Abstract
Antifungal agents account for approximately 3% of Drug-Induced Liver Injury (DILI) cases. Isavuconazole is a novel triazole, and experience with long-term use of it is lacking. We report a case of late-onset DILI occurring after 11 months of isavuconazole therapy in a 55-year old man of Angolan descent on long-term antifungal therapy for the management of chronic pulmonary aspergillosis complicating previously treated pulmonary tuberculosis. The DILI could be described as idiosyncratic as it was not associated with high isavuconazole serum levels and his liver function tests returned to normal following treatment discontinuation.
Keywords: Isavuconazole, Chronic pulmonary aspergillosis, Drug-induced liver injury, Late-onset
1. Introduction
Antimicrobial agents are the commonest class of drugs associated with drug-induced liver injury [DILI] [1]. Isavuconazole, a novel triazole antifungal agent, exhibits in vitro activity against a broad-spectrum of clinically significant filamentous fungi, including Aspergillus spp. and agents of mucormycosis [2], [3], [4]. Isavuconazole is used as a fourth-line agent for the treatment of patients with chronic pulmonary aspergillosis (CPA) who are intolerant of itraconazole, voriconazole and posaconazole or following detection of Aspergillus isolates resistant to these agents [5]. Liver toxicity is usually seen within the first few weeks or months of isavuconazole therapy. Here, a case of hepatotoxicity due to isavuconazole occurring after almost a year of therapy for CPA is described.
2. Case
In April 2016 (day 0), a 55-year old man of Angolan descent was commenced on isavuconazole for the treatment of CPA complicating previously treated pulmonary tuberculosis and severe post-tuberculosis bronchiectasis. The diagnosis of CPA was made in November 2014 and his CT thorax at that time showed a right upper lobe cavity with a large aspergilloma. Prior to commencing isavuconazole, he had undergone several bronchial artery embolization procedures for the control of haemoptysis related to his CPA. His CPA was initially treated with oral itraconazole. Twelve months after initiating itraconazole therapy, isolates of Aspergillus fumigatus which were resistant to both itraconazole and posaconazole were cultured from his sputum and therapy was switched to oral voriconazole. However, after 3 months of voriconazole therapy he had failed to achieve therapeutic serum levels despite escalation to the maximum tolerable dose (300 mg twice daily). Consequently, given his low weight, isavuconazole was initiated at a dose of 100 mg once daily by mouth after induction therapy of 200 mg twice daily by mouth for the first 48 h.
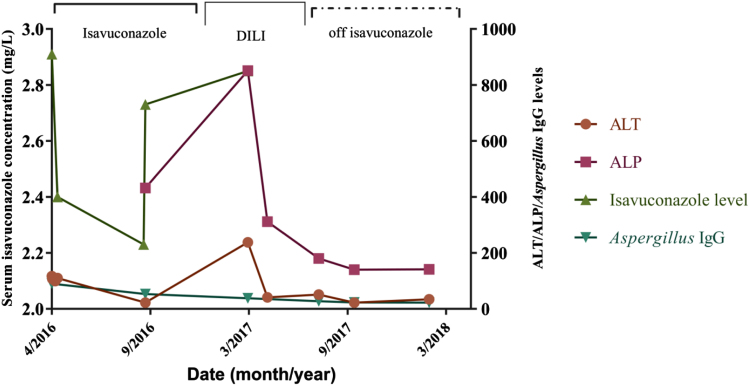
Eleven months into isavuconazole therapy (day +308), he had shown significant clinical improvement as evidenced by weight gain, reduction in the size of both fungal ball and lung cavity on CT imaging with a significant decline in serum Aspergillus IgG levels from 90 to 22 mg/L (normal value <40 mg/l). However, by this time his alanine transferase (ALT) had risen from 34 U/L to 238 U/L and his aspartate transferase (AST) had increased from 22 U/L to 145 U/L (Fig. 1). His alkaline phosphatase (ALP) also rose from 431 U/L to 851 U/L. His total bilirubin levels remained within normal limits. He did not report any abdominal pain. There were no peripheral stigmata of chronic liver disease on examination. His serum isavuconazole levels remained within the therapeutic range (2.23–2.9 mg/L) throughout the entire duration of therapy (Fig. 1). The pattern of liver injury appeared to be cholestatic in nature with an = = 0.66 (<2), where ULN=upper limit of normal.
Fig. 1.
Isavuconazole serum concentration, ALT and ALP levels and Aspergillus IgG levels over a 2-year period. DILI: Drug-induced liver injury.
R = ratio
His serum iron, transferrin, alpha-1 antitrypsin and alpha-fetoprotein levels were within normal limits. Serum hepatitis B virus DNA PCR and hepatitis C RNA viral PCR tests were both negative, as was his HIV test. His international normalised ratio was 1.2 and autoimmune liver screen was negative. As no alternative cause for his deranged liver function tests was found, he was diagnosed with DILI secondary to isavuconazole use and therefore the drug was discontinued.
His past medical history included sarcoidosis for which he was being treated with long term prednisolone. He denied any history of alcohol abuse. Previous liver imaging (in 2014) had shown findings consistent with early mild cirrhosis but no evidence of cholestatic liver disease (Fig. 2).
Fig. 2.
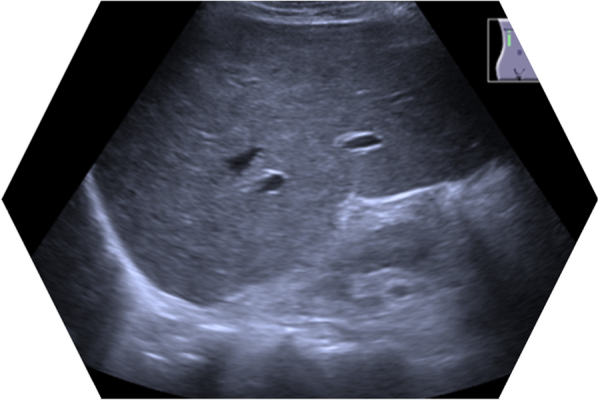
Liver parenchyma is mildly coarse and heterogeneous, with a nodular echotexture suggestive of early cirrhotic changes. No evidence of inter or extrahepatic ductal dilatation. No obvious ductal calculus. No obvious focal intrahepatic lesions evident.
He had an uneventful recovery with normalisation of ALT and ALP 5 months (day +476) following discontinuation of isavuconazole therapy. He is not currently on any antifungal therapy for his CPA and he remains asymptomatic with no further episodes of haemoptysis since the discontinuation of antifungal therapy.
3. Discussion
A systematic review of chemotherapy-induced liver injury showed that antifungal agents, excluding isavuconazole, account for about 3% of DILI [6]. Longer treatment duration may be associated with an increased risk of antifungal agent-induced liver injury [7]
Isavuconazole has very predictable pharmacokinetics, with excellent oral bioavailability and no food effect [8]. No dosage adjustment is required for advanced age, renal impairment (including end-stage renal disease) or for mild to moderate hepatic impairment [4]. Isavuconazole is generally well tolerated and associated with fewer skin, eye and hepatobiliary adverse events than voriconazole [2]. It is exclusively metabolised by the liver, but liver toxicity has been reported as generally mild, rarely requiring discontinuation [9]. In phase III clinical trials, approximately 16% of patients on isavuconazole for invasive aspergillosis or mucormycosis developed hepatobiliary disorders (including DILI) while on therapy [3], [4]. The vast majority of patients in these studies, approximately 90%, received less than 6 months of treatment. In those treated for a longer period, there were no reports of DILI developing more than 6 months after the introduction of isavuconazole therapy. A case of hepatic veno-occlusive disorder requiring isavuconazole discontinuation in a patient with undifferentiated acute myeloid leukaemia has recently been reported [10].
Experience with long-term use of isavuconazole is lacking [3], [4]. To our knowledge, this is the first reported case of late-onset DILI associated with isavuconazole therapy, with deranged liver function tests requiring cessation of treatment developing 11 months after the drug was introduced. The DILI could be described as idiosyncratic as it was not associated with high isavuconazole serum levels.
In conclusion, isavuconazole should be used with caution in patients at risk of idiosyncratic DILI. Furthermore, it is important that regular liver function tests be performed on all patients on long-term isavuconazole therapy, especially those with underlying liver disease.
Acknowledgements
Infectious diseases consultants and specialist nurses at the UK National Aspergillosis Centre
Acknowledgments
Conflict of interest
There are none.
References
- 1.Ahmad J., Odin J.A. Epidemiology and genetic risk factors of drug hepatotoxicity. Clin. Liver Dis. 2017;21:55–72. doi: 10.1016/j.cld.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shirley M., Scott L.J. Isavuconazole: a review in invasive aspergillosis and mucormycosis. Drugs. 2016;76:1647–1657. doi: 10.1007/s40265-016-0652-6. [DOI] [PubMed] [Google Scholar]
- 3.Marty F.M., Ostrosky-Zeichner L., Cornely O.A., Mullane K.M., Perfect J.R., Thompson G.R., Alangaden G.J., Brown J.M., Fredricks D.N., Heinz W.J., Herbrecht R., Klimko N., Klyasova G., Maertens J.A., Melinkeri S.R., Oren I., Pappas P.G., Ráčil Z., Rahav G., Santos R., Schwartz S., Vehreschild J.J., Young J.-A.H., Chetchotisakd P., Jaruratanasirikul S., Kanj S.S., Engelhardt M., Kaufhold A., Ito M., Lee M., Sasse C., Maher R.M., Zeiher B., Vehreschild M.J.G.T. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016;16 doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 4.Maertens J.A., Raad I.I., Marr K.A., Patterson T.F., Kontoyiannis D.P., Cornely O.A., Bow E.J., Rahav G., Neofytos D., Aoun M., Baddley J.W., Giladi M., Heinz W.J., Herbrecht R., Hope W., Karthaus M., Lee D.G., Lortholary O., Morrison V.A., Oren I., Selleslag D., Shoham S., Thompson G.R., Lee M., Maher R.M., Schmitt-Hoffmann A.H., Zeiher B., Ullmann A.J. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 5.Maghrabi F., Denning D.W. The management of chronic pulmonary aspergillosis: the UK National Aspergillosis Centre approach. Curr. Fungal Infect. Rep. 2017 doi: 10.1007/s12281-017-0304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raschi E., Poluzzi E., Koci A., Caraceni P., De Ponti F. Assessing liver injury associated with antimycotics: concise literature review and clues from data mining of the FAERS database. World J. Hepatol. 2014;6:601–612. doi: 10.4254/wjh.v6.i8.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao W.-Y., Su C.-W., Huang Y.-S., Chou Y.-C., Chen Y.-C., Chung W.-H., Hou M.-C., Lin H.-C., Lee F.-Y., Wu J.-C. Risk of oral antifungal agent-induced liver injury in Taiwanese. Br. J. Clin. Pharmacol. 2014;77:180–189. doi: 10.1111/bcp.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slavin M.A., Thursky K.A. Isavuconazole: a role for the newest broad-spectrum triazole. Lancet. 2016;387:726–728. doi: 10.1016/S0140-6736(15)01218-0. [DOI] [PubMed] [Google Scholar]
- 9.Kyriakidis I., Tragiannidis A., Munchen S., Groll A.H. Clinical hepatotoxicity associated with antifungal agents. Exp. Opin. Drug Saf. 2017;16:149–165. doi: 10.1080/14740338.2017.1270264. [DOI] [PubMed] [Google Scholar]
- 10.Mesini A., Cangemi G., Palmisani E., Dufour C., Castagnola E. Hepatic veno-occlusive disease during isavuconazole administration. J. Chemother. 2018;30:63–64. doi: 10.1080/1120009X.2017.1418619. [DOI] [PubMed] [Google Scholar]