Abstract
Objective:
Although numerous studies have documented the effects of sleep loss on executive control (EC) and related abilities, research examining the impact of early EC on subsequent sleep problems is lacking. Therefore, the current study reports on a longitudinal investigation of EC in preschool as a predictor of sleep/wake problems and daytime sleepiness in early adolescence.
Participants:
The participants were 141 children (48.6% female) recruited from the community for a longitudinal study spanning preschool through early adolescence, with an oversampling for high sociodemographic risk (34.1% based on eligibility for public medical insurance, free/reduced lunch status, and/or family income-to-needs below the federal poverty line).
Methods:
Participants completed a battery of developmentally-appropriate tasks assessing major aspects of EC (working memory, inhibitory control, flexible shifting) during a laboratory visit at age 4 years, 6 months. Participants also completed a follow-up session in early adolescence (between ages 11 years and 13.5 years; mean age = 11.82 years, SD = .62 years), during which they completed self-report measures of sleep/wake problems and daytime sleepiness.
Results:
Structural equation modeling results indicate that preschool EC (represented by a single latent construct) significantly negatively predicted both sleep/wake problems and daytime sleepiness in early adolescence, with poorer EC predicting greater subsequent sleep problems.
Conclusions:
Poorer EC abilities during the critical period of preschool may be a risk factor for later sleep problems in adolescence. Given that EC appears to be modifiable, early interventions to promote EC development may help prevent subsequent sleep problems and promote long-term health trajectories.
Keywords: executive control, sleep problems, sleepiness, preschool, early adolescence
A considerable body of literature has examined the association between sleep and cognitive abilities in both children and adults (see Alhola& Polo-Kantola, 2007; De Freitas Araújo&Moraes de Almondes; 2014; Lim &Dinges, 2010). Much of this research has focused on executive control (EC; also known as “executive function”) – a set of critical “top-down” cognitive processes for directing attention and behavior (Diamond, 2013; Garon, Bryson, & Smith, 2008) – and related abilities.1 While the effects of poor sleep on EC are well-established, the inverse effect of poor EC on later sleep problems has received limited attention.
Numerous studies have documented the negative effects of suboptimal sleep – either naturally occurring or experimentally manipulated – on a range of specific executive abilities and related constructs.For example, Bernier and colleagues (2013) found that infant sleep at age 1 year (specifically, higher proportion of sleep at night) was associated with better performance on EC tasks, but not general intelligence, at age 4. Similarly, Sadeh and colleagues (2015) reported that poorer infant sleep quality at age 1 (measured by actigraphy) was associated with more attention and behavior regulation problems in preschool, and Nelson et al. (2015) found that preschool sleep problems significantly predicted poorer performance on working memory and interference suppression inhibition tasks in early elementary school. Karpinski and colleagues (2008) reported that sleep-disordered breathing was associated with poorer EC in a sample of preschoolers. Analyzing data from the large-scale Colorado Longitudinal Twin Study, Friedman and colleagues (2009) found that changes in sleep problems across childhood predicted EC, with those experiencing decreased sleep problems over time showing better EC in late adolescence.In an experimental design, Gruber and colleagues (2012) found that even modest sleep restriction was associated with greater impulsivity in a sample of children ages 7 to 11. These negative cognitive and behavioral effects arebelieved to be due to the deleterious impact of sleep disruption on the prefrontal cortex where EC abilities are centered (Beebe &Gozal, 2002; Dahl, 1996).
Although the influence of poor sleep on EC is relatively well-established, there has been much less research exploring the possibility that poor EC could contribute to the later development of sleep problems. Longitudinal studies with rigorous measurement of EC at critical points in development are particularly lacking. Therefore, the current paper reports on one such study to determine if early EC abilities during the critical period of preschool predict sleep problems in early adolescence.
Despite a lack of empirical studies to date, there is reason to believe that poor EC could lead to subsequent sleep problems. EC underlies the regulation of attention and behavior (Diamond, 2013; Garon, Bryson, & Smith, 2008), and these abilities may contribute to the development of healthy sleep habits. Maintaining a consistent sleep schedule, which is essential to sleep health (Gruber, 2013), requires the planful direction of one’s behavior to “stay on schedule,” particularly in the context of social and academic demands that threaten to interfere with healthy sleep schedules. Individuals who struggle to effectively regulate and direct their behavior, therefore, may be at increased risk for inconsistent sleep because they may have difficulty planning (which emerges from problems with core EC abilities) and efficiently completing daytime activities in time to meet bedtime goals.From a personality perspective, the trait of conscientiousness may be important in establishing, and adhering to, a healthy sleep schedule, and research has found positive associations between conscientiousness and EC (see Williams, Suchy, & Rau, 2009, for review).Further, the process of transitioning from waking activities to sleep requires the individual to down-regulate from the day by reducing physiological arousal and “turning off” cognitive processes (e.g., rumination) that may interfere with sleep onset (Schwartz & Roth, 2008). Individuals with poor EC may struggle to effectively down-regulate in preparation for bedtime, contributing to longer sleep latency and shorter total sleep duration.Similarly, difficulties with inhibition may lead to engaging in immediately rewarding, but sleep-interfering, behaviors at night (e.g., watching television, playing video games), furtherdisrupting sleep patterns. Taken together, poor EC may undermine healthy sleep hygiene, contributing to chronic suboptimal nighttime sleep and excessive daytime sleepiness; however, studies rigorously examining this possibility are currently lacking.
In exploring the potential influence of EC on sleep, it is important to consider the developmental context of these constructs. EC abilities begin to develop early, with preschool commonly acknowledged as a critical period for EC development (Clark et al., in press; Garon et al., 2008), and continue to develop throughout adolescence and into early adulthood (Diamond, 2013). Although sleep habits also begin to develop early in life, adolescence is a particularly important time for developing individual sleep habits given the increased independence that characterizes this period. Adolescents typically take on greater responsibility for a variety of health behaviors (Sawyer et al., 2012), including sleep (Carskadon, 1990), and some may have difficulty managing this responsibility, as evidenced by the high rates of sleep problems among adolescents (Roberts, Roberts, & Duong, 2009; Owens et al., 2014). Further, adolescence is often a time of increasing academic and social demands (Christie & Viner, 2005; Jaworska&MacQueen, 2015), making it even more challenging to navigate this developmental period while maintaining a healthy sleep schedule. The cognitive and regulatory resources (such as EC) that the individual brings to this transition may be particularly importantforthe development and maintenance of healthy sleep habits because these abilities are tested by the challenging context of adolescence. The development of healthy sleep in adolescence, in turn, can affect health trajectories throughout adolescence and into adulthood (Dahl & Lewin, 2002). From this perspective, cognitive abilities such as EC that begin to emerge as early as preschool may have long-term effects on sleep and overall health.
Despite theory proposing an important role for EC in sleep (e.g., Dahl, 1996), empirical studies examining this effect are extremely limited. In one of the only studies in this area, Todd and Mullan (2013) found that better cognitive flexibility, an aspect of EC, was significantly associated with better sleep hygiene in a sample of college students. In terms of children and adolescents, numerous studies link Attention-Deficit/Hyperactivity Disorder (ADHD), which is often characterized by deficits in EC, and poor sleep (e.g., Gruber, Sadeh, &Raviv, 2000; Gruber et al., 2009; Kirov, Pillar, &Rothenberger, 2004; Silvestri et al., 2009).However, we are not aware of any published studies with children or adolescents that specifically examine the effect of earlyEC on subsequent sleep problems.
To address gaps in the current literature, we conducted a unique longitudinal investigation of EC and sleep, employing rigorous performance-based measures of EC during the critical period of preschool to predict sleep problems in adolescence. We hypothesized that EC performance in preschool would predict sleep problems in adolescence (both sleep/wake problems and daytime sleepiness), with poorer EC associated with greater sleep problems. This unprecedented longitudinal study will provide a novel examination of how early-developing executive abilities may affect later-emerging sleep behaviors, with potential implications for long-term health. This study could also point to new strategies for promoting healthy sleep by targeting early EC development. Research indicates that EC is modifiable, and that intervention may be particularly promising in preschool (Diamond& Lee, 2011; Zelazo& Carlson, 2012), although the long-term health effects of EC intervention are not yet established. If EC deficits contribute to later sleep problems, it may be possible that early intervention to improve EC could help promote healthy sleep habits throughout development.
Methods
Participants and Procedures
Participants were 141 children (48.6% female) who participated in a larger longitudinal study spanning preschool to early adolescence. The larger project focused on early EC development, and the sample was recruited through flyer distribution in the community. Given the focus on normative EC development in the broader study, children with a diagnosed developmental or behavioral disorder at the time of the initial preschool recruitment were excluded. Children from families with high socioeconomic risk were oversampled, with 34.1% considered high risk at the time of enrollment based on eligibility for public medical insurance, free/reduced lunch status, and/or income-to-needs below the federal poverty line. The sample was 70.3% Caucasian, 12.3% Hispanic, 2% African American, 1% Asian, and 15.2% multiracial.
All of the children included in the current investigation completed a laboratory-based protocol within two weeks of turning4 years, 6 months. They also participated in a follow-up visit to the laboratory at some point between the ages of 11 and 13.5 years (m = 11.82 years, SD = .62). The preschool visit, which was part of a larger study to describe early EC development, included the child completing an extensive battery of developmentally-appropriate neuropsychological tasks to assess key aspects of EC (see Measures). To examine the associations between early EC and selected health issues in adolescence (including sleep problems), a follow-up study was planned for youth who had participated in the preschool visit and were in fifth grade or beyond at the time of the follow-up. This early adolescence follow-up visit involved the adolescent completingbrief questionnaires measuring aspects of health and behavior, including sleep problems and daytime sleepiness. Adolescent height and weight were also objectively measured by a research assistant using a standard scale and stadiometer at this visit. Participants were not aware of specific study hypotheses, but rather were informed of the general purpose of the follow-up study (i.e., to study factors affecting adolescent health). All procedures were approved by the Institutional Review Board of [BLINDED FOR REVIEW].
A total of 231 children in the larger longitudinal study completed the initial laboratory protocol at ages 4 years, 6 months. Of those 231 children, 151 were eligible for the follow-up appointment in early adolescence based on the requirement that the child be in fifth grade or beyond at the time of follow-up evaluation. (Note: Because the larger study used a lagged cohort sequential design for initially enrolling participants, the remaining 80 children had not yet reached fifth grade and where therefore not eligible to participate in the follow-up which focused on early adolescent health.) Of the 151 children who were eligible to participate in the follow-up, 141 (93.4%) agreed to participate and completed the early adolescent follow-up data collection protocol. For the 141 participants in the final sample, there was no missing data on any of the measures used in this study. Those who participated in the early adolescent follow-up did not significantly differ from those who participated only at age 4 years, 6 months in terms of sex, race/ethnicity or maternal education (ps> .05).
Measures
Preschool Executive Control.
EC in preschool was measured using a battery of nine tasks that were individually administered to children during the age 4 years, 6 months visit. Tasks covered the major areas comprising EC, including working memory, inhibitory control, and flexible shifting. Tests of working memory included Nine Boxes (adapted from Diamond et al., 1997),Delayed Alternation(Espy et al., 1999; Goldman, Rosvold, Vest, &Galkin, 1971), and Nebraska Barnyard (adapted from Noisy Book; Hughes Dunn, & White, 1998). Tasks measuring inhibitory control were Big-Little Stroop (adapted from Kochanska, Murray, & Harlan, 2000),Go/No-Go (adapted from Simpson & Riggs, 2006), Shape School- Inhibit Condition (Espy, 1997; Espy, Bull, Martin, & Stroup, 2006), and a modified Snack Delay (adapted from Kochanska, Murray, Jacques, Koenig, & Vandegeest, 1996; Korkman, Kirk, & Kemp, 1998). Finally, flexible shifting was assessed using Shape School - Switching Condition (Espy, 1997; Espy et al., 2006) and Trails-Switching Condition (modified from Espy & Cwik, 2004). All tasks have demonstrated excellent psychometric properties with preschool children, including considerable variability, minimal missing data, and excellent inter-rater reliability (see James, Choi, Wiebe, & Espy, in press, for summary). Table 1 provides a brief description of each task.
Table 1.
Description of Executive Control Tasks
| EC Task | Brief Description | Source |
|---|---|---|
| Working Memory | ||
| Nine Boxes | Child searches among nine boxes for a reward. Boxes are different colors and shapes, and the child must remember previously searched boxed to be able to find the reward in the fewest number of trials. |
Adapted from Diamond et al. (1997) |
| Delayed Alternation | Child searches for reward by choosing one of two locations on a board. The location of the reward alternates, so the child must remember the last location following a delay. |
Espy et al. (1999); Goldman et al. (1971) |
| Nebraska Barnyard | Child is shown locations of animal figures on a 3×3 field of boxes and then must remember the locations in order to indicate locations of animal sequences after pictures are removed. |
Adapted from Hughes et al. Noisy Book task (1998) |
| Inhibitory Control | ||
| Big-Little Stroup | Child is presented with stimuli made of smaller objects embedded within a larger objects. Child is asked to name the smaller object, which requires suppression of the larger object name. |
Adapted from Kochanska et al. (2000) |
| Go/No-Go | Child is presented a series of fish or shark stimuli on the screen. Child is asked to press a button when a fish is shown but inhibit pressing the button when a shark is shown. |
Adapted from Simpson & Riggs (2006) |
| Shape School – Inhibit | Child is presented with cartoon stimuli with either a happy or sad faces. Child is asked to say the color of the stimulus when the face is happy and to inhibit naming the color when the face is sad. |
Espy (1997); Espy et al. (2006) |
| modified Snack Delay | Child is seated in close proximity to a candy reward and is asked to remain still until a bell rings. |
Adapted from Konchanska et al. (1996); Korkman et al. (1998) |
| Flexible Shifting | ||
| Shape School - Switching | In the switching phase of the Shape School task, the child is presented with cartoon stimuli of different colors and shapes that are either wearing a hat or not. Child is asked to say the color of the stimulus when not wearing a hat and say the shape when wearing a hat |
Espy (1997); Espy et al. (2006) |
| Trails - Switching | Child uses a stamp to mark stimuli on a page, alternating between dog and bone stimuli. |
Adapted from Espy & Cwik (2004) |
Early Adolescent Sleep/Wake Problems.
Early adolescent sleep/wake problems were assessed using the Sleep/Wake Problems Behavior Scale of the Sleep Habits Survey (SWP; Wolfson &Carskadon, 1998). This scale consists of 10 items measuring a variety of problems with sleep onset (e.g., difficulty falling asleep, going to bed late) and waking from sleep (e.g., oversleeping in the morning). Previous research with the SWP scale has demonstrated strong reliability and validity, and this measure was categorized as “Approaching Well-Established” in a review of evidence-based measures of pediatric sleep by Lewandowski and colleagues (2011) based on multiple published studies showing good internal consistency and correlations with sleep diaries and actigraphy.The scale also showed good internal consistency in the current sample (α=.74).
Early Adolescent Daytime Sleepiness.
Daytime sleepiness was measured using the widely-used Epworth Sleepiness Scale-Revised for Children Version (ESS; Melendres, Lutz, Rubin, & Marcus, 2004; Moore et al., 2009). The ESS is an 8-item self-report measure assessing the likelihood of falling asleep in a variety of common daytime situations (e.g., riding in a car, watching television). The ESS has demonstrated good reliability and validity in previous research and was categorized as “Approaching Well-Established” by Lewandowski et al.(2011) based on multiple published studies reporting good internal consistency and validity analyses with theoretically related constructs.Internal consistency for the ESS was good in the current study (α=.70).
Family Socioeconomic Status.
Caregivers (almost always mothers) accompanying the child to laboratory sessions reported onfamily socioeconomic status (SES). Reflecting the conceptualization of SES as a multifaceted construct (Duncan & Magnuson, 2003), multiple variables related to family SES were collected, including years of maternal education (mean = 15.2 years; SD = 2.1 years), eligibility for public assistance (37.3% eligible), and income-to-needs ratio (mean = 2.75; SD = 1.83).
Analysis Plan
Structural equation modeling was used to examine preschool EC as a predictor of later sleep problems in adolescence. EC was modeled using nine behavioral tasks (each of which was standardized) as indicators loading on a single latent EC construct. Previous research using this taskbattery has supported a unitary factor structure as the most appropriate model in preschool. Specifically, Nelson and colleagues (in press) found excellent fit for the unitary factor model using the same nine EC tasks presented in the current paper with children age 4 years, 6 months (RMSEA = .04; CFI = .96). Further, the unitary model was found to be preferable to both 2- and 3-factor models at this age based on fit and parsimony.Therefore, the single latent EC construct was used in our analyses with examination of fit indices to confirmappropriate fit for the entire model (see Results). The two sleep constructs (sleep/wake problems and daytime sleepiness) were modeled as observed variables using total scores from the SWP and the ESS, respectively, and included in the model together with latent preschool EC as the predictor. The model was estimated using maximum likelihood estimation (ML) in Mplus version 7.2 (Muthén&Muthén, 1998–2014). All variables demonstrated acceptable distributional properties.
Results
Preliminary Analyses
We considered several demographic variables as potential covariates, including child sex, child age (at the early adolescence visit), and indicators of family SES (i.e., maternal education, eligibility for public assistance, income-to-needs ratio). These variables were considered because each has been previously associated with both EC and sleep, and therefore were considered as the most plausible covariates requiring examination.Neither child sex nor child age was significantly correlated with either of the sleep outcomes, so these variables were not included in the final model. Neither maternal education nor eligibility for public assistance was significantly related to the sleep outcomes, so they were not included in the model. However, income-to-needs ratio was significantly associated with both sleep/wake problems (r = −.24, p = .006) and daytime sleepiness (r = −.22, p = .011), so it was included as a control variable in the final model. In addition to demographic variables, we also considered adolescent body mass index (BMI) percentile has a potential covariate because some research has found an association between higher BMI and both EC and sleep problems. However, BMI percentile was not significantly correlated with either of the sleep outcomes in this study, so it was not included as a control variable in the final model.
Structural Equation Model
In the predictive model controlling for income-to-needs ratio(Figure 1), latent EC significantly predicted sleep/wake problems, such that poorer EC performance in preschool was associated with more sleep/wake problems in adolescence, β= −.23, SE=.10, t= −2.65, p=.021. Similarly, latent EC significantly predicted daytime sleepiness, such that poorer EC performance in preschool was associated with greater daytime sleepiness in adolescence, β= −.26, SE=.10, t= −2.65, p=.008. The overall model demonstrated good fit [χ2(51)=63.21, p=.12, RMSEA=.042, CFI=.94] and all of the task loadings on the latent EC construct were significant.
Figure 1.
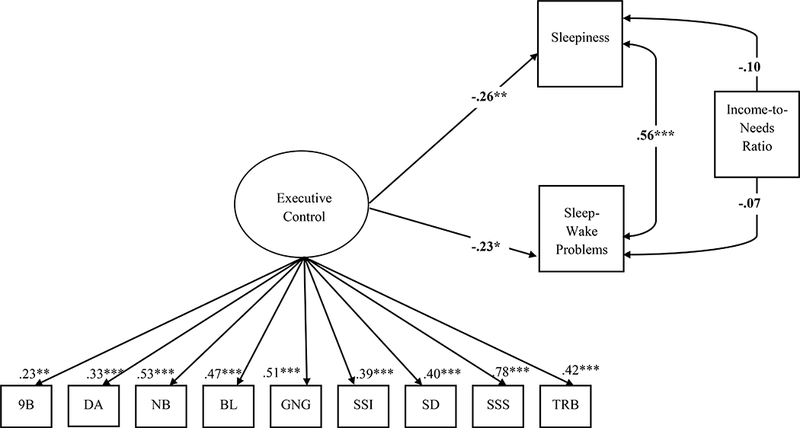
Results of Structural Equation Model
Notes: All results are standardized
*p< .05, **p< .01, ***p< .001
9B = 9 Boxes; DA = Delayed Alternation; NB = Noisy Book; BL = Big Little Stroop; GNG = Go-No-Go; SSI = Shape School Inhibit; SD = Snack Delay; SSS = Shape School Switch; TRB = Trail Making Test
Discussion
The current study examined the role of early EC as a predictor of subsequent sleep problems, finding that preschool EC was inversely associated with sleep problems in early adolescence. To our knowledge, this is the first study to explore EC predicting later sleep problems in a longitudinal pediatric study. The findings were consistent with a priori hypotheses and suggest that certain cognitive abilities that begin to develop very early in life may play an important role in emerging critical health behaviors, such as sleep, years later in adolescence. These findings add to the growing literature indicating that early EC is a powerful predictor of later child and adolescent functioning across various domains (e.g., Anzman& Birch, 2009; Clark, Sheffield, Wiebe, & Espy, 2013; Espy, Sheffield, Wiebe, Clark, &Moehr, 2011), while expanding this literature to include sleep as a domain that may be affected by EC. The current study is also consistent with recent theoretical work (e.g., Hall & Marteau, 2014) proposing EC as an important factor affecting broad health risk through its influence on health behaviors. Deficits in EC may compromise developing healthy sleep habits, which in turn can lead to a host of long-term physical, cognitive, and behavioral risks (Colten &Altevogt, 2006), particularly given the importance of sleep in adolescence for brain development (Dahl & Lewin, 2002).
Although our specific empirical findings are novel, they fit well with earlier conceptualizations of the interplay between self-regulatory abilities and sleep, particularly in the often challenging period of adolescence. Dahl, for example, has highlighted the central role of the prefrontal cortex (PFC) in both higher-order cognitive abilities (such as EC) and the regulation of sleep (e.g., Dahl, 1996; Dahl & Lewin, 2002). Subtle deficits in early PFC development underlying poor EC could compromise a child’s ability to effectively regulate attention and behavior in preparation for sleep. Chronic sleep problems, in turn, could further undermine EC development through deleterious effects on the PFC, leading to a “vicious cycle” of escalating EC and sleep problems across development. This possibility points to the potential value of intervening to improve EC or sleep (or both) to “break the cycle.” While previous studies documenting the negative effects of sleep loss on EC have highlighted sleep as a potential target to improve cognitive functioning, our study suggests the inverse may be worth considering, as well. That is, given that poorer early EC predicts later sleep problems, perhaps interventions to improve early EC development may be a viable strategy for promoting better long-term sleep health. An emerging body of literature suggests that EC can be improved through a variety of interventions (Diamond & Lee, 2011; Hillman et al., 2014) and that early environmental factors may be particularly important for EC development in the context of sociodemographic risk (Nelson, Choi, Clark, James, Fang, Wiebe, & Espy, 2015). Although the long-term health benefits of early intervention to promote EC are not yet established, it is possible that stronger EC might better equip children with the self-regulatory abilities needed to develop life-long healthy habits in sleep and other critical health behaviors.Research building on the current study to examine if early EC intervention might promote better sleep in the long-term is needed.
The current study has some limitations to consider. The primary limitation is the measurement of early adolescent sleep. Although the SWP and ESS are considered evidence-based measures of pediatric sleep, they are limited in both their subjective report format and incomplete coverage of sleep issues. Future studies should seek to replicate our findings using objective sleep measures (e.g., actigraphy, polysomnography) and a broader range of sleep constructs. Another important limitation is the use of single time-point measures of EC (in preschool) and sleep (in early adolescence), which limits our ability to definitively determine causal relations between the constructs. Although our design did preserve the hypothesized temporal ordering of EC preceding sleep problems, we cannot rule out the possibility of sleep problems causing EC deficits or bidirectional effects. It is possible, for example, that early sleep problems (which were not measured in this study) compromised early EC development which, in turn, was associated with later sleep problems. Alternatively, it is plausible that problems in EC and sleep could be caused by a shared underlying (and unmeasured) condition which accounts for their association, or that the two constructs affect each other in sequential bidirectional effects across development. To more rigorously evaluate causal direction, future investigations incorporating repeated measurements of both EC and sleep across key developmental periods (e.g., preschool, adolescence) would be extremely valuable. Finally, the current study did not explicitly explore possible mechanisms explaining the observed EC-sleep association. Future studies might consider how the core EC abilities of working memory, inhibitory control, and flexible shifting early in developmentinfluence later sleep behaviors through possible mechanisms such as deficits in planning, time management, and down-regulation in preparation for sleep.Despite these limitations, the current study provides a rare longitudinal examination of how early EC, measured using a comprehensive battery of rigorous performance-based measures during the critical period of preschool, may contribute to sleep years later in adolescence.
Acknowledgements:
We thank the participating families and acknowledge the invaluable assistance with data collection and coding by research technicians and undergraduate and graduate students of the Developmental Cognitive Neuroscience Laboratory at the University of Nebraska-Lincoln.
Funding:
This work was supported by NIH grant MH065668 and an award from the Office of Research, College of Arts and Sciences and Department of Psychology at the University of Nebraska-Lincoln.
Footnotes
The current study focuses on executive control, which is considered distinct from related constructs such as effortful control. Executive control is a neuropsychological construct focusing on modular cognitive processes underlying the direction of attention and behavior. Effortful control, in contrast, is conceptualized as an aspect of temperament that does not focus as directly on cognitive processes.
References
- Alhola P, & Polo-Kantola P (2007). Sleep deprivation: Impact on cognitive performance. Journal ofNeuropsychiatric Disease and Treatment, 3, 553–567. [PMC free article] [PubMed] [Google Scholar]
- Anzman SL, & Birch LL (2009). Low inhibitory control and restrictive feeding practices predict weight outcomes. Journal of Pediatrics, 155, 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DW, &Gozal D (2002). Obstructive sleep apnea and prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. Journal of Sleep Research, 11, 1–16. [DOI] [PubMed] [Google Scholar]
- Carskadon MA (1990). Patterns of sleep and sleepiness in adolescents. Pediatrician, 17, 5–12. [PubMed] [Google Scholar]
- Christie D, & Viner R (2005). Adolescent development. BMJ, 330, 301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CA, Chevalier N, Nelson JM, James TD, Garza JP, Choi H-J, & Espy KA (in press). The changing nature of executive control in preschool: I. Executive control in early childhood. Monographs of the Society for Research in Child Development. [DOI] [PubMed] [Google Scholar]
- Clark CA, Sheffield TD, Wiebe SA, & Espy KA (2013). Longitudinal associations between executive control and developing mathematical competence in preschool boys and girls. Child Development, 84, 662–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colten HR, &Altevogt BM. (eds). (2006). Sleep disorders and sleep deprivation: An unmet public health problem. National Academies Press: Washington, D.C. [PubMed] [Google Scholar]
- Dahl RE (1996). The impact of inadequate sleep on children’s daytime cognitive function. Seminars in Pediatric Neurology, 3, 44–50. [DOI] [PubMed] [Google Scholar]
- Dahl RE, & Lewin DS (2002). Pathways to adolescent health sleep regulation and behavior. Journal of Adolescent Health, 31, 175–184. [DOI] [PubMed] [Google Scholar]
- De Freitas Araújo D, &Moraes de Almondes K (2014). Sleep and cognitive performance in children and pre-adolescents: A review. Biological Rhythm Research, 45, 193–207. [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology, 64, 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, & Lee K (2011). Interventions shown to aid executive function development in children 4–12 years old. Science, 333, 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Prevor MB, Callender G, &Druin DP. (1997). Prefrontal cortex cognitive deficits in children treated early and continuously for PKU. Monographs of the Society for Research in Child Development, 62, 1–206. [PubMed] [Google Scholar]
- Espy KA (1997). The Shape School: Assessing executive function in preschool children. Developmental Neuropsychology, 26, 379–384. [DOI] [PubMed] [Google Scholar]
- Espy KA, Bull R, Martin J, & Stroup W (2006). Measuring the development of executive control with the Shape School. Psychological Assessment, 18, 373–381. [DOI] [PubMed] [Google Scholar]
- Espy KA, &Cwik MF (2004). The development of a trial making test in young children: The TRAILS-P. Clinical Neuropsychology, 18, 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy KA, Kauffman PM, McDiarmid MD, &Glisky ML (1999). Executive functioning in preschool children: Performance on A-not-B and other delayed response formats. Brain and Cognition, 41, 178–199. [DOI] [PubMed] [Google Scholar]
- Espy KA, Sheffield TD, Wiebe SA, Clark CA, &Moehr MJ (2011). Executive control and dimensions of problem behaviors in preschool children. Journal of Child Psychology and Psychiatry, 52, 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Corley RP, Hewitt JK, & Wright KP Jr. (2009). Individual differences in childhood sleep problems predict later cognitive executive control. Sleep, 32, 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N, Bryson SE, & Smith IM (2008). Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin, 134, 31–60. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE, Vest B, &Galkin TW(1971). Analysis of the delayed alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. Journal of Comparative and Physiological Psychology, 77, 212–220. [DOI] [PubMed] [Google Scholar]
- Gruber R (2013). Making room for sleep: The relevance of sleep to psychology and the rationale for development of preventative sleep education programs for children and adolescents in the community. Canadian Psychology, 54, 62–71. [Google Scholar]
- Gruber R, Cassoff J, Frenette S, Wiebe S, & Carrier J (2012). Impact of sleep extension and restriction on children’s emotional lability and impulsivity. Pediatrics, 130, e1155–e1161. [DOI] [PubMed] [Google Scholar]
- Gruber R, Sadeh A, &Raviv A (2000). Instability of sleep patterns in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 495–501. [DOI] [PubMed] [Google Scholar]
- Gruber R, Xi T, Frenette S, Robert M, Vannasinh P, & Carrier J (2009). Sleep disturbances in prepubertal children with attention deficit hyperactivity disorder: A home polysomnography study. Sleep, 32, 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PA, & Marteau TM (2014). Executive function in the context of chronic disease prevention: Theory, research and practice. Preventive Medicine, 68, 44–50. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Pontifex MB, Castelli DM, Khan NA, Raine LB, Scudder MR,&Kamijo K (2014). Effects of the FITKids randomized controlled trial on executive control and executive function. Pediatrics, 134, e1063–e1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C, Dunn J, & White A (1998). Trick or treat? Uneven understanding of mind and emotion and executive dysfunction in “hard-to-manage” preschoolers. Journal of Child Psychology and Psychiatry, 39, 981–994. [PubMed] [Google Scholar]
- James TD, Choi HJ, Wiebe SA & Espy KA (in press). The changing nature of executive control in preschool: II. The Preschool Problem Solving Study: Sample, data, and statistical methods. Monographs of the Society for Research in Child Development. [DOI] [PubMed] [Google Scholar]
- Jaworska N, &MacQueen G. (2015). Adolescence as a unique developmental period. Journal of Psychiatry and Neuroscience, 40, 291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov R, Pillar G, &Rothenberger A (2004). REM-sleep changes in children with attention-deficit/hyperactivity disorder: Methodologic and neurobiologic considerations. Sleep, 27, 1215. [PubMed] [Google Scholar]
- Kochanska G, Murray K, & Harlan ET (2000). Effortful control in early childhood: Continuity, change, antecedents and implications for social development. Developmental Psychology, 36, 220–232. [PubMed] [Google Scholar]
- Kochanska G, Murray K, Jacques TY, Koenig AL, &Vandegeest KA (1996). Inhibitory control in young children and its role in emerging internalization. Child Development, 67, 490–507. [PubMed] [Google Scholar]
- Korkman M, Kirk U, & Kemp S (1998). NEPSY: A developmental neuropsychological assessment. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Lewandowski AS, Toliver-Sokol M, & Palermo TM (2011). Evidence-based review of subjective pediatric sleep measures. Journal of Pediatric Psychology, 36, 780–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, &Dinges DF (2010). A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychological Bulletin, 136, 375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendres MC, Lutz JM, Rubin ED, & Marcus CL (2004). Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics, 114, 768–775. [DOI] [PubMed] [Google Scholar]
- Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Ancoli-Israel S, & Redline S (2009). Relationships among sleepiness, sleep time, and psychological functioning in adolescents. Journal of Pediatric Psychology, 34, 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, &Muthén BO Mplus user’s guide, 3rd Edition. Los Angeles: Muthén&Muthén, 2004. [Google Scholar]
- Nelson JM, Choi H-J, Clark CA, James TD, Fang H, Wiebe SA, & Espy KA (2015). Sociodemographic risk and early environmental factors that contribute to resilience in executive control: A factor mixture model of 3-year-olds. Child Neuropsychology, 21, 354–378. [DOI] [PubMed] [Google Scholar]
- Nelson JM, James TD, Choi HJ, Clark CAC, Wiebe SA, & Espy KA (in press). The changing nature of executive control in preschool: III. Distinguishing executive control from overlapping foundational cognitive abilities during the preschool period. Monographs of the Society for Research in Child Development. [DOI] [PubMed] [Google Scholar]
- Nelson TD, Nelson JM, Kidwell KM, James TD, & Espy KA (2015). Preschool sleep problems and differential associations with specific aspects of executive control in early elementary school. Developmental Neuropsychology, 40, 167–180. [DOI] [PubMed] [Google Scholar]
- Owens J, Adolescent Sleep Working Group, & Committee on Adolescence (2014). Insufficient sleep in adolescents and young adults: An update on causes and consequences. Pediatrics, 134, e921–e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, & Duong HT (2009). Sleeplessness in adolescence: Prospective data on sleep deprivation, health and functioning. Journal of Adolescence, 32, 1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, & Bates J (2006). Temperament In Eisenberg N, Ed. Handbook of Child Psychology: Social, Emotional, and Personality Development. New York, NY: Wiley & Sons, 99–166. [Google Scholar]
- Sadeh A, de Marcas G, Guri Y, Berger A, Tikotzky L, & Bar-Haim Y (2015). Infant sleep predicts attention regulation and behavior problems at 3–4 years of age. Developmental Neuropsychology, 40, 122–137. [DOI] [PubMed] [Google Scholar]
- Sawyer SM, Afifi RA, Bearinger LH, Blakemore SJ, Dick B, Ezeh AC, & Patton GC (2012). Adolescence: A foundation for future health. The Lancet, 379, 1630–1640. [DOI] [PubMed] [Google Scholar]
- Schwartz JRL, & Roth T (2008). Neurophysiology of sleep and wakefulness: Basic science and clinical implications. Current Neuropharmacology, 6, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri R, Gagliano A, Aricò I, Calarese T, Cedro C, Bruni O,& Bramanti P (2009). Sleep disorders in children with attention-deficit/hyperactivity disorder (ADHD) recorded overnight by video-polysomnography. Sleep Medicine, 10, 1132–1138. [DOI] [PubMed] [Google Scholar]
- Simpson A, & Riggs KJ (2006). Conditions under which children experience inhibitory difficulty with a “button-press” go/no-go task. Journal of Experimental Child Psychology, 94, 18–26. [DOI] [PubMed] [Google Scholar]
- Todd J, &Mullan B (2013). The role of self-regulation in predicting sleep hygiene in university students. Psychology, Health, & Medicine, 18, 275–288. [DOI] [PubMed] [Google Scholar]
- Turnbull K, Reid GJ, & Morton JB (2013). Behavioral sleep problems and their potential impact on developing executive function in children. Sleep, 36, 1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PG, Suchy Y, & Rau HK (2009). Individual differences in executive functioning: Implications for stress regulation. Annals of Behavioral Medicine, 37, 126–140. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, &Carskdon MA (1998). Sleep schedules and daytime functioning in adolescents. Child Development, 69, 875–887. [PubMed] [Google Scholar]
- Zelazo PD, & Carlson SM (2012). Hot and cool executive function in childhood and adolescence: Development and plasticity. Child Development Perspectives, 6, 354–360. [Google Scholar]


