Abstract
Colonic metastasis from lung cancer is rare, generally asymptomatic and usually develop at advanced cancer stages. Here, we report a case with a resected stage IA lung adenocarcinoma in a 51yo male patient that presented two years later with mild abdominal pain due to intestinal obstruction caused by a metastatic colon tumor. The patient underwent colonoscopy followed by surgical resection and the pathologic report was adenocarcinoma which was the same as that from a lung nodule that was excised two years earlier. Immunohistochemistry was cytokeratin 7 (CK7) positive, thyroid transcription factor 1 (TTF1) focally positive and cytokeratin 20 (CK20), caudal-related homeobox transcription factor 2 (CDX2) negative on both lung biopsy and colon surgical specimens. Interestingly there was no obvious lung cancer recurrence both at the time of metastasis and one year following chemotherapy.
Keywords: Lung adenocarcinoma, Colonic metastasis
1. Introduction
Lung cancer is the leading cause of cancer death in the world. In 2008 1.6 million people received a new diagnosis of lung cancer, comprising 13% of all new cancer diagnoses, and 1.4 million died of lung cancer, which was 18% of all cancer deaths [1]. High mortality of lung cancer can be attributed to its late diagnosis, which is usually made after the development of distant metastasis. The brain, liver, adrenal glands, and the bone are the most common sites of metastatic disease in patients with lung cancer [2]. Gastrointestinal (GI) metastases are considered rare and are usually diagnosed in more advanced disease [[3], [4], [5], [6]]. Several autopsy studies reported that gastrointestinal metastasis from primary lung cancer occur in about 0.2–11.9% of cases [3,7,8]; although others have reported higher rates [2,9]. Patients with intestinal metastases may present with symptoms such as abdominal pain, gastrointestinal bleeding, obstruction, perforation, fistula and peritonitis, complications associated with high mortality and poor short-term prognosis.
Here, we present a case of a patient with stage IA adenocarcinoma that presented with a single colonic metastasis two years after resection. Interestingly, the patient at the time of diagnosis had no evidence of local recurrence in the lung and, thus, before obtaining an endoscopic biopsy specimen, the colonic mass was regarded as a new (second) primary tumor.
2. Case presentation
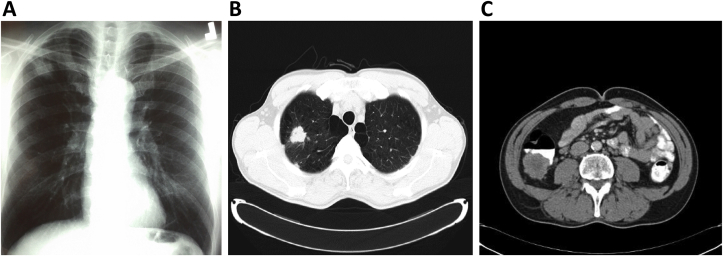
In July 2012, a 49 year old ex-smoker male, with no past history of serious illness, operations or hospitalizations, presented with chest pain over the last 15 days. There was no change of appetite or body weight. Clinical examination revealed no abnormal findings. Chest X-ray showed a nodule on right upper lobe (Fig. 1A) and the subsequent chest CT revealed a 2.8 cm nodule in the right upper lobe with speculated margins without mediastinal lymph node enlargement (Fig. 1B). Contrast enhanced CT of the abdomen and the head had no findings suggestive of metastatic disease. The patient was referred to a thoracic surgeon that performed right upper-middle bilobectomy (due to an anatomic variation with the middle lobe bronchus originating from the right upper lobe bronchus). The tumor was a 3cm primary lung adenocarcinoma and all resected lymph nodes were negative. Immunohistochemistry showed that the carcinoma cells were positive for Carcinoembryonic antigen (CEA), CK7 and TTF1 (focally positive) and negative for CK20, CDX2, p63. Thus, the patient was diagnosed with primary adenocarcinoma of the lung stage IA (T1bNoMo). According to guidelines, no postoperative chemotherapy or radiotherapy was applied to the patient, and he was set on CT surveillance every 3–6 months [10,11].
Fig. 1.
A. Chest X-Ray: Right upper lobe nodule. B. Chest computed tomography which revealed a 2.8cm right upper lobe nodule with speculated margins. C. Abdominal computed tomography which revealed a large soft tissue mass of 4.3 × 3.8 cm in size in the ascending colon.extending from the colonic lumen to the pericolic fat.
For the next two years all serial CTs of the chest, upper abdomen and head had had no findings suggestive of local or distal recurrence of the disease. The patient then complained of mild pain at the right upper quadrant of the abdomen without diarrheas, constipation or blood loss. As there were no abnormal findings at clinical examination we added CT scan for lower abdomen to his scheduled CT surveillance that revealed an ascending colonic mass 4.3 × 3.8 cm extending from the colonic lumen to the pericolic fat (Fig. 1C).
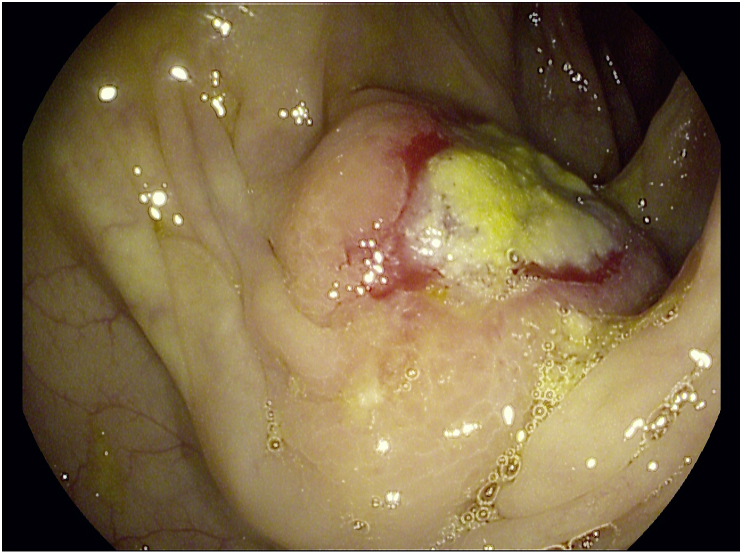
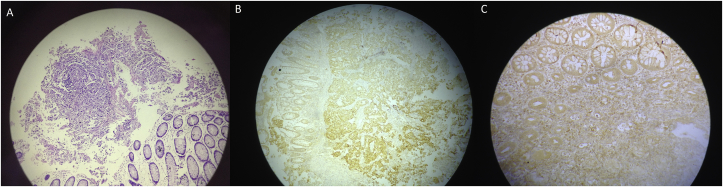
The lesion was initially supposed to be a second primary intestinal tumor. Colonfibroscopy revealed a large protruding lesion with central ulceration in the ascending colon (Fig. 2). Colonic biopsies were examined using hematoxylin and eosin (H&E) staining and showed a poorly differentiated adenocarcinoma with signet ring cells pattern without evidence of colonic dysplastic epithelium. Immunohistochemical staining was negative for anti-cytokeratin 20 antibody, positive for anti-pancytokeratin (AE1-AE3) and anti-cytokeratin 7 antibody and only focally positive for anti-TTF-1 antibody, with moderate intensity nuclear staining (Fig. 3). These results narrowed down the origin of the mass to the lung or the stomach. Gastroscopy did not demonstrate any abnormal findings and the patient was transferred for surgical resection due to the danger of imminent obstruction.
Fig. 2.
Colonofibroscopy image: A large protruding lesion with central ulceration in the ascending colon.
Fig. 3.
A. Poorly-differentiated adenocarcinoma metastatic to the colon [hematoxylin eosin (HE) × 10]. B: Positive immunohistochemical staining for CK 7 (× 10). C: Positive immunohistochemical staining for TTF-1 (× 10).
Right colectomy and ileotransverse anastomosis was performed. Macroscopically, a 3.8 × 3 × 3 cm colonic mass was present four cm away from the ileocolic valve. Immunohistochemistry showed a moderately differentiated adenocarcinoma, CK7 positive and CK20, CDX2, TTF1 negative, results suggestive of metastatic lung carcinoma. Eleven of twenty two mesocolic lymph nodes were infiltrated by cancer cells. Comparison of the intestinal tumor immunohistochemical characteristics to those of the initial pulmonary adenocarcinoma confirmed the metastatic nature of the intestinal tumor Molecular testing for Epidermal Growth Factor Receptor (EGRF) mutations (exons 18.19.20.21) and Anaplastic Lymphoma Kinase (ALK) gene rearrangements were negative.
The patient subsequently received four cycles of chemotherapy with cisplatin and vinorelbine. One year follow-up of the patient revealed hypermetabolic disease in the left adrenal gland and right iliac lymph nodes by means of PET-CT; however, without evidence of intrathoracic disease.
3. Discussion
We present a rare case of colonic metastasis from stage IA adenocarcinoma of the lung diagnosed two years after lobectomy. To our knowledge, this is the first reported case of an isolated metastasis to the intestine from a successfully resected, stage IA lung adenocarcinoma that had no signs of local recurrence or any other common extra-thoracic metastasis two years after resection.
Lung carcinoma is the leading cause of cancer death about 50% having distal metastasis at the time of diagnosis [12]. The preferential sites of extra-pulmonary metastasis are the lymph nodes, liver, adrenal gland, bone and brain. GI metastasis from lung cancer is considered to be rare, although there is about 4.7%–14% prevalence at autopsy [13,14]. Upon searching the medical records of 470 patients with lung cancer confirmed by autopsy, Yoshimoto et al. [15] identified 56 (11.9%) cases with gastrointestinal metastasis. Multiple metastases occurred in 6.2% (29), with metastasis to the stomach in 5.1% (24) and small intestine in 8.1% (38). Large intestine was the rarest site of lung metastasis with an occurrence of only 4.5% (21). Colonic metastasis usually occurs late in locally advanced disease and typically presents after the presence of other extra-pulmonary metastasis.
Immunohistochemistry is a very valuable tool for the differential diagnosis of lung carcinoma. With the use of immunohistochemical markers, it is possible to differentiate most lung neoplasms and classify them into therapeutic categories [12]. Most primary adenocarcinomas are TTF-1 positive, whereas metastatic adenocarcinomas to the lung are almost always TTF-1 negative [16]. Pulmonary adenocarcinomas are usually CK20 negative and CK7 positive, whereas metastatic adenocarcinomas of the colorectum are typically CK20 positive and CK7 negative [13]. CDX-2 is also a highly specific and sensitive marker for GI adenocarcinoma used to differentiate it from primary lung adenocarcinomas [14]. In our case, the pathologic results of lung nodule and colonic tumor were consistent, in which there was metastatic involvement from a primary adenocarcinoma of the lung.
The patient presented here was initially diagnosed at an “ideal” low lung cancer stage (T1bNoMo); however, he developed the most rare out of all possible lung metastasis that became apparent two years after surgical resection. At initial investigation, the patient did not undergo PET-CT scanning due to lack of insurance coverage. Furthermore, PET-CT screening had a low-grade recommendation in previous guidelines for stage IA lung cancer [17,18]. Interestingly, current guidelines do not suggest either staging or routine follow-ups with PET-CT in patients with peripheral stage cIA tumors after curative-intent therapy [19,20]. It is conceivable that at initial evaluation the distant colon metastasis could have been detected by means of PET-CT, however it is highly unlikely due to its small size at the time. Besides, the definite role of FDG PET/CT in the diagnosis of gastrointestinal metastasis from lung cancer is still controversial with only a few cases being reported; solid clinical data are still lacking [21].
In conclusion, lung carcinoma presenting with gastrointestinal metastasis is rather rare even at advanced stages. Such presentations are possibly associated with the extended survival rates that can nowadays be achieved via surgery and/or chemotherapy combined with radiotherapy or newer targeted therapies. Although thorough investigation for distant metastasis is not widely recommended for peripheral stage cIA tumors after curative-intent therapy, clinicians must be aware that even pathological stage IA lung cancer has 73% 5 year survival due to the possibility of local or, most rarely, distal recurrence [22].
Acknowledgements
We are immensely grateful to Eleftherios Zervas (Pulmonologist/Sotiria Hospital for Chest Diseases, Athens, Greece) for assistance in writing this article and Argyro Papathanasiou (Pathologist, Private Practice, Chania, Greece) for her valuable work in immunohistochemical stainings. Both colleagues also shared their pearls of wisdom with us during this case's investigation.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hillers T.K., Sauve M.D., Guyatt G.H. Analysis of published studies on the detection of extrathoracic metastases in patients presumed to have operable non-small cell lung cancer. Thorax. 1994;49:14–19. doi: 10.1136/thx.49.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang C.J., Hwang J.J., Kang W.Y., Chong I.W., Wang T.H., Sheu C.C., Tsai J.R., Huang M.S. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer. 2006;54:319–323. doi: 10.1016/j.lungcan.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Carroll D., Rajesh P.B. Colonic metastases from primary squamous cell carcinoma of the lung. Eur. J. Cardio. Thorac. Surg. 2001;19:719–720. doi: 10.1016/s1010-7940(01)00646-7. [DOI] [PubMed] [Google Scholar]
- 5.Garwood R.A., Sawyer M.D., Ledesma E.J., Foley E., Claridge J.A. A case and review of bowel perforation secondary to metastatic lung cancer. Am. Surg. 2005;71:110–116. [PubMed] [Google Scholar]
- 6.Lou H.Z., Wang C.H., Pan H.M., Pan Q., Wang J. Colonic metastasis after resection of primary squamous cell carcinoma of the lung: a case report and literature review. World J. Gastroenterol. 21-5-2014;20:5930–5934. doi: 10.3748/wjg.v20.i19.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNeill P.M., Wagman L.D., Neifeld J.P. Small bowel metastases from primary carcinoma of the lung. Cancer. 15-4-1987;59:1486–1489. doi: 10.1002/1097-0142(19870415)59:8<1486::aid-cncr2820590815>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Kim M.S., Kook E.H., Ahn S.H., Jeon S.Y., Yoon J.H., Han M.S., Kim C.H., Lee J.C. Gastrointestinal metastasis of lung cancer with special emphasis on a long-term survivor after operation. J. Cancer Res. Clin. Oncol. 2009;135:297–301. doi: 10.1007/s00432-008-0424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di J.Z., Peng J.Y., Wang Z.G. Prevalence, clinicopathological characteristics, treatment, and prognosis of intestinal metastasis of primary lung cancer: a comprehensive review. Surg. Oncol. 2014;23:72–80. doi: 10.1016/j.suronc.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Howington J.A., Blum M.G., Chang A.C., Balekian A.A., Murthy S.C. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 11.Colt H.G., Murgu S.D., Korst R.J., Slatore C.G., Unger M., Quadrelli S. Follow-up and surveillance of the patient with lung cancer after curative-intent therapy: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e437S–e454S. doi: 10.1378/chest.12-2365. [DOI] [PubMed] [Google Scholar]
- 12.Antler A.S., Ough Y., Pitchumoni C.S., Davidian M., Thelmo W. Gastrointestinal metastases from malignant tumors of the lung. Cancer. 1-1-1982;49:170–172. doi: 10.1002/1097-0142(19820101)49:1<170::aid-cncr2820490134>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Antler A.S., Ough Y., Pitchumoni C.S., Davidian M., Thelmo W. Gastrointestinal metastases from malignant tumors of the lung. Cancer. 1-1-1982;49:170–172. doi: 10.1002/1097-0142(19820101)49:1<170::aid-cncr2820490134>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 14.Rossi G., Marchioni A., Romagnani E., Bertolini F., Longo L., Cavazza A., Barbieri F. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J. Thorac. Oncol. 2007;2:115–120. [PubMed] [Google Scholar]
- 15.Yoshimoto A., Kasahara K., Kawashima A. Gastrointestinal metastases from primary lung cancer. Eur. J. Cancer. 2006;42:3157–3160. doi: 10.1016/j.ejca.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Jagirdar J. Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung. Arch. Pathol. Lab Med. 2008;132:384–396. doi: 10.5858/2008-132-384-AOITTD. [DOI] [PubMed] [Google Scholar]
- 17.Silvestri G.A., Gould M.K., Margolis M.L., Tanoue L.T., McCrory D., Toloza E., Detterbeck F. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132:178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 18.Simou E., Koutsogeorgou E. Effects of the economic crisis on health and healthcare in Greece in the literature from 2009 to 2013: a systematic review. Health Pol. 2014;115:111–119. doi: 10.1016/j.healthpol.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Silvestri G.A., Gonzalez A.V., Jantz M.A., Margolis M.L., Gould M.K., Tanoue L.T., Harris L.J., Detterbeck F.C. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e211S–e250S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- 20.Colt H.G., Murgu S.D., Korst R.J., Slatore C.G., Unger M., Quadrelli S. Follow-up and surveillance of the patient with lung cancer after curative-intent therapy: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e437S–e454S. doi: 10.1378/chest.12-2365. [DOI] [PubMed] [Google Scholar]
- 21.Maziak D.E., Darling G.E., Inculet R.I., Gulenchyn K.Y., Driedger A.A., Ung Y.C., Miller J.D., Gu C.S., Cline K.J., Evans W.K., Levine M.N. Positron emission tomography in staging early lung cancer: a randomized trial. Ann. Intern. Med. 18-8-2009;151:221–248. doi: 10.7326/0003-4819-151-4-200908180-00132. [DOI] [PubMed] [Google Scholar]
- 22.Goldstraw P., Chansky K., Crowley J., Rami-Porta R., Asamura H., Eberhardt W.E., Nicholson A.G., Groome P., Mitchell A., Bolejack V. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]