Abstract
OBJECTIVES
We evaluated the associations of obesity and cardiometabolic traits with incident heart failure with preserved vs reduced ejection fraction (HFpEF vs HFrEF). Given known sex differences in HF subtype, we examined men and women separately.
BACKGROUND
Recent studies suggest that obesity confers greater risk of HFpEF vs HFrEF. Contributions of associated metabolic traits to HFpEF are less clear.
METHODS
We studied 22,681 participants from 4 community-based cohorts followed for incident HFpEF vs HFrEF (EF> vs ≤50%). We evaluated the association of body mass index (BMI) and cardiometabolic traits with incident HF subtype using Cox models.
RESULTS
The mean age was 60±13 years, 53% were women. Over a median follow-up of 12 years, 628 developed incident HFpEF and 835 HFrEF. Greater BMI portended higher risk of HFpEF compared with HFrEF (HFpEF: HR 1.34 per 1 standard deviation (SD) increase in BMI; 95% CI 1.24-1.45 vs HFrEF: HR 1.18; 95% CI 1.10-1.27). Similarly, insulin resistance (HOMA- IR) was associated with HFpEF (HR 1.20 per 1 SD, 95% CI 1.05-1.37), but not HFrEF (HR 0.99, 95% CI 0.88–1.11; P<0.05 for difference HFpEF vs HFrEF). We found that the differential association of BMI with HFpEF vs HFrEF was more pronounced among women (P for difference HFpEF vs HFrEF=0.01) when compared with men (P=0.34).
CONCLUSIONS
Obesity and related cardiometabolic traits including insulin resistance are more strongly associated with risk of future HFpEF vs HFrEF. The differential risk of HFpEF with obesity appears particularly pronounced among women and may underlie sex differences in HF subtypes.
Keywords: HFpEF, Heart Failure, Obesity, Insulin Resistance, Sex Differences
INTRODUCTION
Heart failure (HF) is a growing public health concern, with increasing incidence and prevalence, that accounts for >1 million admissions per year, affecting nearly 6 million Americans (1). Of individuals with incident HF, approximately half have preserved rather than reduced ejection fraction (HFpEF vs HFrEF), and the prevalence of HFpEF is projected to exceed that of HFrEF in the near future (1-3). Obesity is a known risk factor for the future development of overall HF (4), and is associated with subclinical alterations in both systolic and diastolic function cross- sectionally (5).
Underlying drivers of cardiac remodeling in HFpEF and HFrEF appear at least partially distinct, with obesity postulated as a significant contributor to systemic inflammation leading to myocardial remodeling and resultant HFpEF, specifically (6). An initial study among women supports a greater population attributable risk of obesity to HFpEF than HFrEF (7). Motivated by these findings, we sought to examine obesity and associated cardiometabolic traits with future HFpEF vs HFrEF by leveraging a unique international collaboration of four longitudinal community-based cohorts including both men and women. Specifically, we examined associated traits including abdominal adiposity, insulin resistance, dysglycemia, and dyslipidemia.
Notably, sex differences have been described in the prevalence of obesity, body fat distribution, and energy homeostasis, with a higher prevalence of obesity among women (8). Further, cardiometabolic disease appears to harbor a greater risk of coronary artery disease and hypertension among women than men (9). While the prevalence of HFpEF is higher among women (8), the role of underlying sex differences in obesity and cardiometabolic dysfunction are unknown. Accordingly, we sought to conduct sex-specific analyses to better understand these differences.
METHODS
Study Sample
Participants from four community-based cohorts with adjudicated incident HF outcomes were included: the Cardiovascular Health Study (CHS) baseline examination (1989–1990; 1992–1993 for supplemental African-American cohort), the Framingham Heart Study (FHS) offspring examination 6 (1995–1998), the Multi-Ethnic Study of Atherosclerosis (MESA) baseline exam (2000–2002)], and the Prevention of Renal and Vascular Endstage Disease (PREVEND) baseline exam (1997–1998).(10-14) Individuals with prevalent HF (n=321), age <30 years at baseline examination (n=134), those with missing covariates (n=1,640) or missing follow-up (n=27), were excluded, leaving 22,681 individuals for analysis. Cohort specific details have been published previously.(15)
Clinical Assessment
All participant-level data were harmonized across the four cohorts and pooled together. Medical history, physical examination, fasting laboratory assessment, electrocardiography, and waist circumference were collected at the baseline examination. Blood pressure was calculated as the average of 2 seated measurements. Body mass index (BMI) was calculated as weight divided by height2 and expressed as kg/m2. Diabetes mellitus was defined using three criteria: fasting glucose ≥126 mg/dL, random glucose ≥200 mg/dL, or the use of hypoglycemic medications. Modest alcohol use was defined as ≥1 drink per day in both men and women. Electrocardiographic left ventricular hypertrophy (LVH) was defined based on accepted voltage and ST-segment criteria. Waist circumference was measured in centimeters. HOMA-IR and triglycerides were log transformed. Metabolic syndrome was defined according to the National Cholesterol Education Program, which includes three or more of the five following criteria: 1. Waist circumference ≥ 101.6 cm (40 inches, men) or ≥ 88.9 cm (35 inches, women); 2. Triglycerides ≥ 150 mg/dL or receiving pharmacologic treatment; 3. HDL cholesterol ≤ 40 mg/dL (men) or ≤ 50 mg/dL (women) or receiving pharmacologic treatment; 4. Blood pressure ≥ 130 mmHg systolic or 85 mmHg diastolic or receiving pharmacologic treatment; 5. Fasting glucose ≥ 100 mg/dL or receiving pharmacologic treatment.
Definition of Incident Heart Failure Subtypes
Individuals were prospectively followed for the first occurrence of incident HF or death within 15 years of the baseline examination. Outcomes were adjudicated using established protocols by study investigators after reviewing all hospital and outpatient medical records. HF was defined using a combination of signs and symptoms as previously reported.(15) Medical records were reviewed for assessment of LV function at or around the time of the first HF. Each incident HF event was categorized as HFpEF (LVEF>50%), HFrEF (LVEF≤50%), or unclassified (no LV function assessment available). Classification was based on echocardiography in over 85% of classified HF cases.
Statistical Analysis
Baseline clinical and laboratory covariates were summarized by cohort and in aggregate. In primary sex-pooled analyses, we examined the association of seven clinical predictors with HF subtype. Cause-specific Cox models were fitted separately for HFpEF and HFrEF, accounting for competing risks of death, other HF subtype, and unclassified HF. Clinical predictors included waist circumference, BMI, waist-to-hip ratio, HOMA-IR, triglyceride to HDL ratio, fasting glucose, and systolic blood pressure. For HOMA-IR analyses, we excluded participants with diabetes mellitus. Covariates known to be associated with HF were entered in the multivariable model, including age, systolic blood pressure (except systolic blood pressure analyses), hypertension treatment, diabetes mellitus status, smoking status, prevalent myocardial infarction, total cholesterol, HDL (except HDL analyses), left bundle branch block and left ventricular hypertrophy. Secondary analyses further adjusted for C-reactive protein and interim myocardial infarction. Hazard ratios were reported per pooled standard deviation increase in continuous predictor and a strata statement was included to specify study cohorts within the analysis. Primary analyses were considered significant using a Bonferroni corrected p-value (P=0.05/7 traits tested=0.007).
In secondary analyses, sex-specific Cox models were used to examine the association of clinical predictors with HF subtype and sex*covariate interaction terms tested in sex-pooled analyses. For each clinical predictor, HF subtype-specific coefficients were also compared using the Lunn- McNeil method (16). Cohort-specific analyses were performed, and a random effects meta- analysis performed to test for potential heterogeneity in the association of BMI with HF subtypes. We then conducted a sensitivity analysis excluding patients with diabetes mellitus in HOMA-IR and fasting glucose analyses. In exploratory analyses, we examined whether HOMA- IR may act as a mediator in the association of BMI and HFpEF. Further, we examined each of the five metabolic syndrome criteria with incident HF subtype using cause-specific Cox models. All statistical analyses were conducted with SAS version 9.4 for Windows (Cary, NC).
RESULTS
Our sample included a total of 22,681 participants from 4 community-based cohorts (23% from CHS, 15% from FHS, 29% from MESA and 32% from PREVEND). The mean age was 60±13 years, and 53% were women. The mean BMI was 27.1±4.9 kg/m2, with mean waist circumference of 94±14cm. A total of 23% of participants had obesity (21% of men, 25% of women), and 37% of participants met criteria for metabolic syndrome (37% among both men and women). Baseline characteristics by cohort are detailed in Table 1. Over a mean follow-up of 12±3 years, we observed a total of 2,081 incident HF events, of which 1,463 (70%) were classified into HF subtypes. There were 628 incident HFpEF (358 among women and 270 among men) and 835 incident HFrEF events (295 among women and 540 among men). As shown in Figure 1, non-obese men and women had similar risk of incident HFpEF. Obese women had the highest cumulative incidence of HFpEF, whereas obese men had intermediate incidence.
Table 1.
Baseline clinical and laboratory covariates by cohort
| CHS n= 5,263 |
FHS n= 3,381 |
MESA n= 6,677 |
PREVEND n= 7,360 |
Total n= 22,681 |
|
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 73 (6) | 59 (10) | 62 (10) | 49 (12) | 60 (13) |
| Women, n (%) | 3031 (58) | 1788 (53) | 3521 (53) | 3696 (50) | 12036 (53) |
| Race | |||||
| White, n (%) | 4456 (85) | 3381 (100) | 2560 (38) | 6992 (95) | 17389 (77) |
| Black, n (%) | 778 (15) | – | 1838 (28) | 65 (1) | 2681 (12) |
| Other, n (%) | 29 (1) | – | 2279 (34) | 248 (3) | 2556 (11) |
| Clinical covariates | |||||
| Systolic blood pressure, mmHg | 136 (21) | 128 (19) | 127 (22) | 129 (20) | 130 (21) |
| Heart rate, bpm | 68 (11) | 64 (10) | 63 (10) | 69 (10) | 66 (11) |
| 2 Body mass index, kg/m | 26.7 (4.7) | 27.9 (5.1) | 28.3 (5.5) | 26.1 (4.2) | 27.1 (4.9) |
| Waist circumference, cm | 94 (13) | 98 (14) | 98 (14) | 88 (13) | 94 (14) |
| Hip circumference | 102 (10) | 104 (10) | 106 (11) | 100 (8) | 103 (10) |
| Hypertension treatment, n (%) | 2389 (45) | 943 (28) | 2478 (37) | 999 (14) | 6809 (30) |
| Diabetes mellitus, n (%) | 816 (16) | 326 (10) | 841 (13) | 271 (4) | 2254 (10) |
| Current smoker, n (%) | 622 (12) | 519 (15) | 872 (13) | 2515 (34) | 4528 (20) |
| Prior myocardial infarction, n (%) | 416 (8) | 110 (3) | 0 (0) | 404 (5) | 930 (4) |
| Laboratory covariates | |||||
| Total cholesterol | 212 (39) | 206 (40) | 194 (36) | 218 (44) | 208 (41) |
| HDL cholesterol | 54 (16) | 51 (16) | 51 (15) | 51 (15) | 52 (15) |
| Triglycerides | 139 (76) | 140 (128) | 132 (89) | 125 (88) | 133 (93) |
| Fasting serum glucose | 110 (36) | 101 (28) | 97 (30) | 87 (20) | 99 (31) |
| HOMA-IR | 5 (13) | 3 (6) | 3 (6) | 2 (2) | 3 (8) |
Continuous variables are reported as mean (SD) and categorical variables are reported as n (%)
Figure 1.
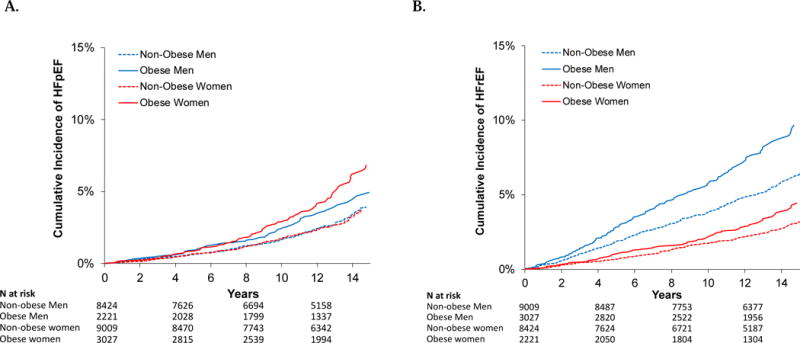
Cumulative incidence of (A) HFpEF and (B) HFrEF in men and women with and without obesity.
Obesity and related traits are associated with HF subtypes
In sex-pooled multivariable-adjusted analyses, BMI, WC, waist to hip ratio, and fasting glucose independently predicted both HFpEF and HFrEF, albeit with larger effect sizes for HFpEF (Table 2). Specifically, a 1-standard deviation (SD) increase in BMI was associated with a 1.34- fold increased hazard of future HFpEF (95% CI 1.24-1.45, P<0.0001), and a 1.18-fold increased hazard of future HFrEF (95% CI 1.10-1.27, P<0.0001). By contrast, systolic BP predicted HFpEF and HFrEF with similar effect sizes. Conversely, HOMA-IR was significantly associated with HFpEF (HR 1.20 per 1-SD increase; 95% CI 1.05 to 1.37, P=0.006) but not HFrEF (HR 0.99; 95%CI 0.88 to 1.11, P=0.81). We directly tested whether a given cardiometabolic trait had a differential effect on the risk of HFpEF vs HFrEF and found that both BMI and HOMA-IR portended greater risk of HFpEF vs HFrEF (P<0.05 for difference in HR using Lunn-McNeil method).
Table 2.
Association of obesity-related traits with heart failure subtypes in sex-pooled analyses
| Predictor | Outcome | HR | Multivariable-adjusted 95% CI | P |
|---|---|---|---|---|
| BMI | Incident HFpEF | 1.34* | (1.24 – 1.45) | <0.0001 |
| Incident HFrEF | 1.18 | (1.10 – 1.27) | <0.0001 | |
| WC | Incident HFpEF | 1.32 | (1.22 – 1.44) | <0.0001 |
| Incident HFrEF | 1.19 | (1.10 – 1.29) | <0.0001 | |
| WHR | Incident HFpEF | 1.19 | (1.10 – 1.29) | <0.0001 |
| Incident HFrEF | 1.14 | (1.06 – 1.22) | 0.001 | |
| HOMA-IR | Incident HFpEF | 1.20* | (1.05 – 1.37) | 0.006 |
| Incident HFrEF | 0.99 | (0.88 – 1.11) | 0.81 | |
| TG/HDL ratio | Incident HFpEF | 1.06 | (0.96 – 1.17) | 0.27 |
| Incident HFrEF | 1.13 | (1.04 – 1.23) | 0.003 | |
| Fasting glucose | Incident HFpEF | 1.15 | (1.08 – 1.23) | <0.0001 |
| Incident HFrEF | 1.07 | (0.99 – 1.16) | 0.08 | |
| SBP | Incident HFpEF | 1.20 | (1.11 – 1.20) | <0.0001 |
| Incident HFrEF | 1.19 | (1.11 – 1.27) | <0.0001 |
P for difference <0.05 using Lunn-McNeil method to compare HR for HFpEF versus HFrEF
BMI, body mass index; WC, waist circumference; WHR, waist-to-hip ratio; HOMA-IR, homeostatic model assessment of insulin resistance; TG/HDL ratio, triglyceride-to-high density lipoprotein ratio; SBP, systolic blood pressure.
Hazard ratio per 1- standard deviation increase in continuous predictor
HOMA-IR, triglycerides, and TG/HDL ratio were log-transformed
The multivariable model was adjusted for age, sex, SBP (except SBP analyses), hypertension treatment, diabetes, smoking, prevalent myocardial infarction, TC, HDL (except TG/HDL analyses), left bundle branch block or left ventricular hypertrophy. HOMA-IR analyses excluded participants with diabetes.
In secondary analyses, we further adjusted for C-reactive protein and interim myocardial infarction, neither of which substantively altered effect estimates (Supplemental Tables 1 and 2). In cohort-specific analyses, the effect size of BMI was numerically greater for HFpEF than for HFrEF across all 4 cohorts, although effect sizes were variable between cohorts, with evidence of heterogeneity between cohorts (Supplemental Table 3).
Differential effects of obesity-related traits on HF subtypes among men and women
We examined the association of obesity-related traits with incident HFpEF and HFrEF in sex- stratified models to better understand sex differences in incident HF subtypes (Table 3). Among men, higher BMI was independently associated with both HF subtypes (HR 1.34 per 1-SD; 95% CI 1.18-1.52, P<0.0001 for HFpEF, and HR 1.24; 95% CI 1.14-1.35, P<0.0001 for HFrEF). By contrast, among women, BMI was associated with incident HFpEF but not HFrEF (HR 1.38 per 1-SD, 95% CI 1.24-1.54, P<0.0001 for HFpEF, vs HR 1.09, 95% CI 0.96-1.24, P=0.18 for HFrEF, P for difference 0.01). We found that sex modified the association of BMI with HFrEF (P=0.03). Additionally, risk of incident HFpEF increased significantly across quartiles of BMI in both men and women, yet the risk of HFrEF increased only among men (P<0.001) but not women (P=0.49) (Figure 2). Similarly, higher waist circumference was associated with both HF subtypes among men, but only with HFpEF and not HFrEF among women (HR 1.35 per 1-SD increase; 95% CI 1.20 to 1.51 for HFpEF vs HR 1.11, 95% CI 0.96-1.27 for HFrEF; P for difference 0.04). We did not find sex differences in the association of HOMA-IR with HF subtypes.
Table 3.
Association of obesity related traits and incident heart failure subtypes among men and women
| Predictor | Outcome | Men Multivariable-adjusted |
Women Multivariable-adjusted |
Pinteraction sex*covariate |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| BMI | Incident HFpEF | 1.34 | (1.18 – 1.52) | <0.0001 | 1.38* | (1.24 – 1.54) | <0.0001 | 0.37 |
| Incident HFrEF | 1.24 | (1.14 – 1.35) | <0.0001 | 1.09 | (0.96 – 1.24) | 0.18 | 0.03 | |
| WC | Incident HFpEF | 1.31 | (1.16 – 1.49) | <0.0001 | 1.35* | (1.20 – 1.51) | <0.0001 | 0.42 |
| Incident HFrEF | 1.23 | (1.13 – 1.33) | <0.0001 | 1.11 | (0.96 – 1.27) | 0.15 | 0.09 | |
| WHR | Incident HFpEF | 1.17 | (1.11 – 1.24) | <0.0001 | 1.17 | (1.06 – 1.30) | 0.003 | 0.42 |
| Incident HFrEF | 1.13 | (1.06 – 1.20) | 0.0003 | 1.07 | (0.94 – 1.21) | 0.32 | 0.40 | |
| HOMA-IR | Incident HFpEF | 1.24* | (1.02 – 1.51) | 0.03 | 1.17 | (0.98 – 1.39) | 0.08 | 0.65 |
| Incident HFrEF | 1.02 | (0.89 – 1.17) | 0.78 | 0.88 | (0.71 – 1.11) | 0.29 | 0.44 | |
| Log-TG | Incident HFpEF | 0.88 | (0.75 – 1.04) | 0.14 | 1.08 | (0.94 – 1.26) | 0.29 | 0.23 |
| Incident HFrEF | 0.98 | (0.87 – 1.09) | 0.68 | 1.08 | (0.92 – 1.26) | 0.34 | 0.18 | |
| HDL | Incident HFpEF | 0.93 | (0.83 – 1.05) | 0.26 | 0.93 | (0.84 – 1.04) | 0.21 | 0.83 |
| Incident HFrEF | 0.88 | (0.81 – 0.97) | 0.01 | 0.87 | (0.76 – 1.00) | 0.05 | 0.91 | |
| Fasting | Incident HFpEF | 1.10 | (0.97 – 1.20) | 0.12 | 1.17 | (1.08 – 1.26) | <0.0001 | 0.14 |
| glucose | Incident HFrEF | 1.07 | (0.98 – 1.17) | 0.12 | 1.08 | (0.92 – 1.26) | 0.36 | 0.15 |
| SBP | Incident HFpEF | 1.18 | (1.06 – 1.32) | 0.003 | 1.21 | (1.09 – 1.35) | 0.001 | 0.49 |
| Incident HFrEF | 1.13 | (1.04 – 1.23) | 0.006 | 1.28 | (1.14 – 1.44) | <0.0001 | 0.048 | |
P for difference <0.05 using Lunn-McNeil method to compare HR for HFpEF versus HFrEF
BMI, body mass index; WC, waist circumference; WHR, waist-to-hip ratio; HOMA-IR, homeostatic model assessment of insulin resistance; TG/HDL ratio, triglyceride-to-high density lipoprotein ratio; SBP, systolic blood pressure.
Hazard ratios are reported as 1 standard deviation increase in continuous predictor.
HOMA-IR, triglycerides, and TG/HDL ratio were log-transformed.
The multivariable model was adjusted for age, SBP (except SBP analyses), hypertension treatment, diabetes, smoking, prevalent myocardial infarction, total cholesterol, HDL (except TG/HDL analyses), left bundle branch block or left ventricular hypertrophy. HOMA-IR analyses excluded participants with diabetes.
Figure 2.
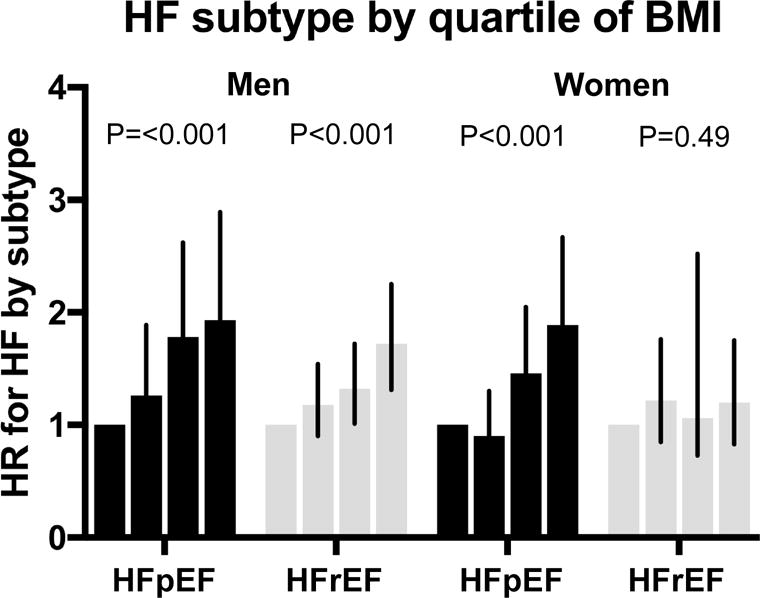
Risk of HFpEF or HFrEF in women and men across quartiles of BMI. P-values represent P for trend.
The remainder of cardiometabolic traits are summarized in Table 3. We found that higher fasting glucose and WHR both predicted incident HFpEF but not HFrEF among women, while among men, WHR was significantly associated with both HFpEF and HFrEF while fasting glucose was not significantly associated with either. Systolic BP was associated with both HF subtypes among men and women. There was an association of lower HDL cholesterol with incident HFrEF among men (P=0.01). We found no association of triglyceride concentrations with incident HF.
Insulin resistance in part mediates the association of BMI with incident HFpEF
In exploratory analyses, we examined whether insulin resistance may in part mediate the association of BMI with incident HFpEF. Among men, we estimate that HOMA-IR accounts for 26% of the total effect, whereas in women, we estimate that HOMA-IR accounts for 29% of the effect on HFpEF risk.
The association of metabolic syndrome with HFpEF and HFrEF
In secondary analyses, we examined the association of each of the metabolic syndrome criteria with HF subtypes. While each of the criteria with the exception of high triglycerides were independently associated with incident HF, effect sizes for elevated waist circumference, hypertension, and fasting glucose were larger for HFpEF than for HFrEF (Supplemental Table 4). By contrast, low HDL cholesterol was associated with incident HFrEF but not HFpEF.
DISCUSSION
Our main study findings are two-fold: first, we demonstrate that obesity and related cardiometabolic traits including insulin resistance are more strongly associated with risk of future HFpEF than HFrEF. Second, we show notable sex differences, wherein obesity in women in particular harbors greater risk of HFpEF vs HFrEF. These findings lend greater granularity to prior studies that have shown an association of obesity and risk of overall HF. We now demonstrate that obesity and cardiometabolic risk predispose to HFpEF, with important sex differences that may underlie the higher prevalence of HFpEF among women.
Obesity has long been described as a major risk factor for the development of overall HF (4), although the differences in HF subtypes have been less clear. More recently, obesity has been proposed as a major driver of systemic inflammation and subsequent myocardial remodeling in HFpEF specifically (6). This is substantiated by prior community-based studies, demonstrating an association of obesity with future HFpEF specifically in participants of the FHS (17) and PREVEND (18), as well as African-American participants of the Atherosclerosis Risk in Communities (ARIC) study (19), although direct comparisons with HFrEF were not performed or limited by sample size. Prior studies and new contributions of the current analysis are summarized in Table 4. Obesity has also been associated with subclinical phenotypes that precede HFpEF, including systolic and diastolic dysfunction, and left ventricular hypertrophy (5,20). We now show that obesity is specifically associated with a higher risk of future HFpEF than HFrEF in a collaboration of 4 large community-based cohorts, leveraging data from over 22,000 individuals followed for incident HF events.
Table 4.
Summary of previous studies and novel aspects of our study
| Prior study | Findings | New in current analysis |
|---|---|---|
| Brouwers FP et al, Eur Heart J, 2013 | Higher BMI was associated with overall HF without differences among HF subtypes among PREVEND participants. | Addition of other cohorts including FHS, CHS, and MESA for a more comprehensive analysis. |
| Eaton CB et al, Circ Heart Fail, 2016 | Higher BMI was associated with incident HFpEF but not HFrEF among post-menopausal women participants of the WHI. | Inclusion of both men and women, and direct comparison of sex-specific effects and differences. |
| Ho JE et al, Circ Heart Fail, 2016 | Higher BMI was associated with incident HFpEF and HFrEF among 28,820 participants from CHS, FHS, and PREVEND, with borderline difference among subtypes (P for equality 0.05). | Addition of MESA cohort for a more comprehensive analysis across 4 cohorts, specific investigation of obesity-related traits previously not analyzed, including waist circumference, insulin resistance, and dyslipidemia. |
| Ingelsson E et al, JAMA, 2005 | Among ULSAM participants, BMI, insulin resistance, and waist circumference independently predicted incident overall HF | Specific evaluation of insulin resistance and BMI and their associations with HF subtypes (HFpEF vs HFrEF) with direct comparisons of effect sizes. |
| Vardeny O et al, JACC Heart Fail, 2013 | Among ARIC participants, insulin resistance and higher BMI were associated with increased risk of overall HF. | Specific evaluation of insulin resistance and BMI and their associations with HF subtypes (HFpEF vs HFrEF) with direct comparisons of effect sizes. |
The mechanisms underlying obesity and HFpEF remain unclear. We specifically investigated obesity-related cardiometabolic traits, in order to shed further light on potential pathways that might lead to HFpEF. We found that obesity (as measured by waist circumference, increased waist-to-hip ratio and increased BMI), and associated cardiometabolic dysfunction, including insulin resistance, abnormal fasting glucose, and hypertension were all associated with incident HFpEF. Our findings are in keeping with prior studies demonstrating the importance of hypertension in the development of both HFpEF and HFrEF, and it may be that hypertension mediates obesity-associated HF.
This extends prior cross-sectional studies demonstrating an association of abdominal and visceral adiposity and diastolic dysfunction (21,22). Of note, the association of insulin resistance and overall HF has been described previously (23,24). Specifically, in the Uppsala Longitudinal Study of Adult Men (ULSAM) study, insulin resistance was an independent predictor of incident HF (23). In ARIC, insulin resistance defined by HOMA-IR levels between 1.0 and 2.0 were associated with incident HF, although values above 2.5 were not (25). Neither study distinguished HFpEF from HFrEF. We now show that HOMA-IR confers a higher risk of future HFpEF, but not HFrEF. Further, our findings suggest that HOMA-IR may in part mediate the association of obesity and HFpEF. While this finding is novel, it is in concert with existing cross- sectional data, demonstrating an association of HOMA-IR with both lower e’ and higher E/e’ ratios suggestive of worse diastolic dysfunction among a population-based sample (26). Our findings fit with the proposed paradigm that cardiometabolic factors including abdominal adiposity and insulin resistance may produce to a systemic inflammatory state (6), including secretion of proinflammatory cytokines (27,28), ultimately predisposing to myocyte remodeling and the development of HFpEF (29).
The second notable finding in our study was focused on sex differences in cardiometabolic risk leading to HFpEF. It has long been observed that the prevalence of HFpEF is greater among women than men (30). Interestingly, among participants of the Women’s Health Study (WHS) (7), obesity was associated with a population attributable risk of future HFpEF that was over 3- fold higher than that of HFrEF. Further, obesity was more common among women than men with existing HFpEF enrolled in the I-PRESERVE trial (31). Motivated by these potential sex differences, we now show that obesity portends a higher risk of HFpEF vs HFrEF among women, whereas this difference is less pronounced in men. The reason for this sex difference remains unclear, but mirrors the differential effect of cardiometabolic risk factors on longitudinal increases in left ventricular mass with aging among women than men (32). Biomarkers such as natriuretic peptides predict incident HF subtypes, and also appear to have sex-specific effects with lower natriuretic peptide levels in abdominal obesity observed among women vs men (33,34). Whether obesity and associated cardiometabolic risk should raise special attention among women requires further study.
Our study had several limitations that deserve mention. Obesity and cardiometabolic disease are known to disproportionately affect different race/ethnic groups (35). While our sample did include ethnic minorities, we did not have enough power to perform race-specific analyses, which will be of high interest in future studies. With respect to the HF endpoint, we were able to classify HFpEF and HFrEF only in individuals who underwent cardiac function assessment at or around the time of their acute HF presentation, which left 30% of cases as unclassified HF. Additionally, once classified by their initial HF presentation, recurrent events and transitions between HFpEF and HFrEF were not captured. The exclusion of individuals missing key covariates may have influenced our results. Furthermore, this was an observational study, limiting potential causal inferences, and further studies are needed to better understand mechanisms underlying obesity and HFpEF. Finally, individual cohorts differed by era of baseline exam and also frequency and timing of follow-up exams. Thus, secular trends including difference in lifestyle or therapies may have confounded results, and serial measures of BMI and other cardiometabolic traits were not taken into account.
In summary, we found that obesity and associated cardiometabolic traits conferred a higher risk of HFpEF than HFrEF, and that obesity among women in particular seemed to be predispose to future HFpEF. These findings add to our current understanding of what predisposes certain patients to developing HFpEF. Whether targeting cardiometabolic disease can prevent HFpEF needs further study, and is particularly important given the current lack of effective therapies once HFpEF has developed.
Supplementary Material
CLINICAL PERSPECTIVES.
Heart failure accounts for a substantial burden of total health care costs world-wide, and about half of individuals presenting with heart failure have heart failure with preserved as opposed to reduced ejection fraction. A better understanding of how obesity and related cardiometabolic traits may lead to each heart failure subtype may inform underlying pathways and guide future preventive strategies.
TRANSLATIONAL OUTLOOK.
Future studies are needed to examine potential pathways that lead from obesity and metabolic dysfunction to the development of heart failure with preserved ejection fraction.
Acknowledgments
None
Funding sources: This work was partially supported by the National Heart, Lung and Blood Institute, including the Framingham Heart Study (contract N01-HC25195 and HHSN268201500001I), the Cardiovascular Health Study (HHSN268201800001C, HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org). MESA and the MESA SHARe project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC- 95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1-TR-001881, and DK063491. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The Prevention of Renal and Vascular End-Stage Disease (PREVEND) study has been made possible by grants from the Dutch Kidney Foundation. Dr. de Boer is supported by the Netherlands Heart Foundation (CVON-DOSIS, grant 2014-40) and the Innovational Research Incentives Scheme program of the Netherlands Organization for Scientific Research (NWO VIDI, grant 917.13.350, to Dr. de Boer). Dr. Nayor received support from K23-HL138260. Dr. Ho is supported by K23-HL116780, R01-HL134893, R01-HL140224, and a Hassenfeld Research Scholar Award (Massachusetts General Hospital, Boston, MA). Dr. Lee is supported by a mid-career award from the Heart and Stroke Foundation of Canada, and is the Ted Rogers Chair in Heart Function Outcomes. Dr. Vasan is supported in parts by the Evans Medical Foundation and the Jay and Louis Coffman Endowment, Boston University School of Medicine. Dr. Ho had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures: Dr. Psaty serves on a DSMB for a clinical trial funded by the manufacturer (Zoll LifeCor) and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. Dr. Kizer has stock ownership in Amgen, Gilead Sciences, Johnson & Johnson, and Pfizer.
ABBREVIATIONS
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- BMI
body mass index
- HOMA-IR
homeostatic model assessment of insulin resistance
- WC
waist circumference
- HDL
high density lipoprotein
- WHR
waist to hip ratio
- TG
triglycerides
- SBP
systolic blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. Jama. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 3.Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Current heart failure reports. 2013;10:401–10. doi: 10.1007/s11897-013-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. The New England journal of medicine. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 5.Wang YC, Liang CS, Gopal DM, et al. Preclinical Systolic and Diastolic Dysfunctions in Metabolically Healthy and Unhealthy Obese Individuals. Circulation Heart failure. 2015;8:897–904. doi: 10.1161/CIRCHEARTFAILURE.114.002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 7.Eaton CB, Pettinger M, Rossouw J, et al. Risk Factors for Incident Hospitalized Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Postmenopausal Women. Circulation Heart failure. 2016:9. doi: 10.1161/CIRCHEARTFAILURE.115.002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2009;10:154–67. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 9.Wenger NK. Coronary heart disease: the female heart is vulnerable. Progress in cardiovascular diseases. 2003;46:199–229. doi: 10.1016/j.pcad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. American journal of epidemiology. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Annals of epidemiology. 1995;5:270–7. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 12.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Annals of the New York Academy of Sciences. 1963;107:539–56. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 13.Diercks GF, Janssen WM, van Boven AJ, et al. Rationale, design, and baseline characteristics of a trial of prevention of cardiovascular and renal disease with fosinopril and pravastatin in nonhypertensive, nonhypercholesterolemic subjects with microalbuminuria (the Prevention of REnal and Vascular ENdstage Disease Intervention Trial [PREVEND IT]) The American journal of cardiology. 2000;86:635–8. doi: 10.1016/s0002-9149(00)01042-0. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. American journal of epidemiology. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 15.Ho JE, Enserro D, Brouwers FP, et al. Predicting Heart Failure With Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Subtypes. Circulation Heart failure. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–32. [PubMed] [Google Scholar]
- 17.Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circulation Heart failure. 2013;6:279–86. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. European heart journal. 2013;34:1424–31. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 19.Gupta DK, Shah AM, Castagno D, et al. Heart failure with preserved ejection fraction in African Americans: The ARIC (Atherosclerosis Risk In Communities) study. JACC Heart failure. 2013;1:156–63. doi: 10.1016/j.jchf.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. Journal of the American College of Cardiology. 2011;57:1368–74. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libhaber CD, Norton GR, Majane OH, et al. Contribution of central and general adiposity to abnormal left ventricular diastolic function in a community sample with a high prevalence of obesity. The American journal of cardiology. 2009;104:1527–33. doi: 10.1016/j.amjcard.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Canepa M, Strait JB, Milaneschi Y, et al. The relationship between visceral adiposity and left ventricular diastolic function: results from the Baltimore Longitudinal Study of Aging. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2013;23:1263–70. doi: 10.1016/j.numecd.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. Jama. 2005;294:334–41. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 24.Wisniacki N, Taylor W, Lye M, Wilding JP. Insulin resistance and inflammatory activation in older patients with systolic and diastolic heart failure. Heart (British Cardiac Society) 2005;91:32–7. doi: 10.1136/hrt.2003.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardeny O, Gupta DK, Claggett B, et al. Insulin resistance and incident heart failure the ARIC study (Atherosclerosis Risk in Communities) JACC Heart failure. 2013;1:531–6. doi: 10.1016/j.jchf.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontes-Carvalho R, Ladeiras-Lopes R, Bettencourt P, Leite-Moreira A, Azevedo A. Diastolic dysfunction in the diabetic continuum: association with insulin resistance, metabolic syndrome and type 2 diabetes. Cardiovascular diabetology. 2015;14:4. doi: 10.1186/s12933-014-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jelic S, Lederer DJ, Adams T, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121:1014–21. doi: 10.1161/CIRCULATIONAHA.109.900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taube A, Schlich R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: links to cardiovascular diseases. American journal of physiology Heart and circulatory physiology. 2012;302:H2148–65. doi: 10.1152/ajpheart.00907.2011. [DOI] [PubMed] [Google Scholar]
- 29.Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. Journal of the American College of Cardiology. 2010;55:2129–37. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. Journal of the American College of Cardiology. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Lam CS, Carson PE, Anand IS, et al. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circulation Heart failure. 2012;5:571–8. doi: 10.1161/CIRCHEARTFAILURE.112.970061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieb W, Xanthakis V, Sullivan LM, et al. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the framingham offspring study. Circulation. 2009;119:3085–92. doi: 10.1161/CIRCULATIONAHA.108.824243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suthahar NMW, Ho JE, Gansevoort RT, Voors AA, van der Meer P, Bakker SJL, Heymans S, van Empel V, Schroen B, van der Harst P, van Veldhuisen DJ, de Boer RA. Sex-specific associations of obesity and N-terminal pro-B-type natriuretic peptide levels in the general population. European Journal of Heart Failure. 2018 doi: 10.1002/ejhf.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Boer RA, Nayor M, deFilippi CR, et al. Association of Cardiovascular Biomarkers With Incident Heart Failure With Preserved and Reduced Ejection Fraction. JAMA cardiology. 2018;3:215–224. doi: 10.1001/jamacardio.2017.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wahi G, Anand SS. Race/Ethnicity, Obesity, and Related Cardio-Metabolic Risk Factors: A Life-Course Perspective. Current cardiovascular risk reports. 2013;7:326–335. doi: 10.1007/s12170-013-0329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


