Abstract
Reinforcement value enhancement by nicotine of non-nicotine rewards is believed to partially motivate smoking behavior. Recently, we demonstrated that the value-enhancing effects of nicotine are well characterized by reinforcer demand models, and that the value-enhancing effects of the smoking cessation aid bupropion (Zyban®) are distinct from those of nicotine and differ between the sexes (Barrett et al., 2017). The present study evaluated potential sex differences in the enhancement effects of nicotine and varenicline (Chantix®) using a reinforcer demand methodology. The role of α4β2* and α7 nicotinic acetylcholine receptors (nAChRs) in the enhancing effects of nicotine and varenicline are also evaluated. Male and female rats (n=12/sex) were trained to lever-press maintained by sensory reinforcement via visual stimulus (VS) presentations. Changes in VS value following nicotine and varenicline administration were assessed using an established reinforcer demand approach. Subsequently, the effects of antagonism of α4β2* and α7 nAChRs on varenicline and nicotine induced enhancement active lever-pressing was assessed using a progressive ratio schedule. Nicotine and varenicline enhanced VS demand equivalently between the sexes as evaluated by reinforcer demand. However, α4β2* receptor antagonism attenuated value enhancement by nicotine and varenicline in females, but only of nicotine in males.
Keywords: Nicotine, Varenicline, Reward Enhancement, Behavioral Economics, Reinforcer Demand, Nicotinic Acetylcholine Receptors, Rat
INTRODUCTION
Among the leading causes of preventable death and disease in the world, tobacco use is the deadliest, accounting for over 480,000 deaths annually in the United States alone (United States Department of Health and Human Services [USDHHS], 2014). Nicotine is commonly accepted to be the primary constituent of tobacco smoke responsible for smoking addiction. However, the primary rewarding effects of nicotine alone are markedly weak relative to the prevalence and tenacity of nicotine dependence (Caggiula et al., 2009; USDHHS, 1988). Rather, it appears that the behavioral and neuropharmacological mechanisms whereby nicotine reinforces smoking are more multifarious than simply primary reinforcement by nicotine (Caggiula et al., 2009). A mounting body of research advises that a complete representation of the mechanisms whereby nicotine maintains smoking must comprise primary reinforcement by nicotine, secondary reinforcement by nicotine-associated environmental stimuli, Pavlovian conditioning of the interoceptive stimulus effects of nicotine with non-nicotine reinforcers, and nicotine-mediated enhancement of the value of non-nicotine reinforcers (for a review see Bevins and Palmatier, 2004).
Research has demonstrated that males and females differ in smoking behavior and nicotine dependence, suggesting that a complete characterization of nicotine reward must also incorporate biological sex (Lynch et al., 2002; Roth et al., 2004; Perkins et al., 1994; 2002; Perkins, 2009; USDHHS, 2001). Specifically, additional research efforts are needed to engaged with determining how males and females may differ regarding the differing degrees to which the primary reinforcing, secondary reinforcing, reinforcer-enhancing and interoceptive stimulus effects of nicotine contribute towards motivating smoking and relapse (Bevins and Palmatier, 2004). Over a decade of research indicates that nicotine enhances the value of non-nicotine rewards, particularly sensory reinforcers, and this enhancement effect partially drives smoking maintenance (for a review, see Caggiula et al., 2009). Additionally, studies indicate that females are more sensitive to the sensory and contextual elements of smoking and less prone to the primary reinforcing effects of nicotine than males (Perkins, 2009). Given the prominence of the enhancing effects of nicotine on sensory reinforcers and the greater contribution of sensory stimuli in the smoking behavior of females, the present study examined whether sex differences are apparent in the reward-enhancing effects of nicotine and varenicline. Varenicline is a compound of interest because it is one of the most commonly prescribed smoking cessation aids (Chantix®), shares a similar mechanism of action with nicotine, and has been shown to produce enhancement of operant behavior analogous to nicotine (Levin et al., 2012; Rollema et al., 2007; Schassburger et al., 2015).
Behavioral economics provides several tools for assessing the value of reinforcers across differing conditions of consumption. In the present study, we employed a reinforcer demand model (Hursh and Silberburg, 2008; Hursh, 2014) to quantifiably compare changes in reinforcement value wrought by nicotine and varenicline between the sexes. The basic framework of this model conceptualizes reinforcement value in terms of reinforcer consumption as a function of its price in units of response cost. Reinforcer consumption decreases as the unit price of the reinforcer increases, and the rate of this decrease defines what is termed elasticity of demand. Demand that is inelastic is relatively insensitive to increases in reinforcer price, and represents consumption that is primarily constrained by satiation. Elastic demand is characterized by proportionally dramatic decreases in consumption relative to increases in price, and represents consumption that is largely constrained by the cost to obtain the reinforcer. Importantly, the rate of change in elasticity of demand provides a metric of condition-specific reinforcement value by representing the sensitivity to escalating reinforcer price. Recently, we demonstrated that reinforcer demand modeling provides a richly detailed characterization of changes in reinforcement value by nicotine and bupropion (Barrett et al, 2017). In the present study, we extend our previous work to the reward enhancing effects of nicotine and varenicline in male and female rats, using a similar reinforcer demand modeling technique.
In the present study, we extend previous research by evaluating the reward-enhancing effects of nicotine alongside those of varenicline, a commonly prescribed and relatively effective smoking cessation aid. The reinforcer demand model was used to quantitatively characterize and compare the reward-enhancing effects of nicotine and varenicline, and to evaluate how the enhancement effects of each drug interact with biological sex. Like nicotine, varenicline acts as an agonist at α4β2-containing nicotinic acetylcholine receptors (nAChRs), though only partially, and acts as a full agonist at α7 and α3β4* (where * indicates additional unspecified subunits) nAChRs (Grady et al., 2010; Ortiz et al., 2012). Considerable evidence suggests that β2-containing nAChRs on dopaminergic neurons in the midbrain regions of the ventral tegmental area and nucleus accumbens mediate the primary reinforcing effects of nicotine (Placzek and Dani, 2009; Picciotto et al., 1998; Picciotto and Mineur, 2014; Brunzell and Picciotto, 2009). Varenicline has mixed agonist and antagonist effects on α4β2-containing nAChRs, which may account for its apparent lack of primary reinforcing effects (Schassburger et al., 2015; Grady et al., 2010; Ortiz et al., 2012). The present study investigated the involvement of the α4β2-containing and α7 nicotinic acetylcholine receptors (nAChRs) in the reward enhancing effects of nicotine and varenicline on progressive ratio-maintained responding in male and female rats, using antagonism via dihydro-β-erythoidine (DHβE) or methyllycaconitine (MLA).
METHODS
Subjects
Twenty-four experimentally naïve Sprague-Dawley rats (n=12 per sex; Harlan, Indianapolis, IN), 9 weeks upon arrival, were individually housed in clear polycarbonate tubs lined with TEK Fresh® cellulose bedding in a temperature- and humidity-controlled colony. Rats were given two days to acclimate to the colony followed by three additional days of handling before initiation of training. Water was continuously available and rats were given 12 (female) or 15 (males) g of laboratory chow daily, unless otherwise specified. Previous work in our laboratory has found this feeding regimen to be sufficient to maintain normal growth rates while being lean enough to stimulate exploratory behavior in both sexes. Sessions were conducted during the light phase of a 12:12 hour light/dark cycle. Experimental protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Apparatus
We used sixteen conditioning chambers (ENV-008CT; Med-Associates, Inc., St. Albans, VT; measuring 30.5 × 24.1 × 21.0 cm, L×W×H), enclosed in light- and sound-attenuating cubicles fitted with an exhaust fan. Sidewalls were aluminum; the ceiling and front and back walls were clear polycarbonate. One sidewall featured a dipper receptacle, occupying a 5.2 × 5.2 × 3.8 cm (LxWxH) recessed space, into which a dipper arm provided 0.1 ml of sucrose solution when raised. Retractable response levers were featured on either side of the dipper receptacle, approximately 5 cm above the rod floor. White 28V DC (100-mA) lamps were located 3 cm above each lever, hereafter termed lever lights. Two 28V DC (100-mA) houselights were also located above the conditioning chamber, but within the sound attenuating cubicle. An infrared emitter/detector unit, positioned 4 cm above the floor, bisected the chamber 14.5 cm from the sidewall featuring the dipper receptacle and functioned to monitor chamber activity. Data collection and presentation of experimental events were controlled via personal computer with Med Associates interface and software (MedPC for Windows, IV).
Acquisition
Rats were trained to lever press over four auto-shaping sessions using 26% (weight/volume) liquid sucrose (cf. Charntikov et al., 2013). Auto-shaping sessions began with random insertion of one of the two levers. After a lapse of 15 s or a lever press, the response lever was immediately retracted and the dipper arm was raised for 4 s. Following a variable length timeout (average 60 s, range=30-90 s), the opposite lever was inserted into the chamber initiating a new trial as just described. The lever inserted on odd numbered trials was always randomly determined, and the opposite lever always followed on even numbered trials. Thus, over a 60-trial session, each lever was inserted 30 times, but never presented more than twice in succession. Each session was conducted in continuous house-light illumination and no other stimuli were presented.
Over the following 10 days, rats were trained to lever press maintained by visual stimuli (VS), consisting of 60-s termination of house-light illumination compounded with 5 s illumination of lever lights. Active and inactive lever assignments were pseudo-randomly determined and counterbalanced. Daily sessions were 60 min. VS reinforcement was delivered on a fixed ratio (FR) 1 schedule (1 response per reinforcer) for responding on the active lever; responses on the inactive lever were recorded but had no programmed consequences. To familiarize the rats to injection procedures and to provide sufficient nicotine pre-exposure to minimize the response suppressant effects of nicotine, each rat received an injection of saline 5 min preceding placement into the chamber and an injection of nicotine 15 min following termination of each session. Previous work in our laboratory has found that rats tolerate to the suppressant effects of nicotine within roughly 3 consecutive days.
Reinforcer Demand Assessment
Following the tenth day of FR1 training, rats continued to lever-press maintained by VS reinforcement in 60-min sessions, as described earlier. The response requirement was now systematically increased after completion of each block of 16 sessions. The sequence of response costs followed an exponential base 2 sequence ranging from FR 1 to FR 256. Over different sessions within each FR block, rats received injections of 0.9 % saline, nicotine (0.4 mg/kg), or varenicline (0.1 or 1.0 mg/kg) before placement in the apparatus. Sessions proceeded with the restriction that each drug condition was experienced once before repeating and no drug condition was experienced two days in succession. Drug and dose administration order was randomized and then counterbalanced across subjects, such that an equal number of subjects experienced each drug or dose condition on any given day. Each drug was tested four times within each FR block. However, only the last three were included in analyses to capture stable performance on each FR schedule (i.e., the terminal 12 sessions of each 16 session FR block). Demand assessment continued for each rat until the last session of FR 256 or until the last session of a FR block in which the mean number of VS presentations earned was <1 across all drug conditions.
The demand model relates reinforcer consumption to unit response cost via the following equation:
| Equation 1 |
in which Q reflects units of reinforcer consumption, Q0 is predicted consumption in the absence of the constraint of cost (i.e., the ordinate intercept), k is a constant reflecting the range of the demand function in log units of consumption, e is the base of the natural logarithm, C is the response cost to obtain reinforcement, and α represents the rate of change in decline of consumption in standardized price (Q0 * C). The values of Q0 and α are permitted to vary to maximize the fit of the demand model and may be conceptualized to represent basal intensity of demand (Q0) and sensitivity to price (α; Hursh and Silberburg, 2008; Hursh, 2014). That is, Q0 represents consumption where the only constraint is satiation, and α reflects the limiting effects of both satiation and price on consumption by representing the rate at which consumption shifts toward being primarily constrained by price rather than by satiation (Hursh and Silberburg, 2008; Bickel et al., 2000; Johnson and Bickel, 2006). Importantly, the essential value of a reinforcer is inversely related to sensitivity to price (α) and can be calculated from the demand model as:
| Equation 2 |
where essential value (EV) is conceptualized as the strength of a reinforcer to maintain behavior independent of scalar manipulations of reinforcer magnitude and accounting for individual sensitivity to response cost (Hursh and Silberburg, 2008; Hursh, 2014). Previous work has demonstrated that reinforcer demand models effectively characterize changes in the reinforcement value of non-nicotine rewards, such as food pellets (Cassidy and Dallery, 2012), food-associated conditioned rewards (Cassidy and Dallery, 2014), and primary reinforcing sensory stimulation (Barrett and Bevins, 2012; Barrett et al., 2017).
Progressive Ratio and Antagonist Testing
This phase began 24 h after the last demand assessment session. Within a single session, lever pressing was reestablished via the same sucrose-maintained auto-shaping procedure previously described. Over the next 15 sessions, responding for VS stabilized on a progressive ratio (PR) schedule of reinforcement. The PR sequence followed an exponential base 2 sequence in one-third logarithmic steps, rounded to the nearest whole number (i.e., 2, 3, 4, 5, 6, 8, 10, 13, 16, etc.). This sequence was chosen because it included the ratios experienced in the demand-assessment phase and progressed slowly enough to minimize ratio strain in the beginning of ratio progression. This progression also afforded the possibility of encountering schedules as high as, or higher than, each rat’s termination schedule in the demand-assessment phase.
The purpose of the PR schedule in this phase was to provide a platform, on which to test the effects of α4β2* or α7 receptor antagonism on the reward-enhancing effects of nicotine and varenicline, that reflected the conditions of the demand-assessment phase, but allowed for rapid assessment over a relatively short period of time. There are advantages and limitations in employing a PR schedule compared to a full demand assessment. Namely, the PR schedule allows for rapid assessment of drug effects on responding constrained by cost within sessions, whereas assessing the same drug effects in the demand approach requires multiple administrations of the drug across multiple sessions at different FR schedules. Both approaches allow for the assessment of drug effects on behavior in the face of escalating response costs; the PR schedule does so within sessions and the demand approach does so across many sessions. The primary limitation of the PR approach is that there can be no assessment of the cost-consumption relationship because consumption at each cost is not free to vary. Because we desired to test the effects of receptor antagonism in the same rats from the preceding demand phase, and in the interest of validating the effects of the demand assessment with a second methodological approach, we opted to employ the PR schedule in this phase.
Over 36 sessions, rats continued to respond on the PR schedule described above. On these sessions, rats received an injection of either DHβE or MLA followed by administration of saline, nicotine (0.4 mg/kg), or varenicline (1.0 mg/kg). Only the higher dose of varenicline from the demand-assessment phase was included in this phase to ensure a high baseline for observing potential decreases in responding brought by dopamine receptor antagonism. Each antagonist was assessed at three different doses (including a vehicle benchmark), and in combination with saline, nicotine, or varenicline across two determinations, requiring 18 days of testing for each antagonist. Testing with DHβE was completed before testing began with MLA. Sessions were a strict 60 min; the assessment of PR breakpoints was not a goal of this phase.
Drugs
(−)-Nicotine hydrogen tartrate [0.4 mg/kg, 5-min injection-to-placement interval (IPI)], varenicline dihydrochloride (0.1 and 1.0 mg/kg; 30-min IPI), dihydro-β-erythoidine (DHβE; 1.0 and 3.0 mg/kg; 45-min injection-to-placement interval; IPI), and methyllycaconitine (MLA; 3.0 and 10 mg/kg; 45-min IPI) were obtained from Sigma Aldrich (St. Louis, MO; nicotine, DHβE, and MLA) or NIDA (Bethesda, MD; varenicline) and dissolved in 0.9% saline. All injections were at 1 mL/kg. As per field standards, nicotine dose is reported as base form; all other drug doses are reported as salt form. The pH for nicotine was adjusted to 7.0±0.2 with a NaOH solution. All doses and IPIs were based on published research, including previous work from our laboratory (Levin et al., 2012, Liu et al., 2010; Pittenger et al., 2017; Wooters et al., 2009). Nicotine was injected s.c; all other drugs were injected i.p.
Dependent Measures and Analyses
Total active and inactive lever-presses, infrared beam breaks (activity), and VS presentations earned within each session were recorded throughout the experiment. Total VS presentations earned over sessions of the demand assessment phase was analyzed using the exponential reinforcer demand model proposed by Hursh and Silberberg (2008) and the values of Q0 (consumption at price-zero) and α (sensitivity to cost) were calculated from the model fits to the consumption data of individual rats using nonlinear least squares regression. To ensure comparability of α estimates, the range parameter, k, was shared across all eight conditions of sex * drug (k=1.968). This value was determined by first fitting the demand equation to the data from all conditions across all subjects with the goal of finding a shared estimate for k, and then using that estimate as a constant when fitting the model to the data of individual rats. Estimates of EV were calculated from α and k using Equation 2. The effects of nicotine and varenicline on Q0 or EV were analyzed via mixed-factorial ANOVA with sex as a between-subjects factor and drug as a within-subjects factor. Post-hoc pairwise comparisons were conducted upon detection of significant main effects of drug or sex * drug interactions.
The primary measures of interest during the antagonist testing phase were total active lever-presses and beam breaks in experimental sessions. These measures were subjected to three-factor, mixed measures ANOVA with sex as a between-subjects factor and with drug and antagonist dose conditions as within-subject factors. Each antagonist testing phase (DHβE and MLA) was analyzed separately. Post-hoc pairwise comparisons were conducted upon detection of significant interactions. All pairwise comparisons throughout all phases of the study corrected family-wise error rates using the Holm-Bonferroni method with significance set at adjusted p-values < 0.05 (Holm, 1979).
RESULTS
Assessment of Demand for VS
The demand functions for VS reinforcement between saline, nicotine, and both varenicline dose conditions for males (Figure 1A) and females (Figure 1B) are displayed in Figure 1. The fits of the demand model presented in Figure 1 are representative curves fit to the data averaged across rats within each condition. All statistical analyses used fits of the demand model to data of individuals. Males and females differed in how quickly they completed the demand assessment phase by reaching termination criteria. The point at which 50% of the males or females had reached termination criteria was FR 64 and FR 128, respectively. One male and one female rat were excluded from demand analyses because the model-generated estimates of Q0 from their data under the saline condition were outside 1.5 times the inter-quartile range (i.e., Tukey’s hinges; Hoaglin et al., 1983).
Figure 1.
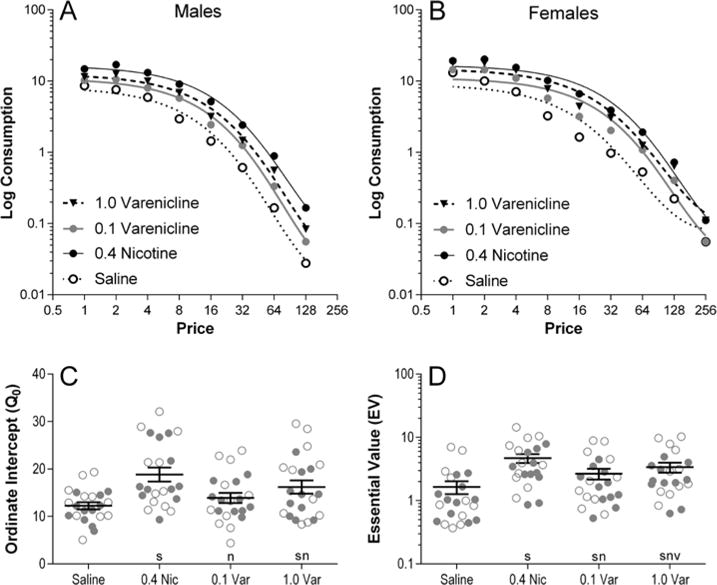
Upper Panels: VS consumption as a function of FR schedule in males (panel A) and females (panel B), and across the four administration conditions of nicotine (filled circles), saline (open circles), 0.1 mg/kg varenicline (open triangles), and 1.0 mg/kg varenicline (closed triangles). The presented demand curves are representative fits to averaged data, and not the curves used to generate metrics for statistical analysis. Because individual rats exited the demand assessment phase upon reaching termination criteria at different FR schedules, data indicating performance at the higher FR schedules is only representative of the subset of the rats who continued through the demand assessment phase. Specifically, the n for FR 64 was 11 females and 9 males, for FR 128 was 6 females and 4 males, and for FR 256 was 5 females. These data are for presentation purposes only; all analyses were conducted on separate fits of the reinforcer demand model to data from each individual. Lower Panels: The vertical axis intercept (Q0; panel C) and essential value (EV; panel D) as estimated by the reinforcer demand model from fits to individual data, presented to represent the significant main effects of drug. Filled circles represent males and open circles represent females. The solid lines and whiskers represent the means and standard error of the means, respectively. Significant differences are denoted as follows: s = different from saline, n = different from nicotine, v = difference between varenicline doses (ps<0.05).
Analysis of Q0 (Figure 1C) discovered a significant main effect of drug [F(3,60)=18.44, p<0.001], but not of sex and no significant interaction [Fs<1]. Further analysis of the main effect of drug found higher Q0 estimates in the nicotine condition than any other condition (ps≤0.033). Q0 was also higher in the 1.0 mg/kg varenicline condition compared to saline (p<0.001). No significant differences were detected between saline and the 0.1 mg/kg varenicline condition (p=0.317). Likewise, no significant differences were detected between 0.1 and 1.0 mg/kg varenicline (p=0.079).
Analysis of EV (Figure 1D) revealed a significant main effect of drug [F(3,60)=108.3; p<0.001], but not of sex and no significant interaction [Fs<1]. Further analysis on the main effect of drug discovered greater EV in the nicotine condition compared to all other conditions (ps<0.001). Both varenicline doses enhanced EV relative to saline (ps<0.001), and EV was greater in the 1.0 mg/kg condition relative to 0.1 mg/kg varenicline (p=0.001).
DHβE Testing on Progressive Ratio
Active lever-pressing and locomotor activity on the PR schedule of VS reinforcement across sessions of the DHβE testing phase are displayed in the upper panels of Figure 2. Nicotine and varenicline increased active lever-pressing (Figure 2A) on the PR schedule relative to saline in males and females. Analysis revealed significant effects of sex [F(1,21)=8.77; p<0.01], drug [F(2,42)=50.15; p<0.001], and dose [F(2,42)=11.65; p<0.001]. The sex * drug [F(2,42)=6.59; p<0.005], sex * dose [F(2,42)=4.63; p<0.02], and drug * dose [F(4,84)=7.31; p<0.001] interactions were all significant, as was the sex * drug * dose interaction [F(4,84)=2.86; p<0.05].
Figure 2.
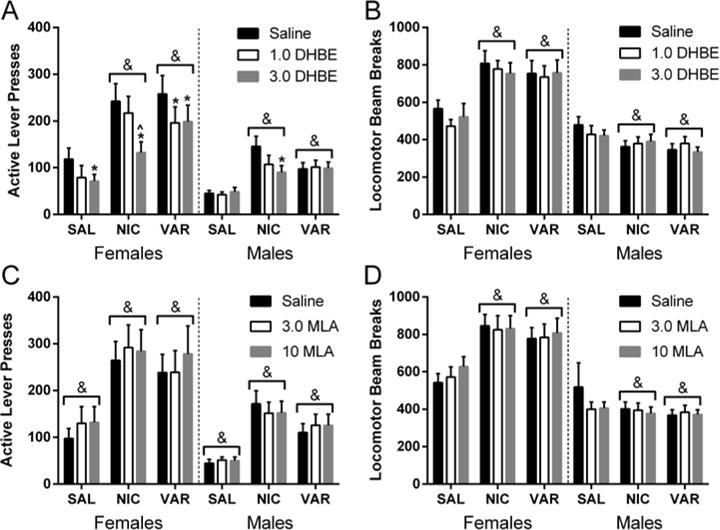
Active lever-pressing (left panels) and locomotor activity (right panels) under the conditions of saline, nicotine, or 1.0 mg/kg varenicline on behavior maintained by a PR schedule of VS reinforcement during the DHβE (upper panels) and MLA (lower panels) testing phases. Significant differences within each condition of drug are denoted as follows: * = difference from the saline condition of antagonist dose, ^ = difference from the 1.0 mg/kg DHβE condition of antagonist. Significant differences between sexes at conditions of drug and antagonist dose are denoted with &.
Post-hoc analysis on the three-factor interaction revealed significantly higher active lever-pressing in females than males in the nicotine and varenicline conditions, regardless of DHβE dose (ps≤0.035). No sex differences were observed under saline conditions, regardless of DHβE dose (ps≥0.219). Furthermore, nicotine and varenicline increased active lever-pressing relative to saline in both sexes, across all DHβE dose conditions (ps≤0.028), except in males at 3.0 mg/kg DHβE (p=0.084). Active lever-pressing differed between the nicotine and varenicline conditions in males, in the absence of DHβE (p=0.039), and in females when administered 3.0 mg/kg DHβE (p=0.004). No differences were observed between nicotine and varenicline conditions under any other conditions of sex or antagonist dose (ps≥0.554). In females, 3.0 mg/kg DHβE suppressed active lever-pressing relative to saline in all conditions of drug (ps≤0.039), as did 1.0 mg/kg DHβE in the varenicline condition (p=0.004), but not the saline or nicotine conditions (p≥0.102). Active lever-pressing was lower following 3.0 mg/kg relative to 1.0 mg/kg DHβE in the nicotine condition for females (p<0.001). In males, no effects of DHβE were detected at either dose in either the saline or varenicline conditions (ps≥0.941). In the nicotine condition, 3.0 mg/kg DHβE decreased active lever-pressing relative to saline (p=0.008), but the lower dose did not (p=0.093); no differences were detected between DHβE doses in the nicotine condition in males (p=0.615).
Analysis of locomotor activity during the DHβE testing phase (Figure 2B) revealed significant effects of sex [F(1,21)=26.51; p<0.001] and drug [F(2,42)=10.09; p<0.001], but not of dose [F(2,42)=1.40; NS]. The sex * drug interaction was significant [F(2,42)=41.08; p<0.001], but neither the sex * dose [F(2,42)=1.12; NS] nor drug * dose [F(4,82)=1.60; NS] interaction were significant. The sex * drug * dose interaction also was not significant [F<1]. Further analysis on the sex * drug interaction revealed significantly higher locomotor activity in females than in males in the varenicline and nicotine conditions (ps<0.001), but not in the saline condition (p=0.217). Additionally, nicotine and varenicline increased locomotor activity relative to saline in females (ps<0.001), with no differences were detected between nicotine or varenicline conditions (p=0.551). Varenicline decreased locomotor activity in males relative to saline (p=0.008), whereas locomotor activity was statistically similar between the nicotine and saline (p=0.064), and nicotine and varenicline conditions (p=0.668).
MLA Testing on Progressive Ratio
Active lever-pressing and locomotor activity on the PR schedule of VS reinforcement across sessions of the MLA testing phase is displayed in the lower panels of Figure 2. Analysis of active lever-pressing (Figure 2C) revealed significant main effects of sex [F(1,21)=7.83; p<0.02] and drug [F(2,42)=53.29; p<0.001], but not of dose [F(2,42)=1.06; NS]. None of the sex * drug [F(2,42)=2.84; p=0.07], sex * dose [F(2,42)=1.09; NS], and drug * dose [F<1] interactions were significant. Neither was the sex * drug * dose interaction significant [F<1]. Post-hoc examination of the significant main effects found that active lever-pressing was higher in females than males (p=0.011), and that nicotine and varenicline increased active lever-pressing relative to saline (ps<0.001), with higher active lever-pressing in the nicotine condition relative to varenicline (p=0.048).
Analysis of locomotor activity during the MLA testing phase (Figure 2D) revealed significant effects of sex [F(1,21)=28.21; p<0.001] and drug [F(2,42)=6.73; p<0.005], but not of dose [F<1]. The sex * drug interaction was significant [F(2,42)=17.52; p<0.001], but none of the sex * dose [F(2,42)=1.51; NS], drug * dose interaction [F<1] or sex * drug * dose [F(4,84)=1.19; NS] interactions were significant. Further analysis on the sex * drug interaction revealed significantly higher locomotor activity in females than in males in the varenicline and nicotine conditions (ps<0.001), but not in the saline condition (p=0.057). Additionally, nicotine and varenicline increased locomotor activity relative to saline in females (ps<0.001); no significant differences were detected between nicotine or varenicline conditions (p=0.536). No significant effects of varenicline or nicotine were detected on locomotor activity in males (ps≥0.214).
DISCUSSION
Nicotine and varenicline increased consumption of VS in males and females across a broad range of response costs. The reinforcer demand analysis found that in both sexes, nicotine and varenicline increased intensity of demand (Q0) and essential value (EV) of VS reinforcement. Note that Q0 and EV represent different facets of the reinforcement value construct; Q0 reflects value as consumption where the only constraint on behavior is satiation, while EV represents value as sensitivity to cost independent of scalar changes in reinforcer quantity yet reflective of reinforcer quality (cf. Barrett and Bevins, 2012; 2017; Hursh and Silberberg, 2008). Therefore, the finding that nicotine and varenicline enhanced the value represented by both Q0 and EV is noteworthy because it suggests that both drugs enhanced VS reinforcement value by decreasing both the impact of satiation and sensitivity to cost in males and females. This finding replicates what we reported in Barrett and Bevins (2017) regarding nicotine, while extending those findings with a side-by-side comparison to varenicline in the present report.
Furthermore, nicotine and varenicline increased active lever-pressing maintained by a PR schedule of VS reinforcement during both antagonist testing phases. Notably, both drugs also produced locomotor activation in females, but not in males. Administration of 3.0 mg/kg DHβE attenuated enhancement of active lever-pressing by nicotine in both sexes, without effecting locomotor activation by either drug. Interestingly, DHβE also decreased active lever-pressing in both the saline and varenicline conditions in females, but only in the nicotine condition in males. Treatment with MLA produced no significant effects on active lever-pressing or locomotor activity in either sex at across all drug conditions. Together with the reinforcer demand analyses, these findings suggest that both nicotine and varenicline enhance the value of sensory reinforcement in males and females, but suggest potential differences in enhancement between nicotine and varenicline and the expression of these effects between the sexes.
Regarding the enhancing effects of varenicline relative to those of nicotine: whilst both drugs demonstrated largely parallel enhancing effects on Q0 and EV of VS reinforcement, the enhancement effects of nicotine were consistently greater than those of varenicline. Additionally, neither the effects of nicotine nor varenicline on the demand metrics of value differed by sex. In contrast, in Barrett and Bevins (2017), we found that the enhancing effects of bupropion on Q0 and EV of VS reinforcement reached equivalent levels to those of nicotine, but showed marked differences regarding sex, with greater enhancement by bupropion in females than in males. Combined, these studies provide a clear demonstration that nicotine reliably enhances the value of sensory reinforcers irrespective of sex. Additionally, both studies show that commonly prescribed smoking cessation aids, namely bupropion (Zyban®) and varenicline (Chantix®), produce value-enhancing effects like those of nicotine, which may account in part for their clinical efficacy. Finally, these findings suggest that important differences likely exist regarding the biological mechanisms of the value-enhancing effects of nicotine, varenicline and bupropion (pharmacologically, behaviorally, or both).
Varenicline has a complex pharmacological profile on nicotinic acetylcholine receptors (nAChRs), but its clinical efficacy as a smoking cessation aid are believed to result from its partial agonist/antagonist activity at α4β2* receptors, both mimicking and antagonizing the effects of nicotine at these receptors (Mihalak et al., 2006). Varenicline acts as a full agonist at α7 receptors with high binding affinity, and activates α6β2* and α3β4* receptors (Bordia et al., 2012; Grady et al., 2010; Ortiz et al., 2012; Rollema et al., 2007; Mihalak et al., 2006). Antagonism by DHβE but not MLA in the present study suggests that activation of α4β2* receptors, but not α7 receptors, plays a critical role in the value-enhancing effects of varenicline. Notably, this is a feature that it shares with nicotine, in which activation of α4β2-containing receptors has been implicated in both the primary reinforcing and value-enhancing effects of nicotine (Palmatier et al., 2009). However, recent findings suggest that varenicline does not appear to have primary reinforcing effects, despite sharing a mechanism of action with nicotine at α4β2* receptors (Schassburger et al., 2015). Additionally, DHβE also works as an antagonist at receptors containing the α6β2 and α3β4 subunits (Harvey and Luetje, 1996; Harvey et al., 1996); the potential role of these other subunits in the value-enhancing effects of varenicline or nicotine has not been investigated. Together, these findings suggest that the α4β2* nAChRs may not be solely responsible for the value-enhancing effects of nicotine and varenicline, but that some other receptor subtype also antagonized by DHβE may also be involved, such as α6β2* or α3β4* nAChRs (Schassburger et al., 2015). Further investigation using more specific receptor antagonists, such as AT-1001 (Toll et al., 2012), or knockout gene expression may be critical in further elucidating the mechanisms by which nicotine and varenicline enhance reinforcement value.
The present report adds to previous findings that the value-enhancing effects of nicotine are distinct from its locomotor activating effects (Barrett and Bevins, 2014; Barrett et al., 2017), and extends these findings to varenicline. Note that while nicotine and varenicline produced robust enhancing effects on active lever-pressing in both sexes throughout the experiment, locomotor activation by nicotine or varenicline was only evident in females. Indeed, nicotine produced no detectable locomotor activation in males, and varenicline even produced locomotor decreases in males while simultaneously increasing active lever-pressing (cf. DHβE testing phase). Together with previous findings that have demonstrated similar dissociations between the value-enhancing effects and locomotor activating effects of nicotine (Barrett and Bevins, 2012; 2014; Barrett et al., 2017), the present findings suggest that locomotor activation is not a necessary component of value-enhancement by nicotine. That is not to say, however, that the locomotor stimulating effects of drugs cannot interact with or modify the expression of its separate value-enhancing effects. It is possible that the combination of locomotor activation and value-enhancement may lead to enhancement of operant behavior above and beyond circumstances where value-enhancement occurs in the absence of locomotor effects. That is, expression of drug-induced value-enhancement may be more pronounced in animals whose locomotor behavior has been substantially activated. Future research may consider investigating the interaction between locomotor activation and value-enhancement by drugs that produce either or both effects (cf. Swalve et al., 2015).
In the present study, no sex differences were observed in the demand metrics of reinforcement value produced by nicotine or varenicline, or under saline conditions. However, sex differences in rates of active lever-pressing under the PR schedule were reliably evident across the nicotine and varenicline conditions, and to a lesser extent in the saline condition. The discrepancy regarding sex differences between the demand metrics and active lever-pressing on the PR schedule likely stems from sex differences in responding that are only evident at sufficiently high constraints of response cost. Indeed, similar trends in active lever-pressing are evident in the nicotine and 1.0 mg/kg varenicline conditions during the demand assessment phase (data not shown). However, because rats exited the demand assessment phase at different FR schedules, based on when they met termination criterion, an unbiased analysis of between-subject effects on active lever-pressing at high response costs is impossible in the present study. To this end, the PR schedule here employed was designed to mimic the FR progression of the demand assessment while providing enough pressure on responding to detect effects on responding when responding was primarily constrained by cost. The present findings suggest that female rats may be more sensitive than males to the sensory reinforcement of VS and the enhancement of VS reinforcement by nicotine and varenicline under sufficiently high constraints of response cost.
In the present study, lever-pressing was maintained by a VS that functioned as sensory reinforcement in individually-housed, Sprague-Dawley rats. There are a variety of benefits to using sensory reinforcement in the present preparation, but also a variety of theoretical quandaries. Namely, issues regarding consumption behavior and motivating operations (satiation or deprivation) are theoretically muddy when using sensory reinforcers whose features and functions deviate from more commonly studied primary reinforcers like food, water, or drugs. Consider the previous statement that the demand metric Q0 reflects consumption when the only constraint on behavior is satiation; this interpretation is intuitive and straightforward with a classic reinforcer like food, but what exactly is satiety to a VS? Notably, the subjects in the present study were individually-housed, albino rats who spend most their time in relatively Spartan environmental conditions. This particular arrangement may represent a form of sensory deprivation that gives the VS its capacity to function as a mild reinforcer. While such theoretical discussions are beyond the scope of this report, for the present we assume that sensory reinforcers follow the same general principles as more traditional reinforcers.
The present study replicates the findings of Barrett and Bevins (2017) for nicotine, which also found no differences in Q0 or EV between the sexes in the saline or nicotine conditions. By contrast, Barrett and Bevins (2017) reported higher Q0 in females than males when administered 10 mg/kg bupropion, and greater EV in females than males in the 10 and 20 mg/kg bupropion conditions. Together, these findings suggest that value enhancement by nicotine is similar between the sexes, and extends that finding to varenicline. Moreover, the present findings demonstrate the critical involvement of α4β2* nAChRs in the value-enhancing effects of nicotine and varenicline. Conversely, the value-enhancing effects of bupropion are sex-sensitive and are mediated by dopaminergic and noradrenergic, and not cholinergic mechanisms (Barrett and Bevins, 2017; Palmatier et al., 2009). Importantly, bupropion and varenicline are effective, FDA-approved pharmacotherapies for smoking cessation. Future research should investigate the role that value-enhancement replacement by bupropion and varenicline may play in the efficacy of both agents as smoking cessation aids. If value enhancement by these agents is critical to their efficacy as pharmacotherapies, then perhaps bupropion and varenicline may be differentially effective treatments for men and women trying to quit smoking. Alternatively, differences in sensitivity to the value-enhancing effects of bupropion and varenicline may also be accompanied by differences in sensitivity to the side-effects of both these agents for tobacco-abstaining patients. Regardless, an extension of the present findings to human populations of smokers with special attention to the biobehavioral mechanisms of value-enhancement promises to be a fruitful endeavor and may provide insights into the best practices toward treating tobacco dependence between the sexes.
Acknowledgments
We also thank Dr. Jeffrey Stevens, Dr. Ming Li, and Dr. Samuel Allgood for providing insightful comments on an earlier version of this manuscript. Finally, we also thank Cindy Pudiak, Olivia Loh, Tiffany Schultz, and Aly Lange for help with conducting daily experimental sessions. The Med-Associates programs used in this research, or a more recent version, are available upon request.
FUNDING DISCLOSURE
The authors and this research were in part supported in part by a grant from the National Institute on Drug Abuse (DA034389).
Footnotes
The authors declare no conflicts of interest in relation to the work herein reported.
References
- Barrett ST, Bevins RA. A quantitative analysis of the reward-enhancing effects of nicotine using reinforcer demand. Behavioural Pharmacology. 2012;23:781–789. doi: 10.1097/FBP.0b013e32835a38d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, Bevins RA. Nicotine enhanced operant responding for qualitatively distinct reinforcers under maintenance and extinction conditions. Pharmacology, Biochemistry and Behavior. 2013;114–115:9–15. [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, Geary TN, Stein AN, Bevins RA. Sex differences and the role of dopamine receptors in the reward-enhancing effects of nicotine and bupropion. Psychopharmacology. 2017;234:187–198. doi: 10.1007/s00213-016-4448-x. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behavioral and Cognitive Neuroscience Reviews. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measure of pharmacological reinforcement with behavioral economics: A theoretical proposal. Psychopharmacology. 2000;153:44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- Bordia T, Hrachova M, Chin M, McIntosh JM, Quik M. Varenicline is a potent partial agonist at α6β2* nicotinic acetylcholine receptors in rat and monkey striatum. Journal of Pharmacology and Experimental Therapeutics. 2012;342:327–334. doi: 10.1124/jpet.112.194852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Picciotto MR. Molecular mechanisms underlying the motivational effects of nicotine. In: Bevins RA, Caggiula AR, editors. The Motivational Impact of Nicotine and its Role in Tobacco Use. New York: Springer Science and Business Media; 2009. pp. 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: A dual reinforcement model. In: Bevins RA, Caggiula AR, editors. The Motivational Impact of Nicotine and its Role in Tobacco Use. Springer Science and Business Media; New York: 2009. pp. 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy RN, Dallery J. Effects of economy type and nicotine on the essential value of food in rats. Journal of the Experimental Analysis of Behavior. 2012;97:183–202. doi: 10.1901/jeab.2012.97-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy RN, Dallery J. Quantifying nicotine’s value-enhancement effect using a behavioral economic approach. Journal of the Experimental Analysis of Behavior. 2014;102:353–362. doi: 10.1002/jeab.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Swalve N, Pittenger S, Fink K, Schepers S, Hadlock GC, Fleckenstein AE, Hu G, Li M, Bevins RA. Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine. Neuropharmacology. 2013;75:138–144. doi: 10.1016/j.neuropharm.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Federov NB, et al. Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 3 beta 4*-, and alpha 7-nicotine acetylcholine receptors. Neuropharmacology. 2010;58:1054–1066. doi: 10.1016/j.neuropharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Leutje CW. Determinants of competitive antagonist sensitivity on neuronal nicotinic receptor beta subunits. Journal of Neuroscience. 1996;16:3798–3806. doi: 10.1523/JNEUROSCI.16-12-03798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. Journal of Neurochemistry. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Hoaglin DC, Mosteller F, Tukey JW. Introduction to more refined estimators. In: Hoaglin DC, Mosteller F, Tukey JW, editors. Understanding robust and exploratory data analysis. John Wiley and Sons; New York: 1983. pp. 283–296. [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Hursh SR. Behavioral economics and the analysis of consumption and choice. In: McSweeny FK, Murphy ES, editors. The Wiley Blackwell Handbook of Operant and Classical Conditioning. John Wiley and Sons; New York: 2014. pp. 275–305. [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychological Review. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Replacing relative reinforcing efficacy with behavioral economic demand curves. Journal of the Experimental Analysis of Behavior. 2006;85:73–93. doi: 10.1901/jeab.2006.102-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Sved AF, Donny EC. Varenicline dose dependently enhances responding for nonpharmacological reinforcers and attenuates the reinforcement-enhancing effects of nicotine. Nicotine and Tobacco Research. 2012;14:299–305. doi: 10.1093/ntr/ntr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF. Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology. 2007;194:463–473. doi: 10.1007/s00213-007-0863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: Preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carrol FI, Luetje CW. Varenicline is a partial agonist at alpha-4-beta-2 and a full agonist at alpha-7 neuronal nicotinic receptors. Molecular Pharmacology. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Ortiz NC, O’Neill HC, Marks MJ, Grady SR. Varenicline blocks β2*-nAChR-mediated response and activates β4*-nAChR-mediated responses in mice in vivo. Nicotine and Tobacco Research. 2012;14:711–719. doi: 10.1093/ntr/ntr284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Levin ME, Mays KL, Donny EC, Caggiula AR, Sved AF. Bupropion and nicotine enhance responding for nondrug reinforcers via dissociable pharmacological mechanisms in rats. Psychopharmacology. 2009;207:381–390. doi: 10.1007/s00213-009-1666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine reinforcement and reward: Influences on the persistence of tobacco smoking. In: Bevins RA, Caggiula AR, editors. The Motivational Impact of Nicotine and its Role in Tobacco Use. Springer Science and Business Media; New York: 2009. pp. 143–169. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Grobe J, Fonte C. Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacology, Biochemistry and Behavior. 1994;47:107–112. doi: 10.1016/0091-3057(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology. 2002;163:194–201. doi: 10.1007/s00213-002-1168-1. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Mineur YS. Molecules and circuits involved in nicotine addition: The many faces of smoking. Neuropharmacology. 2014;76:545–553. doi: 10.1016/j.neuropharm.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pittenger ST, Barrett ST, Chou S, Bevins RA. The effects of varenicline on methamphetamine self-administration and drug-primed reinstatement in male rats. Behavioural Brain Research. 2017;320:195–199. doi: 10.1016/j.bbr.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek AN, Dani JA. Synaptic plasticity within midbrain dopamine centers contributes to nicotine addiction. In: Bevins RA, Caggiula AR, editors. The Motivational Impact of Nicotine and its Role in Tobacco Use. New York: Springer Science and Business Media; 2009. pp. 143–169. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha-4-beta-2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: A review of preclinical studies. Neuroscience and Biobehavioral Reviews. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Schassburger RL, Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Donny EC, Sved AF. Differentiating the primary and reinforcement-enhancing effects of varenicline. Psychopharmacology. 2015;232:975–983. doi: 10.1007/s00213-014-3732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalve N, Barrett ST, Bevins RA, Li M. Examining the reinforcement-enhancement effects of phencyclidine and its interactions with nicotine on lever-pressing for a visual stimulus. Behavioural Brain Research. 2015;291:253–259. doi: 10.1016/j.bbr.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Zaveri NT, Polgar WE, Jiang F, Khrovan TV, Zhou W, et al. AT-1001: a high affinity and selective α3β4 nicotinic acetylcholine receptor antagonist blocks nicotine self-administration in rats. Neuropsychopharmacology. 2012;37:1367–1376. doi: 10.1038/npp.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Humans Services. The Health Consequences of Smoking: Nicotine Addiction: A Report of the Surgeon General. Atlanta: U.S Department of Health and Humans Services, Public Health Service, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1988. 1988. [Google Scholar]
- U.S. Department of Health and Human Services. Women and Smoking: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2001. 2001. [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. 2014. [Google Scholar]
- Wooters TE, Bevins RA, Bardo MT. Neuropharmacology of the interoceptive stimulus properties of nicotine. Current Drug Abuse Reviews. 2009;2:243–255. doi: 10.2174/1874473710902030243. [DOI] [PMC free article] [PubMed] [Google Scholar]


