Abstract
Fire blight, caused by the enterobacterium Erwinia amylovora, is a destructive disease, which can affect most members of the Rosaceae family. Since no significant genomic differences have been found by others to explain differences in virulence, we used here a gel-based proteomic approach to elucidate mechanisms and key players that allow the pathogen to survive, grow and multiply inside its host. Therefore, two strains with proven difference in virulence were grown under controlled conditions in vitro as well as in planta (infected apple rootstocks). Proteomic analysis including 2DE and mass spectrometry revealed that proteins involved in transcription regulation were more abundant in the in planta condition for both strains. In addition, genes involved in RNA processing were upregulated in planta for the highly virulent strain PFB5. Moreover, the upregulation of structural components of the F0F1-ATP synthase are major findings, giving important information on the infection strategy of this devastating pathogen. Overall, this research provides the first proteomic profile of E. amylovora during infection of apple rootstocks and insights into the response of the pathogen in interaction with its host.
Introduction
The Gram-negative enterobacterium Erwinia amylovora is a plant pathogen that causes fire blight, a devastating necrotic disease forming a major threat to pome fruit and other economically relevant species belonging to the Rosacea family1. To date, no efficient and sustainable management strategy is available to fight fire blight. This is due to the limitations on the use of antibiotics, the occurrence of resistance against the existing antibiotics and the limited efficacy of the alternative control agents2.
The molecular basis of survival and propagation of virulent strains of E. amylovora inside its host is largely unknown and poorly understood. Much research has been performed on the host-pathogen interaction at the transcriptome level of the host3–6. Information on virulence factors and defense processes of the pathogen itself against the immune responses of the plant came from mutant screenings under controlled conditions, unable to completely mimic natural conditions. Several genes were shown to be upregulated upon infection of immature pear tissue such as virulence factors already known as components of the type III secretion system and the effector dspE7. This type III secretion system is encoded by genes from the hrp (hypersensitivity response and pathogenicity) gene cluster8 and consists of a pilus-like structure that will deliver effector proteins directly into the plant cells9. This system, together with amylovoran production, motility and biofilm formation is downregulated during infection by the secondary messenger cyclic di-GMP10.
Over the past decade, proteomic approaches have proven to be an excellent tool to characterize and understand the dynamic interplay of host and pathogen11. Therefore, this technique was chosen for investigating the proteome of E. amylovora. Moreover, due to its rather high conserved genome in comparison with other phytopathogenic bacteria, as published by Mann et al., where they did a comparative genomic analysis of 12 strains of E. amylovora and concluded that the chromosomes of Spiraeoideae-infecting strains are highly homogeneous12,13, we opted to look at the proteome of two strains to investigate the reactions the plant induces in the pathogen.
In previous studies of our lab, two-dimensional electrophoresis was successfully used to explore different aspects of the proteome of E. amylovora. We were able to identify differentially expressed proteins and genes between strains differing in virulence14,15. Moreover, we mapped the outer membrane proteome of E. amylovora grown in planta using a low and high virulent strain i.e. LMG2024 and PFB5, respectively16. We showed that the higher virulent strain produced more type III secretion effectors, which are necessary for starting and sustaining a successful infection16. Here, using an identical experimental setup, the focus was on the biochemical processes involved in a successful infection by comparing the CHAPS-urea solubilized protein complement by 2DE of the same two strains when grown in planta.
Results
Proteome analysis of E. amylovora during interaction with apple rootstocks
Cells were grown in vitro in MM2 medium until mid-exponential phase before protein extraction. For the in planta samples, apple rootstock were infected using the scissors method15 and when signs of systemic infection were observed, samples were taken, approximately after 10 to 15 days after infection depending on the strain14. The experimental set-up allowed a comparison between the in vitro and in planta proteome of both strains independently as well as a comparison between strains and conditions (Fig. 1). Thereby unraveling the mechanisms that could be pathogen specific but also highlighting differences between a high and low virulent strains.
Figure 1.
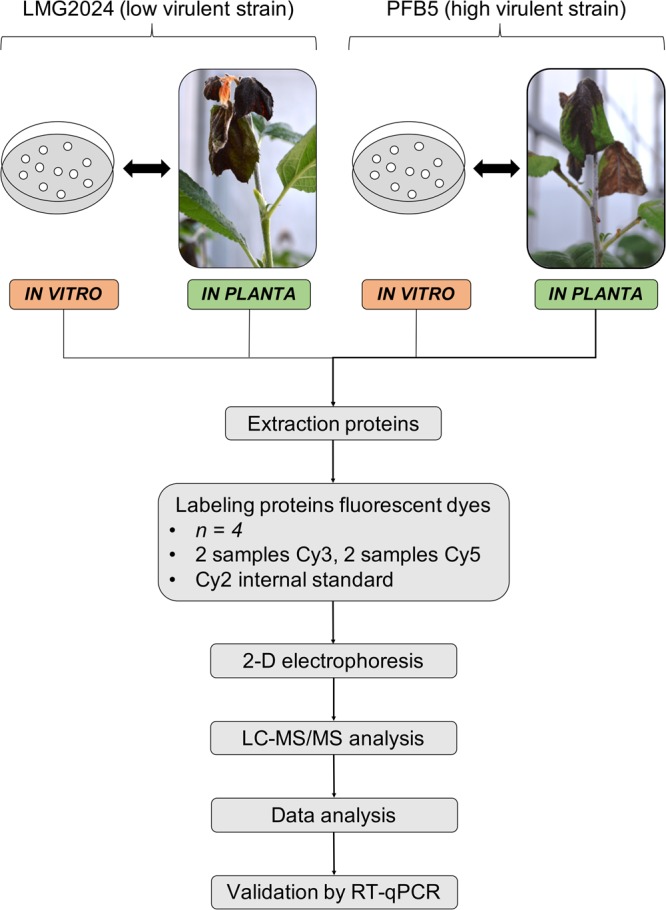
General workflow and experimental design of the proteomic experiment. Two strains differing in virulence were selected, LMG2024 (low virulent strain) and PFB5 (high virulent strain). Both strains were grown both in vitro and in planta. When grown until mid-exponential phase, the in vitro samples were taken. After 10 to 15 days after infection of apple rootstocks with respectively PFB5 and LMG2024, bacterial cells were extracted from the plant tissue. Samples from both strains and both conditions were used in a single 2D electrophoresis experiment so both strains and conditions could be compared using the SameSpot software. LC-MS/MS and data analysis was performed to identify the proteins. Finally, data were validated using RT-qPCR.
Comparison in vitro and in planta proteome of LMG2024
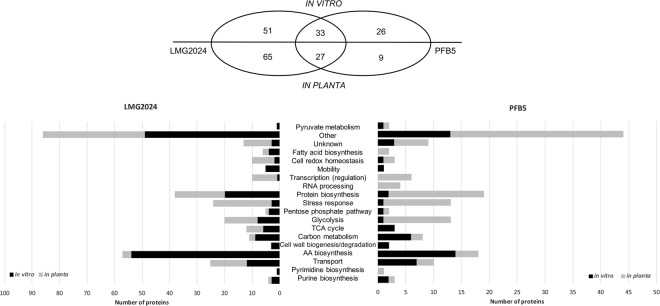
When comparing the proteome in vitro and in planta of LMG2024, 200 spots were selected (Fig. 2A,B), meeting the preset requirements14. From these, 177 spots were identified by mass spectrometry, of which 93 were upregulated in vitro and 84 in planta (See Supplementary Data S1). For some spots, multiple proteins were identified having similar isoelectric point and molecular weight. Because of the uncertainty of which protein caused the difference between conditions, all proteins were considered. The identified proteins were further categorized according to their molecular function or biological process following the annotation of UniProtKB (www.uniprot.org) (Fig. 3). The percentage of each category was determined relative to the total amount of proteins that are identified. Moreover, if the same protein was identified in multiple spots, due to the uncertainty of possible isoforms of this protein, all were considered in the categorization. The main biological processes and molecular functions identified during this research include pyruvate metabolism, fatty acid biosynthesis, cell redox homeostasis, mobility, translation, transcription (regulation), protein biosynthesis, stress response, pentose phosphate pathway, glycolysis, TCA cycle, carbon metabolism, cell wall biogenesis/degradation, amino acid biosynthesis, transport, pyrimidine biosynthesis and purine biosynthesis.
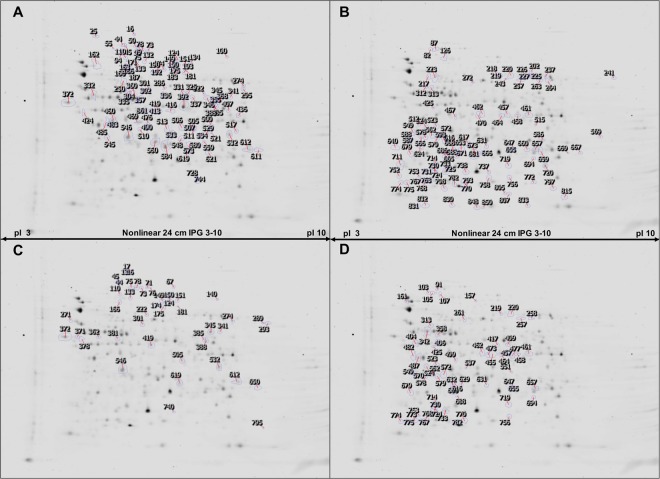
Figure 2.
Representative 2DE gels of proteins extracted from E. amylovora. (A) Spots indicated represent proteins that are upregulated in vitro for LMG2024 in comparison with the in planta condition. (B) Spots upregulated in planta for LMG2024. (C) Indication of the spots upregulated in vitro in PFB5 in comparison with the in planta proteome (D). Upregulated spots from the in planta proteome in comparison with the in vitro proteome of PFB5.
Figure 3.
Functional categorization of the significantly different expressed proteins. Proteins identified in E. amylovora after 2DE are categorized according their biological and molecular function. This categorization was done for both strains and both conditions, in vitro represented in black and in planta in grey. The Venn diagram shows the proteins that were specifically and commonly identified between LMG2024 and PFB5 both in vitro and in planta.
It becomes clear that higher concentrations of flagellin are produced in a synthetic medium and this protein is less recovered in planta. Moreover, we see in vitro a higher amount of proteins involved in biosynthesis of amino acids while in planta more proteins involved in transcription and transcription regulation (RpoA, OmpR, EAMY_1829, GlnK, H-NS and CspG) are more abundant. Also, as expected a rise in proteins related to stress response are observed in higher amounts for the in planta condition.
Comparison in vitro and in planta proteome of PFB5
The same comparison was made for a strain with a higher virulence, PFB5 (Fig. 2C,D). Out of 111 spots that were selected using the SameSpot software and that were picked from the gels, 62 were identified of which 46 were upregulated in vitro and 65 were upregulated in planta (See Supplementary Table S1). Also for this strain, the identified proteins were classified according their biological or molecular function (Fig. 3). In vitro, we saw an upregulation of proteins classified under the TCA cycle, cell wall biogenesis and degradation, amino acid biosynthesis and transport. Whilst for the in planta condition, proteins related to transcription (regulation), RNA processing, protein biosynthesis and stress response were more abundant.
Corresponding results between both strains
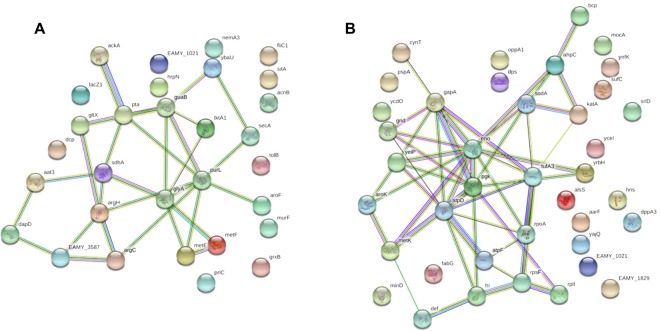
As can be expected, several proteins are shared amongst conditions between both strains. STRING was used to identify relationships between these proteins (Fig. 4, Supplementary Tables S3 and S4). This tool demonstrates the significance of the identified interactions. For the common proteins upregulated in vitro for LMG2024 and PFB5, the program identified 17 proteins as classified under metabolic pathways and 12 were classified as involved in biosynthesis of secondary metabolites. The other classifications included amino acid metabolism (9 proteins), microbial metabolism in diverse environments (9 proteins), carbon metabolism (7 proteins), propanoate metabolism (3 proteins), methane metabolism (3 proteins), taurine and hypotaurine metabolism (2 proteins), 2-oxocarboxylic acid metabolism (3 proteins), one carbon pool by folate (2 proteins), glyoxylate and dicarboxylate metabolism (2 proteins) and the biosynthesis of lysine (2 proteins).
Figure 4.
Interaction network of differentially expressed proteins. For both conditions, in vitro (A) and in planta (B) an interaction network was developed containing all proteins shared by both strains in this condition. Colored nodes represent query proteins and first shell of interactions. Empty nodes indicate proteins of unknown 3D structure. Different line colors indicate the types of protein-protein associations: light blue (from curated databases) and purple (experimentally determined) indicate known interactions. Green (gene neighborhood), red (gene fusions), blue (gene co-occurrence), light green (text mining), black (co-expression) and light purple (protein homology) indicate predicted interactions.
However, for the in planta condition, no functional enrichment in the networks was detected. Although, from this network it becomes clear enolase is interconnected with many other proteins and forms a link between several different proteins.
Validation of the proteomics results by reverse transcription PCR
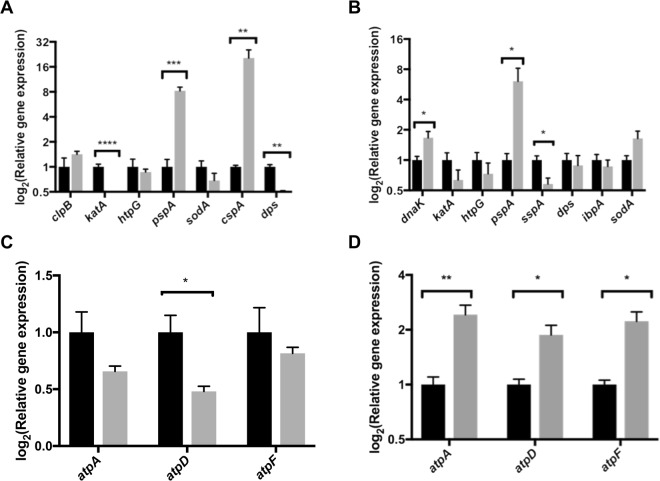
For E. amylovora, it is known that necrosis and the production of reactive oxygen species is induced by the pathogen through the injection of effector molecules and thereby aids in the infection process17. Therefore, we were especially interested in the stress related proteins that were identified during this screen. For the lower virulent strain, 6 stress related proteins were upregulated including ClpB, KatA, HtpG, PspA, SodA and CspG. For the higher virulent strain, we identified seven stress related genes including dnaK, katA, htpG, pspA, sspA, dps and hspB. For both strains, we used RT-qPCR to validate these results (Fig. 5A,B). Results show that for the lower virulent strain, proteomic and transcriptomic results correspond for PspA and CspG. However, for katA a contradictory result was obtained, showing that katA was lower expressed in the in planta condition in comparison with in vitro (Fig. 5A).
Figure 5.
Gene expression profiling by RT-qPCR. Gene expression analysis was performed for genes involved in stress response for (A) LMG2024 and (B) PFB5 and (C) for genes corresponding to structural components of the F0F1-ATP synthase in the low virulent strain (LMG2024) and (D) the high virulent strain (PFB5). Normalized data are presented for the in vitro samples (black) and the in planta samples (grey). Up-or downregulations are presented on a log2 scale y-axis relative to the in vitro samples. Colums represent four biological replicates ± standard errors. Asterisk (*) indicates a statistically significance between conditions. The statistical analysis was done by unpaired student’s t-test, *p < 0,05; **p < 0,01; ***p < 0,001; ****p < 0,0001.
For the higher virulent strain, the data were confirmed for dnaK and pspA. For the other genes, no significant difference was observed between in vitro and in planta samples. However, for sspA, a contradictory result was observed (Fig. 5B).
Proteomic results also showed a higher abundance of structural proteins of the F0F1-ATP synthase including AtpA, AtpD and AtpF, which was observed for both strains. To validate these results, we measured the gene expression of the corresponding genes for both strains and both conditions. For the lower virulent strains, LMG2024, we found differences between proteomic and transcriptomic data (Fig. 5C). However, for PFB5, high correspondence with the proteomics data and significant differences could be observed for all three genes between both conditions (Fig. 5D).
Discussion
The obtained results from this research show that these techniques can be used to extract viable cells from infected plant tissue for further processing using different omics techniques. For this study, a proteomics approach was chosen and interesting protemic data were validated using RT-qPCR.
From this research, we found that proteins related to transcription and transcription regulation are important for E. amylovora for survival and growth inside the host plant. For the low virulent strain, LMG2024, a higher expression of OmpR was observed in planta. This protein is part of the two-component signal transduction system (TCST) OmpR-EnvZ which is widely distributed and well-studied in γ-proteobacteria and which has shown to have a role in virulence, amylovoran biosynthesis and swarming motility in E. amylovora18. In this complex, the transmembrane sensor EnvZ, is activated in response to acidic pH conditions and changes in osmolarity and will next phosphorylate OmpR, which will regulate expression of OmpC and OmpF, two major outer-membrane porins19. This is confirmed by our previous research where we identified OmpF as being more abundantly expressed in the outer membrane of the lower virulent strain LMG2024 when grown in planta16. Moreover, OmpR is also involved in regulation of several cellular processes including gene expression of flagellar components and fatty acid transport20. During our research, we previously found that the lower virulent strain produces a higher amount of flagellin (FliC) than the higher virulent strain in an in vitro comparison14. This raised the hypothesis that the pathogen might use this regulator to lower the amount of flagellin to diminish recognition possibilities by the host, since flagellin can be recognized as a pathogen associated molecular pattern (PAMP)21. GlnK was also identified as more abundantly expressed during the in planta condition for LMG2024. This regulatory protein is differentially expressed in Pseudomonas aeruginosa under swarming conditions22. This could form a link between the proven higher swarming motility of this strain and more virulent strains14.
For both LMG2024 and PFB5, we identified the histon-like nucleoid structuring protein (H-NS), a DNA-binding protein, encoded by hns as being upregulated in the in planta condition. Functions of this protein are well known in E. coli and include organizing and compacting of the DNA but it is also important for the global regulation of the expression of approximately 5% of all genes in E. coli including proteins involved in transcription, translation and in production of components of the cell envelope which are needed for the adaptation to varying environments23. Moreover, research on Shigella spp. has shown that the ecological fitness of this pathogen is closely interwoven with the silencing of virulence genes by H-NS. Also, for this pathogen it is known that within the host, silencing mediated by H-NS orchestrates a precise and hierarchical expression of virulence genes that occur in response to environmental and host related cues24–26. These findings provide evidence for a possible role for H-NS in infection of E. amylovora since this protein is upregulated for both strain in the in planta condition and opens possibilities for further research.
As previous identified, several proteins related to RNA processing were found to be more abundant for the higher virulent strain in comparison with the lower one in an in planta comparison15. Interestingly, during this research, several of these proteins were identified in higher amounts for the in planta condition in PFB5 in comparison with in vitro including enolase and polynucleotide phosphorylase. Therefore, these proteins, indicating a higher RNA processing, may have a major implication on the difference between this low and higher virulent strain in their ability to survive and infect their host.
The design of the experiment allowed us to identity proteins that were corresponding between conditions and between both strains (Fig. 1). Thereby, we were able to extract the proteins that were shared between strains for both the in vitro comparison (Fig. 4A) and in planta comparison (Fig. 4B). In vitro, a strong enrichment of pathways could be found between the shared proteins. For the in planta condition, there was no enrichment in pathways, although, a strong interconnection exists between the proteins (Fig. 4B). Amongst these proteins, we found SrlD, a protein involved in the conversion of sorbitol, the main transport sugar alcohol in apple and pear27, to β-D-fructose-6P. It has been shown that sorbitol provides the pathogen with a good carbon source for the production of amylovoran and increases exopolysaccharide biosynthesis28 and that SrlD is required for the fire blight symptom formation on apple seedlings29. This protein can be of aid for the pathogen to overcome recognition by the immune system of the plant and help it to survive inside the host by allowing it to use sorbitol as a carbon source.
For this research, we were especially interested in the stress related defenses the pathogen uses to cope with the immune system of the plant. In their defense against pathogens, plants rely on the innate immunity of each cell and on systemic signals generated at the infection sites to fight off infection30. Upon infection with E. amylovora, an oxidative stress response is induced in the host including an accumulation of superoxide, lipid peroxidation, electrolyte leakage and enzyme induction, resembling an incompatible interaction17. Results show that several stress mechanisms are addressed for both strains. Similar proteins between both strains are observed of which PspA appears to be an important one. This protein is the major product of the pspABCDE operon in E. coli and in Yersinia enterocolitica, and plays an important role in the perception of membrane stress and consecutively signaling to the transcription apparatus, thereby using an ATP-hydrolysing transcription activator to produce effector proteins to overcome the stress31. Further investigation in E. amylovora would be necessary to prove that the same mechanism is present, however, this further emphasizes the importance of transcription regulation during the infection process. As previously reported, we see an upregulation of CspG, a cold shock protein for the lower virulent strain and an upregulation of DnaK, a heat shock protein, in PFB515.
Remarkably, our proteome analysis showed a higher abundance of structural components of the F0F1-ATP synthase both for LMG2024 as PFB5. Transcriptome analysis only confirmed this for the higher virulent strain, PFB5 (Fig. 5C,D). Differences between proteome and transcriptome data may be observed in some cases32. Proteome data give information on the presence of a specific, final protein, whilst transcriptomics does not include posttranslational modifications and other processes that have an influence on the final protein product. Two important physiological functions have been assigned to the F0F1-ATP synthase in bacteria including oxidative phosphorylation by the synthesis of ATP aerobically from ADP and inorganic phosphate using the energy of an electrochemical ion gradient and the reverse reaction whereby a transmembrane ion gradient or proton motive force (PMF) is generated anaerobically at the expense of ATP hydrolysis when the driving force is low33. The generation of this PMF could be important to overcome an acidic environment by the increase of the intracellular pH34. It is known that pathogenic elicitors can induce a cytosolic acidification due to H+ influx in plants35–37. An upregulation of structural proteins of this F0F1-ATP synthase in E. amylovora during infection, may be important for acid tolerance induced by the plant as a defense mechanism. Moreover, both proteome as transcriptome results show a strong upregulation of these proteins/genes for the higher virulent strain PFB5 (Fig. 5D). Previously, we reported a higher gene expression of nearly all type III secreted effectors for this higher virulent strain16, which are possible inducers of cytoplasmic acidification by the plant. However, this hypothesis must be further investigated to prove the effective role of the F0F1-ATP synthase in acidic tolerance in E. amylovora.
In conclusion, this research highlights the importance of transcription and its regulation for the infection and survival strategy of E. amylovora. Moreover, a possible link was identified between stress perception at the membrane and effector production through PspA. Also, a higher expression of structural components of the F0F1-ATP synthase during infections indicates an effective mechanism against cytoplasmic acidification of the plant. Thereby these results form a firm platform for further investigation.
Materials and Methods
Bacterial strains and growth conditions
During this research, we included two strains of E. amylovora with a proven difference in virulence, namely LMG2024, a low virulent strain and PFB5, a high virulent strain14–16. For the in vitro experiments, the strains were grown overnight in liquid MM2 medium supplemented with 1% sorbitol28, shaking at 100 rpm at 24 °C. The bacteria were grown until the exponential phase (OD600nm = 0.8).
For the in planta experiments, the bacteria were extracted from the infected apple rootstocks as described previously15.
Protein extraction
The isolated bacteria from both in vitro and in planta experiments were washed in PBS; total protein fractions were extracted as described previously14,15. In short, after washing, the cells were disrupted by the addition of a lysis buffer (7 M urea, 2 M thio-urea and 4% (w/v) CHAPS) and sonication using a microtip. After a final centrifugation step (76 000 g for 90 min) the amount of proteins was determined using the 2-D Quant kit (GE Healthcare) according the instructions of the manufacturer.
2D DIGE
Next, analysis of the proteins was done using the differential in-gel electrophoresis (DIGE) for which samples were initially, minimally labeled using cyanine-derived fluors (3 Dyes 2D CYanine Labeling kit from Proteomics Consult) as described previously14,15. The individual Cy3, Cy5 and Cy2 labeled samples were mixed according to the experimental design including a dye swap. Each gel was loaded with 75 µg proteins, 25 µg from each sample and 25 µg from the internal standard. For this experiment, 4 biological replicates were considered for each strain and each condition. The separation in the first dimension was done with an IPGphor isoelectric focusing apparatus (GE Healthcare), using precast immobilized pH gradient (IPG) strips (SERVA; pH 3–10, 24 cm) on which the protein samples were loaded. The separation in second dimension was performed at 18 °C with an HPE-FlatTop Tower (SERVA) using precast, plastic-backed 10–15% polyacrylamide gels (2D-Large-Gel Flatbed NF 10–15% gradient gels) according to the manufacturer’s instructions. The fluorescent labeled proteins were visualized directly by scanning using an Ettan DIGE Imager (GE Healthcare). All gels were scanned at 100 μm (pixel size) resolution. Determination of spot abundance and statistical analysis were performed using the Progenesis SameSpot software. The spots considered, were spots with at least 1.5-fold changes in volume (P < 0.05) in one condition after normalization14–16. Following this analysis, the spots of interest were excised from the 2D gels in 1.5 mm diameter gel plugs using a semi-automated Screen picker (made by Proteomics Consult). Hereafter the plugs were processed for mass spectrometry according the protocol of Shevchenko et al.38.
LC-MS/MS analysis and data analysis
The tryptic peptide mixture was further analyzed using an Easy-nLC 1000 liquid chromatograph (Thermo Scientific), on-line coupled to a mass calibrated LTQ-Orbitrap Velos Pro (Thermo Scientific) via a Nanospray Flex ion source (Thermo Scientific) using sleeved 30 µm ID stainless steel emitters (spray voltage +2.3 kV, capillary temperature: 200 °C) as described previously14. Next, the analysis of the mass spectrometric raw data was carried out using Proteome Discoverer software v.1.2 (Thermo Scientific) with build-in Sequest v.1.3.0339 and interfaced with an in-house Mascot v.2.4 server (Matrix Science). The Scaffold protein and peptide scoring and identification data obtained for each sample were exported and subsequently, merged into a Microsoft Excel summary report (Supplementary Data S1).
The UniprotKB database (www.uniprot.org) was used to annotate the identified proteins, using the GO sets ‘biological process’ and ‘molecular function’. Moreover, the proteins that were shared between both strains for each condition were presented in a graphical form using the Search Tool for Retrieval of Interacting Genes (STRING) (https://string-db.org/). This is a database and web resource dedicated to understanding the relationship among differentially expressed proteins. The protein interactions can be displayed according their confidence, evidence and actions or interactions.
RNA extraction and gene expression analysis by quantitative RT-PCR
For the in vitro experiments, the E. amylovora strains were grown in liquid MM2 medium until the mid-exponential phase before adding 2 volumes of RNAprotect Bacteria Reagent (Qiagen, Venlo, The Netherlands). The in planta samples were treated with this reagent after the final wash step with PBS. Hereafter, the bacteria were collected by centrifugation (5000 g, 10 min) and the RNA was extracted using the RNeasy Mini Kit (Qiagen, Venlo, The Netherlands) as described previously14. Quantitative PCR (qPCR) was performed using Fast SYBR Green chemistry according to the manufacturer’s instructions on an ABI Prism 7500 Fast Real-Time PCR System (Applied Biosystems, Belgium). Relative gene expression was calculated as 2−∆Cq and was normalized with a normalization factor determined using the GrayNorm algorithm according to Remans et al.39. For LMG2024, reference genes proC and rpoS were adequate whilst for PFB5 rpsL and gyrA provided the best outcome. Gene-specific primers based on proteins of interest indicated by the proteomics study were developed using Primer3 (Whitehead Institute/MIT Center for Genome Research) (Supplementary Table S2).
Electronic supplementary material
Acknowledgements
Partial funding by project No. 101513 of the Agency of Innovation by Science and Technology (IWT-Flanders, Belgium) is acknowledged. Michelle Holtappels was indebted to the IWT for a predoctoral fellowship during this work. We thank Erik Royackers for technical assistance. We acknowledge the financial support from the Hercules Foundation in the framework of the project R-3986 ‘LC-MS@UHasselt: Linear TrapQuadrupool-Orbitrap mass spectrometer’. We acknowledge the use of the core facilities at PC Fruit in Kerkom (Belgium). Greet Clerx, Inge Hermans, Hilde Schoofs and Robin Wozniak are acknowledged for their technical support. The authors have no conflict of interest.
Author Contributions
M.H. and R.V. designed the experiments. M.H. conducted the experiments, interpreted the data and wrote the manuscript. Mass spectrometry and identification of the proteins was done by J.P.N. All authors were involved in discussion of the results and reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vanneste, J. L. In Fire blight: the disease and its causative agent, Erwinia amylovora. 1–6 (CABI Publishing, 2000).
- 2.Acimovic SG, et al. Control of fire blight (Erwinia amylovora) on apple trees with trunk-injected plant resistance inducers and antibiotics and assessment of induction of pathogenesis-related protein genes. Frontiers in Plant Science. 2015;6:16. doi: 10.3389/fpls.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norelli JL, et al. Rapid transcriptional response of apple to fire blight disease revealed by cDNA suppression subtractive hybridization analysis. Tree Genetics & Genomes. 2009;5:27–40. doi: 10.1007/s11295-008-0164-y. [DOI] [Google Scholar]
- 4.Baldo A, et al. Identification of genes differentially expressed during interaction of resistant and susceptible apple cultivars (Malus x domestica) with Erwinia amylovora. BMC Plant Biology. 2010;10:1. doi: 10.1186/1471-2229-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarowar S, et al. Transcriptome Analysis of Apple Blossom after Challenging with Fire Blight Pathogen Erwinia amylovora Wild Type and Mutant Strains. Xii International Workshop on Fire Blight. 2011;896:245–251. [Google Scholar]
- 6.Kamber T, et al. Fire blight disease reactome: RNA-seq transcriptional profile of apple host plant defense responses to Erwinia amylovora pathogen infection. Scientific Reports. 2016;6:21600. doi: 10.1038/srep21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao YF, Blumer SE, Sundin GW. Identification of Erwinia amylovora genes induced during infection of immature pear tissue. Journal of Bacteriology. 2005;187:8088–8103. doi: 10.1128/JB.187.23.8088-8103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfano JR, Collmer A. The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. Journal of Bacteriology. 1997;179:5655–5662. doi: 10.1128/jb.179.18.5655-5662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogdanove AJ, Wei ZM, Zhao L, Beer SV. Erwinia amylovora secretes harpin via a type III pathway and contains a homolog of yopN of Yersinia spp. Journal of Bacteriology. 1996;178:1720–1730. doi: 10.1128/jb.178.6.1720-1730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edmunds AC, Castiblanco LF, Sundin GW, Waters CM. Cyclic Di-GMP modulates the disease progression of Erwinia amylovora. Journal of Bacteriology. 2013;195:2155–2165. doi: 10.1128/JB.02068-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jean Beltran PM, Federspiel JD, Sheng X, Cristea IM. Proteomics and integrative omic approaches for understanding host-pathogen interactions and infectious diseases. Molecular Systems Biology. 2017;13:922. doi: 10.15252/msb.20167062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann RA, et al. Comparative Genomics of 12 Strains of Erwinia amylovora Identifies a Pan-Genome with a Large Conserved Core. PloS One. 2013;8:e55644. doi: 10.1371/journal.pone.0055644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smits THM, et al. Erwinia Amylovora in the Genomics Era: From Genomes to Pathogen Virulence, Regulation, and Disease Control Strategies. Journal of Plant Pathology. 2017;99:7–23. [Google Scholar]
- 14.Holtappels M, et al. A comparative proteome analysis reveals flagellin, chemotaxis regulated proteins and amylovoran to be involved in virulence differences between Erwinia amylovora strains. J Proteomics. 2015;123:54–69. doi: 10.1016/j.jprot.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 15.Holtappels M, et al. The in planta proteome of wild type strains of the fire blight pathogen, Erwinia amylovora. J Proteomics. 2016;139:1–12. doi: 10.1016/j.jprot.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Holtappels M, Noben JP, Valcke R. Virulence of Erwinia amylovora, a prevalent apple pathogen: Outer membrane proteins and type III secreted effectors increase fitness and compromise plant defenses. Proteomics. 2016;16:2377–2390. doi: 10.1002/pmic.201500513. [DOI] [PubMed] [Google Scholar]
- 17.Venisse JS, Gullner G, Brisset MN. Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiology. 2001;125:2164–2172. doi: 10.1104/pp.125.4.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao YF, et al. Systems level analysis of two-component signal transduction systems in Erwinia amylovora: Role in virulence, regulation of amylovoran biosynthesis and swarming motility. BMC Genomics. 2009;10:245. doi: 10.1186/1471-2164-10-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forst S, Delgado J, Inouye M. Phosphorylation of Ompr by the Osmosensor Envz Modulates Expression of the Ompf and Ompc Genes in Escherichia-Coli. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:6052–6056. doi: 10.1073/pnas.86.16.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park D, Forst S. Co-regulation of motility, exoenzyme and antibiotic production by the EnvZ-OmpR-FlhDC-FliA pathway in Xenorhabdus nematophila. Molecular Microbiology. 2006;61:1397–1412. doi: 10.1111/j.1365-2958.2006.05320.x. [DOI] [PubMed] [Google Scholar]
- 21.Ali GS, Reddy ASN. PAMP-triggered immunity Early events in the activation of FLAGELLIN SENSITIVE2. Plant Signaling & Behavior. 2008;3:423–426. doi: 10.4161/psb.3.6.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overhage J, Bains M, Brazas MD, Hancock REW. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. Journal of Bacteriology. 2008;190:2671–2679. doi: 10.1128/JB.01659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hommais F, et al. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Molecular Microbiology. 2001;40:20–36. doi: 10.1046/j.1365-2958.2001.02358.x. [DOI] [PubMed] [Google Scholar]
- 24.Stoebel DM, Free A, Dorman CJ. Anti-silencing: overcoming H-NS-mediated repression of transcription in Gram-negative enteric bacteria. Microbiology. 2008;154:2533–2545. doi: 10.1099/mic.0.2008/020693-0. [DOI] [PubMed] [Google Scholar]
- 25.Marteyn B, Gazi A, Sansonetti P. Shigella: a model of virulence regulation in vivo. Gut Microbes. 2012;3:104–120. doi: 10.4161/gmic.19325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Picker MA, Wing HJ. H-NS, Its Family Members and Their Regulation of Virulence Genes in Shigella Species. Genes (Basel) 2016;7:112. doi: 10.3390/genes7120112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aldridge P, Metzger M, Geider K. Genetics of sorbitol metabolism in Erwinia amylovora and its influence on bacterial virulence. Molecular and General Genetics. 1997;256:611–619. doi: 10.1007/s004380050609. [DOI] [PubMed] [Google Scholar]
- 28.Bellemann P, Bereswill S, Berger S, Geider K. Visualization of Capsule Formation by Erwinia-Amylovora and Assays to Determine Amylovoran Synthesis. International Journal of Biological Macromolecules. 1994;16:290–296. doi: 10.1016/0141-8130(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 29.Geider, K. In Fire Blight: The Disease and its Causitative Agent, Erwinia amylovora. (ed. Vanneste, J. L.) Ch. 7, 117–140 (CABI Publishing, 2000).
- 30.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 31.Joly N, et al. Managing membrane stress: the phage shock protein (Psp) response, from molecular mechanisms to physiology. FEMS Microbiology Reviews. 2010;34:797–827. doi: 10.1111/j.1574-6976.2010.00240.x. [DOI] [PubMed] [Google Scholar]
- 32.Hack CJ. Integrated transcriptome and proteome data: the challenges ahead. Brief Funct Genomic Proteomic. 2004;3:212–219. doi: 10.1093/bfgp/3.3.212. [DOI] [PubMed] [Google Scholar]
- 33.Deckers-Hebestreit G, Altendorf K. The F0F1-type ATP synthases of bacteria: Structure and function of the F-0 complex. Annual Review of Microbiology. 1996;50:791–824. doi: 10.1146/annurev.micro.50.1.791. [DOI] [PubMed] [Google Scholar]
- 34.Cotter PD, Gahan CG, Hill C. Analysis of the role of the Listeria monocytogenes F0F1 -AtPase operon in the acid tolerance response. International Journal of Food Microbiology. 2000;60:137–146. doi: 10.1016/S0168-1605(00)00305-6. [DOI] [PubMed] [Google Scholar]
- 35.Mathieu Y, et al. Membrane Responses Induced by Oligogalacturonides in Suspension-Cultured Tobacco Cells. Plant Journal. 1991;1:333–343. doi: 10.1046/j.1365-313X.1991.t01-10-00999.x. [DOI] [PubMed] [Google Scholar]
- 36.Mathieu Y, et al. Cytoplasmic acidification as an early phosphorylation-dependent response of tobacco cells to elicitors. Planta. 1996;199:416–424. doi: 10.1007/BF00195734. [DOI] [Google Scholar]
- 37.He DY, et al. Gene activation by cytoplasmic acidification in suspension-cultured rice cells in response to the potent elicitor, N-acetylchitoheptaose. Molecular Plant-Microbe Interactions. 1998;11:1167–1174. doi: 10.1094/MPMI.1998.11.12.1167. [DOI] [Google Scholar]
- 38.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver stained polyacrylamide gels. Analytical Chemistry. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 39.Remans T, et al. Reliable gene expression analysis by reverse transcription-quantitative PCR: reporting and minimizing the uncertainty in data accuracy. Plant Cell. 2014;26:3829–3837. doi: 10.1105/tpc.114.130641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.