Abstract
The physiological function of the pancreas is controlled by the circadian clock. The aim of this study was to determine whether aging-induced changes in glucose homeostasis affect properties of the circadian clock in the pancreas and/or its sensitivity to disturbances in environmental lighting conditions. mPer2Luc mice aged 24–26 months developed hyperinsulinemic hypoglycaemia, which was likely due to the Pclo-mediated insulin hyper-secretion and Slc2a2-mediated glucose transport impairment in the pancreas, and due to the alterations in Pp1r3c-related glycogen storage and Sgk1-related glucose transport in the liver. In the pancreatic tissue, aging affected clock gene expression only marginally, it upregulated Bmal1 and downregulated Clock expression. Whereas aging significantly impaired the circadian clock in lung explants, which were used as a control tissue, the properties of the pancreatic clock in vitro were not affected. The data suggest a non-circadian role of Bmal1 in changes of pancreatic function that occur during aging. Additionally, the pancreatic clock was more sensitive to exposure of animals to constant light conditions. These findings provide an explanation for the previously demonstrated relationship between disturbances in the circadian system and disordered glucose homeostasis, including diabetes mellitus type 2, in subjects exposed to long-term shift work.
Introduction
The pancreas, a part of the gastrointestinal tract essential for glucose homeostasis, changes during the lifespan and aging affects the morphology of its exocrine as well as endocrine parts. However, the age-dependent changes in morphology of the exocrine pancreas were not associated with changes in its secretory capacity1,2. The endocrine pancreas also undergoes morphological changes with aging3–5, however, the reported effects of aging on insulin levels in blood have not been consistent (reviewed in6). Nevertheless, in all studied models (humans, rats and mice) aging is associated with impaired glucose tolerance, insulin resistance and altered pancreatic secretory capacity. Whether and how these changes might be caused by aging per se, or by other factors, such as body composition, diet or level of physical activity, is still not known7.
Pancreatic function is under control of the endogenous circadian system, and the circadian clocks residing in the pancreas are involved in optimizing pancreatic function in accordance with changing demands during the day8,9. The pancreas harbours the circadian clocks in its exocrine10 as well as endocrine11 parts; the latter has been extensively studied mainly because of its role in regulation of insulin release from β-cells forming the islets of Langerhans12. The circadian system is organized in the body hierarchically such that peripheral clocks in various tissues, including the pancreas, are synchronized by the central clock in the suprachiasmatic nuclei (SCN) of the hypothalamus13. The SCN receives information about the external light/dark (LD) cycle via a connection with the retina and sends multiple signals to the periphery (reviewed in14) adjusting the autonomous rhythmicity of the pancreatic clock to rhythmicity of external environment. The generation of the rhythmic signal is based on the core molecular clock mechanism formed by transcriptional-translational feedback loops that drive circadian expression of clock genes, namely, Per1/Per2, Cry1/Cry2, Clock/Npas2, Bmal1/Bmal2, Nr1d1/Nr1d2 (Rev-erbα/Rev-erbβ) (for review see15). Most components of the core clock mechanism, also demonstrated in the pancreas9, are rhythmically expressed and serve thereby as downstream transcription factors rhythmically switching on and off expression of a great array of tissue-specific, clock-controlled genes. The functional significance of the clock in the pancreas was confirmed in mice with clock gene deletions (Bmal1−/−) or mutation (ClockΔ19/Δ19) that also led to a reduction in the percentage of large islets of Langerhans11,16. Additionally, mice with disruption of Bmal1 selectively in the β-cells exhibited altered glucose tolerance in vivo and in vitro11. Moreover, frequent disruption of the circadian system, e.g., due to night shifts, has been associated with development of diabetes mellitus type II (T2DM) in humans17,18.
The impact of aging on the circadian clocks has not been resolved yet. For the central SCN clock, the accumulated data favour the hypothesis that the core oscillatory mechanism is not impaired19 but the aged SCN loses the ability to drive the output rhythms at the level of the SCN neuronal activity20 or at the systemic behavioural level19,21,22. It is not known whether the weakened signals from the SCN to the periphery in aging may impair the internal synchrony among peripheral clocks within the body, although such a mechanism seems plausible from recent evidence for attenuated sympathetic tonus in aged animals23. Studies on aging of the peripheral clocks also have not been conclusive. In a study using transgenic Per1-luc rats, a tissue-specific effect of aging was reported in vitro24. In transgenic mPer2Luc mice, aging changed the dynamics of re-entrainment of bioluminescence rhythms to a shift in LD cycle also in a tissue-dependent manner25. Recently, a study on the effect of aging on the circadian transcriptome revealed changes in cyclic protein acetylation in the liver26. Because none of the previous studies addressed aging of the clock in the pancreas, we used transgenic mPer2Luc mice with age-induced impairment in glucose homeostasis to reveal the effect of aging on properties of the circadian clocks in the pancreas. We used lungs as a control tissue not involved in glucose homeostasis. Moreover, we tested the effect of aging on these clocks in conditions where, in addition to aging, the output of the SCN was disrupted via exposure of mice to a disturbance in the environmental LD cycle. These experiments aimed to answer the questions whether the peripheral clock in the pancreas changed with age and whether the rhythmic environment may contribute to the functioning of the pancreatic circadian clock in the elderly.
Results
Aging affects pancreatic function and glucose homeostasis mechanisms in vivo
In aged mPer2Luc mice, basal plasma glucose levels (Fig. 1A) were lower (P = 0.0117) and insulin levels were higher (P = 0.0033) than in adults. Additionally, the IPGTT revealed that glucose levels 30 min after glucose administration, as well as the incremental area under the curve (AUC), were significantly higher in aged animals (P < 0.0001 and P = 0.0045, respectively). In the pancreatic tissue, aging had a significant effect on gene expression (Fig. 1B). It upregulated piccolo (Pclo) mRNA levels (P < 0.0001) throughout the 24 h cycle. Expression of pancreatic and duodenal homeobox 1 (Pdx1) gene was also upregulated significantly by aging (P = 0.0022), although post hoc analysis did not find significant differences at individual time points. In contrast, expression profile of solute carrier family 2 member 2 (Slc2a2) gene for glucose transporter GLUT2 was downregulated by aging (P = 0.0003), although post hoc analysis did not detect significant differences at individual time points. Aging did not significantly affect maximal expression levels of transcription factor E4bp4 (P = 0.1255) and D-box binding protein (Dbp) (P = 0.1111) in the pancreas.
Figure 1.
Glucose homeostasis and pancreatic gene expression in adult (black triangles) and aged (green circles) animals maintained under standard light/dark conditions. (A) Basal plasma glucose and insulin levels for each group are depicted as individual levels as well as the mean ± SD. Basal insulin levels are expressed relative to body weight (BW). Glucose tolerance test (IPGTT) was performed by measuring plasma glucose levels 15, 30, 60, 120 and 180 min after intraperitoneal injection of glucose (levels normalized to basal glucose levels), and values of the incremental area under the curve (AUC) were calculated. (B) Daily profiles in relative expression of Pclo, Pdx1 and Slc2a2 were detected by RT qPCR in samples of pancreas collected from adult (black triangles and dashed line) and aged (green circles and full line) animals every 4 h during the 24 h profile. Animals were sacrificed in darkness; circadian time 0 corresponds to lights on of the previous LD12:12 regime. At each time point, 5 (occasionally 4) animals were sacrificed and data are expressed as the mean ± SD. The results of a post hoc analysis from a 2-way ANOVA comparison between expression levels in adult and aged animals are depicted. (C) The expression of E4bp4 and Dbp in the pancreas of adult (black) and aged (green) animals was detected at circadian times 0 and 12, respectively, i.e., at the time of their maximal levels; for each group, data are depicted as individual levels as well as the mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
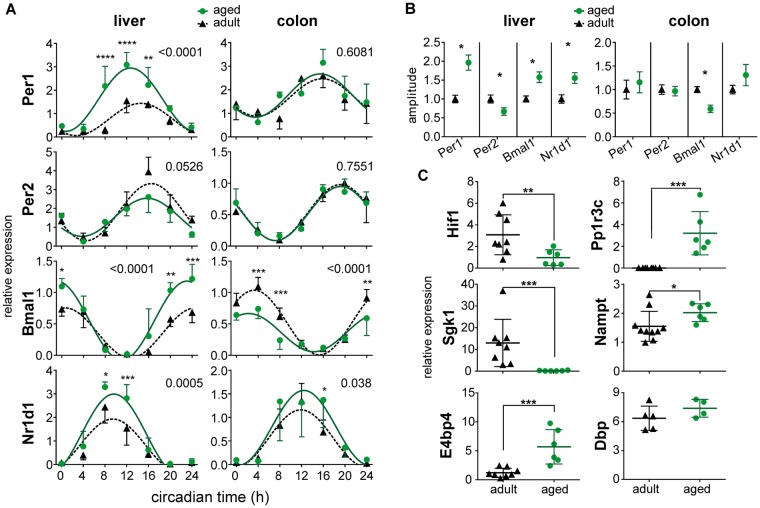
Aging affects amplitudes of Bmal1 and Clock rhythms in a gene-specific and peripheral tissue-specific manners in vivo
The daily expression profiles of the clock genes Per1, Per2, Bmal1, Nr1d1 and Clock were compared between adult and aged animals (Fig. 2A). In the pancreatic tissue, aging had a significant and selective effect on the amplitude of the Bmal1 expression profile (Fig. 2A,B), whereas it had no effect in the lungs (Fig. 2A,B). Interestingly, the amplitude of the Bmal1 expression profile was also significantly elevated in the liver of the aged animals (Fig. 3A), i.e., in another glucose metabolism-relevant tissue, but not in the colon, where the amplitude was rather decreased in aged animals. In contrast, the Clock expression was significantly downregulated in both pancreas (P < 0.0001) and lungs (P = 0.0001) of aged animals (Fig. 2A,B). However, the number of BMAL1-immunopositive cells within the islets of Langerhans did not differ between adult and aged mice (Suppl. Figure 2).
Figure 2.
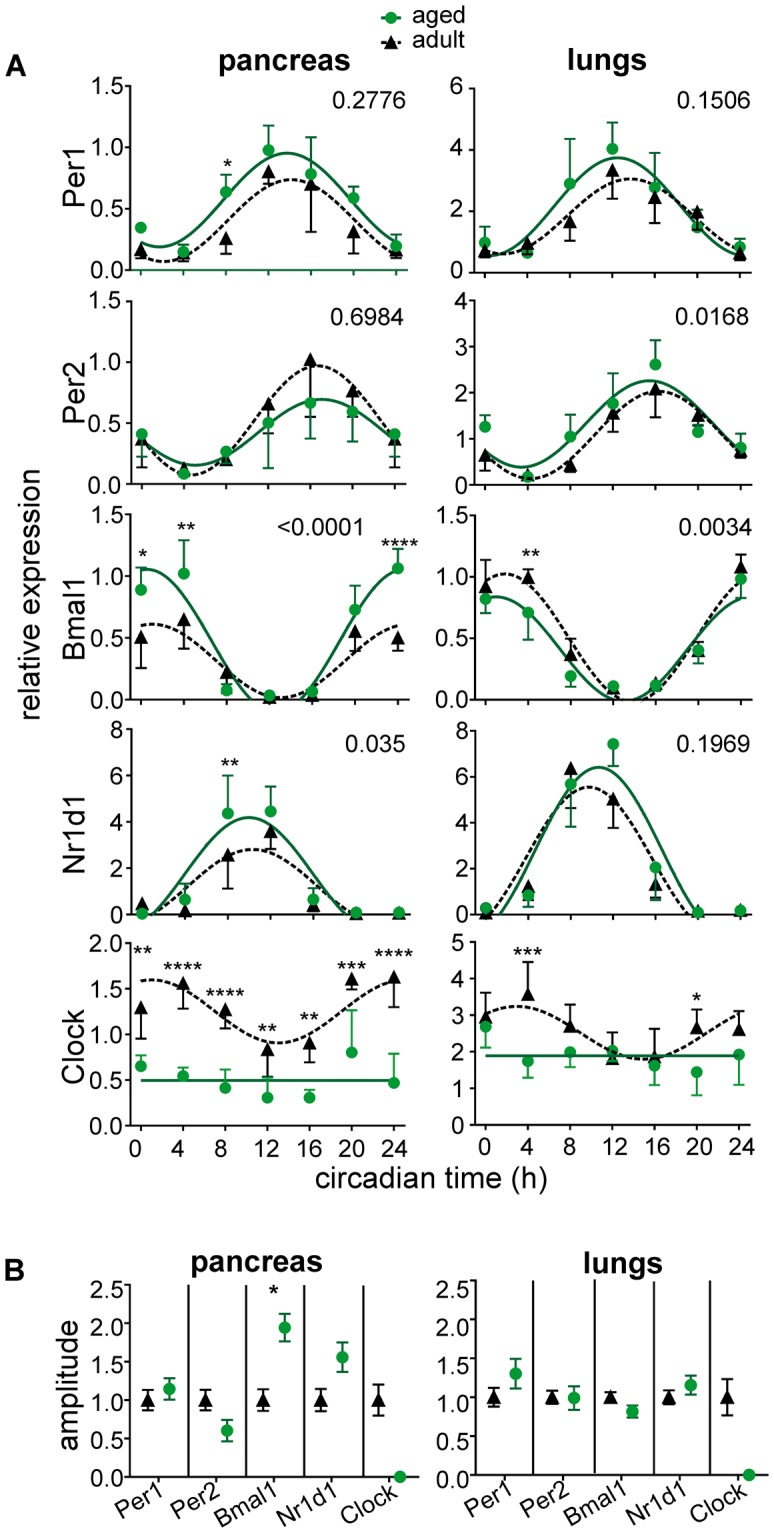
Effect of aging on expression of clock genes in the pancreas and lungs in vivo. (A) Daily profiles of relative expression of Per1, Per2, Bmal1, Nr1d1 and Clock were detected by RT qPCR in samples of adult (black triangles, dashed line) and aged (green circles, solid line) animals. The tissue samples were collected every 4 h during the 24 h profile. Animals were sacrificed in darkness; circadian time 0 corresponds to lights on of the previous LD12:12 regime. At each time point, 5 (occasionally 4) animals were sacrificed and data are expressed as the mean ± SD. For each gene and tissue, the results of a 2-way ANOVA comparison between the profiles of adult and aged animals are depicted as the P value. The data were fitted with cosine curves. (B) Comparison of amplitudes of the rhythmic gene expression in adult (black triangles, dashed line) and aged (green circles, solid line) animals depicted in A) detected by cosine analysis. Data are expressed at mean ± SEM. *P < 0.05; **P < 0.01; ****P < 0.0001.
Figure 3.
Effect of aging on expression of clock genes in the liver and colon in vivo. (A) Daily profiles of relative expression of Per1, Per2, Bmal1 and Nr1d1 were detected by RT qPCR in samples of adult (black triangles, dashed line) and aged (green circles, solid line) animals. Collection of the tissue samples was performed as described in Fig. 2. For each gene and tissue, the results of a 2-way ANOVA comparison between the profiles of adult and aged animals are depicted as the P value. The data were fitted with cosine curves. (B) Comparison of amplitudes of the cosine fits of rhythmic gene expression profiles in adult (black triangles, dashed line) and aged (green circles, solid line) animals depicted in A). Data are expressed as the mean ± SEM. (C) The expression in the liver of adult (black triangles, dashed line) and aged (green circles, solid line) animals; expression of Hif1, Pp1r3c, Sgk1, Nampt, and E4bp4 was detected at circadian time 0 and expression of Dbp at circadian time 12. For each group, data are depicted as individual levels as well as the mean ± SD. *P < 0.05; **P < 0.01; ****P < 0.0001.
Aging affected expression of some metabolism- and clock-related genes in the liver (Fig. 3B); namely, it suppressed expression of hypoxia-inducible factor 1 Hif1a (P = 0.008) and elevated expression of protein phosphatase 1 (Pp1r3c) (P = 0.0007), nicotinamide phosphoribosyltransferase (Nampt) (P = 0.0312) and nuclear factor (E4bp4) (P = 0.0007) but did not affect D-box binding protein (Dbp) (P = 0.2857). Notably, aging completely suppressed expression of serine/threonine protein kinase 1 (Sgk1) (P = 0.0007).
Circadian clocks in pancreas and lungs in vitro differ in their resilience to aging
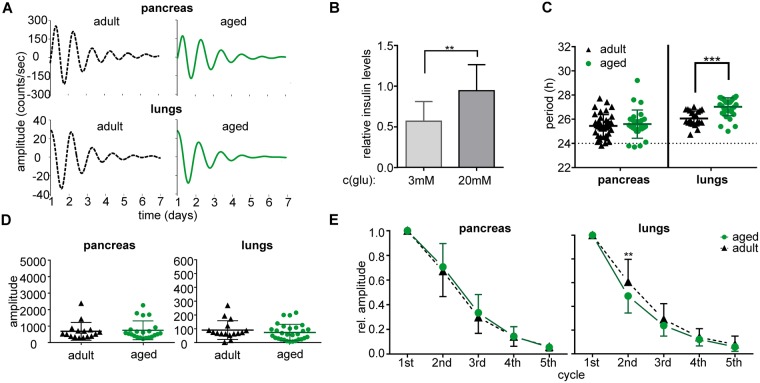
Representative traces of bioluminescence of pancreatic and lung explants from adult and aged mice maintained under LD12:12 are depicted in Fig. 4A. First, we determined that the organotypic explants of the pancreas retained their ability to respond to glucose application with a significant increase in insulin in the media (P = 0.0063) (Fig. 4B). Analyses of the bioluminescence rhythms (Fig. 4C–E) revealed tissue-specific differences: In the pancreas, aging did not affect the period (P = 0.6260), amplitude (P = 0.7245), or rate of the amplitude dampening (P = 0.4502) of the rhythm. In lungs, aging significantly lengthened the period (P = 0.0002), and although the amplitude of the first peak was not affected by aging (P = 0.081), the amplitude dampened faster (P = 0.0088) in aged mice. Altogether, in animals maintained under standard LD12:12 conditions, aging did not affect the robustness and persistence of the pancreatic clock in vitro whereas it had a negative impact on the clock in the lungs.
Figure 4.
Effect of aging on properties of the circadian clocks in vitro in animals maintained in a light/dark regime (LD12:12). Bioluminescence rhythms were recorded from organotypic explants of pancreas and lungs prepared from mPer2Luc adult (black triangles and/or dashed line) and aged (green circles and/or solid line) mice. (A) Representative bioluminescence rhythms of organotypic explants of pancreas and lungs recorded without changing the culture medium for 7 days. (B) Functional test of the pancreatic explant viability. The organotypic explants of the pancreas retained the ability to respond to glucose stimulation (from 3 mM to 20 mM glucose) by significant elevation of insulin release into the culture media in vitro (normalized to basal insulin secretion in medium with 11 mM glucose). Comparison of (C) periods and (D) amplitudes of the bioluminescence rhythms of organotypic explants of pancreas and lungs of adult (black triangles) and aged (green circles) mice. For each group, data are depicted as individual levels and mean ± SD. (E) Comparison of the amplitude dampening rate of the bioluminescence rhythms recorded from pancreas and lungs of adult (black triangles and/or dashed line) and aged (green circles and/or solid line) animals. Amplitudes of 5 subsequent cycles were detected and normalized to the amplitude of the first cycle. Data are expressed as the mean ± SD (n = 14–30). **P < 0.01; ***P < 0.001.
LL affects properties of the circadian clock in the pancreas independently of age
Because the circadian clock in the pancreas was resilient to aging in vitro, we tested whether the exposure of animals to LL, which has deleterious effect on SCN output rhythms, affects its properties and whether that effect is influenced by aging.
Explants of pancreas and lungs from adult and aged animals maintained in LL exhibited circadian rhythms (Fig. 5A). For the pancreas, exposure to LL slightly shortened the mean circadian period when compared to LD12:12 both in adult (24.8 ± 0.4 h compared to 25.5 ± 0.9 h; respectively; P = 0.0108) and in aged (25.0 ± 0.5 h compared to 25.6 ± 1.2 h; respectively; P = 0.0359) mice. Aging had no effect additional to LL on the period length of the rhythm (P = 0.6260) (Fig. 5B); although it decreased the amplitude of the rhythm (Fig. 5C), it had rather a decelerating effect on the rate of its dampening (P = 0.0048) (Fig. 5D). In fact, exposure to LL had a much larger effect than aging, because the rhythms dampened significantly faster in the pancreas of adult as well as aged mice compared to LD12:12 conditions (Fig. 5E). In lungs, exposure to LL significantly lengthened the periods of the rhythms compared to LD12:12 in adult (27.0 ± 0.7 h compared to 26.1 ± 0.6 h; respectively; P = 0.0004) and aged (28.3 ± 1.3 h compared to 27.0 ± 0.7 h; respectively; P < 0.0001) animals. Aging thus had an additional effect on the LL-modulated period of the clock in the lungs in that it significantly further lengthened the LL-prolonged period (Fig. 5B) of the rhythm and suppressed its amplitude (Fig. 5C). However, the rate of the amplitude dampening in lung explants was affected neither by age (P = 0.2985) nor by exposure to LL (P = 0.2995) (Fig. 5D,E).
Figure 5.
Effect of aging on properties of the circadian clocks in vitro in animals maintained in constant light (LL). Bioluminescence rhythms were recorded from organotypic explants of pancreas and lungs prepared from mPer2Luc adult (black triangles and/or dashed line) and aged (green circles and/or solid line) mice. (A) Representative PER2-driven bioluminescence rhythms of organotypic explants of pancreas and lungs recorded without changing the culture medium for 7 days. Comparison of (B) periods and (C) amplitudes of the bioluminescence rhythms of organotypic explants of pancreas and lungs of adult (black triangles) and aged (green circles) mice. For each group, data are depicted as individual levels and mean ± SD. (D) Comparison of the amplitude dampening rate of the bioluminescence rhythms recorded from pancreas and lungs of adult (black triangles and/or dashed line) and aged (green circles and/or solid line) animals on LL. Amplitudes of 5 subsequent cycles were detected and normalized to the amplitude of the first cycle. Data are expressed as the mean ± SD (n = 7–11). (E) Comparison between amplitude dampening rates of the bioluminescence rhythms of explants from animals maintained in LD12:12 and LL; explants of adult animals on LD (filled black triangle) and LL (open black triangle), and explants of aged animals on LD (filled green circle) and LL (open green circles). Amplitudes of 5 subsequent cycles were detected and normalized to the amplitude of the first cycle. Data are expressed as the mean ± SD (n = 7–30). **P < 0.01; ****P < 0.0001.
The next question was whether LL exposure, which impaired coherence of the explants (Fig. 5E), affected the ability of these explants to be synchronized by rhythmic cues. A rhythmic signal was imposed on the explants via the in vitro treatment described in the Materials and Methods, and the treatment was considered to have entraining effect if it caused a significant change in the period lengths and/or a decrease in their inter-individual variability (Fig. 6). In pancreatic explants (Fig. 6A) of mice maintained in LD12:12, the treatment significantly decreased the mean period values and/or their individual variability in adult animals (periods before: 29.4 ± 6.3 h and after: 24.9 ± 0.7, P = 0.0006; variability: F test, P < 0.0001), as well as in aged animals (periods before: 25.3 ± 1.5 h, after: 25.6 ± 0.6 h; P = 0.4071; variability: F test, P = 0.0022). Importantly, after exposure to LL, the same treatment was not potent enough to entrain the clock in the pancreas. Although the mean period length had changed after the treatment in adult (period before: 24.5 ± 0.8 h, after: 25.8 ± 0.9 h, P = 0.0002) as well as aged (period before: 24.8 ± 0.6 h, after: 27.5 ± 3.3 h, P = 0.039) animals, its variability either did not change in adults (F test: P = 0.5825) or was even larger in aged animals (F test: P < 0.0001). For comparison, in lung explants (Fig. 6B) of animals maintained in LD12:12, the period did not change after the treatment in adults (before: 25.9 ± 0.8 h, after: 26.1 ± 0.9 h, P = 0.4297; variability: F test, P = 0.7631) but significantly shortened in aged (before 27.7 ± 0.5 h; after: 26.3 ± 0.3 h; P = 0.0003; variability: F test, P = 0.1212) animals. In LL, the treatment had a significant effect in explants from adult animals (period before: 27.1 ± 0.9 h; after: 25.9 ± 0.4 h, P = 0.0001; variability: F test: P = 0.0036) and a less robust effect in explants from aged animals (period before 27.8 ± 1.0 h; after: 25.8 ± 1.3 h, P = 0.0013; variability: F test, P = 0.4919). Notably, LL exposure significantly impaired the ability of the pancreatic, but not the lung, explants to be entrained by treatment in vitro.
Figure 6.
Effect of in vitro treatment on periods of organotypic explants of adult (depicted in black) and aged (depicted in green) animals maintained under light/dark (LD12:12) or constant light (LL) conditions. The periods were detected before and after the treatment performed during 5 days consisting of application of 1 µl solution followed by medium exchange (open and filled circles apply to different solutions preceding the medium exchange as described in Methods), and the effects of the treatment on the period were statistically evaluated. The resulting t-test P values for differences between the periods before and after the treatment are depicted by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001), while the F test P values for differences between inter-individual period variances are depicted by hash marks (##P < 0.01; ####P < 0.0001).
Discussion
Our results revealed that in aged (24–26 months old) mPer2Luc mice the glucose homeostasis was significantly altered compared to adults (8–13 months old); their pancreas produced higher levels of insulin in spite of lower basal glucose levels; additionally, they had a lower rate of glucose clearance measured by IPGTT. The effect of aging on basal insulin levels has been controversial; both an increase27 and a decrease28 in insulin release have been reported. Additionally, age-dependent impairment in glucose clearance has been described5,28,29. The mechanism behind the elevated insulin levels in our aged mice might relate to a previously reported age-dependent increase in the ratio of large islets3,4, which have a higher insulin secretion rate5. It also could be due to a possible age-dependent decline in the insulin removal rate30, which could be due to a decline in insulin-degrading enzyme (IDE) levels in the liver31. In humans, the effect of aging on increase in insulin levels has not been confirmed after correction for visceral fat or diet32–34. However, in our study, significantly higher insulin levels were found in aged mice fed with the same diet as adult mice even after correction for their body weight. Higher insulin levels in our aged animals correlated with increased Pclo mRNA levels in the pancreas. Although we did not analyse levels of PCLO protein or activity, the functional relationship could be expected because Pclo is one of the crucial factors regulating pancreatic cAMP-dependent exocytosis and downregulation of its expression in β-cells significantly impaired insulin secretion35. Expression of Pdx1, a transcriptional factor involved in glucose- but not non-glucose-stimulated insulin secretion36, was also higher in our aged animals, as previously reported37. Expression of Slc2a2, a β-cell glucose transpoter GLUT2 required for normal glucose-stimulated insulin release, was lower in our aged animals, which may result in the decrease in pancreatic glucose sensitivity. The lower basal levels of plasma glucose we observed in aged animals were likely a consequence of increased glycogen storage in the liver as a compensatory mechanism to the higher insulin levels because we found significantly higher expression of Pp1r3c, which is a gene coding protein phosphatase 1, which promotes hepatic glycogen synthesis38. In line with this, expression of Sgk1, previously shown to influence glucose transport rate39, was dramatically suppressed in the liver of our aged animals. Therefore, downregulation of Sgk1 expression may participate in the lower glucose uptake as well as in the lower rate of glucose clearance that we observed in aged mice by IPGTT. Additionally, in the liver of aged animals we detected significant changes in expression of metabolism-related genes (Nampt, Hif1a and E4bp4) in accordance with recently described aging-induced changes in the liver transcriptome26. Altogether, our aged animals exhibited signs of hyperinsulinemic hypoglycaemia that indicates changes in their pancreatic function and related glucose homeostasis.
These findings provided us with a rationale to study the effect of aging on the pancreatic clock. Comparing the daily profiles of clock gene expression in various peripheral tissues, we were unable to detect gross suppression of amplitudes of clock gene expression profiles due to aging in any of those studied tissues. Instead, we detected only selective gene- and tissue-specific downregulation, such as for the Clock expression in the pancreas and lungs and the Bmal1 expression in the colon and lungs. Surprisingly, expression of some clock genes was rather upregulated in aging. Remarkably, both in the pancreas and liver (but not in the lungs), Bmal1 was rhythmic with significantly higher amplitude. In accordance, previous study revealed gene specific up- and down-regulation due to aging in the liver26. The apparent age-related decrease in Clock expression we found in the pancreas did not seem to affect the overall quality of oscillations of other clock gene expression. This is possibly due to BMAL1 protein being the rate limiting partner in the transcription heterodimer40. The consequence of the effect of aging on Bmal1 expression in the pancreatic tissue is not clear because it did not lead to a significant effect on clock-controlled gene expression. Nevertheless, the Bmal1 gene, apart from its major role in the circadian timekeeping mechanism15, may have also a role beyond this function41 and thus participate in the age-dependent deterioration of the pancreatic physiology. In other tissues and cell types, such as in epidermal and muscle stem cells, circadian oscillations were also reported to remain robust during aging but to undergo extensive reprogramming42.
Because we found expressions of both clock genes coding the positive elements of the molecular feedback loop mechanism to be affected by aging, that is upregulated (Bmal1) and downregulated (Clock), we considered providing evidence for changes in properties of the in vitro dissected aged clock might answer the question whether clock-dependent or clock-independent pathways are involved in aging of the pancreas. Resilience of the clock to aging would favour an additional non-circadian role of Bmal1 in the pancreas. To test this hypothesis, we used organotypic explants of the pancreas from mPer2Luc mice that allowed us to monitor the circadian clock in real time for several weeks. The explants represent an integral tissue composed of all cell types, i.e., the endocrine and exocrine pancreas, but β-cells in the explants are viable as we confirmed by the ability of the explants to produce insulin upon glucose stimulation. Isolated β-cells are not a suitable model for this type of experiment because their much shorter viability would preclude the long-term recording necessary for the study of their clock properties.
When animals were maintained under LD12:12 conditions, aging had no effect on properties of the circadian clock in the pancreas in vitro; there was no effect on the period and dampening rate in clock-driven bioluminescence rhythms. This was in remarkable contrast to the clock in the lungs that became less persistent due to aging, because the rhythms ran with prolonged periods and dampened faster. An effect of aging on the clock in the lungs was reported earlier24, although lung explants of the aged Per1-luc rats completely lost their rhythmicity.
The high resilience to aging of the circadian clock in the pancreas led us to explore whether and how the clock is sensitive to a situation that compromises the rhythmic inputs from the central clock in the SCN, because exposure to an environmental LD cycle may facilitate rhythmicity of peripheral clocks via SCN-independent mechanisms43. Previously, we found that rhythmicity of the SCN in vitro could be disturbed by exposure of animals to LL19, which may uncouple the SCN from peripheral clocks and impair rhythmicity in clocks in the tissues of the gastrointestinal tract (liver, duodenum and colon)44. Here, we provide evidence that exposure of mice to LL changes properties of the clock more robustly in the pancreas than in the lungs. In the lungs, the LL-induced lengthening in periods of the bioluminescence rhythms was fully reversible by in vitro daily treatment (simulating the regular rhythmic stimuli from the central clock), demonstrating a high sensitivity of the clock in the lungs to systemic rhythmic signals. In contrast, in the pancreatic explants, exposure to LL accelerated dampening of the clock-driven bioluminescence rhythms, and the LL-induced effect on their periods was not reversible by the treatment. Therefore, the pancreatic clock properties were changed due to the previous exposure to disturbed lighting conditions, rather than simply due to an absence of rhythmic signals.
In conclusion, from the comparison between properties of the pancreatic clock with the clock in a tissue not involved in glucose homeostasis (lungs), we provide evidence for apparently high resilience to aging of the core molecular clock mechanism in the pancreas in animals maintained under standard lighting conditions. We also demonstrate a significantly higher sensitivity of the pancreatic clock to disturbances in external lighting conditions that might provide a basis for previously demonstrated consequences of disturbances in the circadian system on disordered glucose homeostasis (including T2DM) in human subjects exposed to shift work18.
Methods
Experimental animals and procedures
Male and female mPer2Luc mice (strain B6.129S6-Per2tm1Jt/J, JAX, USA)45 and Wistar rats (Czech Republic and Institute of Physiology, Czech Academy of Sciences) were housed individually in a temperature-controlled (21 ± 2 °C) animal facility and had free access to food and water throughout the experiment. They were maintained under a light/dark cycle with 12 h of light and 12 h of darkness (LD12:12), with lights (150–300 lx, depending on cage position) on between 06:00 and 18:00. Time is expressed as circadian time, i.e., time 0 corresponds to lights on, and time 12 corresponds to lights off during the LD regime. For constant light (LL), the mice were exposed continuously to light of the same intensity as during the LD12:12 cycle for up to 9 months. At the time of sampling, mPer2Luc mice up to the age of 8–13 months were designated “adult”, and mice 24–26 months old were designated “aged”. For rats, the group of “adult” animals was 6 months old and that of “aged” animals was 22–26 months old. The daily profiles in the expression of clock gene Bmal1 detected in the pancreas of adult Wistar rats and mPer2Luc mice did not reveal any significant inter-species differences (Suppl. Figure 1) and, therefore, the results of both animal strains were involved in the study.
The Animal Care and Use Committee of the Institute of Physiology, in agreement with the Animal Protection Law of the Czech Republic as well as European Community Council directives 86/609/EEC, approved all experiments. All efforts were made to alleviate the suffering of the animals.
Detection of BMAL1 protein in the pancreas by immunohistochemistry
12-μm-thick sections of mPer2Luc mouse pancreas were cut, mounted on slides, fixed in 4% paraformaldehyde in PBS and processed for immunohistochemistry using the standard avidin-biotin method with diaminobenzidine as the chromogen (Vector Laboratories, Peterborough, UK) as previously described46. For more details, see Suppl. Figure 2.
Detection of gene expression in vivo using real-time RT qPCR
For detection of gene expression daily profiles, rats were released into constant darkness, and starting at circadian time 0, they were sacrificed under deep anaesthesia (thiopental, 50 mg/kg b.w., i.p.) every 4 h during a 24-h cycle. At each time point, samples of liver, lung, pancreas and colon were collected from 5 rats. The pancreatic tissue was homogenized using ceramic beads (1.4 mm, MoBio Laboratories, Carlsbad, CA, USA; MagNa Lyser, Roche, Basel, Switzerland) in isolation buffer with β-mercaptoethanol according to manufacturer’s instructions (GenElute Mammalian Total RNA Miniprep Kit; Sigma-Aldrich, St. Louis, MO, USA) immediately after tissue dissection. The method of sampling of other tissues was described previously46.
The RNA isolation and real-time RT qPCR were performed as previously described46. For specific primers, see Table 1. The ΔΔCt method was used for the quantification of relative cDNA concentration. Relative expression was achieved by normalizing the expression to the mean relative expression of two housekeeping genes; for colon, lungs and liver these were β2-microglobulin (B2m) and glyceraldehyde 3-phosphate dehydrogenase (Gapdh), and for the pancreas, they were TATA binding protein (Tbp) and ribosomal protein S18 (Rps18). Expression of these reference genes has been proven to be stable throughout the 24 h cycle47. For each tissue, samples from adult and aged animals were assayed in the same real-time RT qPCR run.
Table 1.
List of primers.
| primer - RefSeq | sequence |
|---|---|
| Gapdh - NM_017008 |
Forward: 5′-TGATTCTACCACGGCAAG-3′ Reverse: 5′-TGATGGGTTTCCCATTGAT-3′ |
| Rps18- NM_213557.1 |
Forward: 5′-ACTGCCATTAAGGGTGTG-3′ Reverse: 5′-GTCAGGGATCTTGTATTGTC-3′ |
| Pclo - NM_020098.1 |
Forward: 5′-GATCGATTCAGAAGAAAGATG-3′ Reverse: 5′-CCCAGATTCAAGCTAAAACG-3′ |
| Pdx1 - NM_022852.3 |
Forward: 5′-TCATCTCCCTTTCCCGTGGAT-3′ Reverse: 5′-TATTCTCCTCCGGTTCTGCTG-3′ |
| Dbp - NM_012543 |
Forward: 5′-GCTAATGACCTTTGAACCTG-3′ Reverse: 5′-AGTACTTCTCATCCTTCTGTTC-3′ |
| Nampt - NM_177928 |
Forward: 5′-CTTTGGTTCTGGTGGCGCTTTGCTAC-3′ Reverse: 5′-GCCGGCCCTTTTTCGACCTTTTGTT-3′ |
| Hif1a - NM_024359 |
Forward: 5′-AGCGGCTGGGGACACGAT-3′ Reverse: 5′-TGGCTTTGGAGTTTCAGAGGCAGGTAA-3′ |
| Sgk1- NM_001193569.1 |
Forward: 5′-AAGAAGATCACGCCCCCATTTA-3′ Reverse: 5′-AGGCTTCCGCGGCTTTCAC-3 |
| PP1r3c - NW_047565 |
Forward: 5′-CCGCTAAGTGCGTGGTGCGA-3′ Reverse: 5′-GGGGTGGTGAATGTGCCAAGCA-3′ |
| E4bp4 - NM_053727 |
Forward: 5′-ACCGTGTGAAGGGCGTGGAT-3′ Reverse: 5′-GTGGCGGTGGGGGTGCTC-3′ |
Intraperitoneal glucose tolerance test (IPGTT)
IPGTT was performed in mPer2Luc mice maintained in LD12:12, as previously described48. Mice were fasted for 3 h before the IPGTT, and testing started at 12:00. Blood glucose was measured by glucose meter (GlucoLab, Infopia Co., Ltd., Korea) before injection (basal) and then 15, 30, 60, 120 and 180 min after glucose injection (2 g/kg b.w., i.p.). The incremental area under curve (AUC) was calculated49. For detection of basal insulin levels, blood was drawn after 3 h fasting before the glucose injection, and insulin levels in plasma were assessed by ELISA (BioVendor Brno, Czech Republic).
Tissue explant preparation and bioluminescence monitoring
The mPer2Luc mice were maintained in LD12:12 or LL and were sacrificed between 12:00 and 13:00 via rapid cervical dislocation under isoflurane anaesthesia; their lung and pancreas were removed, rinsed in HBSS buffer (Sigma-Aldrich), and individual explants were placed onto Millicell Culture Inserts (Merck, Darmstadt, Germany) inside 35-mm Petri dishes with 1 ml of air-buffered recording medium supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, 10 µg/ml gentamicin, 1x GlutaMAX (Thermo Fisher, Waltham, MA, USA), 10% foetal calf serum (Sigma-Aldrich) and 0.1 mM D-Luciferin (Biosynth, Staad, Switzerland). Pancreatic explants were pre-incubated for 1.5 h in 1 ml air-buffered recording medium and then placed onto fresh recording medium. The bioluminescence was recorded in a LumiCycle apparatus (Actimetrics, Wilmette, IL, USA) beginning immediately after setting the explant cultures. The raw bioluminescence traces were analyzed using LumiCycle Analysis software (Actimetrics). The data were baseline-subtracted using the 24-h running average and smoothed prior to fitting a damped sine wave to calculate the period and amplitude before and after the treatments.
To measure the dampening rate of the amplitude of the bioluminescence rhythms, the explants were left untreated for at least 5 days. The amplitudes of the subsequent peaks were expressed relative to the 1st peak. The in vitro test of entrainment of the circadian clock was performed in another group of explants according to a previously published procedure19: the rhythms were recorded for the first 4 days after setting the in vitro culture, and then, the explants were exposed to a treatment that involved addition of 1 µl solution [1% ethanol/saline phosphate buffer with or without melatonin (Sigma-Aldrich); final melatonin concentration in the recording medium was 1 µM] at 08:00 and followed by fresh medium replacement 8 h later. The treatment was originally designed to test the effect of 8-h melatonin exposure on the explants. However, after completion of the experiment, it appeared that the effect of treatment on the period length was independent of the treatment with 1 µl solution (with or without melatonin) preceding medium exchange. The medium exchange itself had a strong effect whereas application of the solution without media exchange had no effect on the period length. Therefore, the effect of the treatment could be attributed solely to the medium exchange, and composition of the solution was not considered in the final analyses of the data. This treatment was performed every day on 5 subsequent days, and then, the explants were left in the recording medium for another 5 days. Only the explants that survived the treatment (exhibited measurable oscillations after the treatment) were considered for subsequent statistical analyses (approximately 90%) (Suppl. Figure 3).
Insulin secretion from pancreatic explants
To test the viability of the islets of Langerhans within the organotypic explants of the pancreas from mPer2Luc mice during the in vitro incubation, we used a modified glucose- stimulated insulin secretion (GSIS) protocol. Explants were cultivated in air-buffered recording medium (as described above) that was enriched with 11 mM glucose. After 5 days of cultivation, the concentration of glucose was decreased to 3 mM for 24 h and then increased to 20 mM to stimulate insulin secretion. Insulin concentrations in media were assessed by ELISA (BioVendor).
Statistical analysis
Differences between daily gene expression profiles were analyzed by two-way ANOVA followed by the Holm-Sidak post hoc multiple-comparison test. The amplitudes of these profiles were determined using cosine analysis as described elsewhere50; for comparison, the amplitudes were normalized relative to the value of the adult group and compared by multiple t-tests with the Holm-Sidak correction, where applicable.
Differences between means in (i) the periods of the bioluminescence rhythms in vitro (ii) the plasma glucose and insulin levels, (iii) the insulin levels in media after GSIS, and (iv) the metabolic-relevant gene expression were compared using Student’s t-test with Welch’s correction; when the data did not show a normal distribution (according to the D’Agostino-Pearson omnibus normality test), the Mann-Whitney test was used. The F test was used to assess significant differences in the periods of bioluminescence rhythms before and after the treatment. P < 0.05 (adjusted for multiple comparisons when relevant) was set as the threshold of significance for all statistical tests. Statistical analyses were implemented in Prism 6 software (GraphPad, Inc., La Jolla, CA, USA).
Electronic supplementary material
Acknowledgements
The study was supported by the Czech Science Foundation grant P30412G069 (to A.S.), the Grant Agency of the Charles University 198215 (to Z.N.), the OPPK Brain View CZ.2.16/3.1.00/21544 and Research Project RV0: 67985823.
Author Contributions
Z.N. performed experiments and drafted the manuscript. L.P. performed experiments. M.S. contributed to the discussion and reviewed the manuscript. A.S. designed the study and wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chantarojanasiri T, et al. Evolution of pancreas in aging: degenerative variation or early changes of disease? J. Med. Ultrason. 2015;42:177–183. doi: 10.1007/s10396-014-0576-2. [DOI] [PubMed] [Google Scholar]
- 2.Majumdar AP, Jaszewski R, Dubick MA. Effect of aging on the gastrointestinal tract and the pancreas. Proc. Soc. Exp. Biol. Med. 1997;215:134–144. doi: 10.3181/00379727-215-44120. [DOI] [PubMed] [Google Scholar]
- 3.Chen M, Bergman RN, Pacini G, Porte D. J. Pathogenesis of age-related glucose intolerance in men: insulin resistance and decreased β-cell function. J. Clin. Endocrinol. Metab. 1985;60:13–20. doi: 10.1210/jcem-60-1-13. [DOI] [PubMed] [Google Scholar]
- 4.Hellman B. The Total Volume of the Pancreatic Islet Tissue At Different Ages of the Rat. Acta Pathol. Microbiol. Scand. 1959;47:35–50. doi: 10.1111/j.1699-0463.1959.tb03419.x. [DOI] [PubMed] [Google Scholar]
- 5.Kitahara A, Adelman RC. Altered regulation of insulin secretion in isolated islets of different sizes in aging rats. Biochem. Biophys. Res. Commun. 1979;87:1207–13. doi: 10.1016/S0006-291X(79)80035-2. [DOI] [PubMed] [Google Scholar]
- 6.Adelman RC. Secretion of Insulin During Aging. J. Am. Geriatr. Soc. 1989;37:983–990. doi: 10.1111/j.1532-5415.1989.tb07287.x. [DOI] [PubMed] [Google Scholar]
- 7.Evans, W. J. & Farrell, P. A. In Comprehensive Physiology 969–998 (John Wiley & Sons, Inc.), 10.1002/cphy.cp070232 (2011).
- 8.Pulimeno P, et al. Autonomous and self-sustained circadian oscillators displayed in human islet cells. Diabetologia. 2013;56:497–507. doi: 10.1007/s00125-012-2779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perelis M, et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350:aac4250. doi: 10.1126/science.aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchiyama Y, Saito K. A morphometric study of 24-hour variations in subcellular structures of the rat pancreatic acinar cell. Cell Tissue Res. 1982;226:609–620. doi: 10.1007/BF00214788. [DOI] [PubMed] [Google Scholar]
- 11.Marcheva B, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saini, C. et al. A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells. Diabetes. Obes. Metab. 355–365, 10.1111/dom.12616 (2015). [DOI] [PubMed]
- 13.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annu. Rev. Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dibner C, Schibler U, Albrecht U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi JS. Molecular components of the circadian clock in mammals. Diabetes, Obes. Metab. 2015;17:6–11. doi: 10.1111/dom.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamia KA, Storch K-F, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA. 2008;105:15172–7. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalsbeek A, la Fleur S, Fliers E. Circadian control of glucose metabolism. Mol. Metab. 2014;3:372–83. doi: 10.1016/j.molmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bescos R, et al. Four days of simulated shift work reduces insulin sensitivity in humans. Acta Physiol. 2018;12:e13039. doi: 10.1111/apha.13039. [DOI] [PubMed] [Google Scholar]
- 19.Polidarová L, Sládek M, Novosadová Z, Sumová A. Aging does not compromise in vitro oscillation of the suprachiasmatic nuclei but makes it more vulnerable to constant light. Chronobiol. Int. 2016;00:1–13. doi: 10.1080/07420528.2016.1242491. [DOI] [PubMed] [Google Scholar]
- 20.Farajnia S, Deboer T, Rohling JHT, Meijer JH, Michel S. Aging of the suprachiasmatic clock. Neuroscientist. 2014;20:44–55. doi: 10.1177/1073858413498936. [DOI] [PubMed] [Google Scholar]
- 21.Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am. J. Physiol. 1997;273:R1957–64. doi: 10.1152/ajpcell.1997.273.6.C1957. [DOI] [PubMed] [Google Scholar]
- 22.Polidarová L, Houdek P, Sumová A. Chronic disruptions of circadian sleep regulation induce specific proinflammatory responses in the rat colon. Chronobiol. Int. 2017;34:1273–1287. doi: 10.1080/07420528.2017.1361436. [DOI] [PubMed] [Google Scholar]
- 23.Tahara Y, et al. Age-related circadian disorganization caused by sympathetic dysfunction in peripheral clock regulation. npj Aging Mech. Dis. 2017;3:16030. doi: 10.1038/npjamd.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki S, et al. Effects of aging on central and peripheral mammalian clocks. Proc. Natl. Acad. Sci. USA. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sellix MT, et al. Aging Differentially Affects the Re-entrainment Response of Central and Peripheral Circadian Oscillators. J. Neurosci. 2012;32:16193–16202. doi: 10.1523/JNEUROSCI.3559-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato S, et al. Circadian Reprogramming in the Liver Identifies Metabolic Pathways of Aging. Cell. 2017;170:664–677.e11. doi: 10.1016/j.cell.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregg T, et al. Pancreatic β-cells from mice offset age-associated mitochondrial deficiency with reduced K ATPchannel activity. Diabetes. 2016;65:2700–2710. doi: 10.2337/db16-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santulli G, et al. Age-related impairment in insulin release: The essential role of β2-adrenergic receptor. Diabetes. 2012;61:692–701. doi: 10.2337/db11-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold G, Karoly K, Freeman C, Adelman RC. A possible role for insulin in the altered capability for hepatic enzyme adaptation during aging. Biochem. Biophys. Res. Commun. 1976;73:1003–1010. doi: 10.1016/0006-291X(76)90222-9. [DOI] [PubMed] [Google Scholar]
- 30.Reaven GM, Greenfield MS, Mondon CE, Rosenthal M, Wright D. Does insulin removal rate from plasma decline with age? Diabetes. 1982;31:670–673. doi: 10.2337/diab.31.8.670. [DOI] [PubMed] [Google Scholar]
- 31.Kochkina EG, et al. Effects of ageing and experimental diabetes on insulin-degrading enzyme expression in male rat tissues. Biogerontology. 2015;16:473–484. doi: 10.1007/s10522-015-9569-9. [DOI] [PubMed] [Google Scholar]
- 32.Imbeault P, et al. Aging per se does not influence glucose homeostasis: In vivo and in vitro evidence. Diabetes Care. 2003;26:480–484. doi: 10.2337/diacare.26.2.480. [DOI] [PubMed] [Google Scholar]
- 33.DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981;4:493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- 34.Kohrt WM, et al. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42:273–281. doi: 10.2337/diab.42.2.273. [DOI] [PubMed] [Google Scholar]
- 35.Fujimoto K, et al. Piccolo, a Ca2+sensor in pancreatic β cells: Involvement of cAMP-GEFII-Rim2-Piccolo complex in cAMP-dependent exocytosis. J. Biol. Chem. 2002;277:50497–50502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, et al. β-Cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes. 2005;54:482–491. doi: 10.2337/diabetes.54.2.482. [DOI] [PubMed] [Google Scholar]
- 37.Avrahami D, et al. Aging-Dependent Demethylation of Regulatory Elements Correlates with Chromatin State and Improved β Cell Function. Cell Metab. 2015;22:619–632. doi: 10.1016/j.cmet.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Printen JA, Brady MJ, Saltiel AR. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science. 1997;275:1475–8. doi: 10.1126/science.275.5305.1475. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, et al. Hepatic serum- and glucocorticoid-regulated protein kinase 1 (SGK1) regulates insulin sensitivity in mice via extracellular-signal-regulated kinase 1/2 (ERK1/2) Biochem. J. 2014;464:281–9. doi: 10.1042/BJ20141005. [DOI] [PubMed] [Google Scholar]
- 40.Lee C, Etchegaray JP, Cagampang FRA, Loudon ASI, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/S0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 41.Yang G, et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci. Transl. Med. 2016;8:324ra16. doi: 10.1126/scitranslmed.aad3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solanas G, et al. Aged Stem Cells Reprogram Their Daily Rhythmic Functions to Adapt to Stress. Cell. 2017;170:678–692.e20. doi: 10.1016/j.cell.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 43.Husse J, Leliavski A, Tsang AH, Oster H, Eichele G. The light-dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock. FASEB J. 2014;28:4950–60. doi: 10.1096/fj.14-256594. [DOI] [PubMed] [Google Scholar]
- 44.Polidarová L, Sládek M, Soták M, Pácha J, Sumová A. Hepatic, duodenal, and colonic circadian clocks differ in their persistence under conditions of constant light and in their entrainment by restricted feeding. Chronobiol. Int. 2011;28:204–15. doi: 10.3109/07420528.2010.548615. [DOI] [PubMed] [Google Scholar]
- 45.Yoo S-H, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sládek M, et al. Insight into the circadian clock within rat colonic epithelial cells. Gastroenterology. 2007;133:1240–9. doi: 10.1053/j.gastro.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 47.Polidarová L, et al. Mechanisms of hormonal regulation of the peripheral circadian clock in the colon. Chronobiol. Int. 2017;34:1–16. doi: 10.1080/07420528.2016.1231198. [DOI] [PubMed] [Google Scholar]
- 48.Ayala JE, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3:525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Floch J-PL, Escuyer P, Baudin E, Baudon D, Perlemuter L. Blood Glucose Area Under the Curve: Methodological Aspects. Diabetes Care. 1990;13:172–175. doi: 10.2337/diacare.13.2.172. [DOI] [PubMed] [Google Scholar]
- 50.Polidarová L, et al. Development and entrainment of the colonic circadian clock during ontogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306:G346–56. doi: 10.1152/ajpgi.00340.2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.