Abstract
Microalgae biotechnologies are rapidly developing into new commercial settings. Several high value products already exist on the market, and systems development is focused on cost reduction to open up future economic opportunities for food, fuel and freshwater production. Light is a key environmental driver for photosynthesis and optimising light capture is therefore critical for low cost, high efficiency systems. Here a novel high-throughput screen that simulates fluctuating light regimes in mass cultures is presented. The data was used to model photosynthetic efficiency (PEµ, mol photon−1 m2) and chlorophyll fluorescence of two green algae, Chlamydomonas reinhardtii and Chlorella sp. Response surface methodology defined the effect of three key variables: density factor (Df, ‘culture density’), cycle time (tc, ‘mixing rate’), and maximum incident irradiance (Imax). Both species exhibited a large rise in PEµ with decreasing Imax and a minimal effect of tc (between 3–20 s). However, the optimal Df of 0.4 for Chlamydomonas and 0.8 for Chlorella suggested strong preferences for dilute and dense cultures respectively. Chlorella had a two-fold higher optimised PEµ than Chlamydomonas, despite its higher light sensitivity. These results demonstrate species-specific light preferences within the green algae clade. Our high-throughput screen enables rapid strain selection and process optimisation.
Introduction
Green algae are oxygenic photosynthetic organisms which, like higher plants and cyanobacteria, have evolved over 3 billion years to tap into the huge energy resource of the sun. This energy is used to fix CO2, releasing O2 as a by-product and producing biomass rich in proteins, lipids, starch, bioactive compounds and phytonutrients. Consequently, single celled green algae (microalgae) are increasingly being integrated into industrial production systems to realise solar driven biotechnologies. Microalgae technologies are already being exploited commercially to produce high value commodities (e.g. functional foods, feeds, protein therapeutics and chemicals)1–3 and the knowledge gained is driving down production costs toward the levels required to expand low value market opportunities including fuels and fertilisers as well as ecosystem services (e.g. water treatment and CO2 sequestration)4–6. The first step of all solar driven microalgae processes is light capture and conversion to chemical energy (ATP, NADPH), and the optimisation of this step is therefore essential to develop high-efficiency economic solutions7–9. In outdoor mass cultures, the light reaching the surface of the pond or bioreactor is highly variable over the day, ranging from light limiting during early/late hours of the day or periods of high cloud cover, to photo-inhibiting conditions (up to 2,000 µmol m−2 s−1) during mid-day in locations receiving high solar radiation. Within the culture itself, cells are exposed to high light gradients as they cycle from the illuminated surface (often inhibitory light levels) to deep within the culture (light limiting or dark conditions). This fluctuating light regime within the mass culture is governed by the optical properties of the culture (based on cell size, cell number and pigment content) while the frequency with which cells cycle between the light and dark zones is regulated by mixing rate as well as the photobioreactor geometry which influences the light distribution through the optical pathlength and the surface to volume ratio. The relatively rapid light fluctuations within the culture affect the photo-regulatory response, while the relatively slow environmental light fluxes affect photoacclimation, both leading to changes in the overall productivity of the culture10–12.
Defining and optimising the effects and interactions of the variables that govern a given light regime is a challenge that requires comparatively large experimental datasets which can be laborious and expensive to obtain using traditional pilot- or even laboratory-scale bioreactors. The high-throughput light screen method presented here has been designed to simulate light regimes encountered in mass cultured photobioreactors under ‘typical’ outdoor production conditions to enable process optimisation, model guided system design, species selection and a better extrapolation of laboratory results to field trials.
The light screen collected data from LED illuminated microwells, and Response Surface Methodology was employed to predictively model photosynthetic efficiency (PEµ), to define both main effects and the pair-wise interactions between the light factors that govern it and to identify the conditions that yield optimum productivity. As fluctuating light can effect photoregulation and photoacclimation, we also investigated some of these underlying mechanisms to assess the extent of their effect on PEµ.
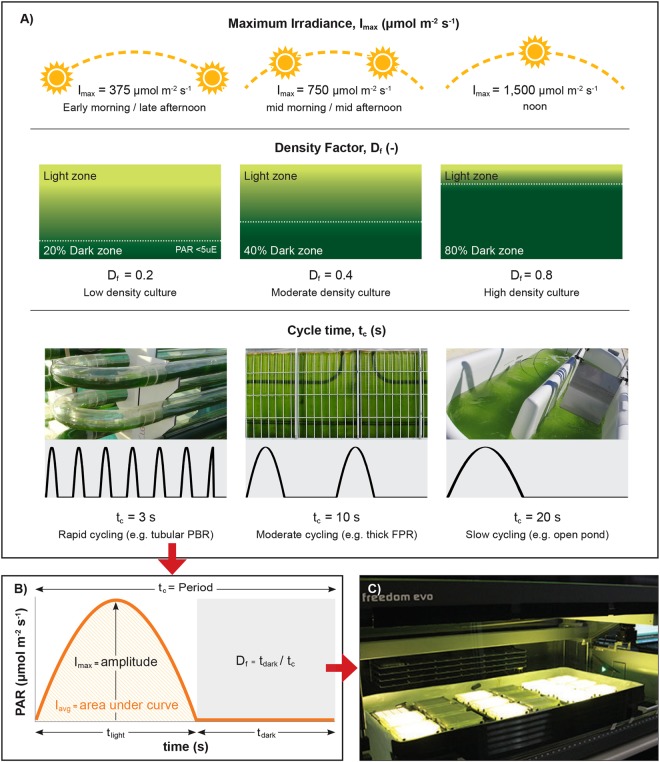
A full factorial experimental design was employed, with quadratic models fitted to the data to measure the PEµ in response to variations of three key factors that govern the light regime to which cells in mass culture are exposed: density factor (‘Df’, -), defined as the proportion of the time that cells are in the dark zone (tdark, s) compared with the total time in both light (tlight, s) and dark zones; cycle time (‘tc’, s), which is defined by the mixing rate, or the total time of a cell’s fluctuation between light and dark zones for one cycle along the culture depth; and maximum irradiance (Imax, µmol photons m−2 s−1) defined as the irradiance entering the photobioreactor at the illuminated surface (Fig. 1A). Dark was defined as <5 μmol PAR at which respiration typically exceeds photosynthesis (the compensation point)13,14. The three factors (Df, tc, Imax) affect the average irradiance (Iavg), which is the integration of light experienced by the cells over the entire light cycle (Fig. 1B). Our miniaturised and automated screen enables the analysis of the interactions between the three light-dependent factors and generates a strain-specific model that can be used to optimise production conditions or predict productivities for different production scenarios.
Figure 1.
Experimental design for high-throughput light simulations of cells cycling in outdoor microalgae mass cultures. (A) Depicts the 3 factors that affect the light regime experienced by cells cycling in mass cultures: Imax, Df and tc, and the levels used for the full factorial experimental design which are based on ‘typical’ outdoor conditions. (B) Each combination of light factors was programmed by changing the light intensity of the LEDs over the cycle time, assuming cell cycling occurs in a sinusoidal trajectory. Here, Imax, is the amplitude of the sine, simulating the maximum irradiance that a cell would receive when at the ‘surface’ of a mass culture, Df, is the proportion of time that PAR is below 5 µmol m−2 s−1 in one period; this simulates the fraction of time that a cell spends in the dark, depending on the culture density, and tc is the period of one sine wave, that simulates the time required for a cell to cycle through the reactor. Iavg is the integration of light received, simulating the average irradiance or light dose received the by cell. Here tlight and tdark are the time cells receive PAR (>5 µmol m−2 s−1) and no PAR (<5 µmol m−2 s−1) respectively. (C) The programmed LEDs form part of an 18-plate microwell robotic system. Chlamydomonas and Chlorella were incubated in 96-well plates placed on LED arrays with one LED per microwell and one unique light regime per plate. All light regimes occurred over a photoperiod of 16 h day−1 and a dark period of 8 h day−1.
This empirical model is an alternative approach to traditional models based on photosynthetic irradiance (P–I) curves. It only requires knowledge of the density factor, incident irradiance and mixing rate. The Df for a given species and reactor geometry can be easily found (indoor or outdoor) for a given incident irradiance by measuring the depth of culture at the point where light is reduced to <5 µmol m−2 s−1 (i.e. start of the “dark zone”) and calculating the ratio of this depth to the total culture depth (usually fixed). This can be correlated to a range of optical densities (or biomass dry weights) to provide a simple method to establish what Df a reactor will have at a known culture density, pathlength and incident irradiance. Since Df has been determined as a critical factor in this and other studies, we believe that this is another useful modelling tool for process design.
Two biotechnologically relevant microalgae strains were analysed in this study: Chlamydomonas reinhardtii (Chlamydomonas), the model alga most used in photosynthetic studies15,16 and for heterologous protein expression17,18, and a strain of Chlorella sp, 11_H5 (Chlorella) isolated in Australia which was found to have high biomass productivity at laboratory and pilot scale19,20. Chlamydomonas (originally isolated from soil)21 has successfully transitioned from land to water in laboratory conditions, arguably owing to its robust and evolved photosynthetic machinery that protects it from oxidative stress and changing environmental conditions22. Hence, understanding the interplay between photosynthetic regulation, photoacclimation and its effect on growth and biomass productivity would determine the feasibility of delivering functional microalgae biotechnologies. This paper presents a high-throughput miniaturised light optimisation screen (allowing up to 18 different combinations of light regime and up to 1,728 conditions), designed to identify species-specific illumination conditions that maximise photosynthetic efficiency and productivity to fast track systems optimisation.
Results
High-throughput screen (HTS) of simulated light regimes in mass cultures
To analyse the effects of varying levels of Imax, Df and tc (Fig. 1B) on the PEµ of microalgae, light simulations were performed on dilute 150 μl microwell cultures (5 mm pathlength)23, each illuminated using individual LEDs (Fig. 1C). The intensity of photosynthetically active radiation (400–700 nm, PAR) emitted by the LEDs was programmed (Arduino® integrated circuit and controller) to mimic a sinusoidal trajectory of a cell cycling in a one-dimensionally illuminated culture (i.e. an open pond) between the illuminated surface and the dark zone (Fig. 1B)10. In this way, the light regime encountered by the incubated cells in each well was a function of the LED’s illumination profile, thereby allowing tight control of the levels of each factor (Imax, Df and tc), (Fig. 1A). A robotic arm was programmed to take the plates to a reader at determined time intervals where rapid measurements of optical density and fluorescence can be taken. Here, two strains were analysed for the initial HTS light simulations, however, this method can rapidly be used to model up to 32 strains run in triplicate in one experiment.
Figure 1A depicts the three levels of each factor (Imax, Df, tc) and the real-world phenomena they represent based on information from literature24–26 and on experimental data27–29. A low (0.2) or high (0.8) Df represents a low or high cell/biomass density respectively (e.g. dilute cultures at the beginning of cultivation versus dense cultures at harvest in a batch production regime). The system is able to analyse any range between 10 ms fluctuations to constant light. The cycle time of 3–20 s represents typical ‘mixing’ cell cycle rates through the optical pathlength of photobioreactors, where a tc of 3, 10, and 20 s represents rapid, moderate or slow mixing, as might occur in a tubular PBR, thick flat panel PBR and open pond respectively. The tc is influenced by mixing and/or sparging rates, reactor pathlength, or a combination of the two, which can vary for individual reactors depending on cultivation regime. The Imax values represent the incident solar radiation in the early morning and late afternoon (375 µmol m−2 s−1), mid-morning and -afternoon (750 µmol m−2 s−1), and noon (1500 µmol m−2 s−1) respectively. Imax values are based on the average annual solar radiation levels for Brisbane, Australia30,31, and are representative of other high solar regions that are suitable for outdoor microalgae production. The simulation of these three factors at three levels each via programmed changes in LED light flux over time are depicted in Fig. 1B. This approach provided a complete factorial design (33) of 27 combinations for model fitting of the main response variable, PEµ (Table 1) and underlying responses at the level of PSII (Table 2).
Table 1.
PEµ of Chlamydomonas and Chlorella under the experimental matrix of light regimes.
| Imax | Df | tc | Iavg | PEµ (mol photon−1 m2) | ||||
|---|---|---|---|---|---|---|---|---|
| Actual (µmol m −2 s −1 ) | Coded | Actual (−) | Coded | Actual (s) | Coded | (mol m −2 h −1 ) | Chlamydomonas | Chlorella |
| 375 | −1 | 0.2 | −1 | 3 | −1.73 | 0.619 | 0.118 ± 0.0030 | 0.136 ± 0.017 |
| 10 | 0 | 0.099 ± 0.0093 | 0.142 ± 0.018 | |||||
| 20 | 1 | 0.107 ± 0.0031 | 0.151 ± 0.026 | |||||
| 0.4 | 0 | 3 | −1.73 | 0.490 | 0.174 ± 0.0070 | 0.183 ± 0.012 | ||
| 10 | 0 | 0.133 ± 0.0079 | 0.149 ± 0.001 | |||||
| 20 | 1 | 0.094 ± 0.0070 | 0.132 ± 0.018 | |||||
| 0.6* | — | 3 | −1.73 | 0.367 | 0.088 ± 0.0066 | 0.176 ± 0.007 | ||
| 10 | 0 | 0.099 ± 0.0010 | 0.167 ± 0.011 | |||||
| 20 | 1 | 0.084 ± 0.0100 | 0.149 ± 0.007 | |||||
| 0.8 | 1 | 3 | −1.73 | 0.18 | 0.040 ± 0.0028 | 0.277 ± 0.022 | ||
| 10 | 0 | 0.048 ± 0.0000 | 0.197 ± 0.014 | |||||
| 20 | 1 | 0.047 ± 0.0107 | 0.159 ± 0.006 | |||||
| 750 | 0 | 0.2 | −1 | 3 | −1.73 | 1.242 | 0.078 ± 0.0037 | 0.039 ± 0.003 |
| 10 | 0 | 0.063 ± 0.0013 | 0.054 ± 0.002 | |||||
| 20 | 1 | 0.053 ± 0.0022 | 0.076 ± 0.001 | |||||
| 0.4 | 0 | 3 | −1.73 | 0.979 | 0.060 ± 0.0121 | 0.087 ± 0.004 | ||
| 10 | 0 | 0.061 ± 0.0040 | 0.087 ± 0.006 | |||||
| 20 | 1 | 0.049 ± 0.0020 | 0.095 ± 0.008 | |||||
| 0.6* | — | 3 | −1.73 | 0.738 | 0.079 ± 0.0030 | 0.099 ± 0.005 | ||
| 10 | 0 | 0.061 ± 0.0016 | 0.082 ± 0.006 | |||||
| 20 | 1 | 0.049 ± 0.0030 | 0.182 ± 0.003 | |||||
| 0.8 | 1 | 3 | −1.73 | 0.360 | 0.063 ± 0.0073 | 0.134 ± 0.012 | ||
| 10 | 0 | 0.046 ± 0.0023 | 0.072 ± 0.022 | |||||
| 20 | 1 | 0.020 ± 0.0027 | 0.097 ± 0.008 | |||||
| 1500 | 1 | 0.2 | −1 | 3 | −1.73 | 2.480 | 0.051 ± 0.0027 | 0.021 ± 0.0004 |
| 10 | 0 | 0.067 ± 0.0109 | 0.025 ± 0.002 | |||||
| 20 | 1 | 0.049 ± 0.0021 | 0.047 ± 0.006 | |||||
| 0.4 | 0 | 3 | −1.73 | 1.958 | 0.053 ± 0.0021 | 0.037 ± 0.004 | ||
| 10 | 0 | 0.052 ± 0.0035 | 0.055 ± 0.001 | |||||
| 20 | 1 | 0.045 ± 0.0026 | 0.072 ± 0.011 | |||||
| 0.6* | — | 3 | −1.73 | 1.472 | 0.050 ± 0.0138 | 0.067 ± 0.001 | ||
| 10 | 0 | 0.041 ± 0.0074 | 0.057 ± 0.006 | |||||
| 20 | 1 | 0.030 ± 0.0080 | 0.092 ± 0.003 | |||||
| 0.8 | 1 | 3 | −1.73 | 0.713 | 0.051 ± 0.0053 | 0.072 ± 0.001 | ||
| 10 | 0 | 0.031 ± 0.0088 | 0.043 ± 0.006 | |||||
| 20 | 1 | 0.030 ± 0.0170 | 0.043 ± 0.007 | |||||
All data are the mean of 3 replicates ± standard deviation. *Indicates data used for model validation. ‘Coded’ refers to the normalised values used for the quadratic model (Equation 2).
Table 2.
Comparison of the factor coefficients of the quadratic model obtained from analysis of variance (ANOVA) for A) PEµ, B) ΦPSII and C) Fv/Fm parameters for Chlamydomonas and Chlorella. *Represents significant effects at p-value < 0.05. n = 3 (PEµ), n = 2 (ΦPSII & Fv/Fm).
| Coefficients from the quadratic non-linear model | ||||||
|---|---|---|---|---|---|---|
| PEµ (10−3) | ΦPSII (10−3) | Fv/Fm (10−3) | ||||
| Chlamydomonas | Chlorella | Chlamydomonas | Chlorella | Chlamydomonas | Chlorella | |
| Df | −21.0* | 20.5* | −35.7* | −8.1* | 16.4* | 16.6* |
| Imax | −20.0* | −61.2* | −3.2 | −4.4 | 22.1* | −54.2* |
| tc | −6.6 | −5.5* | −3.3 | −2.0 | −0.9 | −6.8* |
| Df − Imax | 16.0* | −10.3* | −29.6* | −6.8* | −6.1 | 9.7* |
| Df − tc | −1.1 | −14.7* | 0.8 | 0.9 | −3.6 | 3.9 |
| Imax − tc | 3.2 | −9.5* | −5.0* | 3.9 | 3.0 | −6.5* |
| Df2 | −24.6* | 2.4 | −26.8* | −4.7 | 10.4 | 1.8 |
| Imax2 | 14.2* | 28.0* | 19.1* | −4.9 | 31.8* | 1.7 |
| tc2 | 1.0 | 2.8 | −0.2 | 0.6 | 1.7 | −3.8 |
| Intercept | 67.6 | 71.1 | 236.5 | 194.3 | 655.6 | 647.1 |
| R2 | 0.67 | 0.93 | 0.89 | 0.44 | 0.74 | 0.91 |
A further dataset with a Df of 0.6 (at each level of Imax and tc) provided 9 independent data points used for model validation and goodness of fit (Table 1, validation data are indicated by ‘*’. See section (Model validation shows that the light factors Df, Imax and tc can be used to predict PEμ accurately in Chlorella and moderately in Chlamydomonas) for results). For all treatments, the combination of each Df and Imax also resulted in 12 unique integrated average irradiance levels, Iavg (mol photons m−2 h−1). Additional experiments compared the PEµ between cells exposed to fluctuating regimes with cells exposed to constant illumination (control) with the same Iavg to compare the effect of light regime and light dose (Fig. 2C, Supplementary Table S1, Supplementary Fig. S4).
Figure 2.
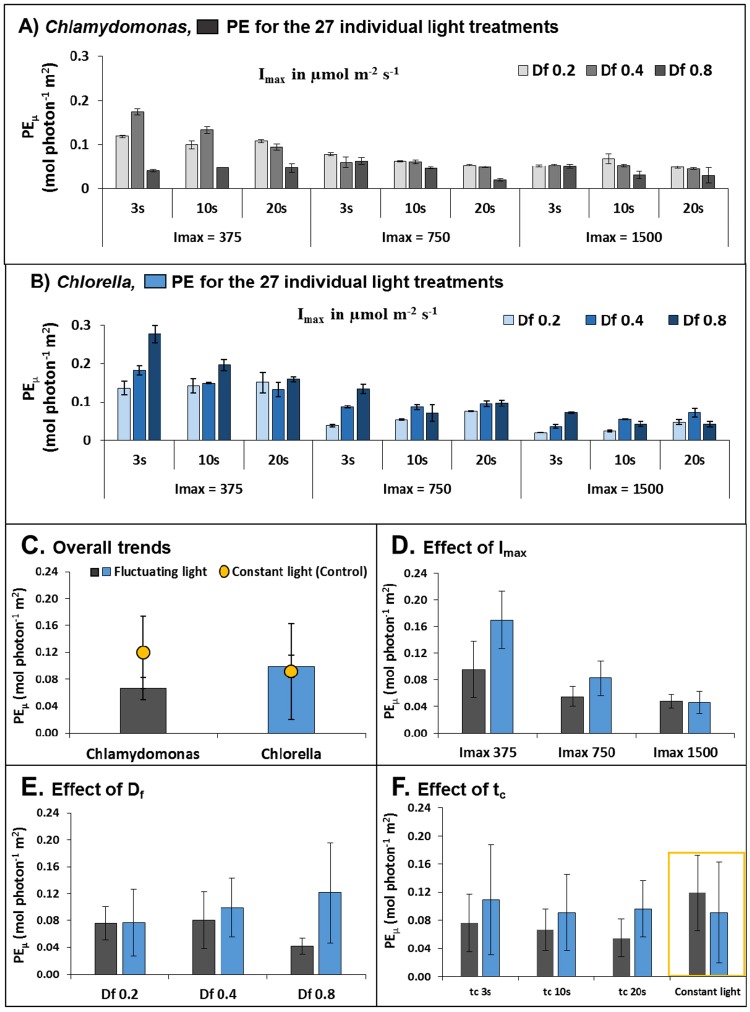
Trends in photosynthetic efficiency (PEµ) under different light regimes of Chlamydomonas (grey bars) and Chlorella (blue bars). (A and B) individual PEµ data of the 27 light treatments for Chlamydomonas and Chlorella, respectively (n = 3), (C) the overall trends in averaged PEµ values over all conditions of Df, Imax and tc tested (n = 27), (D) the averaged PEµ values of Df and tc combined to show effect of Imax (n = 9), (E) the averaged PEµ values of Imax and tc combined to show effect of Df (n = 9) and (F) the averaged PEµ values of Df and Imax combined to show effect of tc (n = 9). Error bars represent the standard deviation (SD) of individual treatments within biological triplicates (A,B) and between different treatments (C–F).
Light screen experiments were conducted over 3 days in a controlled semi-continuous cultivation regime. As light acclimation occurs on a timescale of several hours to days, sufficient time was given for the cells to acclimate to the light regime that they were exposed to. To minimise cell shading effects with increasing OD, cultures were diluted back to the same initial OD750 of 0.1 (pathlength 5 mm) each day. Quasi-steady-state growth rates, μ (h−1) were calculated (Equation 3) from 3-hourly OD750 measurements (Supplementary Figs S1 and S2) on Day 2 during the exponential phase (after ~38 hours of light regime exposure) and normalised to the light received to estimate the photosynthetic efficiency (PEμ) (Equation 4).
Photosynthetic efficiency under different light regimes
The PEµ of Chlamydomonas and Chlorella under all 27 fluctuating light regimes are shown in Fig. 2A and B. Some similarities in the general trends of Chlamydomonas and Chlorella are evident, such as the effect of Imax, where a large increase in PEµ occurred with decreasing Imax. To better depict PEµ trends, individual treatments were averaged for each species over all factors (Fig. 2C), and over all but one factor (Fig. 2D–F). Overall, Chlorella exhibited a ~50% higher PEµ than Chlamydomonas (average PEµ of 0.099 ± 0.060 mol photon−1 m2 and 0.066 ± 0.034 mol photon−1 m2 respectively, Fig. 2C), in line with previous reports32.
Figure 2C also shows the mean PEµ obtained under constant light was ~80% higher in Chlamydomonas but approximately the same for Chlorella (−7.5%) than that obtained under fluctuating light of the same Iavg. For Chlamydomonas, this result concurs with other studies showing a negative impact of fluctuating light on time-integrated photosynthesis and growth rates10,12,33,34. Interestingly, for this strain of Chlorella, fluctuating light had little effect compared to constant light conditions.
For main effects of each factor, Fig. 2D shows at the lowest Imax value, the mean PEµ increased up to two-fold for Chlamydomonas and 3.67-fold for Chlorella, respectively, indicating that photosynthetic light utilisation is compromised under high incident light (i.e. at noon under outdoor conditions)35–37, especially for Chlorella.
The trends of Df (Fig. 2E) resulted in diametrically opposing responses: PEµ in Chlamydomonas performed best at a low Df (increasing up to 83% from Df = 0.8 to Df = 0.2) while Chlorella at a high Df (PEµ increased up to 58% from Df = 0.2 to Df = 0.8). Since mass cultures operating under high cell densities is advantageous to reduce downstream processing costs, these results suggest that Chlorella is more suited to mass cultivation than Chlamydomonas.
For both species, the effect of tc seemed minor (Fig. 2F). Cell cycling in the range analysed (tc = 3, 10, 20 s) exhibited a modest increase in PEµ with decreasing tc values (39% for Chlamydomonas and 13% for Chlorella). While large improvements of PEµ have been reported under sub-second cycle times approaching the ‘flashing light effect’28,38,39, this is in line with other studies that have reported similar modest improvements for Chlamydomonas below cycle times of 10 s12 and little effect in the seconds range for other Chlorella sp. and other algae11,38.
Modelling light factor interactions using response surface methodology
Response surface methodology of the complete factorial design40–45 was next employed to model and explore the interactions between the three input factors (Imax, Df and tc) to PEµ. Furthermore, to determine the influence of photoregulation under fluctuating light on PEµ, supporting parameters at the level of PSII regulation for Chlamydomonas and Chlorella were also modelled from chlorophyll fluorescence data. These are: the operating efficiency of PSII (ϕPSII) – a measure of the proportion of absorbed light used for photochemistry; maximum quantum efficiency of PSII photochemistry (Fv/Fm) – an indicator of PSII inactivation via photoinhibition; and non-photochemical quenching (NPQ) – the apparent rate constant for heat loss from PSII44. These parameters provide clues as to the underlying mechanisms of the observed PEμ.
The three levels of each factor (Table 1) were coded with the mid-point (coded as ‘0’) and this was halved and doubled in the experimental design such that the coded factors of the independent variables were calculated using the logarithmic equation,
| 1 |
where, x is the coded factor level, X is the actual value of the factor, i = 1, 2, 3; A is the intercept value of the logarithmic function for each factor with A1 = 1.3219, A2 = −9.5507 and A3 = −3.3219 for Df, Imax and tc respectively.
Quadratic models (Equation 2) were fitted to the data:
| 2 |
In Equation 2, Y is the predicted response variable (PEµ, ϕPSII, Fv/Fm or NPQ); β0, βi, βij and βii are the coefficients for intercept, linear, interaction and quadratic effects respectively; x1, x2 … xk are the coded values of the input factors (i ≠ j); and k = 3. Multiple regression of the data was used to obtain the regression coefficients.
Model validation shows that the light factors Imax, Df and tc can be used to predict PEµ accurately in Chlorella and moderately in Chlamydomonas
For the primary response, PEµ, the quadratic model demonstrated a moderate and high goodness of fit for Chlamydomonas (R2 = 0.67) and Chlorella (R2 = 0.93), respectively.
To assess whether the model fit was adequate to predict PEµ within the range analysed, the quadratic models were validated using an additional set of experimental data at Df = 0.6 at each level of Imax and tc (9 experimental sets for each strain) (Table 1). Comparing the fitted models against the actual data gave a low R2 of 0.456 for Chlamydomonas and a high R2 of 0.882 for Chlorella (Supplementary Fig. S5). In general, the residuals showed a normal distribution and the Cook’s distance plot showed only a small number of outliers for Chlamydomonas and Chlorella (Supplementary Fig. S5).
For Chlorella, these results indicated that the three light factors accounted for a high proportion of variation in PEµ observed and can be used to adequately predict their relationship to PEµ. For Chlamydomonas, it seems there are more complex regulations of the photosynthetic machinery, which cannot be modelled with these factors alone.
The light factors of Imax and Df significantly affect PEµ under fluctuating light
The coefficient terms tabulated in Table 2 show the relative size and direction that effect each factor has on the response variables, while the three dimensional (3D) response surface plots and 2D contour plots graphically depict the interactions of two factors on the primary response of PEµ, where the third factor is set to the midpoint (Fig. 3).
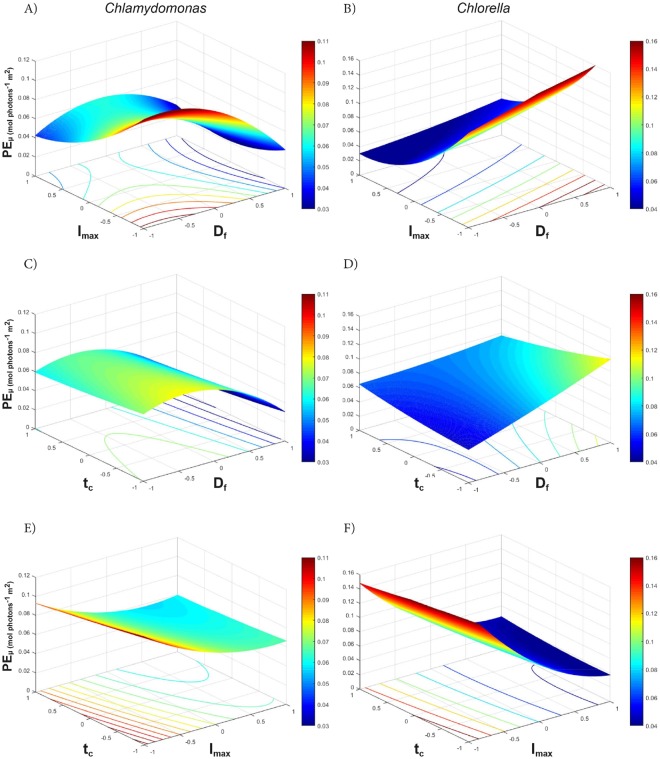
Figure 3.
Response surface (3D) and contour (2D) plots of two-way interactions of factors affecting the PEµ (mol photon−1 m2) of Chlamydomonas (A,C,E) and Chlorella (B,D,F). The colour bar depicts high PEµ values in red and lower PEµ values in blue.
For Chlamydomonas, the most significant factors affecting PEµ were: Imax (p-value = 3.83E−08), Df (p-value = 1.04E−08), and the interaction of Df-Imax (p-value 1.05E−04) (Table 2). Here, both high Df and high Imax had similar negative impacts on PEµ, yet the interaction of Df-Imax had a positive effect, suggesting that dense cultures may offer some protection under high light whilst dilute cultures may improve PEµ under low light. As expected, the 3D plots show the highest PEµ values at a combination of low Df (i.e. not light limited) and low Imax (i.e. not photo-inhibited) (Fig. 3A), however, the slight saddle shape of the interaction plot at high Imax shows that the optimal Df is around 0.4 (at the mid-point) for Chlamydomonas.
The PEµ of Chlorella was most significantly adversely affected by high Imax (p-value 9.92E−37), and unlike Chlamydomonas, showed a significant positive response for increasing Df (p-value 4.67E−12). The Imax-Df interaction showed an exponential increase in PEµ with a reduction of Imax and an increase in Df (Fig. 3B). However, the significant negative interaction of Df-tc (Table 2) suggests that long cycle times could adversely affect productivity in high density cultures (Fig. 3D). Overall, for Chlamydomonas a low Imax and low Df (Fig. 3A) and for Chlorella a low Imax and high Df (with moderate benefits of low tc) (Fig. 3 B and D) resulted in the highest PEµ.
PSII regulation has a strong effect on PEµ under fluctuating light
To assess some underlying mechanisms that may affect PEµ, chlorophyll fluorescence measurements were taken to assess levels of stress and photo-inhibition (Fv/Fm), the operating efficiency of PSII (ΦPSII) and non-photochemical quenching (NPQ). The data was fitted to the quadratic model (Equation 2) to compare the magnitude of effect of the three light factors. Additionally, changes in the ratio of OD680/OD750 were used as a high-throughput proxy to determine photoacclimation via changes in chlorophyll content.
A high goodness of fit to the quadratic model was observed in Chlamydomonas for ΦPSII (R2 = 0.89) and Fv/Fm (R2 = 0.74) and, in Chlorella, for Fv/Fm (R2 = 0.91), suggesting that PSII regulation is highly affected by the three light factors examined in this study and is a contributing factor to the observed PEµ. Remarkably, all treatments for both species showed low NPQ (<0.3) relative to average values reported in literature (up to ~2 for Chlamydomonas and ~1.5 for Chlorella)15,46–48 and a poor goodness of fit to the quadratic model for both strains (see Supplementary Table S2). Other stressors, such as nutrient limitation, are also known to increase NPQ49. Since both strains were cultivated on previously optimised nutrients this may have contributed to the overall reduced NPQ in this study.
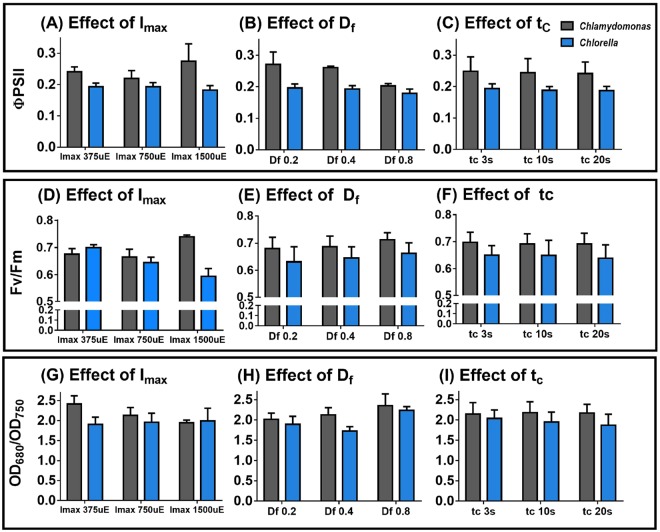
For Chlamydomonas, a significant (p-value = 1.79E-17) reduction in ΦPSII occurred at high Df (Table 2, Fig. 2E). This suggests that efficient electron transfer is compromised under high dark fractions for this alga and links ΦPSII to the reduced PEµ trends under high Df observed. Furthermore, increased OD680/750 measurement (a proxy for chlorophyll content per cell) was prominent with increasing Df (Fig. 4H), suggesting high dark fractions lead to increased cellular chlorophyll levels typical for low-light acclimation, which may further explain the lower efficiency of light utilisation (i.e. PE) at high Df (Fig. 2E). Remarkably, a high Imax actually improved both ΦPSII (Fig. 4A) and Fv/Fm (Fig. 4D) and lowered OD680/750 (Fig. 4G), despite a reduction in PEµ (Fig. 2D). This suggests that while photosynthetic rates improved in Chlamydomonas under high light, the over-saturating irradiance could not be fully utilised by the Calvin-Benson cycle, suggesting other downstream mechanisms such as alternative electron sinks50 could become relevant under high light.
Figure 4.
Trends in underlying photosynthetic mechanisms. Plots depict averaged effects of Imax, Df and tc on ΦPSII (A,B and C) (n = 2); Fv/Fm (D,E and F) and OD680/OD750 (G,H and I) respectively for Chlamydomonas (grey bars) and Chlorella (blue bars) (n = 3, Error bars represent standard deviation).
For Chlorella, the most significant factor corresponding directly to PEµ was the effect of Imax on Fv/Fm, which gave a large negative coefficient in the model (Table 2) and showed a noticeable decline in Fv/Fm with increasing Imax (Fig. 4D). Like Chlamydomonas, increasing Df was found to have a positive effect on Fv/Fm (Fig. 4E), also seen by the relative magnitudes of coefficients and their significance (p-value = 3.09E-07), and a significant positive interaction between Df − Imax (p-value = 5.19E-03). Similar to Chlamydomonas, Chlorella exhibited an up-regulation of OD680/750 (indicative of higher chlorophyll) at high Df (Fig. 4H, Supplementary Table S2).
In summary, these results suggest that Chlorella is sensitive to high light as seen by PSII inactivation but less sensitive to light/dark fluctuations. In contrast, Chlamydomonas is sensitive to strong light/dark fluctuations due to disrupted electron transport flows but seems to have better acclimisation strategies to cope with high light. These results suggest that maintaining Chlamydomonas at relatively dilute cultures is beneficial, whereas operating Chlorella at high densities is preferable, especially under high light.
Optimisation predicts a two-fold higher maximum PEµ for Chlorella compared to Chlamydomonas
It is evident from the 3D surface plots (Fig. 3) showing PEµ response that the maxima occur at the extremes in most instances. The maximum PEµ values (at the mid-point, i.e. level 0) and their corresponding factor levels were used to obtain the maximum PEµ and optimum conditions. For both Chlamydomonas and Chlorella, the maximum PEµ values occurred at the minimum Imax (375 µE) and the minimum value of tc (Table 3). Using this combination of Imax and tc, the optimal Df values were found to be 0.24 and 0.8 for Chlamydomonas and Chlorella respectively. These combination of factor values results in a theoretical maximum PEµ of 0.126 and 0.226 mol photon−1 m2 (Table 3), predicting a nearly 2-fold higher maximum PEµ for Chlorella than Chlamydomonas. As discussed in the section 3.3.1, the three light factors modelled only explains two thirds of the variation in PEµ for Chlamydomonas and these results are indicative only for this species.
Table 3.
Optimisation of PEµ and the respective factor levels around the mid-point of each factor, and around the optimised point for total predicted maximum PEµ within the ranges of the full factorial design.
| Species | Condition | Predicted max PEµ | Df | Imax | tc | |||
|---|---|---|---|---|---|---|---|---|
| (mol photon−1m2) | Coded | (−) | Coded | (µmol m−2 s−1) | Coded | (s) | ||
| Chlamydomonas | tc midpoint | 0.116 | −0.75 | 0.24 | −1 | 375 | 0 | 10 |
| Imax midpoint | 0.079 | −0.4 | 0.30 | 0 | 750 | −1 | 5 | |
| Df midpoint | 0.113 | 0 | 0.40 | −1 | 375 | −1 | 5 | |
| Optima | 0.126 | −0.73 | 0.24 | −1 | 375 | −1 | 5 | |
| Chlorella | tc midpoint | 0.194 | 1 | 0.80 | −1 | 375 | 0 | 10 |
| Imax midpoint | 0.117 | 1 | 0.80 | 0 | 750 | −1 | 5 | |
| Df midpoint | 0.178 | 0 | 0.40 | −1 | 375 | −1 | 5 | |
| Optima | 0.226 | 1 | 0.80 | −1 | 375 | −1 | 5 | |
Concluding Remarks
The HTS coupled with response surface methodology delivers a working statistical design for simultaneous light optimisation of several species of microalgae. This platform has been used to screen growth responses to nutrients and organic carbon sources20,23, and can be extended to screen other parameters such as CO2 or growth contaminants (e.g. herbicides, antibiotics, bacteria or predating organisms), and could monitor other response variables such as lipid accumulation (e.g. Nile Red) and protein expression using fluorescence tags. Some limitations imposed by the microwell HTS can include high variation between replicates when trialled at conditions that give very low growth rates; and some evaporation losses that limit the duration of the experiment due to the low culture volume. Radzun, K. A. et al. have reported that despite some evaporative losses observed in the TECAN robotic system, the RSD values were considerably lower than can be achieved through manual measurement.
As the OD measurements in the plate reader are made vertically rather than horizontally, the reduction of depth due to evaporation is compensated for by the concomitant increase in cell concentration to maintain the same optical pathlength23. Furthermore, variation can be reduced by adding additional technical replicates (as done in this study), while evaporation can be addressed by using a humidifier in the enclosed chamber system (currently being developed) and/or reducing the frequency of measurement readings which requires lid removal. Despite this, the HTS provides a cost-effective, rapid and efficient platform to obtain large data-sets for a wide array of solar driven microalgae applications, which would otherwise require significant investment of time, money and resources.
In most mass cultures, particularly those of outdoor raceway ponds, severe light limitation exists, typically where light penetrates only the first millimetres or centimetres at most and high dark fractions of 90% or greater are normal24,30. These dark fractions and cycling between light/dark zones can be detrimental for redox imbalances, as was shown to be the case for Chlamydomonas. Therefore, species such as the strain of Chlorella tested here, have a selective advantage for mass culture, as productivity was found to be unaffected by light fluctuations. Furthermore, it opens up new insights for the design of high efficiency cell lines, capable of handling both high light intensities and strong light/dark fluctuations. Improving light distribution deeper within the culture depth with minimal transmittance losses (e.g. by increasing surface to volume ratios or using specially designed light guides51) may be another strategy to improve PEµ, rather than adjusting cycle time (by increasing mixing rates, gas sparging) particularly as the latter would require higher energy inputs with minimal gains in PEµ. Another important deduction of strain-specific characterisation for scale up was the detrimental effect of cycle time on PEµ for Chlamydomonas (~−46%) versus a similar effect for Chlorella as compared to constant light. This signifies the application of our HTS outcomes toward strain selection as well as growth platform selection (i.e. open pond (slow mixing) versus tubular PBRs (faster mixing) or other designs) when going from laboratory (constant light) to outdoor systems (fluctuating light). In both alga, as is typical of other species, high incident light has the most detrimental effect on PEµ. Therefore, efforts to diffuse light sources, such as done through the use of reflectors, or to use vertical flat panels or vertically stacked tubular photobioreactors to avoid direct sunlight at high light periods, may benefit from the ‘light dilution effect’.
Previous transcriptomic and proteomic studies in Chlamydomonas have shown that acclimation to environmental stimuli is achieved by remodelling photosystem I and II antenna complexes, further highlighting the flexibility of their photosynthetic machinery52. While Chlamydomonas may possess the survival strategies required to acclimate to changing light conditions, typically for soil environments, they may not be tuned for high biomass productivity, unlike fast-growing strains like the Chlorella strain used in this study, which despite seemingly lacking the level of regulatory sophistication, might be better suited for mass cultivation.
In conclusion, the HTS method developed here enables a rapid approach to optimise systems design, scale up operational conditions and species selection to advance feasible solar-driven biotechnologies.
Materials and Methods
Strains and pre-culture conditions
Liquid pre-cultures were prepared in triplicate (40 mL culture in 100 ml flasks) and inoculated with either C. reinhardtii WT strain CC12553 or Chlorella sp. 11_H519 (Australian isolate) maintained on TAP54 agar (1.5%) plates. To ensure nutrients were non-limiting, photoautotrophic medium previously optimised for each species was used for C. reinhardtii (PCM55, N source NH4+) and Chlorella sp (OpM220, N source urea). Flasks were maintained on shakers (200 rpm) in an enclosed incubation system at 23 °C, 1% CO2 and a 16/8 hour light/dark cycle, illuminated with 100 µmol m−2 s−1 of overhead white fluorescent light for 5 days.
To ensure that the cultures were well synchronised to the light conditions being tested, flask pre-cultures first acclimated to a 16/8 h light/dark cycle were inoculated into microwell plates (150 µL), and gradually acclimated to the light intensity close to the mean Iavg before the first measurement. For the higher light intensity experiments (Imax = 1500 µmol m−2 s−1), care was taken not to shock the low density cultures by subjecting them to a step-wise gradually increasing light regime (a detailed summary of the acclimation regimes is provided in Supplementary Table S3).
Automated HTS and lighting design
The design, structure and operation of the HTS system (Tecan Freedom Evo 150, Tecan Group Ltd., Männedorf, Switzerland) is as previously described20,23. Briefly, the HTS system is an enclosed chamber fitted with three orbital shakers which hold six microwell plates each, a robotic manipulator arm that removes the plate lid and carries the plates to a reader (Infinite M200 PRO, Tecan Group Ltd., Männedorf, Switzerland, Fig. 1C) and atmospheric CO2 control. Each of the 18 microwell plate positions is fitted with 96 ‘warm white’ LEDs positioned directly under each well of a 96-well plate. Each of the LED arrays is controlled by user defined scripts on an Adruino® integrated circuit controller and software, permitting 18 different light conditions to be tested in parallel. LEDs were fitted with a low pass LC filter to smooth the intensity signal from pulse width modulation to variable voltage, thereby eliminating ‘flashing light’ phenomena due to on/off signals. The spectrum of wavelengths of LEDs is compared against that of natural sunlight (see Supplementary Fig. S6). For simplicity, a sinusoidal mixing regime was assumed to allow tight control of the factors of Imax, Df and tc, as has been used in previous studies56,57. Pre-cultures were centrifuged (500 g, 20 min, 18 °C) and the pellet re-suspended in fresh medium. To minimise cell shading effects and ensure tight light control, a volume of 150 µl was chosen for a short pathlength of 5 mm and a semi-continuous cultivation regime was applied by daily culture dilutions back to a starting OD750 of 0.1. Each of the three biological replicates per species was inoculated into each well of a 96-well plate. Since only two strains were tested in this study, all wells were inoculated, providing 14 technical replicates per biological replicate. Of these, 10 wells were used for automated OD750 and OD680 readings, the remaining wells (of two biological replicates) were extracted on day 2 for manual PSII measurements. The final row of 12 wells contained 150 µl pure media to use as blank controls.
Growth rate and photosynthetic efficiency (PEµ) measurements
Growth rates were calculated from 3-hourly OD750 measurements. High-throughput automated measurements of OD750 were used as a proxy for growth from which growth rates, µ (h−1), were calculated as the rate of change of OD750,
| 3 |
where, t1 and t2 are the time points at which OD750(t1) and OD750(t2) were measured.
A 3-hour measuring frequency during the light period was used for the growth curve calculations. This frequency was chosen to limit evaporation and contamination issues. A detailed description of the growth curves, sampling points and lighting schedule can be found as Supplementary Figs S1 and S2.
The main response variable, PEµ, was assumed to be indicative of light utilisation efficiency of the microalgae, where the growth rate normalised to the average integrated PAR received,
| 4 |
And the Iavg is,
| 5 |
In Equation 5, tc is the cycle time, I(t) is the irradiance (µmol photons m−2 s−1) at a given time of tc, and 3.6*10−9 is the conversion factor from µmol photons m−2 s−1 to mol photons m−2 h−1.
Chlorophyll fluorescence of photosystem II measurements
Photosystem II (PSII) kinetics were measured as a function of PSII chlorophyll fluorescence10,58,59. Biological duplicates of each sample (dilution factor of 5) was added to a Fluorimeter cuvette (Sigma), dark adapted for 20 minutes and processed using the FluoroWin software (Photon Systems Instruments, Czech Republic). The quenching analysis protocol had the following settings: measuring light: 20% V; saturating pulse: 0.9 s, 80% V; actinic light: 51 s, 18.3 V (~800 µmol m−2 s−1). Weak infrared pulses (730 nm) were applied for 5 s prior to measurement to quench QA. The PSII parameters calculated from the quenching analysis were: Fv/Fm (maximum quantum efficiency of PSII), ΦPSII (PSII operating efficiency), and NPQ (Non photochemical Quenching) using respectively,
| 6 |
| 7 |
| 8 |
Photoacclimation via OD680/750
Chlorophyll a has a maximum absorbance at 680 nm. Therefore, OD680 measurements were normalised to OD750 (OD680/750) as a proxy of changes in chlorophyll absorption between different light regimes.
Statistical Analysis
All data are expressed as Mean ± SD of three biological replicates (for automated readings) and two biological replicates (for the manual PSII measurements), each with multiple technical replicates as mentioned in section 5.2. MATLAB was used for the design and analysis of the response surface methodology. A p-value <0.05 was used for determining significant effects. Both contour and surface plots were developed for visualisation of the data and to predict the relationship and interaction effects on the light utilisation efficiency. Regression coefficient (R2) was used to resolve the goodness of fit. The fitted model using the regression coefficients was validated with an additional experimental dataset.
Electronic supplementary material
Supplementary information for Manuscript
Acknowledgements
We would like to recognise the work of John Srnka who collaborated on the design of the TECAN’s LED lighting system and conducted all related programming and electrical work; Dr Nick Hamilton and James Lefevre for their input on the statistical analysis reported in the manuscript. We also gratefully acknowledge the support of the Australian Research Council (Linkage grant LP150101147), University of Queensland International Scholarship (UQI) and the Science and Industry Endowment Fund (John Stocker Postdoctoral Fellowship PF16-087).
Author Contributions
Jennifer Yarnold, Juliane Wolf and Ben Hankamer conceived and designed the experiments. Shwetha Sivakaminathan and Jennifer Yarnold performed the experiments. Shwetha Sivakaminathan, Jennifer Yarnold and Juliane Wolf analysed the data. Shwetha Sivakaminathan, Jennifer Yarnold and Juliane Wolf drafted the article. Ben Hankamer revised it critically and provided the funds and infrastructure for conducting the research at the Institute for Molecular Bioscience.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Juliane Wolf, Email: j.wolf@imb.uq.edu.au.
Jennifer Yarnold, Email: j.yarnold@imb.uq.edu.au.
References
- 1.Singh S, Kate BN, Banerjee UC. Bioactive Compounds from Cyanobacteria and Microalgae: An Overview. Critical Reviews in Biotechnology. 2005;25:73–95. doi: 10.1080/07388550500248498. [DOI] [PubMed] [Google Scholar]
- 2.Borowitzka, M. High-value products from microalgae—Their development and commercialisation. Vol. 25 (2013).
- 3.Carrera Pacheco SE, Hankamer B, Oey M. Optimising light conditions increases recombinant protein production in Chlamydomonas reinhardtii chloroplasts. Algal Research. 2018;32:329–340. doi: 10.1016/j.algal.2018.04.011. [DOI] [Google Scholar]
- 4.Koutra E, Economou CN, Tsafrakidou P, Kornaros M. Bio-Based Products from Microalgae Cultivated in Digestates. Trends in Biotechnology. 2018 doi: 10.1016/j.tibtech.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Chew KW, et al. Microalgae biorefinery: High value products perspectives. Bioresource Technology. 2017;229:53–62. doi: 10.1016/j.biortech.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Béchet, Q., Plouviez, M., Chambonnière, P. & Guieysse, B. In Microalgae-Based Biofuels and Bioproducts (ed. Raúl Muñoz) 505–525 (Woodhead Publishing, 2017).
- 7.Stephens E, et al. An economic and technical evaluation of microalgal biofuels. Nat Biotech. 2010;28:126–128. doi: 10.1038/nbt0210-126. [DOI] [PubMed] [Google Scholar]
- 8.Ringsmuth AK, Landsberg MJ, Hankamer B. Can photosynthesis enable a global transition from fossil fuels to solar fuels, to mitigate climate change and fuel-supply limitations? Renewable and Sustainable Energy Reviews. 2016;62:134–163. doi: 10.1016/j.rser.2016.04.016. [DOI] [Google Scholar]
- 9.Mussgnug JH, et al. Engineering photosynthetic light capture: impacts on improved solar energy to biomass conversion. Plant Biotechnology Journal. 2007;5:802–814. doi: 10.1111/j.1467-7652.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- 10.Yarnold J, Ross IL, Hankamer B. Photoacclimation and productivity of Chlamydomonas reinhardtii grown in fluctuating light regimes which simulate outdoor algal culture conditions. Algal Research. 2016;13:182–194. doi: 10.1016/j.algal.2015.11.001. [DOI] [Google Scholar]
- 11.Barbosa MJ, Hoogakker J, Wijffels RH. Optimisation of cultivation parameters in photobioreactors for microalgae cultivation using the A-stat technique. Biomolecular Engineering. 2003;20:115–123. doi: 10.1016/S1389-0344(03)00033-9. [DOI] [PubMed] [Google Scholar]
- 12.Takache H, Pruvost J, Marec H. Investigation of light/dark cycles effects on the photosynthetic growth of Chlamydomonas reinhardtii in conditions representative of photobioreactor cultivation. Algal Research. 2015;8:192–204. doi: 10.1016/j.algal.2015.02.009. [DOI] [Google Scholar]
- 13.Senge M, Senger H. Response of the Photosynthetic Apparatus during Adaptation of Chlorella and Ankistrodesmus to Irradiance Changes. Journal of plant physiology. 1990;136:675–679. doi: 10.1016/S0176-1617(11)81343-X. [DOI] [Google Scholar]
- 14.Hosni T, Gwendoline C, Jean‐François C, Jérémy P. Experimental and theoretical assessment of maximum productivities for the microalgae Chlamydomonas reinhardtii in two different geometries of photobioreactors. Biotechnology progress. 2010;26:431–440. doi: 10.1002/btpr.356. [DOI] [PubMed] [Google Scholar]
- 15.Peers G, et al. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature. 2009;462:518–521. doi: 10.1038/nature08587. [DOI] [PubMed] [Google Scholar]
- 16.Merchant SS, et al. The Chlamydomonas Genome Reveals the Evolution of Key Animal and Plant Functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayfield SP, et al. Chlamydomonas reinhardtii chloroplasts as protein factories. Current Opinion in Biotechnology. 2007;18:126–133. doi: 10.1016/j.copbio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Oey M, Ross IL, Hankamer B. Gateway-Assisted Vector Construction to Facilitate Expression of Foreign Proteins in the Chloroplast of Single Celled Algae. PLOS ONE. 2014;9:e86841. doi: 10.1371/journal.pone.0086841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf J, et al. Multifactorial comparison of photobioreactor geometries in parallel microalgae cultivations. Algal Research. 2016;15:187–201. doi: 10.1016/j.algal.2016.02.018. [DOI] [Google Scholar]
- 20.Wolf J, et al. High-throughput screen for high performance microalgae strain selection and integrated media design. Algal Research. 2015;11:313–325. doi: 10.1016/j.algal.2015.07.005. [DOI] [Google Scholar]
- 21.Harris, E. H. The Chlamydomonas Sourcebook: Introduction to Chlamydomonas and Its Laboratory Use. (Elsevier Science, 2009).
- 22.Moejes FW, et al. A systems-wide understanding of photosynthetic acclimation in algae and higher plants. Journal of Experimental Botany. 2017;68:2667–2681. doi: 10.1093/jxb/erx137. [DOI] [PubMed] [Google Scholar]
- 23.Radzun KA, et al. Automated nutrient screening system enables high-throughput optimisation of microalgae production conditions. Biotechnology for Biofuels. 2015;8:65. doi: 10.1186/s13068-015-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richmond, A. In Handbook of Microalgal Culture 169–204 (John Wiley & Sons, Ltd, 2013).
- 25.Larkum AWD. Limitations and prospects of natural photosynthesis for bioenergy production. Current Opinion in Biotechnology. 2010;21:271–276. doi: 10.1016/j.copbio.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Masojídek, J., Sergejevová, M., Malapascua, J. R. & Kopecký, J. In Algal Biorefineries: Volume 2: Products and Refinery Design (eds Aleš Prokop, Rakesh K. Bajpai, & Mark E. Zappi) 237–261 (Springer International Publishing, 2015).
- 27.Janssen M, et al. Scale-up aspects of photobioreactors: effects of mixing-induced light/dark cycles. Journal of Applied Phycology. 2000;12:225–237. doi: 10.1023/A:1008151526680. [DOI] [Google Scholar]
- 28.Janssen M, Slenders P, Tramper J, Mur LR, Wijffels R. Photosynthetic efficiency of Dunaliella tertiolecta under short light/dark cycles. Enzyme Microb Tech. 2001;29:298–305. doi: 10.1016/S0141-0229(01)00387-8. [DOI] [Google Scholar]
- 29.Janssen M, et al. Efficiency of light utilization of Chlamydomonas reinhardtii under medium-duration light/dark cycles. Journal of Biotechnology. 2000;78:123–137. doi: 10.1016/S0168-1656(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 30.Yarnold, J. Photosynthesis of microalgae in outdoor mass cultures and modelling its effects on biomass productivity for fuels, feeds and chemicals PhD thesis, The University of Queensland (2016).
- 31.Bureau of Meteorology, www.bom.gov.au (2016).
- 32.Janssen M, et al. Specific growth rate of Chlamydomonas reinhardtii and Chlorella sorokiniana under medium duration light/dark cycles: 13–87 s. Journal of biotechnology. 1999;70:323–333. doi: 10.1016/S0168-1656(99)00084-X. [DOI] [Google Scholar]
- 33.Külheim C, Ågren J, Jansson S. Rapid regulation of light harvesting and plant fitness in the field. Science. 2002;297:91–93. doi: 10.1126/science.1072359. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser E, Morales A, Harbinson J. Fluctuating Light Takes Crop Photosynthesis on a Rollercoaster Ride. Plant Physiology. 2018;176:977–989. doi: 10.1104/pp.17.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonente G, Pippa S, Castellano S, Bassi R, Ballottari M. Acclimation of Chlamydomonas reinhardtii to different growth irradiances. Journal of Biological Chemistry. 2012;287:5833–5847. doi: 10.1074/jbc.M111.304279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacIntyre HL, Kana TM, Anning T, Geider RJ. Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria1. J Phycol. 2002;38:17–38. doi: 10.1046/j.1529-8817.2002.00094.x. [DOI] [Google Scholar]
- 37.de Winter L, Cabanelas ITD, Martens DE, Wijffels RH, Barbosa MJ. The influence of day/night cycles on biomass yield and composition of Neochloris oleoabundans. Biotechnology for Biofuels. 2017;10:104. doi: 10.1186/s13068-017-0762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vejrazka C, Janssen M, Benvenuti G, Streefland M, Wijffels RH. Photosynthetic efficiency and oxygen evolution of Chlamydomonas reinhardtii under continuous and flashing light. Applied Microbiology and Biotechnology. 2013;97:1523–1532. doi: 10.1007/s00253-012-4390-8. [DOI] [PubMed] [Google Scholar]
- 39.Sforza E, Simionato D, Giacometti GM, Bertucco A. & Morosinotto, T. Adjusted Light and Dark Cycles Can Optimize Photosynthetic Efficiency in Algae Growing in Photobioreactors. Plos One. 2012;7:e38975. doi: 10.1371/journal.pone.0038975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Box GEP, Behnken DW. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics. 1960;2:455–475. doi: 10.1080/00401706.1960.10489912. [DOI] [Google Scholar]
- 41.Belhaj D, et al. Box-Behnken design for extraction optimization of crude polysaccharides from Tunisian Phormidium versicolor cyanobacteria (NCC 466): Partial characterization, in vitro antioxidant and antimicrobial activities. International Journal of Biological Macromolecules. 2017;105:1501–1510. doi: 10.1016/j.ijbiomac.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy M, Krouse D. Strategies for improving fermentation medium performance: a review. Journal of Industrial Microbiology and Biotechnology. 1999;23:456–475. doi: 10.1038/sj.jim.2900755. [DOI] [Google Scholar]
- 43.Wang B, Lan CQ. Optimising the lipid production of the green alga Neochloris oleoabundans using box–behnken experimental design. The Canadian Journal of Chemical Engineering. 2011;89:932–939. doi: 10.1002/cjce.20513. [DOI] [Google Scholar]
- 44.Zhao L-C, et al. Response Surface Modeling and Optimization of Accelerated Solvent Extraction of Four Lignans from Fructus Schisandrae. Molecules. 2012;17:3618. doi: 10.3390/molecules17043618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasiri S, Abdulsalam S, Ulrich A, Prasad V. Optimization of CO2 fixation by Chlorella kessleri using response surface methodology. Chemical Engineering Science. 2015;127:31–39. doi: 10.1016/j.ces.2015.01.008. [DOI] [Google Scholar]
- 46.Garcia-Mendoza E, Matthijs HCP, Schubert H, Mur LR. Non-photochemical quenching of chlorophyll fluorescence in Chlorella fusca acclimated to constant and dynamic light conditions. Photosynthesis Research. 2002;74:303. doi: 10.1023/A:1021230601077. [DOI] [PubMed] [Google Scholar]
- 47.Finazzi, G. & Minagawa, J. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria 445–469 (Springer, 2014).
- 48.Masojídek J, et al. Photoadaptation of two members of the Chlorophyta (Scenedesmus and Chlorella) in laboratory and outdoor cultures: changes in chlorophyll fluorescence quenching and the xanthophyll cycle. Planta. 1999;209:126–135. doi: 10.1007/s004250050614. [DOI] [PubMed] [Google Scholar]
- 49.Petroutsos D, et al. The Chloroplast Calcium Sensor CAS Is Required for Photoacclimation in Chlamydomonas reinhardtii. The Plant Cell. 2011;23:2950–2963. doi: 10.1105/tpc.111.087973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Depège N, Bellafiore S, Rochaix J-D. Role of Chloroplast Protein Kinase Stt7 in LHCII Phosphorylation and State Transition in <em> Chlamydomonas </em>. Science. 2003;299:1572–1575. doi: 10.1126/science.1081397. [DOI] [PubMed] [Google Scholar]
- 51.Kommareddy, A. & Gary Anderson, D. Study of Light as a parameter in the growth of algae in a Photo-Bio Reactor (PBR) (ASAE, St. Joseph, MI, 2003).
- 52.Eberhard S, Finazzi G, Wollman F-A. The Dynamics of Photosynthesis. Annual Review of Genetics. 2008;42:463–515. doi: 10.1146/annurev.genet.42.110807.091452. [DOI] [PubMed] [Google Scholar]
- 53. Chlamydomonas Resource Centre, https://www.chlamycollection.org (2016).
- 54.Gorman DS, Levine R. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proceedings of the National Academy of Sciences. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oey M, et al. RNAi knock-down of LHCBM1, 2 and 3 increases photosynthetic H 2 production efficiency of the green alga Chlamydomonas reinhardtii. PLoS One. 2013;8:e61375. doi: 10.1371/journal.pone.0061375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flameling IA, Kromkamp J. Photoacclimation of Scenedesmus protuberans (Chlorophyceae) to fluctuating irradiances simulating vertical mixing. Journal of Plankton Research. 1997;19:1011–1024. doi: 10.1093/plankt/19.8.1011. [DOI] [Google Scholar]
- 57.Ibelings BW, Mur LR. Acclimation of Photosystem II in a Cyanobacterium and a Eukaryotic Green Alga to High and Fluctuating Photosynthetic Photon Flux Densities, Simulating Light Regimes Induced by Mixing in Lakes. New Phytologist. 1994;128:407–424. doi: 10.1111/j.1469-8137.1994.tb02987.x. [DOI] [PubMed] [Google Scholar]
- 58.Murchie EH, Lawson T. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. Journal of Experimental Botany. 2013;64:3983–3998. doi: 10.1093/jxb/ert208. [DOI] [PubMed] [Google Scholar]
- 59.Baker NR. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annual Review of Plant Biology. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information for Manuscript