studies.
In sensitivity analysis, meta-analysis was reconducted after omitting each study in turn. Potential publication bias was evaluated with Begg test [14] and Egger test.[15] Statistical analyses were completed with STATA 11.0 (StataCorp, College Station, TX), using 2-sided P values (set at .05). All analyses of this meta-analysis were based on previous published studies, and this meta-analysis did not have original data. Thus, no ethical approval and patient consent are required.
3. Results
3.1. Study search and characteristics
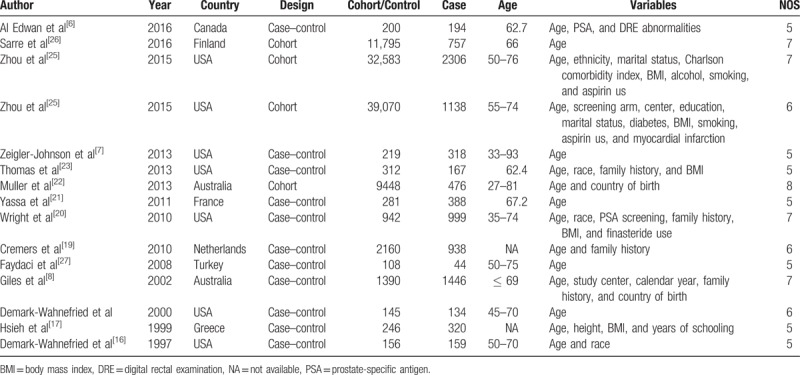
The detailed process of literature search is shown in Fig. 1. A total of 15 eligible studies [6–8,16–27] were finally included in the present meta-analysis. These studies were carried out in the following geographical regions: Europe (n = 5), North America (n = 8), and Oceania (n = 2). There were 4 cohort studies and 11 case–control studies, which were published between 1997 and 2016. Information on exposure (male pattern baldness) was collected by face-to-face interview or self-reported questionnaire. Study outcome (prostate cancer) was histopathologically proved in most of the included studies. Quality scores evaluated by the Newcastle–Ottawa Scale (NOS) ranged from 5 to 8. The main characteristics of all included studies have been summarized in Table 1.
Figure 1.
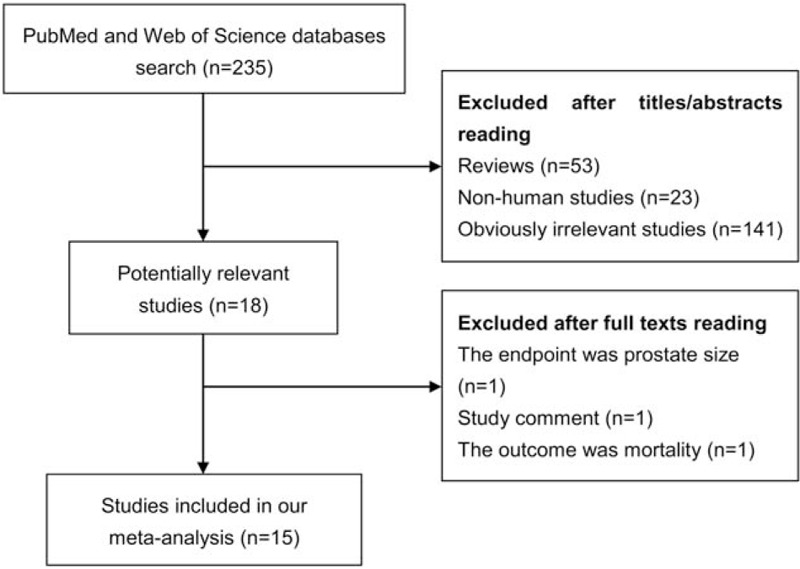
Literature search and study selection.
Table 1.
Summary characteristics of the studies included in this meta-analysis.
3.2. Overall analysis and heterogeneity assessment
Multivariable adjusted RRs with their 95% CIs extracted from each included study that evaluated baldness (any pattern) and prostate cancer risk are present in Fig. 2. Overall, in a random effects model combining these studies, no statistically significant association between baldness (any pattern) and prostate cancer risk was identified (RR: 1.03, 95% CI 0.96–1.11). There was statistically significant heterogeneity across included studies (P < .078 for heterogeneity, I2 = 36.4%). Thus, we adopted the Galbraith plot to explore the studies that led to the heterogeneity. Consequently, studies by Al Edwan et al,[6] Zeigler-Johnson et al,[7] and Giles et al[8] were the major sources of heterogeneity (Fig. 3). Overall analysis was reconducted after omitting these 3 studies. The heterogeneity was disappeared (P = .908, I2 = 0.0%), while the trend direction of combined risk estimate was not changed (RR: 0.99, 95% CI 0.94–1.04).
Figure 2.
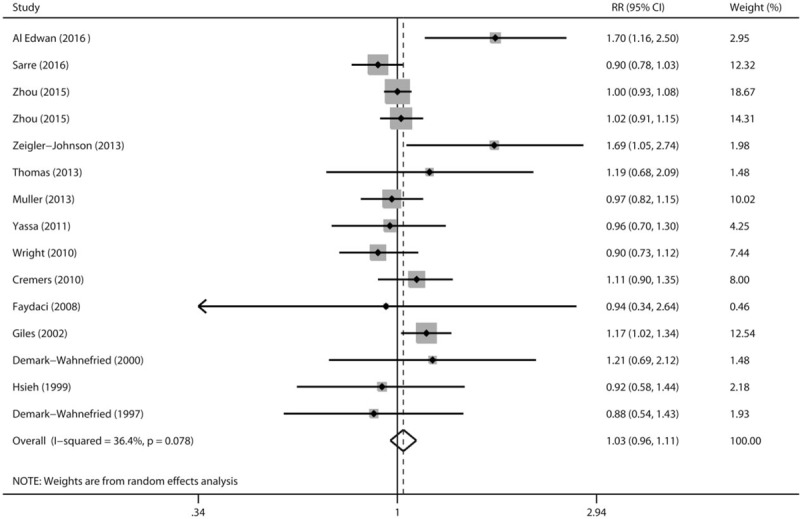
A forest plot showing effect size estimates from case–control and cohort studies evaluating the association between male pattern baldness and prostate cancer risk.
Figure 3.
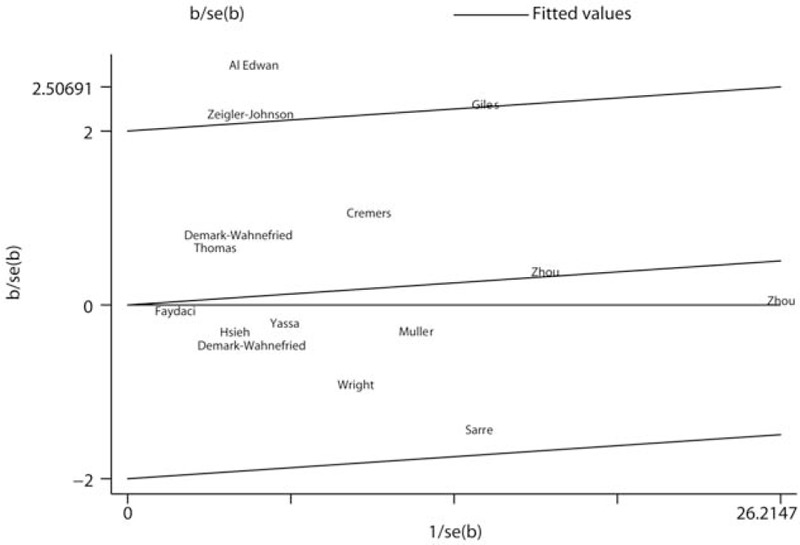
Galbraith plot analysis was performed to detect potential sources of heterogeneity.
3.3. Subgroup analysis
Next, we performed stratified analyses according to various study characteristics (Table 2). When stratified analysis by geographical regions, the RRs with 95% CIs were 0.96 (0.86–1.06), 1.07 (0.95–1.20), and 1.07 (0.89–1.29) for Europe, North America, and Oceania, respectively. When further subgroup analysis was done by types of baldness, statistically significant association was observed for vertex baldness (RR 1.24, 95% CI 1.05–1.46) but not for other types of baldness. We then conducted stratified analysis by study design, the analysis limited to cohort studies yielded an RR of 0.99 (95% CI 0.93–1.04), and the analysis for case–control studies yielded an RR of 1.11 (95% CI 0.98–1.25). In the subgroup analysis by stage of prostate cancer, the RRs with 95% CIs were 1.04 (0.92–1.17) and 1.14 (0.96–1.35) for nonaggressive and aggressive prostate cancer, respectively. Finally, in the stratified analysis by the age of baldness onset, male pattern baldness at age 20 years, age 30 years, and age 40 years were not associated with overall prostate cancer risk.
Table 2.
Subgroup analysis of the association between male pattern baldness and risk of prostate cancer.
3.4. Sensitivity analysis and publication bias
Sensitivity analysis was performed to determine the influence of each included study on the pooled RR by repeating the meta-analysis after removing each study in turn. As shown in Fig. 4, no individual study obviously dominated the pooled RR. The 15 study-specific RRs ranged from a low of 1.01 (95% CI 0.94–1.08) to a high of 1.05 (95% CI 0.98–1.13) after omitting the study by Giles et al [8] and the study by Sarre et al,[26] respectively. Finally, no significant publication bias was detected by Begg test (Fig. 5, P = .428) or Egger test (P = .627).
Figure 4.
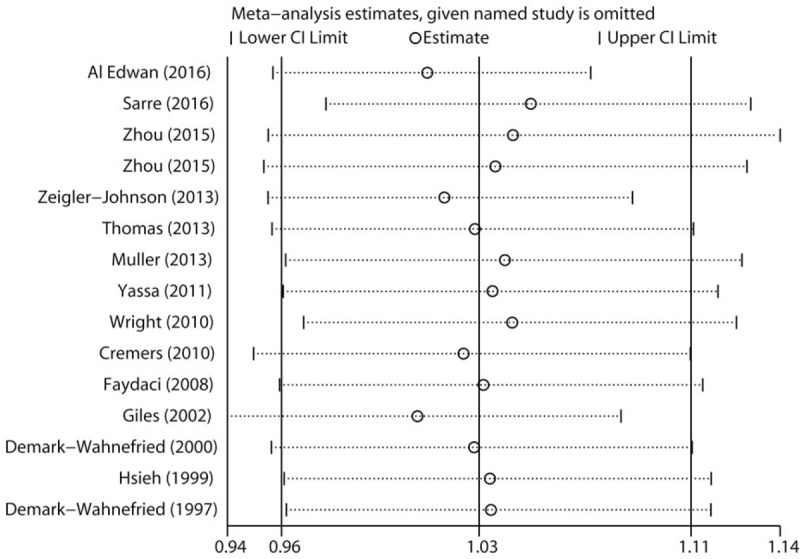
Sensitivity analysis was carried out by omitting each study in turn and recalculating the pooled risk estimates.
Figure 5.
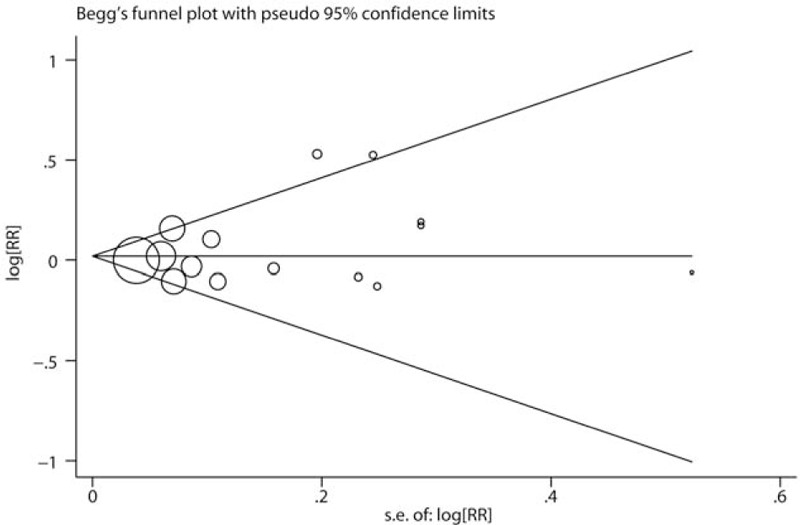
Publication bias was evaluated by Begg test.
4. Discussion
The present systematic review and meta-analysis summarized the relationship between male pattern baldness and incidence of prostate cancer based on 4 cohort studies and 11 case–control studies. Overall, there was no statistically significant association between baldness (any pattern) and prostate cancer risk (RR: 1.03, 95% CI 0.96–1.11). However, in the subgroup analysis by types of baldness, a statistically significant association was observed for vertex baldness (RR 1.24, 95% CI 1.05–1.46).
The results of our meta-analysis were basically in accordance with a previous meta-analysis by Amoretti et al published in 2013,[28] which only included 7 case–control studies. They also found that any pattern baldness was not associated with a significant increase in the risk of prostate cancer, but individuals with vertex baldness had a higher incidence of prostate cancer. After their study was reported, emerging studies, especially cohort studies, on this topic have been published with conflicting results. Hence, we performed the present updated meta-analysis to summarize all available evidence. Furthermore, we also analyzed age-specific baldness in relation to prostate cancer risk and the association of male pattern baldness with different stage of prostate cancer, which were not presented in the previous meta-analysis.
Male pattern baldness is considered as a common progressive hair-loss process in a well-defined pattern and seems to share common pathophysiological mechanisms with prostate cancer (i.e., age, heritability and endogenous hormones).[4,5] Both incidences of male pattern baldness and prostate cancer increase with advancing age. Inherited genetic factors contribute to 81% of male pattern baldness [5] and 42% of prostate cancer risk.[4] A multistage genome-wide association study has identified more than 40 prostate cancer susceptibility loci, explaining ∼25% of the familial risk in this disease.[29] Several independent studies indicated that some androgen receptor (AR) polyglycine repeat polymorphisms might confer susceptibility to androgenetic alopecia.[30–32] As for endogenous hormones, both hair follicles and the prostate gland respond to stimulation of androgen. Individuals with baldness seem to have a higher level of circulating androgens than those without.[33] High circulating androgens have been reported to be associated with an increased risk of prostate cancer,[16,34] although the evidence in epidemiologic studies are still conflicting.[35] Considering that male pattern baldness happens decades earlier than prostate cancer, baldness may serve as a potential noninvasive indicator for screening of prostate cancer if a clear association between male pattern baldness and prostate cancer could be confirmed by large well-designed cohort studies.
The present study had some strengths. A total of 15 epidemiologic studies were included in this systematic review and meta-analysis, which improved the statistical power and reported more reliable estimates compared with any individual study. Various subgroup analyses according to key characteristics were performed, which provided a comprehensive and in-depth evaluation of the relationship between male baldness and prostate cancer. Sensitivity analyses and Galbraith plot were carried out to confirm the robustness of pooled risk estimate. The effect size estimates were extracted from fully adjusted models in each study, which might minimize the potential confounding. No obvious publication bias was observed in Begg test or Egger test.
However, several important limitations should also be acknowledged in this study. First, statistical significantly heterogeneity was detected across included studies (P < .078 for heterogeneity, I2 = 36.4%), which might affect the robustness of the pooled risk estimates. We removed the studies that contributed to the heterogeneity via the Galbraith plot and recalculated the combined RR, which did not remain significant (RR: 0.99, 95% CI 0.94–1.04). This result implied that the overall analysis was not substantially affected by the heterogeneity. Second, most of the eligible studies were case–control studies, which were less conclusive than cohort studies and might introduce the select bias and recall bias. Third, although the majority of the included studies adopted the Norwood–Hamilton scale to assess the baldness, differences in exposure assessment still existed, which might distort the combined risk estimates. Finally, gray literature (e.g., conference abstracts) and studies reported in languages other than English or Chinese were less likely to be retrieved, which might lead to a certain degree of publication bias.
In conclusion, this systematic review and meta-analysis allowed us to perform overall analysis of male pattern baldness with prostate cancer risk, as well as various subgroup analyses with strong statistical power. As a result, we found that individuals with vertex baldness had an increased risk of prostate cancer. Given the obvious heterogeneity and null results in overall analysis and most of subgroup analyses, further large well-designed prospective cohort studies are warranted to confirm our preliminary findings.
Author contributions
Conceptualization: Huadong He, Bo Xie, Liping Xie.
Data curation: Huadong He, Bo Xie, Liping Xie.
Formal analysis: Huadong He, Bo Xie, Liping Xie.
Funding acquisition: Liping Xie.
Investigation: Huadong He, Bo Xie, Liping Xie.
Methodology: Huadong He, Bo Xie, Liping Xie.
Project administration: Huadong He, Bo Xie, Liping Xie.
Resources: Huadong He, Bo Xie, Liping Xie.
Software: Huadong He, Liping Xie.
Supervision: Huadong He, Bo Xie, Liping Xie.
Validation: Huadong He, Bo Xie, Liping Xie.
Visualization: Huadong He, Bo Xie, Liping Xie.
Writing – original draft: Huadong He, Bo Xie, Liping Xie.
Writing – review & editing: Huadong He, Bo Xie, Liping Xie.
Footnotes
Abbreviations: CI = confidence interval, NOS = Newcastle–Ottawa Scale, OR = odds ratio, RR = relative risk.
The authors of this work have nothing to disclose.
The authors declare no conflict of interest.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Potosky AL, Miller BA, Albertsen PC, et al. The role of increasing detection in the rising incidence of prostate cancer. JAMA 1995;273:548–52. [PubMed] [Google Scholar]
- [3].Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet 2016;387:70–82. [DOI] [PubMed] [Google Scholar]
- [4].Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer: analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000;343:78–85. [DOI] [PubMed] [Google Scholar]
- [5].Nyholt DR, Gillespie NA, Heath AC, et al. Genetic basis of male pattern baldness. J Invest Dermatol 2003;121:1561–4. [DOI] [PubMed] [Google Scholar]
- [6].Al Edwan G, Bhindi B, Margel D, et al. The association of male pattern baldness and risk of cancer and high-grade disease among men presenting for prostate biopsy. Can Urol Assoc J 2016;10:E424–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zeigler-Johnson C, Morales KH, Spangler E, et al. Relationship of early-onset baldness to prostate cancer in African-American men. Cancer Epidemiol Biomarkers Prev 2013;22:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Giles GG, Severi G, Sinclair R, et al. Androgenetic alopecia and prostate cancer: findings from an Australian case-control study. Cancer Epidemiol Biomarkers Prev 2002;11:549–53. [PubMed] [Google Scholar]
- [9].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [11].Hartemink N, Boshuizen HC, Nagelkerke NJ, et al. Combining risk estimates from observational studies with different exposure cutpoints: a meta-analysis on body mass index and diabetes type 2. Am J Epidemiol 2006;163:1042–52. [DOI] [PubMed] [Google Scholar]
- [12].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [13].Bax L, Ikeda N, Fukui N, et al. More than numbers: the power of graphs in meta-analysis. Am J Epidemiol 2009;169:249–55. [DOI] [PubMed] [Google Scholar]
- [14].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [15].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Demark-Wahnefried W, Lesko SM, Conaway MR, et al. Serum androgens: associations with prostate cancer risk and hair patterning. J Androl 1997;18:495–500. [PubMed] [Google Scholar]
- [17].Hsieh CC, Thanos A, Mitropoulos D, et al. Risk factors for prostate cancer: a case-control study in Greece. Int J Cancer 1999;80:699–703. [DOI] [PubMed] [Google Scholar]
- [18].Denmark-Wahnefried W, Schildkraut JM, Thompson D, et al. Early onset baldness and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2000;9:325–8. [PubMed] [Google Scholar]
- [19].Cremers RG, Aben KK, Vermeulen SH, et al. Androgenic alopecia is not useful as an indicator of men at high risk of prostate cancer. Eur J Cancer 2010;46:3294–9. [DOI] [PubMed] [Google Scholar]
- [20].Wright JL, Page ST, Lin DW, et al. Male pattern baldness and prostate cancer risk in a population-based case-control study. Cancer Epidemiol 2010;34:131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yassa M, Saliou M, De Rycke Y, et al. Male pattern baldness and the risk of prostate cancer. Ann Oncol 2011;22:1824–7. [DOI] [PubMed] [Google Scholar]
- [22].Muller DC, Giles GG, Sinclair R, et al. Age-dependent associations between androgenetic alopecia and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2013;22:209–15. [DOI] [PubMed] [Google Scholar]
- [23].Thomas JA, Antonelli JA, Banez LL, et al. Androgenetic alopecia at various ages and prostate cancer risk in an equal-access multiethnic case-control series of veterans. Cancer Causes Control 2013;24:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhou CK, Littman AJ, Levine PH, et al. Male pattern baldness in relation to prostate cancer risks: an analysis in the VITamins and lifestyle (VITAL) cohort study. Prostate 2015;75:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhou CK, Pfeiffer RM, Cleary SD, et al. Relationship between male pattern baldness and the risk of aggressive prostate cancer: an analysis of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. J Clin Oncol 2015;33:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sarre S, Maattanen L, Tammela TL, et al. Postscreening follow-up of the Finnish Prostate Cancer Screening Trial on putative prostate cancer risk factors: vitamin and mineral use, male pattern baldness, pubertal development and non-steroidal anti-inflammatory drug use. Scand J Urol 2016;50:267–73. [DOI] [PubMed] [Google Scholar]
- [27].Faydaci G, Bilal E, Necmettin P, et al. Baldness, benign prostate hyperplasia, prostate cancer and androgen levels. Aging Male 2008;11:189–92. [DOI] [PubMed] [Google Scholar]
- [28].Amoretti A, Laydner H, Bergfeld W. Androgenetic alopecia and risk of prostate cancer: a systematic review and meta-analysis. J Am Acad Dermatol 2013;68:937–43. [DOI] [PubMed] [Google Scholar]
- [29].Kote-Jarai Z, Olama AA, Giles GG, et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Genet 2011;43:785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ellis JA, Scurrah KJ, Cobb JE, et al. Baldness and the androgen receptor: the AR polyglycine repeat polymorphism does not confer susceptibility to androgenetic alopecia. Hum Genet 2007;121:451–7. [DOI] [PubMed] [Google Scholar]
- [31].Ellis JA, Stebbing M, Harrap SB. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J Invest Dermatol 2001;116:452–5. [DOI] [PubMed] [Google Scholar]
- [32].Hillmer AM, Hanneken S, Ritzmann S, et al. Genetic variation in the human androgen receptor gene is the major determinant of common early-onset androgenetic alopecia. Am J Hum Genet 2005;77:140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bang HJ, Yang YJ, Lho DS, et al. Comparative studies on level of androgens in hair and plasma with premature male-pattern baldness. J Dermatol Sci 2004;34:11–6. [DOI] [PubMed] [Google Scholar]
- [34].Hyde Z, Flicker L, McCaul KA, et al. Associations between testosterone levels and incident prostate, lung, and colorectal cancer. A population-based study. Cancer Epidemiol Biomarkers Prev 2012;21:1319–29. [DOI] [PubMed] [Google Scholar]
- [35].Gill JK, Wilkens LR, Pollak MN, et al. Androgens, growth factors, and risk of prostate cancer: the multiethnic cohort. Prostate 2010;70:906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


