Abstract
This study investigated the effect of transcutaneous electrical nerve stimulation (TENS) for the treatment of patients with chronic pain after ankylosing spondylitis (AS).
A total of 72 eligible patients with chronic pain following AS were included. All included patients received exercise and were assigned to a treatment group and a control group equally. In addition, patients in the treatment group also underwent TENS therapy. All patients were treated for a total of 6 weeks. The primary outcome of pain intensity was measured by visual analog scale (VAS). The secondary outcomes included degree of functional limitation, as assessed by Bath Ankylosing Spondylitis Functional Index (BASFI); and quality of life, as evaluated by Ankylosing Spondylitis Quality of Life (ASQoL) questionnaire. All outcomes were assessed before and after 6 weeks treatment. Furthermore, adverse events were also recorded.
After 6-week treatment, patients in the treatment group did not show more promising outcomes in pain reduction, as measured by VAS (P = .08); functional evaluation, as evaluated by BASFI (P = .19); as well as quality of life, as assessed by ASQoL (P = .18), compared with patients in the control group. No adverse events occurred in both groups.
This study did not exert encouraging outcomes in patients with chronic pain following AS after 6-week treatment.
Keywords: ankylosing spondylitis, effect, exercise, transcutaneous electrical nerve stimulation
1. Introduction
Ankylosing spondylitis (AS) is a chronic systemic inflammatory disease that mainly impacts the axial skeleton, peripheral joints, and others.[1–3] It manifests with the back pain, loss of spinal mobility, joint stiffness, and fatigue.[4,5] If such condition cannot be treated effectively and timely, it may result in spinal deformity and ankylosis.[6] It has been reported that its prevalence ranges from 0.5% to 1.6%, and such condition occurred more commonly in men than women.[7,8]
The availability of treatment options are still limited for AS. Although several medications are utilized to treat this condition, their efficacy is still not satisfied, and lots of adverse events are accompanied after long term taken.[2,9–12] Thus, alternative therapy is urgently needed to treat this condition.
Complementary and alternative therapy (CAT) is a potential candidate to treat AS.[13,14] It has been reported that CAT intervention may benefit for patients with AS.[13,14] Such intervention mainly consists of acupuncture, moxibustion, Chinese herbal medicine, ultrasound therapy, physical therapy, and transcutaneous electrical nerve stimulation (TENS).[15–24] Of these, TENS is reported to treat patients with AS effectively. However, limited data are still available concerning its effect patients with chronic pain after AS. Therefore, in this retrospective study, we explored the effect of TENS in patients with chronic pain following AS.
2. Methods
2.1. Ethics
This study was approved by the Ethical Committee of the Fourth People's Hospital of Shaanxi. Written informed consent was required from each patient.
2.2. Design
The present study was a retrospective study, and 72 patients with chronic pain following AS were assigned to a treatment group and a control group equally. The data was collected during March 2016 to November 2017 in the Fourth People's Hospital of Shaanxi. The patients in the treatment group received exercise plus TENS therapy, while the subjects in the control group received exercise only. All outcome measurements were evaluated before and after 6 week treatment.
2.3. Patients
In this study, a total of 72 eligible patients with the confirmed diagnosis of chronic pain after AS were included, based on the modified New York criteria for AS.[25] All patients suffered from chronic spinal pain more than 6 months before this study, measured by score on the visual analog scale (VAS; 0–10 scale) ≥ 4. All patients aged from 23 to 75 years old. The cases were excluded if the patients had severe organ diseases, administered corticosteroids, joint injections, exercise, or TENS in the past 1 month. In addition, the case was also excluded if it had incomplete information.
2.4. Intervention
Patients in both groups received exercise.[26] This intervention consisted of postural exercises and analytic flexibility exercises. All the exercises worked at the cervical, thoracic, and lumbar spine. Moreover, this schedule also included the stretching of the erector spinae, hamstrings, and shoulder muscles, as well as the exercises of chest expansion, and breathing.
In addition, patients in the treatment group also underwent TENS therapy. It was applied by TENS stimulator device to the painful area TENS stimulator (ENS 931; Enraf Nonius, Rotterdam, South Holland, the Netherlands) with 2 electrodes. It delivered frequency of 100 Hz, pulse duration of 100 μs for 30 minutes each time. The current intensity was gradually increased to the maximum tolerance of individuals. It was performed 2 times weekly for a total of 6 weeks.
2.5. Outcomes
The primary outcome was pain intensity. It was assessed by the VAS, ranges from 0, no pain, to 10, severest pain.[27] The secondary outcomes consisted of degree of functional limitation, measured by Bath Ankylosing Spondylitis Functional Index (BASFI), varies from 0 to 10, with higher score indicating heavier functional limitation;[28] and quality of life, measured by Ankylosing Spondylitis Quality of Life (ASQoL) questionnaire, ranges from 0 to 18, with higher score indicating worse quality of life.[29] In addition, adverse events were also documented. All the outcomes measurements were assessed before and after 6 weeks treatment.
2.6. Statistical analysis
All data were analyzed by the statistician using SPSS software (SPSS V.17.0, IBM Corp., Armonk, NY). Chi-square test was applied to analyze the categorical data, while t test or Mann–Whitney U test were utilized to analyze the continuous data. The statistical significance level was defined as P < .05.
3. Results
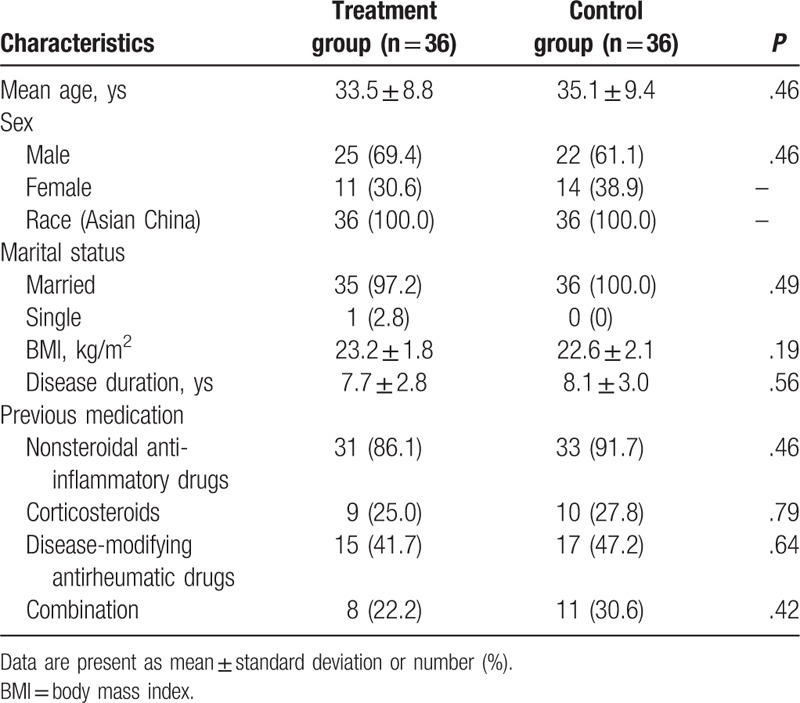
In this retrospective study, all characteristics of subjects in both groups were demonstrated in Table 1. The comparison of all those characteristics did not differ significantly between 2 groups. The characteristics included age, sex, race, marital status, body mass index, disease duration, and previous medication (Table 1).
Table 1.
Patients demographics and characteristics.
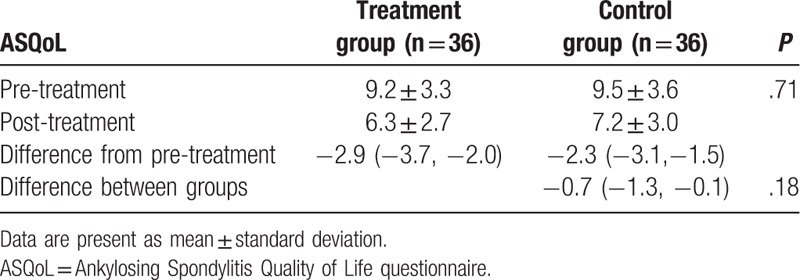
After 6-week treatment, patients in the treatment group did not show better outcomes in reduction of pain intensity, as measured by VAS (P = .08, Table 2); functional improvement, as assessed by BASFI (P = .19, Table 3); and enhancement of quality of life, as evaluated by ASQoL (P = .18, Table 4), compared those patients in the control group.
Table 2.
Comparison of pain intensity pre/post treatment.
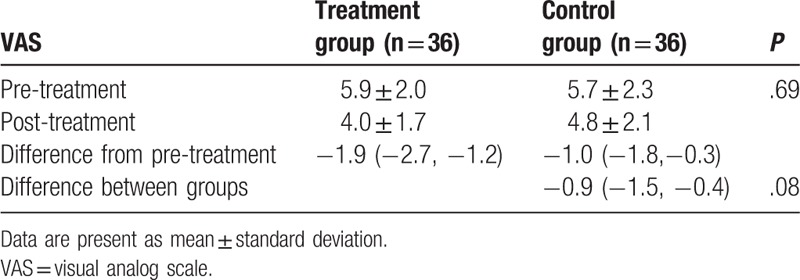
Table 3.
Comparison of degree of functional limitation pre/post treatment.
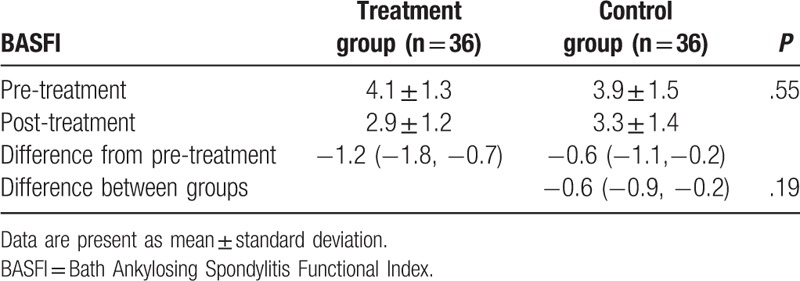
Table 4.
Comparison of quality of life pre/post treatment.
No adverse events were recorded in this study. No death related to the treatment occurred in both groups.
4. Discussion
Two previous published studies addressed investigating the efficacy of TENS for the treatment of patients with AS.[23,24] They found that TENS is significantly better than placebo in treating lumbar pain and stiffness following AS.[23,24] Although their results were still insufficient statistically significance, it was recommended to lower the analgesic medications.
The results of this retrospective study are inconsistent with the previous study.[23,24] This study found that patients in the treatment group did not exert greater effectiveness in chronic pain relief, measured by VAS, compared with patients in the control group. In addition, patients received TNES also did not enhance the degree of functional limitation, measured by BASFI; and failed to improve the quality of life, measured by ASQoL questionnaire. However, no adverse events occurred in both groups. The results of this study indicated that TENS may not benefit for patients with chronic pain, and quality of life caused by AS after 6-week treatment.
This study has several limitations: first, the dose of this study may be insufficient for the chronic pain relief following AS, because it only utilized TENS twice weekly for a total of 6 weeks. Second, the sample size of this retrospective is quite small, which may impact the effectiveness evaluation of TENS for chronic pain after AS. Third, the current study may consist of incomprehensive outcome assessments, due to the only available data of this retrospective study.
5. Conclusion
The results of this study did not exert that TENS may benefit for patients with chronic pain following AS after 6-week treatment.
Author contributions
Conceptualization: Deng-Feng Wang, Fu-Chun Chen, Zhen-Ling Jin.
Data curation: Deng-Feng Wang, Fu-Chun Chen, Zhen-Ling Jin.
Formal analysis: Fu-Chun Chen.
Investigation: Deng-Feng Wang.
Methodology: Fu-Chun Chen.
Project administration: Deng-Feng Wang.
Software: Fu-Chun Chen.
Supervision: Deng-Feng Wang, Zhen-Ling Jin.
Validation: Fu-Chun Chen, Zhen-Ling Jin.
Visualization: Fu-Chun Chen, Zhen-Ling Jin.
Writing – original draft: Deng-Feng Wang, Fu-Chun Chen, Zhen-Ling Jin.
Writing – review & editing: Deng-Feng Wang, Fu-Chun Chen, Zhen-Ling Jin.
Footnotes
Abbreviations: AS = ankylosing spondylitis, ASQoL = Ankylosing Spondylitis Quality of Life, BASFI = Bath Ankylosing Spondylitis Functional Index, TENS = transcutaneous electrical nerve stimulation, VAS = visual analog scale.
The authors declare no conflicts of interest.
References
- [1].Moon KH, Kim YT. Medical treatment of ankylosing spondylitis. Hip Pelvis 2014;26:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wendling D. An overview of investigational new drugs for treating ankylosing spondylitis. Expert Opin Investig Drugs 2016;25:95–104. [DOI] [PubMed] [Google Scholar]
- [3].Hanson A, Brown MA. Genetics and the causes of ankylosing spondylitis. Rheum Dis Clin North Am 2017;43:401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Qian Q, Xu X, He H, et al. Clinical patterns and characteristics of ankylosing spondylitis in China. Clin Rheumatol 2017;36:1561–8. [DOI] [PubMed] [Google Scholar]
- [5].Hinze AM, Louie GH. Osteoporosis management in ankylosing spondylitis. Curr Treatm Opt Rheumatol 2016;2:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dewantoro O, Gianawaty I, Setiyohadi B. Severe ankylosing spondylitis. Acta Med Indones 2006;38:224–5. [PubMed] [Google Scholar]
- [7].Gran JT, Husby G. Klippel JH, Dieppe PA. Ankylosing Spondylitis: Prevalence and Demography. Rheumatology. 2nd ed.London: Mosby; 1998. 1–6. 15. [Google Scholar]
- [8].Gran JT, Husby G. The epidemiology of ankylosing spondylitis. Semin Arthritis Rheum 1993;22:319–34. [DOI] [PubMed] [Google Scholar]
- [9].Elyan M, Khan MA. The role of nonsteroidal anti-inflammatory medications and exercise in the treatment of ankylosing spondylitis. Curr Rheumatol Rep 2006;8:255–9. [DOI] [PubMed] [Google Scholar]
- [10].Fleischmann R, Iqbal I. Risk: benefit profile of etanercept in elderly patients with rheumatoid arthritis, ankylosing spondylitis or psoriatic arthritis. Drugs Aging 2007;24:239–54. [DOI] [PubMed] [Google Scholar]
- [11].Jia C, Liu H, Li M, et al. Effects of icariin on cytokine-induced ankylosing spondylitis with fibroblastic osteogenesis and its molecular mechanism. Int J Clin Exp Pathol 2014;7:9104–9. [PMC free article] [PubMed] [Google Scholar]
- [12].Li H, Guo F, Luo YC, et al. Efficacy of tripterygium glycosides tablet in treating ankylosing spondylitis: a systematic review and meta-analysis of randomized controlled trials. Clin Rheumatol 2015;34:1831–8. [DOI] [PubMed] [Google Scholar]
- [13].Chatfield SM, Dharmage SC, Boers A, et al. Complementary and alternative medicines in ankylosing spondylitis: a cross-sectional study. Clin Rheumatol 2009;28:213–7. [DOI] [PubMed] [Google Scholar]
- [14].Family H, Jordan A, Blaxall K, et al. A truly complementary approach: a qualitative exploration of complementary and alternative medicine practitioners’ views of treating ankylosing spondylitis. Musculoskeletal Care 2018;16:96–102. [DOI] [PubMed] [Google Scholar]
- [15].Huang ZX, Guo XQ, Deng WM, et al. Efficacy of Yisaipu tapering in the treatment of ankylosing spondylitis. Zhonghua Yi Xue Za Zhi 2018;98:1158–61. [DOI] [PubMed] [Google Scholar]
- [16].Lv ZT, Zhou X, Chen AM. Acupuncture therapy versus disease-modifying antirheumatic drugs for the treatment of ankylosing spondylitis: a meta-analysis. Forsch Komplementmed 2015;22:395–402. [DOI] [PubMed] [Google Scholar]
- [17].Jia J, Wang Q, Zhang T, et al. Treatment of ankylosing spondylitis with medicated moxibustion plus salicylazosulfapyridine and methotrexate: a report of 30 cases. J Tradit Chin Med 2006;26:26–8. [PubMed] [Google Scholar]
- [18].Emery P, Lythgoe S. The effect of acupuncture on ankylosing spondylitis. Br J Rheumatol 1986;25:132–3. [DOI] [PubMed] [Google Scholar]
- [19].Sun YY, Cui HJ, Dong JN, et al. Randomized, controlled trial: efficacy of ultrasound and exercise in patients with ankylosing spondylitis. Altern Ther Health Med 2018;[Epub ahead of print]. [PubMed] [Google Scholar]
- [20].Gunay SM, Keser I, Bicer ZT. The effects of balance and postural stability exercises on spa based rehabilitation programme in patients with ankylosing spondylitis. J Back Musculoskelet Rehabil 2018;31:337–46. [DOI] [PubMed] [Google Scholar]
- [21].Pécourneau V, Degboé Y, Barnetche T, et al. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil 2018;99:383–9. [DOI] [PubMed] [Google Scholar]
- [22].Tyrrell JS, Redshaw CH. Physical activity in ankylosing spondylitis: evaluation and analysis of an eHealth tool. J Innov Health Inform 2016;23:169. [DOI] [PubMed] [Google Scholar]
- [23].Nienhuis RL, Hoekstra AJ. Transcutaneous electronic nerve stimulation in ankylosing spondylitis. Arthritis Rheum 1984;27:1074–5. [DOI] [PubMed] [Google Scholar]
- [24].Gemignani G, Olivieri I, Ruju G, et al. Transcutaneous electrical nerve stimulation in ankylosing spondylitis: a double-blind study. Arthritis Rheum 1991;34:788–9. [DOI] [PubMed] [Google Scholar]
- [25].Martins NA, Furtado GE, Campos MJ, et al. Exercise and ankylosing spondylitis with New York modified criteria: a systematic review of controlled trials with meta-analysis. Acta Reumatol Port 2014;39:298–308. [PubMed] [Google Scholar]
- [26].O’Dwyer T, O'Shea F, Wilson F. Exercise therapy for spondyloarthritis: a systematic review. Rheumatol Int 2014;34:887–902. [DOI] [PubMed] [Google Scholar]
- [27].Wickström K, Edelstam G. Minimal clinically important difference for pain on the VAS scale and the relation to quality of life in women with endometriosis. Sex Reprod Healthc 2017;13:35–40. [DOI] [PubMed] [Google Scholar]
- [28].Calin A, Garrett S, Whitelock H, et al. Anew approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing spondylitis functional index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- [29].Doward LC, Spoorenberg A, Cook SA, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis 2003;62:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


