Abstract
Purpose
There are no standardized prognostication algorithms for metastatic radioiodine refractory (RAI-R) differentiated thyroid cancer (DTC). We hypothesize that [18F]-FDG PET/CT may predict progression vs. stability of disease based on quantitative analysis of metabolic tumor volume (MTV) and total lesion glycolysis (TLG).
Methods
Retrospective study of 62 patients with metastatic RAI-R DTC to determine clinical outcomes with median follow up from initial diagnosis of 11.1 years (8.38,14.1), range 1.2 – 20 years. Baseline [18F]-FDG PET/CT scans were evaluated qualitatively for regional and distant metastases, and quantitatively for tumor burden based on MTV and TLG obtained by gradient segmentation method.
Results
After diagnosis of metastatic RAI-R disease was established, the five-year OS probability was 34% and median OS was 3.56 years (2.87, infinity). The five-year PFS probability was 19% and median PFS was 1.31 years (1.03, 2.38). TSH-suppressed thyroglobulin (Tg) levels >100 ng/mL and Tg doubling time (Tg-DT) <6 months were significantly associated with worse OS and PFS. Higher than median values of MTV and TLG were associated with worse OS (p = 0.06) and PFS (p = 0.007). Higher hazard of death was noted for higher values of log-MTV and log-TLG (HR 1.17 [95% CI 0.99, 1.39], p = 0.05 and HR 1.14 [95% CI 1.00, 1.31], p = 0.05, respectively).
Conclusions
[18F]-FDG PET/CT metabolic parameters can help define the volume and biologic variations of metastatic tumor burden. MTV and TLG can be used for dynamic risk stratification of patients with metastatic RAI-R DTC regarding PFS and complement Tg-DT for prognosis of clinical disease course.
Keywords: [18F]-Fluorodeoxyglucose (positron emission tomography scans), Differentiated Thyroid Cancer, Radioiodine Refractory Thyroid Cancer, PET/CT imaging thyroid cancer, MTV, TLG
Introduction
Current prognostic variables for differentiated thyroid cancer (DTC) at initial diagnosis include patient age, gender, tumor characteristics, extra-thyroidal extension and clinical stage, including the presence of regional and distant metastases. However, there are no standardized prognostication algorithms when metastases are subsequently diagnosed, particularly for radioiodine refractory (RAI-R) disease (1-2). A large study found that characterization into risk groups did not accurately identify populations; paradoxically, it found that some patients in the lowest risk group in each strategy died of cancer (3). Two studies demonstrated the poor predictive value of staging approaches, including the widely used TMN system (4,5). Thyroglobulin doubling time (Tg-DT) has been associated with prognosis in papillary thyroid carcinoma, with Tg-DT <1 year portending poor prognosis and Tg-DT >2 years signifying good prognosis. (6). However, Tg-DT cannot be calculated in the presence of anti-Tg antibodies, which invalidate Tg measurements, and cannot inform regarding the metabolic phenotype and spatial distribution of metastatic lesions.
The lack of standardized prognostication is complicated by the large variability in survival time for patients with metastatic DTC, ranging from 1 year to 30 years (2). Reported 10-year survival estimates for metastatic DTC vary between 29% (7) and 38% (8). The lack of accurate estimates of survival for the individual patient has important implications for treatment decisions, especially with the advent of targeted therapies (9-12). Due to the toxicity profile of these medications, current NCCN guidelines advocate active surveillance as standard of care in metastatic disease until evidence of symptomatic or clinically progressive disease, as outlined by the RECIST criteria (13-17). Ultimately, the inability to prognosticate hinders clinicians’ ability to stratify patients by risk and deliver appropriate therapies (18).
[18F]Fluorodeoxyglucose (FDG) positron emission tomography (PET) has been studied for prognostic value in metastatic DTC (19). For simplicity, [18F]-FDG PET/CT will be synonymous with PET/CT, FDG-PET, or FDG scan for remainder of article. Robbins et al. demonstrated that patients with a positive FDG scan had a 7 fold increased risk of mortality compared with thyroid cancer survivors who had a negative FDG scan (20). Specifically, metabolic tumor volume (MTV) and total glycolytic activity (also referred to as total lesion glycolysis, TLG) have been proposed as markers to improve prognostication and enhance clinical staging in human solid tumors (21).
We hypothesized that the metabolic activity of metastatic lesions determined by FDG-PET will correlate with survival and identify patients who may benefit from emerging therapies for metastatic thyroid cancer. The primary end-point of this study was overall survival (OS) and progression free survival (PFS) in patients diagnosed with non-iodine avid distant metastatic disease who underwent baseline evaluation with [18F]-FDG PET/CT imaging in our clinic.
Material and Methods
Patients and Data Collection
This study was approved by the University of Michigan Institutional Review Board (HUM00100626). Informed consent requirements were waived for this study. Patient data was extracted from a database [EMERSE] of all patients treated for metastatic DTC from 1995 though 2014 at our center (n = 2460). We reviewed the records of all thyroid cancer patients who underwent a PET/CT (n =332). Patients with anaplastic, medullary, undifferentiated thyroid carcinoma or other active non-thyroid malignancies (n=11) were excluded. DTC patients were referred for PET/CT imaging at the treating physician’ discretion for evaluation of residual or recurrent thyroid carcinoma in the setting of elevated serum thyroglobulin (Tg) level with a negative radioiodine (RAI) whole-body scan (WBS) after prior postoperative 131I therapy. All patients included in this study had received postoperative 131I therapy and underwent follow-up in our clinic at 12 months after RAI treatment with diagnostic RAI WBS and stimulated thyroglobulin (Tg) levels to assess interval response to RAI treatment. For this evaluation the patients underwent preparation with a low-iodine diet for 2 weeks, and stimulation with either rhTSH (Thyrogen) injections or thyroid hormone withdrawal (THW) as follows: either levothyroxine deprivation for 4 weeks, or triiodothyronine treatment for 4 weeks, with subsequent discontinuation for 2 weeks prior to WBS. Urinary iodine levels were obtained in all patients to determine the adequacy of iodine deprivation, demonstrating spot urine iodine levels < 100 mcg/L, consistent with adequate preparation. For patients with negative WBS and elevated Tg levels, PET/CT imaging was performed to identify non-iodine avid metastatic disease. In accord with other studies, in our center empiric 131I therapeutic challenge is used only in patients with elevated Tg levels and without significant FDG uptake detected on PET/CT scan, (22) thus avoiding repeated courses of 131I therapy in patients unlikely to benefit from such treatment. (23)
We removed from analysis the DTC patients with negative PET/CT scans (n =111) and false positive PET/CT scans (n = 13). Patients with loco-regional disease identified on PET/CT scan who were treated with surgical resection and/or external beam radiation therapy (n = 101) resulting in complete therapeutic response were excluded from analysis. Furthermore, a group of patients with indolent, small burden of loco-regional disease (n = 11) conservatively managed on active clinical surveillance with evidence of stable disease were not included in the study. Patients who were lost to follow up and patients whose scans were irretrievable for review or subsequent follow-up scans were performed at other institutions (n =23) were also excluded. We ultimately analyzed the PET/CT scans of 62 patients with metastatic RAI-R DTC.
[18F]-FDG PET/CT
The [18F]-FDG PET/CT scan was performed in a standard fashion from mid-skull to proximal thighs on a flat tabletop using a hybrid PET/CT scanner (Biograph Classic; Siemens Medical Solutions, Hoffman Estates, IL), with patients’ arms down. The CT images (5-mm slices) for the PET/CT study typically were obtained during quiet ventilation. Emission PET images were obtained beginning 60 minutes after administration of 8 to 10 mCi of [18F]FDG and required blood glucose level < 150 mg/mL at the time of radiotracer injection. The PET/CT scans were evaluated qualitatively for the presence of regional and distant metastatic tumor by a nuclear medicine physician (A.M.A) and by objective quantification of FDG uptake in regions of interest (ROI). Qualitative image review and radiologic interpretation was performed using MedView software. Subsequently the archived PET/CT studies were transferred to a MIM workstation (software version 6.x. MIM Software Inc., Cleveland, OH, USA) for MTV and TLG quantification based on gradient segmentation method using MIM PET Edge software. The gradient segmentation method used in MIM 6.x. software calculates spatial derivatives along the tumor radii and then defines the tumor edge on the basis of derivative levels and continuity of the tumor edge. The software relies on an operator-defined starting point near the center of the lesion. As the operator drags the cursor from the center of the lesion, six axes extend out, providing visual feedback for the starting point of gradient segmentation. Spatial gradients are calculated along each axis interactively, and the length of an axis is restricted when a large spatial gradient is detected. The six axes define an ellipsoid that is then used as an initial bounding region for gradient detection. Gradient segmentation method reduces operator variability by detecting the largest spatial gradient in metabolic activity for definition of lesional MTV. For each patient total MTV was calculated by summing the MTV of all metastatic lesions detected in the patient. The TLG was defined as (MTV) × Mean Standardized Uptake value (SUV mean) and was semi-automatically calculated by the MIM software (21).
Clinical Pathology, Laboratory Markers, and Staging
Diagnosis of all tumors was performed by pathologists and classified according to the World Health Organization (WHO) classification. Demographic, clinical, and pathological data was obtained from hospital records. Disease was staged at the time of diagnosis using the 2010 American Joint Committee on Cancer (AJCC) 7th Edition classification staging system (24). Thyroglobulin (Tg) levels at the time of PET/CT scan were obtained from chart review. Two patients did not have Tg levels at time of initial PET scan. Twelve patients had anti-Tg antibodies, interfering with measurements of Tg and precluding them from analysis. Tg-DT was calculated using the Doubling Time and Progression Calculator created by Miyauchi et al (6). As serum Tg values can vary with TSH values, the calculation of Tg-DT time only included TSH-suppressed Tg values. For 3 patients who underwent surgical resection of cervical metastatic disease, the calculation of Tg-DT resulted in negative Tg-DT values indicating decreased tumor burden, thus invalidating the predictive value of Tg-DT calculation.
Statistical Analysis
The primary analysis consisted of 62 patients with metastatic RAI-R DTC. First we used Wilcoxon Signed Rank tests to explore the relationship between MTV/TLG value and variables of histology, gender, prior therapy, Tg, Tg-DT, and sites of metastases. We use the Kaplan-Meier method to estimate OS and PFS probabilities within groups defined by MTV/TLG and the aforementioned variables. We tested differences between the survival curves using logrank tests and used Cox proportional hazards regression models to study the unadjusted associations between continuous MTV/TLG and OS/PFS. All analyses were conducted using R package, version 3.3.0 (Vienna, Austria).
Results
Patient Characteristics
The clinical, histo-pathological and laboratory information are summarized in Table 1. All patients underwent total thyroidectomy; prophylactic neck dissection and/or therapeutic central and/or unilateral or bilateral neck dissections were performed at the discretion of the endocrine surgeon. Post-operatively, all patients received RAI therapy for ablation of thyroid remnant and/or treatment of metastatic disease. All patients included in this study had negative follow-up post-therapy RAI WBS and elevated Tg levels, which prompted evaluation with FDG PET/CT for localization of non-iodine avid RAI-R metastatic disease. In addition to surgery and post-operative RAI therapy, external beam radiation therapy (EBRT) was performed in 17 patients (27.4%), chemotherapy in 2 patients (3.2%) and 1 patient (1.6%) had received both EBRT and chemotherapy. At the time of initial FDG-PET scan, the median serum Tg was 272 ng/mL (range 1.8 - 23,990). FDG-PET scan demonstrated both distant and regional metastases in 63% of our patients.
Table 1.
Patient Demographics, Tumor Histology and Laboratory Results (N = 62)
| Characteristic | Number of patients (%) |
|---|---|
| Gender | |
| Female | 25 (40.3) |
| Male | 37 (59.6) |
| Age: 63.2 ± 13.1 y, 16-89a | |
| <45 | 3 (4.8) |
| >45 | 59 (95.1) |
| Histology | |
| Follicular | 4 (6.4) |
| Hurthle | 14 (22.5) |
| Papillary | |
| Classical Papillary Thyroid Cancer | 37 (59.6) |
| Insular | 1 (1.6) |
| Macrofollicular Variant | 1 (1.6) |
| Tall Cell Variant | 5 (8.0) |
| Baseline Thyroglobulin [ng/mL] | |
| <10 | 5 (8.0) |
| 10-100 | 10 (16.1) |
| 100-1000 | 17 (27.4) |
| >1000 | 16 (25.8) |
| Anti-thyroglobulin antibodies present | 12 (19.5%) |
| Missing | 2 (3.2%) |
| Thyroglobulin Doubling Time (TgDT) | |
| Minimum TgDT: 1.8 Months | |
| Maximum TgDT: 62.9 Months | |
| TgDT between 0 and 6 months | 17 (27.4) |
| TgDT between 6 and 12 months | 17 (27.4) |
| TgDT between 12 and 24 months | 10 (16.1) |
| TgDT>24 months | 3 (4.8) |
| Anti-thyroglobulin antibodies present | 12 (19.5%) |
| Negative TgDT due to local treatmentb | 3 (4.8%) |
| Stage at Diagnosis | |
| I | 3 (4.8) |
| II | 3 (4.8) |
| III | 18 (29.0) |
| IVA | 13 (20.9) |
| IVB | 6 (9.6) |
| IVC | 19 (30.6) |
| Prior Treatments | |
| Surgery, RAI | 42 (67.7) |
| Surgery, RAI, Chemo | 2 (3.2) |
| Surgery, RAI, EBRT | 17 (27.4) |
| Surgery, RAI, Chemotherapy, EBRT | 1 (1.6) |
| Site of Metastatic Disease | |
| Regional Lymph Node | 27 (43.5) |
| Distant Lymph Node | 19 (30.6) |
| Lung | 44 (70.9) |
| Bone | 17 (27.4) |
| Other | 2 (3.2) |
|
| |
| Results of Initial PET Scan | |
| Regional Mets | 6 (9.6) |
| Distant Mets | 17 (27.4) |
| Both | 39 (62.9) |
Presented as mean ± SD, range
Surgical resection of cervical metastases resulted in negative TgDT
RAI, Radioiodine; EBRT, External Beam Radiation Therapy
Relationship with Metabolic Parameters
The median MTV was 9.08 mL and median TLG was 49.1. In order to create a more normal distribution and minimize the effect of outliers, we formed analysis using log-transformed measurements. The median log-MTV and log-TLG values were 2.18 mL and 3.89, respectively (Table 2). There is a strong correlation between log-MTV and log-TLG as demonstrated by the scatterplot in Supplemental Figure 1. The Spearman correlation coefficient between MTV and TLG is 0.94.
Table 2.
Baseline Values of MTV, TLG, log-MTV, and log-TLG Among All Subjects (N=62)
| Mean | Median | Minimum | Maximum | Standard Deviation | |
|---|---|---|---|---|---|
| MTV | 50.9 | 9.08 | 0.06 | 473.2 | 97.1 |
| TLG | 582.7 | 49.1 | 0.25 | 7485 | 1364 |
| Log-MTV | 2.20 | 2.18 | -2.80 | 6.15 | 2.14 |
| Log-TLG | 3.94 | 3.89 | -1.37 | 8.92 | 2.53 |
MTV, Metabolic tumor volume; TLG, total lesion glycolysis.
In our group of patients, 17 received tyrosine kinase inhibitor (TKI) therapy following their diagnosis of metastatic RAI-R DTC after the initial positive FDG-PET scan. For patients on TKI therapy the median MTV was 31.2 mL and TLG was 125.2, reflective of higher tumor burden with aggressive metabolic characteristics. Median log-MTV and log-TLG for these patients were 3.44 mL and 4.83, respectively. Our analysis showed that patients that received TKI tended to have higher log-MTV values, although this association did not reach the threshold of statistical significance (p = 0.28).
We explored the relationship between log-MTV/log-TLG and variables of histology, gender, prior therapy, Tg, Tg-DT, and sites of metastases. Patents with bone metastases had significantly higher values for log-MTV and log-TLG (p = 0.004), shown in Supplemental Figure 2. Higher baseline Tg levels were significantly associated with higher log-MTV and log-TLG with a Spearman correlation coefficient of 0.59 and 0.58 respectively (p <0.001 from linear regression). Higher values for log-MTV and log-TLG were associated with a lower Tg-DT, but the trend did not reach the threshold for statistical significance (p = 0.38 and p = 0.16, respectively). Specifically, we notice a trend that Tg-DT <12 months relates to larger log-MTV and log-TLG.
Clinical Outcome
From time of initial diagnosis, median follow up for OS was 11.1 years (8.38,14.1), with a range from 1.2 to 20 years. Median overall survival (OS) from time of diagnosis was 9.22 years (7.19, Infinity) (Figure 1, panel A). After diagnosis of metastatic RAI-R disease was established based on a negative radioiodine (RAI) whole-body scan (WBS) after prior postoperative 131I therapy with elevated Tg and positive FDG-PET imaging, median follow up for OS was 3.91 years (3.70, 5.60) with a range of 0.32 - 12 years. The patients underwent regular clinical follow-up with biochemical testing for Tg levels (tumor biomarker) and computer tomography (CT) imaging surveillance every 6 months to assess for stability versus disease progression and determine clinical disease status. At the time of last follow up, 32 patients had died with evidence of disease progression and 30 were alive with persistent disease. After the diagnosis of metastatic RAI-R disease the five year OS probability was 0.34 (0.21, 0.54) and median OS was 3.56 years (2.87, infinity) (Figure 1, panel B). Median follow up for the PFS was 3.70 years (2.53, Infinity). In our study, 43 patients had evidence of disease progression with a five year PFS probability of 0.19 (0.10, 0.35) and a median PFS after the diagnosis of metastatic RAI-R disease of 1.31 years (1.03, 2.38) (Figure 1, panel C).
Fig. 1.
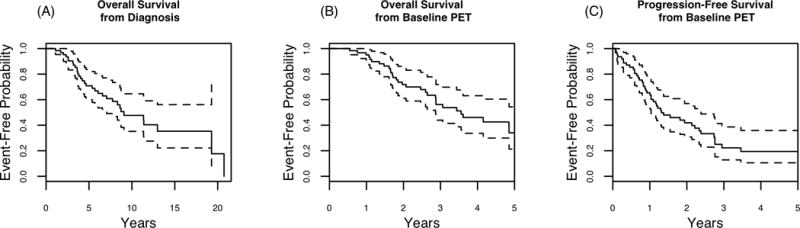
Overall Survival of Metastatic RAI-R DTC Patients (N=62) from initial diagnosis (A). Kaplan Meier plots of OS (B) and PFS (C) from initial FDG-PET scan.
Figure 2 shows estimated OS and PFS event-free probabilities based on whether TLG or MTV values were greater than, or less than median for each variable. There was a trend towards significance for improved OS with TLG and MTV values less than the median (p = 0.06). There was a statistically significant improvement in PFS with TLG and MTV values less than the median (p = 0.007). Additionally, we used regression trees to determine a value at which the OS is most different between the two groups. This yielded a threshold for MTV of 57 mL for OS and PFS (p = 0.015 and 0.023, respectively) and a TLG of 6.3 for OS and PFS (p = 0.009 and 0.016, respectively).
Fig. 2.
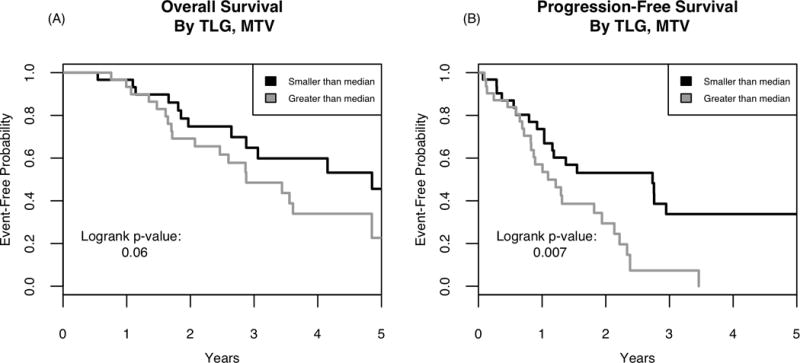
Kaplan Meier curves for OS (A) and PFS (B) based on metabolic parameters of TLG and MTV.
We further used Cox regression modeling to study the unadjusted relationship between MTV/TLG and OS/PFS (Table 3). We observed significantly higher hazard of death for higher values of log-MTV and log-TLG (both with p-value = 0.05). Furthermore, we observed higher rates of progression or death for higher values of log-MTV (HR 1.21 [1.05, 1.39] p = 0.006) and log-TLG (HR 1.19 [1.05, 1.34] p = 0.006). Of note, greater than median value for either MTV or TLG was significantly associated with worse PFS (HR 2.35 [1.24, 4.47], p = 0.009).
Table 3.
Unadjusted Cox Modeling of OS and PFS using Baseline PET Scan Values
| Predictor | Overall Survival | Progression-Free Survival | ||
|---|---|---|---|---|
| HR, (95% CI) | P-value | HR, (95% Cl) | P-Value | |
| Log-MTV | 1.17 (0.99, 1.39) | 0.05 | 1.21 (1.05, 1.39) | 0.005 |
| Log-TLG | 1.14 (1.00, 1.31) | 0.048 | 1.19 (1.05, 1.34) | 0.004 |
| Greater than Median Value | 1.96 (0.94, 4.09) | 0.07 | 2.35 (1.24, 4.47) | 0.008 |
HR, Hazard Ratio; MTV, Metabolic tumor volume; TLG, total lesion glycolysis.
Sub-Analysis: Other Covariates
Our analysis demonstrated that the log-MTV values for patients on TKI therapy tended to be higher, but this association was not significant. Kaplan-Meier plots for OS among subjects that did and did not receive TKI therapy is shown in Supplemental Figure 3. TKI therapy was associated with significantly higher overall survival rates (0= 0.04).
We did not note differences in OS when examining histology subtypes or specific metastatic sites such as lung, bone or both. We observed a trend towards worse PFS for subjects with bone metastases. However this did not reach the threshold of statistical significance (p = 0.068) (Supplemental Figure 4).
TSH-suppressed Tg levels >100 ng/mL were significantly associated with worse OS and PFS with p<0.001 (Figure 3, panels A and B). Tg-DT <6 months was significantly associated with worse OS and PFS, as noted in Figure 3, panels C and D. Tg-DT >12 months trended towards better OS and PFS, but was not significant (Figure 3, panels E and F).
Fig. 3.
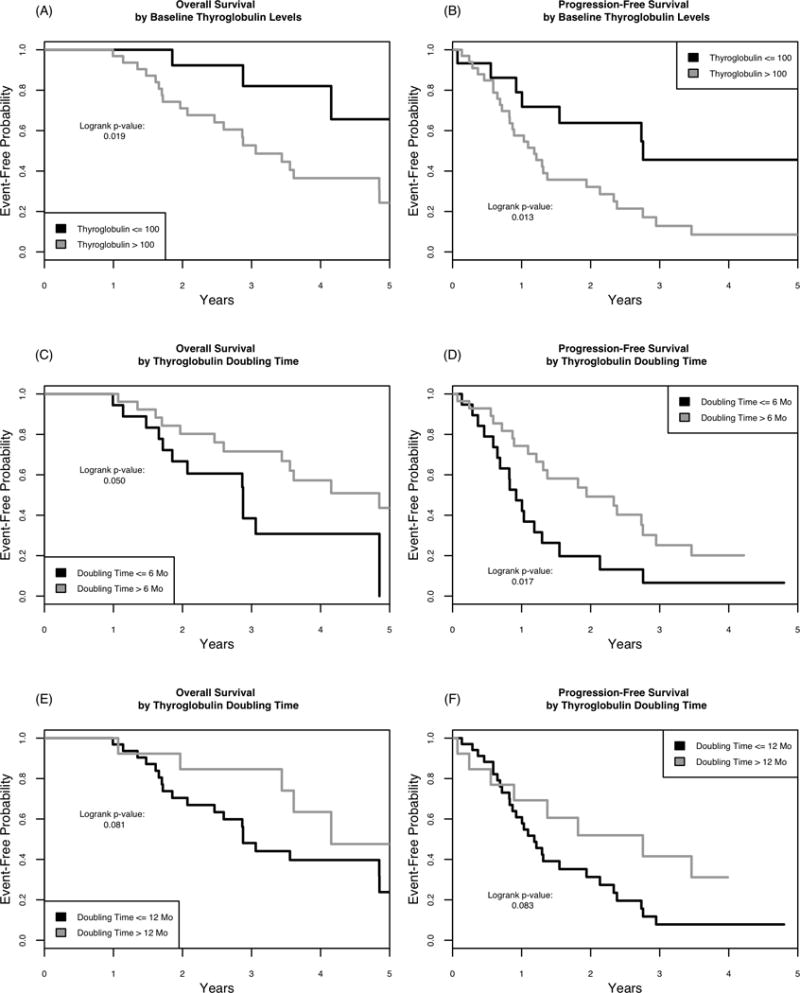
Kaplan-Meier plots for OS (A) and PFS (B) based on baseline Tg levels. Kaplan-Meier plots for OS (C) and PFS (D) based on Tg-DT < or > 6 months and OS (E) and PFS (F) based on Tg-DT < or > 12 months.
Discussion
Our study demonstrates the utility metabolic imaging with FDG PET/CT for prognosis in patients with RAI-R DTC. Prior research has shown the OS variability in thyroid cancer patients, distinguishing two phenotypes in metastatic thyroid cancer - indolent and aggressive. On a molecular level, this is largely driven by genetic patterns with specific mutations associated with aggressiveness of thyroid cancer (25). Approximately 5-15% of thyroid cancer patients will become RAI-R during the course of their disease (26, 27). It is estimated that overall survival in RAI-R DTC ranges between 2.5 years and 4.5 years (20, 27). Our analysis is consistent with these estimates, demonstrating a median OS of 3.56 years and median PFS of 1.91 years. Furthermore, our study is supported by Robbins et al, which demonstrated PFS of 53 months in patients with FDG-PET positive disease (20). Prior studies have shown that thyroid cancers concentrating RAI tend to have more favorable prognosis boasting of 10-year survival greater than 90%, while RAI negative (non-iodine avid) thyroid cancers have a dire 10-year survival of 10% (27). Our results concur with prior literature regarding the guarded prognosis for FDG-avid metastatic DTC, with a calculated 5-year OS probability of 34% and 5-year PFS probability of 19% in our study. This strengthens our proposed hypothesis that FDG-PET positivity can serve as a prognostic marker by identifying the imaging phenotype of patients with aggressive disease.
Tg levels and Tg-DT have been studied for prognostic value in prior studies (6, 28). Independent of many classical prognostication factors (TMN stage, age, and gender), Tg-DT remained a predictor of survival, distant metastases, and loco-regional recurrence on multi-variate analysis [6]. In our study, higher TSH-suppressed Tg levels (>100) were significantly associated with worse OS and PFS and also significantly correlated with higher levels of log-MTV and log TLG. Classically, Tg-DT <12 months has been associated with worse prognosis (6). Our analysis revealed that Tg-DT <6 months (p = 0.05) could be used as a new indicator of poor prognosis. Furthermore, we noted that there was an association with higher log-TLG and log-MTV values and lower Tg-DT. Although this association did not reach the threshold for statistical significance, the observed trend supports that FDG-PET metabolic parameters can be used in a prognostication algorithm.
Over 10 years ago Robbins et al. evaluated a similar hypothesis, that FDG-PET/CT imaging can be used as a prognostic tool for metastatic non-iodine avid DTC. They determined that total FDG tumor volume and lesional SUVmax correlate with survival. Wang et al noted a FDG volume > 125 ml. was associated with worse survival (19). A recent study demonstrated the prognostic value of FDG-PET in 37 patients with metastatic differentiated thyroid cancers, showing that only the metabolic parameters of MTV and TLG were predicative of survival. The authors noted a TLG <154 associated with improved PFS and OS in a univariate analysis (29). Our study confirms this hypothesis demonstrating a trend towards improved OS and statistically significant improvement in PFS in patients with lower than median values for TLG and MTV. In our analysis, we used the median for MTV and TLG as a threshold to create risk categories. Furthermore, we showed that MTV threshold of 57 mL and TLG threshold of 6.3 appear to significantly separate the groups in regard to OS and PFS. The difference in the actual measurement of MTV and TLG between our study and prior studies likely relates to different tumor segmentation methods employed for tumor quantification. In our study we used gradient edge detection algorithm for tumor segmentation, as opposed to manual tumor segmentation or threshold segmentation methods based on arbitrary cutoff values of 30%, 40% or 50% SUVmax thresholds for MTV determination. Based on both in-vitro studies (tumor phantom experiments) and clinical studies, gradient segmentation has been shown to be the most accurate and consistent technique for tumor volume definition, demonstrating superior correlation and reliability with the pathology-based tumor volume as compared to threshold segmentation methods (30, 31). Further prospective studies using gradient tumor segmentation methods can help determine specific targets to categorize patients in higher and lower risk phenotypes.
Using the metabolic parameters of MTV and TLG as surrogate markers for tumor burden and biologic aggressiveness, FDG-PET/CT imaging can serve as an imaging biomarker for prognosis of OS and PFS. Prospective studies can be designed to elicit if FDG-PET can detect phenotypically aggressive tumor types, serving to identify patients that would benefit from early initiation of TKI therapy. Indeed, recently Lin et al. studied changes in metastatic tumor size and glucose metabolism (SUVmax) in a prospective study of ten patients receiving apatinib therapy for RAI-refractory metastatic DTC. They noted a significant decrease in the tumor volume, as well as tumor SUVmax, demonstrating that FDG-PET/CT imaging can also be used to assess treatment response (32).
The limitations of our study largely arise from the retrospective nature of the study. While our sample size is moderate, there is still a concern for type II error due to lack of power. Due to the sample size, we could not adjust for potential confounders. Our study population only included patients with metastatic DTC who were referred for FDG-PET imaging at their physician’s discretion. Currently, active surveillance utilizes serial CT scans to identify progression of disease in patients with metastatic disease. At this time, FDG-PET imaging is approved with Medicare reimbursement to aid in management of thyroid cancer patients with elevated Tg levels and negative radioactive iodine scans. However, it is not integrated into the management of metastatic disease as a method for prognostication. Though a few of our patients did indeed have repeat FDG-PET scans, there were not enough data points to analyze the utility of serial FDG-PET scans to reassess disease status and potentially treatment response, which remains an area to explore for future studies.
Conclusion
Advances in molecular diagnostic imaging and the clinical availability of PET/CT produced a paradigm shift in the management of thyroid cancer by allowing characterization of tumor biology. 18F-FDG PET/CT metabolic parameters can be used to define the volume and biologic variations of metastatic tumor burden. MTV and TLG can be used for dynamic risk stratification of patients with metastatic RAI-R DTC regarding PFS, and complement Tg-DT for prognosis of clinical disease course. In the era of emerging treatment options, there is an impetus to generate prognostication algorithms for this patient population. Our study demonstrates the use of FDG-PET/CT imaging for predicting the course of metastatic RAI-refractory DTC.
Supplementary Material
Acknowledgments
We acknowledge the contribution of Adam Nelson, clinical support engineer, regarding software support from MIM software Inc., Cleveland, Ohio for metabolic parameters analysis.
Footnotes
Disclosure: The authors do not have any conflicts of interest.
References
- 1.Hay I. Prognostic Factors in thyroid carcinoma. Thyroid Today. 1989;12:1–9. [Google Scholar]
- 2.Ain KB. Papillary thyroid carcinoma. Endorincol Metab Clinics North Am. 1995;24:711–760. [PubMed] [Google Scholar]
- 3.DeGroot LJ, Kaplan EL, Straus FH, et al. Does the method of management of papillary thyroid carcinoma make a difference in outcome? World J Surg. 1994;18:123–130. doi: 10.1007/BF00348202. [DOI] [PubMed] [Google Scholar]
- 4.Sherman SI, Brierley JD, Sperling M, et al. Prospective multicenter study of thyroiscarcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–1021. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Brierley JD, Panzarella T, Tsang RW, et al. A comparison of different staging systems predictability of patient outcome. Thyroid carcinoma as an example. Cancer. 1997;79:2414–2423. [PubMed] [Google Scholar]
- 6.Miyauchi A, Kudo T, Miya A, et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid. 2011;21:707–716. doi: 10.1089/thy.2010.0355. [DOI] [PubMed] [Google Scholar]
- 7.Hoie J, Stenwig AE, Kullmann G, et al. Distant metastases in papillary thyroid cancer. A review of 91 patients. Cancer. 1988;61:1–6. doi: 10.1002/1097-0142(19880101)61:1<1::aid-cncr2820610102>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Schlumberger M, Tubiana M, De Vathaire F, et al. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986;63:960–967. doi: 10.1210/jcem-63-4-960. [DOI] [PubMed] [Google Scholar]
- 9.Perez CA, Santos ES, Arango BA, et al. Novel molecular targeted therapies for refractory thyroid cancer. Head Neck. 2012;34:736–745. doi: 10.1002/hed.21755. [DOI] [PubMed] [Google Scholar]
- 10.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 13.Klein Hesselink EN, Steenvoorden D, Kapiteijn E, et al. Therapy of endocrine disease: response and toxicity of small-molecule tyrosine kinase inhibitors in patients with thyroid carcinoma: a systematic review and meta-analysis. Eur J Endocrinol. 2015;172:R215–225. doi: 10.1530/EJE-14-0788. [DOI] [PubMed] [Google Scholar]
- 14.Burtness B, Anadkat M, Basti S, et al. NCCN Task Force Report: Management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Canc Netw. 2009;7(Suppl 1):S5–21. doi: 10.6004/jnccn.2009.0074. quiz S2-4. [DOI] [PubMed] [Google Scholar]
- 15.Brose MS, Frenette CT, Keefe SM, et al. Management of sorafenib-related adverse events: a clinician’s perspective. Semin Oncol. 2014;41(Suppl 2):S1–S16. doi: 10.1053/j.seminoncol.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Carhill AA, Cabanillas ME, Jimenez C, et al. The noninvestigational use of tyrosine kinase inhibitors in thyroid cancer: establishing a standard for patient safety and monitoring. J Clin Endocrinol Metab. 2013;98:31–42. doi: 10.1210/jc.2012-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Network NCC. Thyroid Cancer. https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf2016 [Available from: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf.
- 19.Wang W, Larson SM, Fazzari M, et al. Prognostic value of [18F]fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000;85:1107–1113. doi: 10.1210/jcem.85.3.6458. [DOI] [PubMed] [Google Scholar]
- 20.Robbins RJ, Wan Q, Grewal RK, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91:498–505. doi: 10.1210/jc.2005-1534. [DOI] [PubMed] [Google Scholar]
- 21.Dibble EH, Alvarez AC, Truong MT, et al. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med. 2012;53:709–715. doi: 10.2967/jnumed.111.099531. [DOI] [PubMed] [Google Scholar]
- 22.Leboulleux S, El Bez I, Borget I, Elleuch M, Deandreis D, Al Ghuzlan A, et al. Postradioiodine treatment whole-body scan in the era of 18-fluorodeoxyglucose positron emission tomography for differentiated thyroid carcinoma with elevated serum thyroglobulin levels. Thyroid. 2012;22(8):832–838. doi: 10.1089/thy.2012.0081. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Larson SM, Tuttle RM, Kalaigian H, Kolbert K, Sonenberg M, et al. Resistance of [18f]-fluorodeoxyglucose-avid metastatic thyroid cancer lesions to treatment with high-dose radioactive iodine. Thyroid. 2001;11(12):1169–1175. doi: 10.1089/10507250152741028. [DOI] [PubMed] [Google Scholar]
- 24.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 25.Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381:1058–1069. doi: 10.1016/S0140-6736(13)60109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlumberger M, Brose M, Elisei R, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014;2:356–358. doi: 10.1016/S2213-8587(13)70215-8. [DOI] [PubMed] [Google Scholar]
- 27.Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 28.Rossing RM, Jentzen W, Nagarajah J, et al. Serum Thyroglobulin Doubling Time in Progressive Thyroid Cancer. Thyroid. 2016;26:1712–1718. doi: 10.1089/thy.2016.0031. [DOI] [PubMed] [Google Scholar]
- 29.Masson-Deshayes S, Schvartz C, Dalban C, et al. Prognostic value of (18)F-FDG PET/CT metabolic parameters in metastatic differentiated thyroid cancers. Clin Nucl Med. 2015;40:469–475. doi: 10.1097/RLU.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 30.Werner-Wasik M, Nelson AD, Choi W, et al. What is the best way to contour lung tumors on PET scans? Multiobserver validation of a gradient-based method using a NSCLC digital PET phantom. Int J Radiat Oncol Biol Phys. 2012;82:1164–1171. doi: 10.1016/j.ijrobp.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sridhar P, Mercier G, Tan J, et al. FDG PET metabolic tumor volume segmentation and pathologic volume of primary human solid tumors. AJR Am J Roentgenol. 2014;202:1114–1119. doi: 10.2214/AJR.13.11456. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, Wang C, Gao W, et al. Overwhelming rapid metabolic and structural response to apatanib in radioiodine refractory differentiated thyriod cancer. Oncotarget. 2017;8:42252–42261. doi: 10.18632/oncotarget.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


