Abstract
DNA damage is presumed to be one type of stochastic macromolecular damage that contributes to aging, yet little is known about the precise mechanism by which DNA damage drives aging. Here, we attempt to address this gap in knowledge using DNA repair-deficient C. elegans and mice. ERCC1-XPF is a nuclear endonuclease required for genomic stability and loss of ERCC1 in humans and mice accelerates the incidence of age-related pathologies. Like mice, ercc-1 worms are UV sensitive, shorter lived, display premature functional decline and they accumulate spontaneous oxidative DNA lesions (cyclopurines) more rapidly than wild-type worms. We found that ercc-1 worms displayed early activation of DAF-16 relative to wild-type worms, which conferred resistance to multiple stressors and was important for maximal longevity of the mutant worms. However, DAF-16 activity was not maintained over the lifespan of ercc-1 animals and this decline in DAF-16 activation corresponded with a loss of stress resistance, a rise in oxidant levels and increased morbidity, all of which were cep-1/ p53 dependent. A similar early activation of FOXO3A (the mammalian homolog of DAF-16), with increased resistance to oxidative stress, followed by a decline in FOXO3A activity and an increase in oxidant abundance was observed in Ercc1-/- primary mouse embryonic fibroblasts. Likewise, in vivo, ERCC1-deficient mice had transient activation of FOXO3A in early adulthood as did middle-aged wild-type mice, followed by a late life decline. The healthspan and mean lifespan of ERCC1 deficient mice was rescued by inactivation of p53. These data indicate that activation of DAF-16/FOXO3A is a highly conserved response to genotoxic stress that is important for suppressing consequent oxidative stress. Correspondingly, dysregulation of DAF-16/FOXO3A appears to underpin shortened healthspan and lifespan, rather than the increased DNA damage burden itself.
Keywords: DNA damage, Aging, Reactive oxygen species, Oxidative stress, DAF-16/FOXO3A, Stress resistance
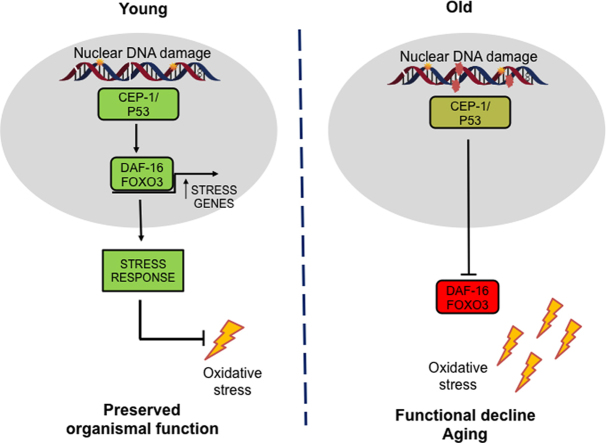
Graphical abstract
1. Introduction
Aging is the principal risk factor for numerous chronic diseases [1] and macromolecular damage is implicated as a key driver [2]. There is evidence that DNA damage is one type of macromolecular damage that drives aging. For example, humans with inherited mutations in genes required for the repair of DNA damage, have accelerated aging or progeroid syndromes [3], [4]. Furthermore, age-related debilitating diseases occur decades earlier in cancer survivors treated with genotoxic agents compared to their untreated siblings [5]. In addition, levels of endogenous DNA lesions appear higher in older or diseased individuals compared to younger, healthier subjects [6]. However, the mechanism(s) by which DNA damage contributes to aging and disease is not fully elucidated.
In replicating cells, DNA damage activates a robust DNA damage response (DDR) program that leads to cell cycle arrest to prevent mutagenesis caused by replicating a damaged genome [7]. If the DNA damage is extensive or irreparable, apoptosis ensues. Apoptosis can contribute to aging by depleting stem cell function and tissue regeneration [8]. Alternatively, the cell cycle arrest can become essentially irreversible causing the cell to senesce [9]. Senescent cells contribute to aging and age-related diseases [10] at least in part via the secretion of proinflammatory factors and metalloproteinases [11]. Many cell types in the body replicate slowly, if at all [12] and recent evidence suggests that DNA damage may also play a prominent role in degenerative disease in post-mitotic tissues. For example, elevated levels of DNA damage (oxidative lesions and strand breaks) are observed in Alzheimer's patients compared to age-matched controls [13], [14]. The genotoxic stress is detected before neurodegeneration is evident, suggesting a causative role, at least in mice [15]. Yet, the mechanism by which DNA damage promotes age-related disease in non-replicating tissues is unknown.
To address this gap in knowledge, we exploited Caenorhabditis elegans (C. elegans), an organism that has yielded a wealth of information on mechanisms that regulate lifespan [16]. Genetic studies in C. elegans led to the identification of numerous molecular pathways that regulate longevity, including nutrient signaling, proteostasis, mitochondrial function and regulation of insulin/IGF signaling. DNA repair pathways are well conserved in C. elegans, but contradictory studies exist about their impact on lifespan [17], [18], [19], [20], [21], [22], [23]. Moreover, there is no consensus as to the longevity pathways that are dysregulated by DNA damage [24].
ERCC1-XPF is a structure-specific endonuclease involved in at least three DNA repair pathways that protect the nuclear genome [3]. Mutations in either gene that result in a reduction of ERCC1-XPF expression result in accelerated accumulation of endogenous oxidative DNA damage [25] and accelerated aging in humans and mice [26], [27]. Here we demonstrate for the first time that ercc-1 mutant worms recapitulate many of the phenotypes of ERCC1-XPF deficient mice, including increased oxidative DNA damage and reduced healthspan/lifespan. Then we asked which stress response mechanisms were activated in response to endogenous DNA damage. We found the DAF-16/FOXO3A transcription factor was activated in ercc-1 worms, leading to increased stress resistance. However, DAF-16 activation was not sustained throughout the lifespan in ercc-1 worms. Decline in DAF-16 activation was cep-1 dependent and was associated with a decrease in stress resistance and a concomitant rise in reactive oxygen species (ROS). Importantly, these events were recapitulated in ERCC1-XPF deficient mammalian cells and mice, as well as naturally aged mice. Thus, we propose that it is not DNA damage per se that drives aging, but rather it is the cellular stress response to this damage that leads to aging phenotypes.
2. Results
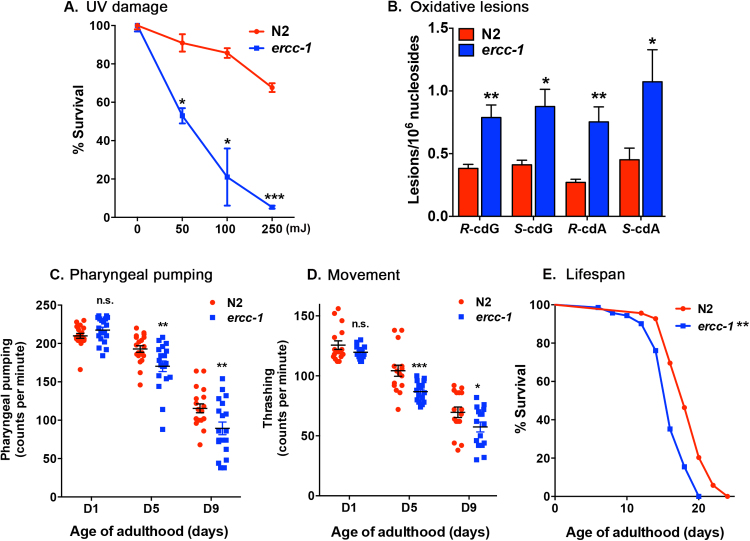
Mutations causing reduced expression of the nuclear DNA repair endonuclease ERCC1-XPF cause an accelerated accumulation of spontaneous DNA damage, the same lesions that accumulate over time with normal aging [28]. It is presumed that it is the increased damage burden that drives the shortened healthspan and lifespan in ERCC1-depleted humans and mice [26], yet the precise mechanism remains enigmatic. Here, we used the nematode C. elegans to identify conserved mechanisms that might explain the effects of ERCC1-XPF deficiency across species. In worms, the ercc-1(tm1981) mutation generates a 619 bp in-frame deletion, whereas, xpf-1(tm2842) generates a 343 bp deletion, and both are considered null alleles [29]. We found ercc-1 worms were hypersensitive to UV irradiation (Fig. 1A), had an increased fraction of unhatched eggs, higher frequency of males and delayed growth (Fig S1), all phenotypes associated with genomic instability in nematodes [20], [29], [30], [31], [32], [33], [34], [35]. Similar phenotypes have been previously shown for the xpf-1 mutant [17], [29]. Furthermore, ercc-1 worms accumulated spontaneous oxidative DNA lesions more rapidly than N2 (Fig. 1B). These observations clearly establish ercc-1 / xpf-1 worms as nucleotide excision repair-deficient.
Fig. 1.
Characterization of DNA repair deficientercc-1worms. (A) Quantification of eggs laid by N2 and ercc-1 adult worms after UV irradiation at various doses relative to mock treatment (0 mJ UV, set at 100% survival). Mean ± S.D. of n = 50–60 worms/genotype; 4 biological replicates. Student's two-tailed t-test *p < 0.05, * **p < 0.001. (B) Levels of endogenous oxidative DNA lesions (R-cdG, S-cdG, R-cdA, and S-cdA cyclopurines) in N2 and ercc-1 worms at day 9 of adulthood (mean ± S.D. from DNA isolated from 1500 to 2000 worms; n = 3 independent populations per genotype). Student's two-tailed t-test *p < 0.05 and * *p < 0.01. (C) Number of pharyngeal contractions and (D) thrashing in liquid per minute for D1, 5 and 9 adult N2 and ercc-1 worms. Mean ± S.D, n = 15–20 worms/group; 3 replicas performed. Student's two-tailed t-test *p < 0.05, * *p < 0.01, * **p < 0.001, n.s. not significant. (E) The lifespan of N2 and ercc-1 mutant worms. Kaplan-Meier survival curves were calculated from populations of 40–70 animals/genotype. Log-rank (Mantel-Cox) test * *p < 0.05.
Consistent with the accelerated aging observed in DNA repair mutant mice and humans harboring mutations in ERCC1 or XPF [26], [27], [36], [37], [38], we found significantly reduced pharyngeal pumping and thrashing frequency in ercc-1 and xpf-1 mutants compared with N2 at day 5 and 9 of adulthood, but not day 1 (Fig. 1C, D, S2A & B), Additionally, backcrossed ercc-1 and xpf-1 mutants exhibited a reproducible reduction in lifespan (Fig. 1E and Fig S2C) compared to N2. Moreover, we observed a reduction in lifespan in the homozygous mutant progeny from a genetically balanced ercc-1 strain (Fig S2D), indicating that these phenotypes are a consequence of ercc-1 deletion. Collectively, these data establish ercc-1 worms DNA repair deficient and prematurely aged, analogous to ERCC1-XPF deficient mammals.
2.1. DNA damage activates DAF-16/FOXO and confers stress resistance
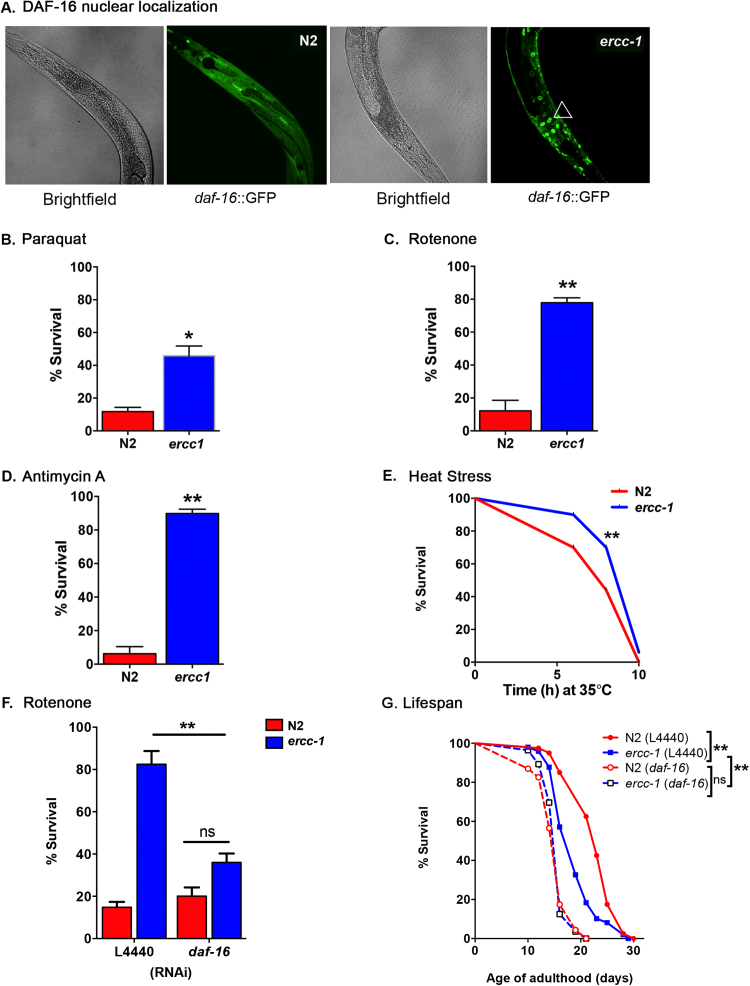
Having confirmed the validity of the worm as an accurate representation of mammalian systems, we used the ercc-1 background to identify pathways activated by DNA damage that may drive aging. We introduced the ercc-1 deletion into multiple transgenic GFP strains that act as reporters of pathways involved in modulating lifespan [39] to identify which were altered by endogenous DNA damage. The screen included reporters for the unfolded protein response (UPR) in endoplasmic reticulum (UPRER–hsp-4::GFP), cytoplasmic heat shock response (hsp-16.2::GFP), the Nrf2 (skn-1::GFP) and FOXO3A (daf-16::GFP) stress defense pathways. Under basal conditions, DAF-16 is maintained in the cytoplasm, but upon exposure to stress, post-translational modifications of DAF-16 leads to its nuclear localization and activation of transcriptional programs that mediate stress resistance and promote longevity [40], [41]. In wild-type worms, DAF-16::GFP was primarily cytoplasmic in D1 adults (Fig. 2A). In contrast, in D1 ercc-1 animals, DAF-16::GFP was nuclear (Fig. 2A). This suggests that this stress defense pathway is activated in adult ercc-1 animals in response to unrepaired, spontaneous, endogenous DNA damage, as previously reported for larvae exposed to UV irradiation [20].
Fig. 2.
DAF-16 activation inercc-1worms leads to multi-stress resistance. Representative images of D1 adult N2 and ercc-1 worms expressing the daf-16::GFP reporter. (B) Viability of day 1 adult N2 and ercc-1 worms 12 h after treatment with 100 mM paraquat (mean ± S.E.M., n = 8 groups of 40–60 worms/genotype). Student's two-tailed t-test *p < 0.05. (C) Viability of day 1 adult worms 16 h after treatment with 12.5 µM rotenone (mean ± S.E.M., n = 6 groups of 40–60 worms/genotype). Student's two-tailed t-test * *p < 0.01. (D) Viability of day 1 adult N2 and ercc-1 worms 12 h after treatment with 25 µM antimycin A (mean ± S.E.M., n = 6 groups of 40–60 worms/genotype). Student's two-tailed t-test * *p < 0.01. (E) Survival of day 1 adult N2 and ercc-1 under acute thermal stress at 35 °C; 3 replicas. Kaplan-Meier survival curves were calculated. Log-rank (Mantel-Cox) test * *p < 0.01. (F) Stress resistance of day 1 adult N2 and ercc-1 worms fed RNAi against control (L4440) or daf-16, 16 h after treatment with 12.5 µM rotenone (mean viability ± S.E.M., n = 4 groups of 40–60 worms/genotype). Student's two-tailed t-test * *p < 0.01; ns not significant. (G) Lifespan of N2 and ercc-1 worms after knockdown of daf-16 with RNAi. Kaplan-Meier survival curves were calculated from populations of 40–70 animals/genotype. Log-rank (Mantel-Cox) test * *p < 0.01; ns not significant.
As a functional readout of DAF-16 activation, we measured survival after administration of various exogenous stressors in ercc-1 mutants. At D1 of adulthood, ercc-1 mutants were more resistant to paraquat than N2 (Fig. 2B). ercc-1 mutants also showed a significant increase in survival following exposure to rotenone, a mitochondrial complex I inhibitor (Fig. 2C), antimycin A, a mitochondrial complex III inhibitor (Fig. 2D), as well as in response to heat stress (Fig. 2E). Importantly, daf-16 RNAi completely abrogated the rotenone resistance of ercc-1 worms (Fig. 2F). Surprisingly, although DAF-16 confers stress resistance in ercc-1 worms, it did not significantly impact lifespan of the animals, since knock-down of daf-16 dramatically shortened lifespan of N2 worms, but did not further shorten ercc-1 lifespan (Fig. 2G).
2.2. Loss of DAF-16 activation is associated with organismal decline
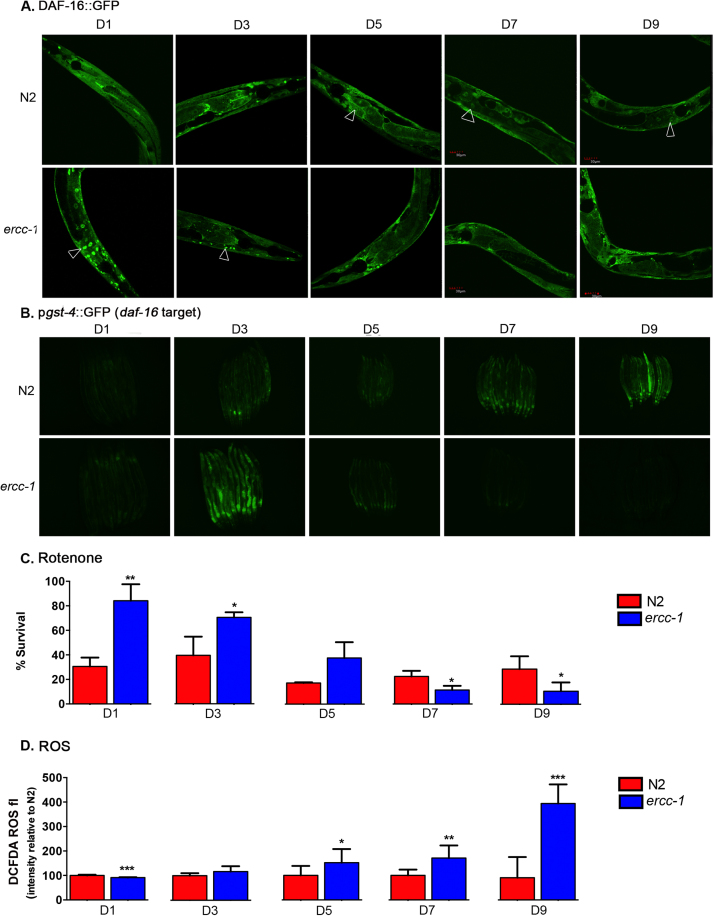
Despite DAF-16 activation in early adulthood, ercc-1 mutants are not long-lived. This is surprising because typically DAF-16 activation and an elevated stress response correlate with increased longevity [42], [43]. We therefore carried out a temporal analysis of DAF-16 activation in N2 worms and ercc-1 mutants. In N2, DAF-16::GFP was predominantly cytoplasmic until D5, after which nuclear localization became evident (Fig. 3A), consistent with previous reports [20], [44]. In contrast, in ercc-1 worms, nuclear localization of DAF-16::GFP was evident at D1 and D3 of adulthood, but by D5, DAF-16::GFP was primarily in the cytoplasm (Fig. 3A & S3). The antioxidant GST-4 is a downstream target of the transcription factor, daf-16. A GFP reporter for gst-4 [41], [45] was robustly induced in ercc-1 mutant animals at D3 compared to N2 worms, but was significantly reduced by D5, and further suppressed by D7 and D9 (Fig. 3B). A similar pattern of DAF-16 nuclear localization was observed in the xpf-1 mutant background (Fig. S4). Thus, DAF-16 and daf-16-modulated antioxidant program is only transiently activated in response to endogenous DNA damage. Interestingly, while UV irradiation of D9 ercc-1 mutants did not restore DAF-16::GFP nuclear localization (Fig S5A), a brief period of starvation did (Fig S5B), demonstrating that older adult ercc-1 worms are able to activate DAF-16 in response to other forms of stress, but no longer in response to genotoxic stress.
Fig. 3.
Loss of DAF-16-dependent stress resistance in DNA repair deficientercc-1worms. (A) Representative images of expression of the daf-16::GFP reporter in N2 and ercc-1 worms at D1, 3, 5, 7 and 9 of adulthood. Arrows indicate nuclear localization of DAF-16::GFP. (B) Representative images of the expression of gst-4::GFP, a reporter of DAF-16 activity, in N2 and ercc-1 worms with age. (C) Viability of N2 and ercc-1 worms 16 h after treatment with 12.5 µM rotenone measured at multiple ages (mean ± S.E.M., n = 3 groups of 40–60 worms/genotype). Student's two-tailed t-test *p < 0.05, * *p < 0.01. (D) Nonspecific oxidant levels measured by staining with H2- DCFDA in N2 and ercc-1 worms at multiple ages (mean ± S.D. of n = 3 groups of 8–15 worms/genotype). Student's one-tailed t-test *p < 0.05, * *p < 0.01, * **p < 0.001.
Since DAF-16 activation declines with age, we predicted that this should coincide with an attrition of stress resistance in ercc-1 worms. We therefore systematically analyzed stress response with age, in N2 and ercc-1 worms exposed to rotenone, a mitochondrial complex I inhibitor that induces oxidative stress, in D 1, 3, 5, 7 and 9 adults at a dose that leads to ~30–40% survival of N2. In this paradigm, we found ercc-1 mutants were significantly more resistant to rotenone than N2 as D1 and 3 adults (Fig. 3C). However, by D5, this resistance was lost, and with increasing age, the ercc-1 worms became hypersensitive to rotenone compared to N2. These results were confirmed in ercc-1 mutant worms derived from a balancer strain, as well as in xpf-1 mutants (Fig S6).
The loss of DAF-16 nuclear localization and reduced expression of gst-4 in the ercc-1 worms was correlated with an increase in endogenous oxidant abundance as assessed by the nonspecific and potentially metal-catalyzed, one-electron oxidation of DCFH to DCF (Fig. 3D). ercc-1 mutants had lower oxidant levels as D1 adults than N2 worms, but by D5 of adulthood there was significantly more oxidants in the ercc-1 worms and this became exaggerated with increasing age of the worms (Fig. 3D). By D9, when ercc-1 mutants were stress sensitive, there was an increase in oxidants (~3.5 fold higher) (Fig. 3D) compared to wild-type worms. Taken together, these data invoke a scenario in which endogenous DNA damage initially triggers activation of a well-described stress response/longevity assurance mechanism, but that activation declines over time, at which point increased oxidant levels, damage, and functional decline ensue.
2.3. cep-1 mediates the effect of DNA damage on DAF-16 dynamics
To begin to understand the mechanism by which DNA damage leads to activation of DAF-16, we performed a targeted RNAi screen for suppressors of stress resistance in ercc-1 mutant worms. We selected 50 candidate genes on the basis of their involvement in processes such as DNA repair signaling, mitochondrial function, metabolism, autophagy and signal transduction. From this screen, we observed that knock-down of cep-1 led to a significant suppression of paraquat resistance in ercc-1 mutants (Fig S7A). cep-1 is the ortholog of the tumor suppressor gene, p53 [46] which is known to be activated in response to DNA damage and activates FOXO3A in response to genotoxic stress [47]. We therefore generated a cep-1 ercc-1 double mutant carrying the daf-16::GFP transgene to examine the effect of cep-1 deletion on DAF-16 activation.
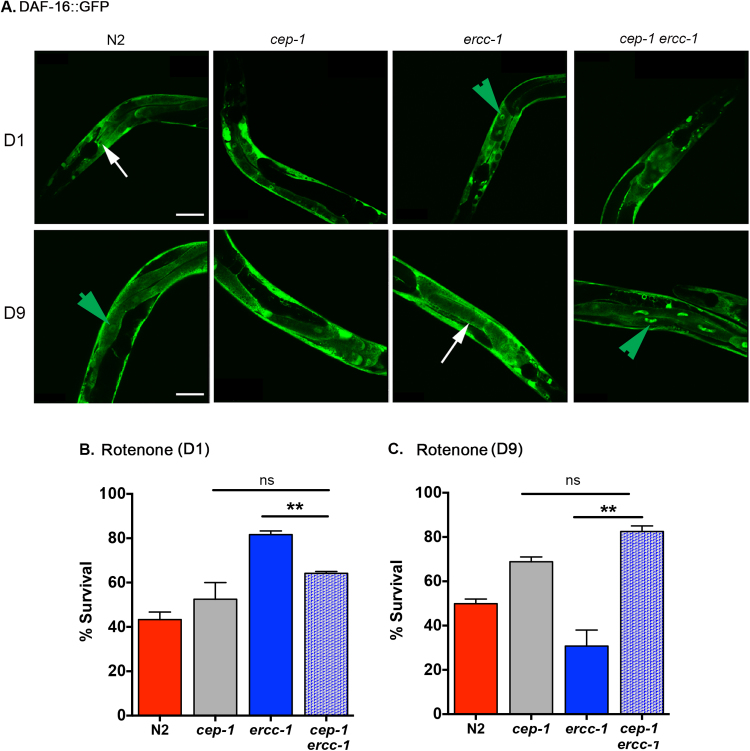
Unlike ercc-1 worms, the cep-1 ercc-1 D1 adults did not have nuclear localization of DAF-16::GFP, but there was strong nuclear localization of DAF-16::GFP in the double mutants at D9, effectively reverting the ercc-1 worms to a WT phenotype in terms of daf-16 activation (Fig. 4A & S7B & C). Consistent with these changes in DAF-16 localization, cep-1 deletion in an ercc-1 background suppressed stress resistance in D1 animals (Fig. 4B & S7D & E). But at D9, cep-1 ercc-1 double mutants were stress resistant compared to the ercc-1 single mutant (Fig. 4C). These results confirm a role for cep-1 in regulating the stress response elicited by endogenous DNA damage. Surprisingly, this also included deactivating DAF-16 in older organisms.
Fig. 4.
Temporal dynamics of DAF-16 iscep-1dependent. (A) Representative images of DAF-16::GFP in N2, cep-1, ercc-1 and cep-1 ercc-1 worms at D1 and D9 of adulthood. Green arrows indicate nuclear localization of DAF-16::GFP and white arrows indicate lack thereof. (B-C) Percent survival of D1 or D9 adult N2, cep-1, ercc-1 and cep-1 ercc-1 worms 16 h after treatment with rotenone (mean ± S.E.M., n = 4 groups of 40–60 worms/genotype; 3 replicas). Student's two-tailed t-test * *p < 0.01; ns not significant.
2.4. Nuclear DNA damage prematurely activates FOXO3A in mammalian cells
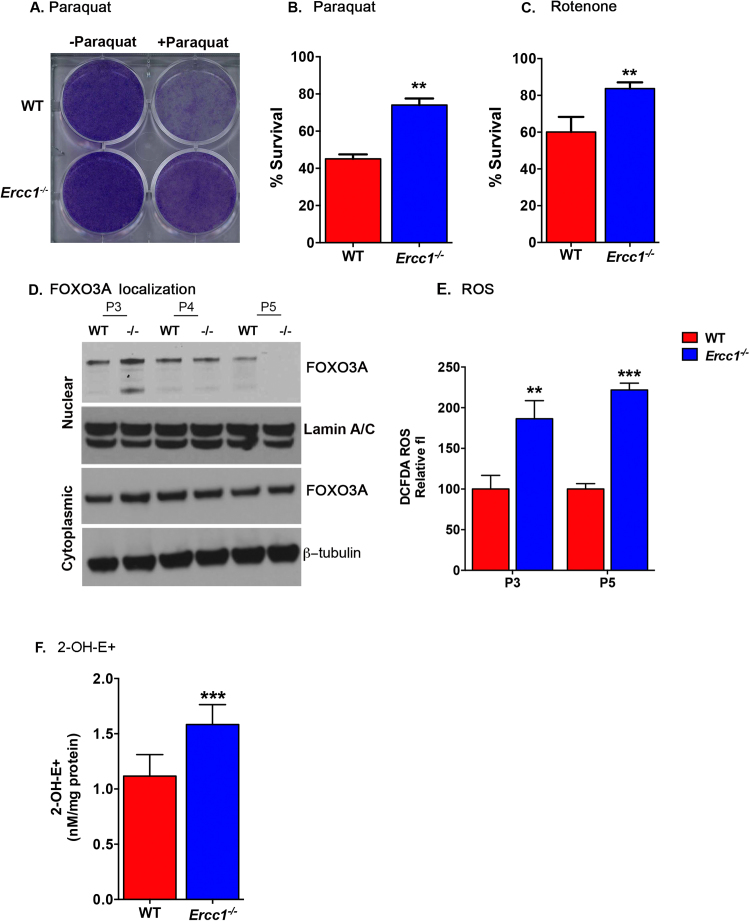
We next turned to mammalian systems to determine if this response is conserved by examining the dynamics of FOXO3A, the mammalian homolog of DAF-16 [48]. We found early passage 3 (P3) Ercc1-/- mouse embryonic fibroblasts (MEFs) were resistant to paraquat and rotenone compared to WT cells (Fig. 5A-C) and, like worms, there was increased nuclear localization of FOXO3A. However, by P5, FOXO3A was no longer observed in the nucleus of cells (Fig. 5D and S8), while increased H2-DCFDA fluorescence was observed (Fig. 5E). Specifically, superoxide (O2•-) levels were increased (Fig. 5F). These data demonstrate that in primary mammalian cells, like in nematodes, unrepaired spontaneous DNA damage activates FOXO3A stress response programs, but that this activation is not sustained over time, leading to a rise in oxidative stress.
Fig. 5.
Activation of FOXO3A inErcc1-/-mammalian cells mimics worms. (A) Representative image of crystal violet staining of cultures of passage 3 WT and Ercc1-/- primary MEFs 24 h after treatment with 0.5 mM paraquat. (B) Quantification of cell viability 24 h after treatment of passage 4 WT and Ercc1-/- MEFs with 0.5 mM paraquat (mean percent survival compared to untreated cells ± S.E.M.; n = 5 independent cultures/genotype). Student's two-tailed t-test * *p < 0.01. (C) Quantification of cell viability 24 h after treatment of passage 3 WT and Ercc1-/- MEFs with 1 µM rotenone (mean percent survival compared to untreated cells ± S.E.M. n = 5 replicas). Student's two-tailed t-test, * *p < 0.01. (D) Immunodetection of FOXO3A in nuclear (top) or cytosolic fractions (bottom) of primary MEFs at increasing passage number. -/- indicates Ercc1-/- cells. Lamin A/C is used as a loading control for nuclear proteins; β-tubulin is used for cytosolic. (E) Nonspecific oxidant levels in WT and Ercc1-/- MEFs measured with 2’,7’-dichlorofluorescein (H2-DCFDA) dye and flow cytometry at early (P3) and late (P5) passage of cells (n = 5 independent cell lines; data are represented as mean ± S.D.). Two-tailed Student's t-test * *p < 0.01, * **p < 0.05. (F) Detection of 2-hydroxyethidium (2-OH-E+) in WT and Ercc1-/- primary MEFs at passage 5 (mean ± S.D. of n = 8 independent cell pellets/genotype). Student's two tailed t-test ***p < 0.001.
2.5. Nuclear DNA damage regulates timing of FOXO3A activation in mammals
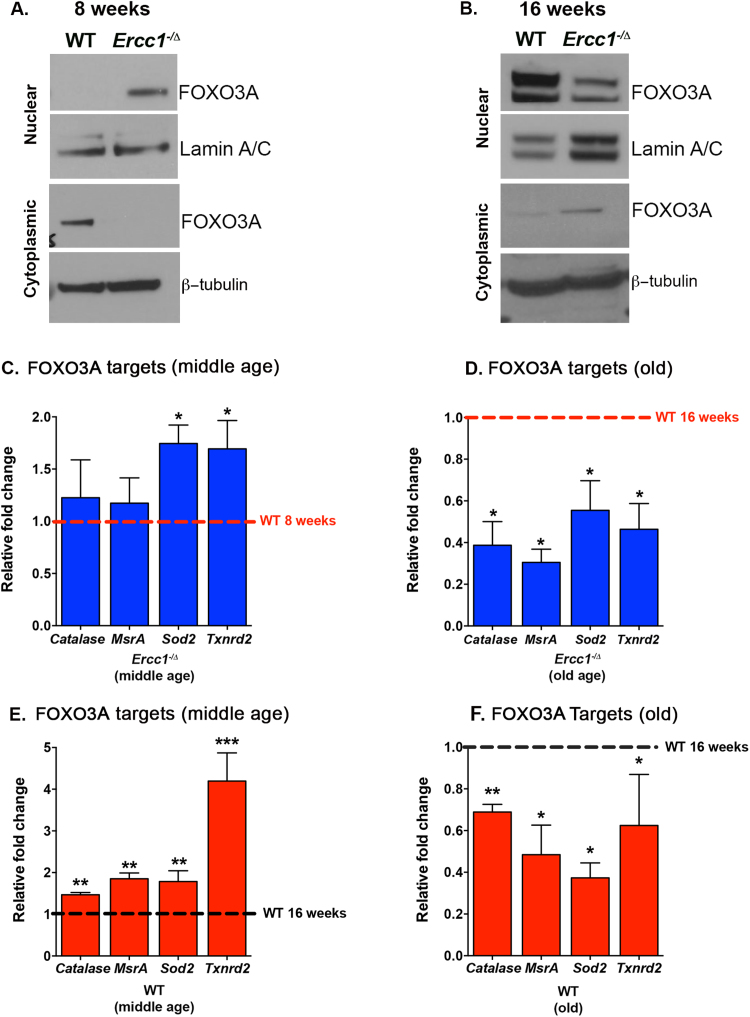
To determine whether the FOXO3A response to endogenous DNA damage occurred in vivo, we next examined liver tissue from DNA repair deficient Ercc1-/Δ mice. FOXO3A was increased in the nuclear fraction of livers of young adult [8 weeks - median lifespan 20 weeks [27]] and reduced in older Ercc1-/Δ mice [16 weeks] compared to same-age WT controls (Fig. 6A & B). Additionally, analysis of the transcriptome from the liver of 16-week-old Ercc1-/Δ mice revealed reduced expression of numerous FOXO3A transcriptional targets compared to WT mice (Fig S9 A). This was validated by qRT-PCR in liver from young adult (8 weeks) but old (16 weeks) Ercc1-/Δ animals. Expression of FOXO3A targets catalase, MsrA, Sod2 and Txrnd2 were increased in Ercc1-/Δ mice at 8 weeks compared to WT, but significantly reduced in older mutant animals (Fig. 6C & D). The antioxidant methionine sulfoxide reductase A (MSRA) is a direct target of FOXO3A, while MSRB is not [49]. Correspondingly, MSRA, but not MSRB, activity was significantly reduced in livers of 16-week-old Ercc1-/Δ mice compared to WT animals (Fig S9 B-C). Collectively, these data demonstrate that the activation of FOXO3A in response to endogenous DNA damage is conserved in mammals, as is the inability to sustain its activation.
Fig. 6.
Activation of FOXO3A is accelerated in DNA repair deficientErcc1-/Δmice. Immunoblot detection of FOXO3A in nuclear (top) and cytosolic (bottom) extracts from liver lysates isolated from 8 (A) and (B) 16-week-old mice WT or ERCC1-deficient mice. (C-D) Expression of FOXO3A-regulated genes measured by qRT-PCR in livers of WT and Ercc1-/Δ mice at 8 weeks (middle-age) and 16 weeks of age (old). WT at the same age is set as 1; red dashed line. n = 6–8 per age; Student's one-tailed t-test, *p < 0.05. (E-F) Expression of FOXO3A-regulated genes measured by qRT-PCR in liver of WT mice at 50 weeks (middle-age, E) and 110–120 weeks of age (old, F). WT at 16 weeks of age is set at 1; black dashed line. n = 4–6 per age; Student's one-tailed t-test, *p < 0.05, * *p < 0.01, * **p < 0.001.
Since WT mice accumulate oxidative DNA lesions as they age [25], we hypothesized that FOXO3A activation and subsequent inactivation should be evident in normal mice with aging, albeit with a less compressed time course than ERCC1-deficient mice. Indeed, compared to young adult animals (16 weeks), expression of FOXO3A targets was significantly higher in middle-aged WT mice (50 weeks) (Fig. 6E) but significantly reduced in liver of old WT mice (2 years-old; Fig. 6F). These data demonstrate that an age-related decline in FOXO3A activation is part of the normal aging process in mammals.
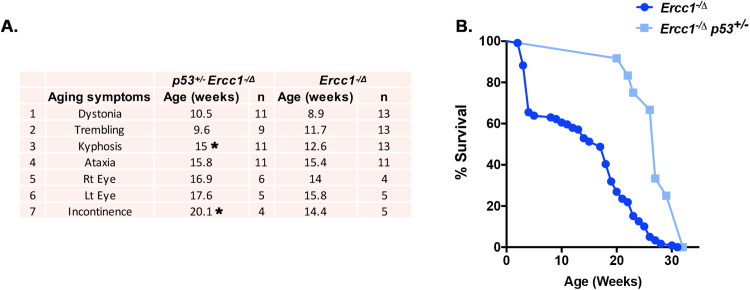
Finally, as we observed that deletion of p53 homolog, cep-1, rescued loss of DAF-16 nuclear localization in older ercc-1 worms (Fig. 4), we hypothesized that suppression of p53 in Ercc1-/Δ mice would improve healthspan and lifespan. Ercc1-/Δp53-/- mice were born at sub-Mendelian frequency. However, p53 heterozygosity delayed the onset of several aging symptoms in Ercc1-/Δ mice (Fig. 7A). Moreover, the median lifespan of Ercc1-/∆ p53+/- mice was significantly increased compared to Ercc1-/∆ animals (Fig. 7B) indicative of increased healthspan [10]. Taken together, these results demonstrate that genetic reduction of p53 delays aging in DNA repair deficient mice, consistent with its role in attenuating stress resistance late in life.
Fig. 7.
Genetic depletion of p53 in DNA repair deficientErcc1-/Δmice improves healthspan and lifespan. (A) Average age (weeks) of onset for several aging symptoms in Ercc1-/Δ and Ercc1-/Δp53+/- mice. n = 11 Ercc1-/Δp53+/- mice and n = 13 Ercc1-/Δ mice. (B) Kaplan-Meier survival curves of Ercc1-/Δ (n = 119) and Ercc1-/Δp53+/- (n = 11) mice were calculated. Log-rank (Mantel-Cox) test * **p < 0.001.
3. Discussion
DNA damage has long been implicated as a driver of aging, although the mechanism(s) are poorly defined. In part, this is because deciphering exactly how endogenous DNA damage affects post-mitotic cells and contributes to organismal aging is challenging in mammalian systems. Here, we set out to elucidate this mechanism using nematodes and then confirm is evolutionary conservation using mice in which ERCC1-XPF endonuclease, an enzyme required for nucleotide excision, interstrand crosslink and double-strand break repair, is genetically depleted. In these DNA repair-deficient organisms, the type and amount of DNA damage that is incurred is unchanged compared with wild type animals. However, since DNA repair is compromised, there is an accelerated accumulation of the same DNA lesions that accumulate over the lifespan of wild-type, repair proficient organisms [25]. This enables study of the mechanisms that are triggered in response to physiologically relevant types and levels of genotoxic stress.
Most mammalian DNA repair genes are maintained in C. elegans, although lifespan data in some of these mutants has yielded contradictory results. For example, another NER mutant, xpa-1 has been reported to have a shortened lifespan while others have observed no differences [17], [19], [23] Previous studies had demonstrated that UV-induced DNA damage leads to activation of DAF-16/FOXO3A [20]. Our results build on this observation to demonstrate that endogenous DNA damage also induces DAF-16/FOXO3A activation, and a corresponding increase in stress resistance, not only in adult worms, but also in murine cells and tissues. The activation of DAF-16/FOXO3A in ERCC1-deficient cells and tissues, suggests a coping mechanism in response to endogenous damage to the nuclear genome. We also found that the FOXO3A stress response pathway is activated in DNA repair-proficient mice, albeit at a later age when damage has accumulated [25].
The activation of DAF-16/FOXO3A is typically associated with longevity. Indeed, FOXO3A variants are linked to longevity in humans, but the functional implications of the sequence variants are not known [50], [51], [52], [53]. Our results add to existing evidence that DAF-16/FOXO3A activity is critical for suppressing oxidative stress [54]. Unexpectedly, we found that oxidant abundance becomes elevated in response to genotoxic stress creating a vicious cycle of escalating damage [55]. Here, we establish that loss of FOXO3A activation likely plays a role in triggering this vicious cycle of increased oxidative stress and damage.
Suppression of FOXO3A activity promotes senescence in vitro [56] and in vivo [57], and leads to changes in cell morphology, reduced proliferation, an increase in senescence associated-β-galactosidase staining and oxidant abundance. Ercc1-/- MEFs senesce prematurely [58], while Ercc1-/Δ mice accumulate senescent cells in multiple tissues [59]. We have previously demontrated that the increase in senescence in Ercc1-/Δ mice is due to elevated oxidative stress. However the mechanism that leads to the surge in oxidants upon endogenous DNA damage was unknown. Similarly, suppression of msra-1, a key downstream target of DAF-16/FOXO3A, leads to sensitivity to oxidative stress and ~30% decrease in median lifespan in C. elegans [60], whereas overexpression of MsrA in cells lowers oxidant levels [61] and increases lifespan in D. melanogaster and yeast [62], [63]. Taken together, our observations suggest that it is the temporal response of the DAF-16/FOXO pathway to unrepaired DNA damage that contributes to deleterious effects on healthspan and lifespan.
What remains unclear is why DAF-16/FOXO3A activity wanes in the face of chronic genotoxic stress. Chronic nuclear localization of DAF-16 is observed in several long-lived mutants [64], indicating that sustained activation of this pathway is not only possible, but is also beneficial. Although the regulation of FOXO3A is not fully elucidated [65], [66], cep-1/p53, ATM, IGF-1, sirtuins and IKK all contribute to it's regulation [47], [67], [68], all of which are dysregulated in Ercc1 mutants [26], [69]. We found that in younger worms, cep-1 promotes DAF-16 activation to invoke a protective stress response. However, upon chronic genotoxic stress in older animals, there is a loss of DAF-16/FOXO3A nuclear retention that is cep-1 dependent. Under conditions of oxidative stress, p53 is modified and inhibits FOXO3A activity [70], [71]. Although, the role of cep-1/p53 in healthspan and lifespan is complex [72], [73], [74], our results indicate that its suppression attenuates age-related decline. Thus, therapeutic strategies aimed at maintaining DAF-16/FOXO3A activity could provide a means of ameliorating the effects of DNA damage-induced accelerated aging, such as occurs in cancer patients [5]. In this respect, it is noteworthy that starvation of aged ercc-1 worms leads to increased DAF-16::GFP nuclear localization (Fig S7B). This is in accordance with a recent report demonstrating that caloric restriction extends the healthspan and lifespan of Ercc1-/Δ mice [75]. It will therefore be important to determine whether such interventions are effective in other systems.
4. Experimental procedures
*Detailed experimental procedures in supplemental data
4.1. C. elegans strains
Wild-type (N2), TG1663 [ercc-1(tm1981)], TG1660 [xpf-1(tm2842)], TJ1 [cep-1(gk138)], TJ356 [zIs356(daf-16p::daf-16a/b::GFP+rol-6), CL2166 [dvIs19((pAF15)gst-4p::GFP::NLS)], XMN617 [hT2/+ [bli-4(e937) let-?(q782) qIs48] (I;III); muIs32 (Pmec-7::GFP)].
C. elegans strains were cultured at 20 °C on Nematode Growth Media (NGM) agar plates seeded with Escherichia coli strain OP50 unless stated otherwise [76]. Strains were provided by the Caenorhabditis Genetics Center (University of Minnesota).
4.2. Cells and mice
Primary mouse embryonic fibroblasts (MEFs) were isolated on embryonic day 12.5–13.5. In brief, mouse embryos were isolated from yolk sac followed by removal of viscera, lung and heart. Embryos were then minced into fine chunks, covered with media, cultured at 3% oxygen to reduce stresses and serially passaged. MEFs were grown in 1:1 of Dulbecco's Modification of Eagles Medium (with 4.5 g/L glucose and L-glutamine) and Ham's F10 medium, supplemented with 10% fetal bovine serum, penicillin and streptomycin and non-essential amino acids. To induce oxidative stress and oxidative DNA damage, MEFs were switched to 20% oxygen at passage 3 Ercc1+/- and Ercc1+/Δ mice from C57BL/6 J and FVB/NJ backgrounds were crossed to generate Ercc1-/Δ F1 hybrid mice. p53+/- mice were crossed to Ercc1+/- from C57BL/6 J background to generate Ercc1+/-p53+/- mice, which were then bred with Ercc1+/Δ mice from FVB/NJ background to generate F1 Ercc1-/∆ p53+/- mice. Breeders were backcrossed for ten generations yielding F1 mice that are genetically identical. All animal studies were conducted in compliance with the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and were approved by The Scripps Research Institute (TSRI) and University of Pittsburgh Institutional Animal Care and Use Committee.
4.3. Confocal microscopy and images processing
Worms were immobilized with 6 mM tetramisole hydrochloride (Sigma) in M9 and mounted on 6% agarose pads on glass slides. Images were acquired using Zeiss LSM 700 Upright confocal microscope (Carl Zeiss AG).
4.4. Statistics
Survival analyses were performed using the Kaplan-Meier method and the significance of differences between survival curves calculated using the log rank test. Other analyses are listed in figure legends.
Acknowledgements
We thank the Niedernhofer, Robbins and Gill lab members for scientific discussions and reading of the manuscript. A.U.G. is supported by NIH/NIAK99 AG049126. M.S.G. supported by NIH/NIA R21 AG049447 and R21 AG050172. L.J.N., E.E.K, C.M.S. and P.R. are supported by NIH/NIAP01 AG043376. L.J.N. and P.R. are additionally supported by NIH/NIAU19 AG056278 and NIH/NCI P30AG024827. L.J.N. was also supported by NIH/NIEHS ES016114. Some nematode strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Acknowledgments
Author contributions
A.U.G. and L.J.N. conceived the study. C. elegans studies were designed and performed with the help of M.S.G. A.U.G. designed and performed most of the experiments with the help of A.W. and M.M. Y.C. and Y.W. measured cyclopurine adducts on DNA. A.U.G., C.M.S. performed IF experiments. C.M.S. performed quantitative image analysis. MsrA and MsrB studies contributed by S.K.A. and H.W. A.U.G., L.J.N., and M.S.G. wrote the manuscript with contributions from other authors and H.W. edited the final manuscript. L.J.N. and M.S.G. supervised the research.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.06.005.
Contributor Information
Laura J. Niedernhofer, Email: lniedern@umn.edu.
Matthew S. Gill, Email: mgill@scripps.edu.
Appendix A. Supplementary material
Supplementary material
References
- 1.Kirkwood T.B. Understanding the odd science of aging. Cell. 2005;120(4):437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy B.K. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregg S.Q., Robinson A.R., Niedernhofer L.J. Physiological consequences of defects in ERCC1-XPF DNA repair endonuclease. DNA Repair (Amst.) 2011;10(7):781–791. doi: 10.1016/j.dnarep.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray M.D. The Werner syndrome protein is a DNA helicase. Nat. Genet. 1997;17(1):100–103. doi: 10.1038/ng0997-100. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong G.T. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J. Clin. Oncol. 2014;32(12):1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob K.D., Noren Hooten N., Trzeciak A.R., Evans M.K. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech. Ageing Dev. 2013;134(3–4):139–157. doi: 10.1016/j.mad.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb. Perspect. Med. 2016;6(3):a026104. doi: 10.1101/cshperspect.a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpless N.E., DePinho R.A. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol. 2007;8(9):703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 9.Rodier F. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009;11(8):973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker D.J. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–p189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppe J.P. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5(2):e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milo R.Pa.R. Cell biology by the numbers. 2015. How Quickly Do Different Cells in the Body Replace Themselves? (Garland Science) [Google Scholar]
- 13.Mullaart E., Boerrigter M.E., Ravid R., Swaab D.F., Vijg J. Increased levels of DNA breaks in cerebral cortex of Alzheimer's disease patients. Neurobiol. Aging. 1990;11(3):169–173. doi: 10.1016/0197-4580(90)90542-8. [DOI] [PubMed] [Google Scholar]
- 14.Madabhushi R., Pan L., Tsai L.H. DNA damage and its links to neurodegeneration. Neuron. 2014;83(2):266–282. doi: 10.1016/j.neuron.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron. 2008;60(5):803–817. doi: 10.1016/j.neuron.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapierre L.R., Hansen M. Lessons from C. elegans: signaling pathways for longevity. Trends Endocrinol. Metab.: TEM. 2012;23(12):637–644. doi: 10.1016/j.tem.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lans H. DNA damage leads to progressive replicative decline but extends the life span of long-lived mutant animals. Cell death Differ. 2013;20(12):1709–1718. doi: 10.1038/cdd.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaposhnikov M., Proshkina E., Shilova L., Zhavoronkov A., Moskalev A. Lifespan and stress resistance in drosophila with overexpressed DNA repair genes. Sci. Rep. 2015;5:15299. doi: 10.1038/srep15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyun M. Longevity and resistance to stress correlate with DNA repair capacity in Caenorhabditis elegans. Nucleic Acids Res. 2008;36(4):1380–1389. doi: 10.1093/nar/gkm1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller M.M. DAF-16/FOXO and EGL-27/GATA promote developmental growth in response to persistent somatic DNA damage. Nat. Cell Biol. 2014;16(12):1168–1179. doi: 10.1038/ncb3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyd W.A. Nucleotide excision repair genes are expressed at low levels and are not detectably inducible in Caenorhabditis elegans somatic tissues, but their function is required for normal adult life after UVC exposure. Mutat. Res. 2010;683(1–2):57–67. doi: 10.1016/j.mrfmmm.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Astin J.W., O'Neil N.J., Kuwabara P.E. Nucleotide excision repair and the degradation of RNA pol II by the Caenorhabditis elegans XPA and Rsp5 orthologues, RAD-3 and WWP-1. DNA Repair. 2008;7(2):267–280. doi: 10.1016/j.dnarep.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Fensgard O. A two-tiered compensatory response to loss of DNA repair modulates aging and stress response pathways. Aging (Albany NY) 2010;2(3):133–159. doi: 10.18632/aging.100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A.U. Gurkar, M.S. Gill, L.J. Niedernhofer, Genome stability and ageing. ed Olsen MSGaA (Springer), 2016.
- 25.Wang J., Clauson C.L., Robbins P.D., Niedernhofer L.J., Wang Y. The oxidative DNA lesions 8,5'-cyclopurines accumulate with aging in a tissue-specific manner. Aging Cell. 2012;11(4):714–716. doi: 10.1111/j.1474-9726.2012.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niedernhofer L.J. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444(7122):1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 27.Dolle M.E. Broad segmental progeroid changes in short-lived Ercc1(-/Delta7) mice. Pathobiol. Aging Age Relat. Dis. 2011;1 doi: 10.3402/pba.v1i0.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J. Quantification of oxidative DNA lesions in tissues of Long-Evans Cinnamon rats by capillary high-performance liquid chromatography-tandem mass spectrometry coupled with stable isotope-dilution method. Anal. Chem. 2011;83(6):2201–2209. doi: 10.1021/ac103099s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agostinho A. Combinatorial regulation of meiotic holliday junction resolution in C. elegans by HIM-6 (BLM) helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 nucleases. PLoS Genet. 2013;9(7):e1003591. doi: 10.1371/journal.pgen.1003591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craig A.L., Moser S.C., Bailly A.P., Gartner A. Methods for studying the DNA damage response in the Caenorhabdatis elegans germ line. Methods Cell Biol. 2012;107:321–352. doi: 10.1016/B978-0-12-394620-1.00011-4. [DOI] [PubMed] [Google Scholar]
- 31.Saito T.T., Youds J.L., Boulton S.J., Colaiacovo M.P. Caenorhabditis elegans HIM-18/SLX-4 interacts with SLX-1 and XPF-1 and maintains genomic integrity in the germline by processing recombination intermediates. PLoS Genet. 2009;5(11):e1000735. doi: 10.1371/journal.pgen.1000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lans H. Involvement of global genome repair, transcription coupled repair, and chromatin remodeling in UV DNA damage response changes during development. PLoS Genet. 2010;6(5):e1000941. doi: 10.1371/journal.pgen.1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Neil N.J. Joint molecule resolution requires the redundant activities of MUS-81 and XPF-1 during Caenorhabditis elegans meiosis. PLoS Genet. 2013;9(7):e1003582. doi: 10.1371/journal.pgen.1003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito T.T., Lui D.Y., Kim H.M., Meyer K., Colaiacovo M.P. Interplay between structure-specific endonucleases for crossover control during Caenorhabditis elegans meiosis. PLoS Genet. 2013;9(7):e1003586. doi: 10.1371/journal.pgen.1003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ermolaeva M.A. DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature. 2013;501(7467):416–420. doi: 10.1038/nature12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashiyama K. Malfunction of nuclease ERCC1-XPF results in diverse clinical manifestations and causes Cockayne syndrome, xeroderma pigmentosum, and Fanconi anemia. Am. J. Hum. Genet. 2013;92(5):807–819. doi: 10.1016/j.ajhg.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian M., Shinkura R., Shinkura N., Alt F.W. Growth retardation, early death, and DNA repair defects in mice deficient for the nucleotide excision repair enzyme XPF. Mol. Cell Biol. 2004;24(3):1200–1205. doi: 10.1128/MCB.24.3.1200-1205.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori T. ERCC4 variants identified in a cohort of patients with segmental progeroid syndromes. Hum. Mutat. 2018;39(2):255–265. doi: 10.1002/humu.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shore D.E., Carr C.E., Ruvkun G. Induction of cytoprotective pathways is central to the extension of lifespan conferred by multiple longevity pathways. PLoS Genet. 2012;8(7):e1002792. doi: 10.1371/journal.pgen.1002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson S.T., Johnson T.E. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 2001;11(24):1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- 41.Murphy C.T. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424(6946):277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 42.Lithgow G.J., White T.M., Melov S., Johnson T.E. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl. Acad. Sci. USA. 1995;92(16):7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harper J.M. Fibroblasts from long-lived bird species are resistant to multiple forms of stress. J. Exp. Biol. 2011;214(Pt 11):1902–1910. doi: 10.1242/jeb.054643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bansal A. Transcriptional regulation of Caenorhabditis elegans FOXO/DAF-16 modulates lifespan. Longev. Health. 2014;3:5. doi: 10.1186/2046-2395-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tullet J.M. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132(6):1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schumacher B., Hofmann K., Boulton S., Gartner A. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 2001;11(21):1722–1727. doi: 10.1016/s0960-9822(01)00534-6. [DOI] [PubMed] [Google Scholar]
- 47.Renault V.M. The pro-longevity gene FoxO3 is a direct target of the p53 tumor suppressor. Oncogene. 2011;30(29):3207–3221. doi: 10.1038/onc.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolff S. SMK-1, an essential regulator of DAF-16-mediated longevity. Cell. 2006;124(5):1039–1053. doi: 10.1016/j.cell.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 49.Shchedrina V.A. Analyses of fruit flies that do not express selenoproteins or express the mouse selenoprotein, methionine sulfoxide reductase B1, reveal a role of selenoproteins in stress resistance. J. Biol. Chem. 2011;286(34):29449–29461. doi: 10.1074/jbc.M111.257600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willcox B.J. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. USA. 2008;105(37):13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anselmi C.V. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12(2):95–104. doi: 10.1089/rej.2008.0827. [DOI] [PubMed] [Google Scholar]
- 52.Flachsbart F. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc. Natl. Acad. Sci. USA. 2009;106(8):2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soerensen M. Replication of an association of variation in the FOXO3A gene with human longevity using both case-control and longitudinal data. Aging Cell. 2010;9(6):1010–1017. doi: 10.1111/j.1474-9726.2010.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morris B.J., Willcox D.C., Donlon T.A., Willcox B.J. FOXO3: a major gene for human longevity--a mini-review. Gerontology. 2015;61(6):515–525. doi: 10.1159/000375235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.R A.R. Spontaneous DNA damage to the nuclear genome promotes senescence, T redox imbalance and aging. Redox Biol. 2018;17:259–273. doi: 10.1016/j.redox.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kyoung Kim H. Down-regulation of a forkhead transcription factor, FOXO3a, accelerates cellular senescence in human dermal fibroblasts. J. Gerontol. A Biol. Sci. Med Sci. 2005;60(1):4–9. doi: 10.1093/gerona/60.1.4. [DOI] [PubMed] [Google Scholar]
- 57.Yao H. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J. Clin. Invest. 2012;122(6):2032–2045. doi: 10.1172/JCI60132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuhrmann-Stroissnigg H. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 2017;8(1):422. doi: 10.1038/s41467-017-00314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gregg S.Q. A mouse model of accelerated liver aging caused by a defect in DNA repair. Hepatology. 2012;55(2):609–621. doi: 10.1002/hep.24713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minniti A.N. Methionine sulfoxide reductase A expression is regulated by the DAF-16/FOXO pathway in Caenorhabditis elegans. Aging Cell. 2009;8(6):690–705. doi: 10.1111/j.1474-9726.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- 61.Yermolaieva O. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc. Natl. Acad. Sci. USA. 2004;101(5):1159–1164. doi: 10.1073/pnas.0308215100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruan H. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc. Natl. Acad. Sci. USA. 2002;99(5):2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koc A., Gasch A.P., Rutherford J.C., Kim H.Y., Gladyshev V.N. Methionine sulfoxide reductase regulation of yeast lifespan reveals reactive oxygen species-dependent and -independent components of aging. Proc. Natl. Acad. Sci. USA. 2004;101(21):7999–8004. doi: 10.1073/pnas.0307929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berman J.R., Kenyon C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell. 2006;124(5):1055–1068. doi: 10.1016/j.cell.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 65.Daitoku H., Sakamaki J., Fukamizu A. Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochim. Et. Biophys. Acta. 2011;1813(11):1954–1960. doi: 10.1016/j.bbamcr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 66.Rinner O. An integrated mass spectrometric and computational framework for the analysis of protein interaction networks. Nat. Biotechnol. 2007;25(3):345–352. doi: 10.1038/nbt1289. [DOI] [PubMed] [Google Scholar]
- 67.Nemoto S., Fergusson M.M., Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306(5704):2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 68.Wang F. Biochemical and structural characterization of an intramolecular interaction in FOXO3a and its binding with p53. J. Mol. Biol. 2008;384(3):590–603. doi: 10.1016/j.jmb.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 69.Tilstra J.S. NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Invest. 2012;122(7):2601–2612. doi: 10.1172/JCI45785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyaguchi Y., Tsuchiya K., Sakamoto K. P53 negatively regulates the transcriptional activity of FOXO3a under oxidative stress. Cell Biol. Int. 2009;33(8):853–860. doi: 10.1016/j.cellbi.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 71.Jayaraman L. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997;11(5):558–570. doi: 10.1101/gad.11.5.558. [DOI] [PubMed] [Google Scholar]
- 72.Liu D., Xu Y. p53, oxidative stress, and aging. Antioxid. Redox Signal. 2011;15(6):1669–1678. doi: 10.1089/ars.2010.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ventura N. p53/CEP-1 increases or decreases lifespan, depending on level of mitochondrial bioenergetic stress. Aging Cell. 2009;8(4):380–393. doi: 10.1111/j.1474-9726.2009.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baruah A. CEP-1, the Caenorhabditis elegans p53 homolog, mediates opposing longevity outcomes in mitochondrial electron transport chain mutants. PLoS Genet. 2014;10(2):e1004097. doi: 10.1371/journal.pgen.1004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vermeij W.P. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537(7620):427–431. doi: 10.1038/nature19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material