Abstract
Purpose
We report a patient with severe multi-organ dysfunction of unknown origin who presented with bilateral orbital and chorioretinal manifestations that led to the diagnosis of Erdheim-Chester Disease (ECD).
Observations
ECD is a rare, histiocytic, proliferative disorder characterized by multi-systemic organ involvement that has historically lacked effective therapy. Our patient underwent genetic testing that was positive for the BRAF V600E mutation; therefore, the patient was treated with vemurafenib.
Conclusions and importance
This case demonstrates the rare orbital and intraocular manifestations of ECD and the unfortunate impact of a delayed diagnosis, the importance of early gene therapy testing for management decisions, and the utilization of targeted directed therapy to improve visual outcomes and quality of life.
Keywords: Histiocytosis, Vemurafenib, Orbital, Chorioretinal, Xanthomatous, Lipogranulomatous, BRAF
1. Introduction
We report an unusual bilateral chorioretinal and orbital presentation of Erdheim-Chester disease (ECD), which is a rare, non-Langerhans’ cell histiocytosis with various clinical manifestations that can involve many organs, including the skeleton, pericardium, lungs, endocrine, and central nervous systems. The pathogenesis of the disease is part of a dysregulated mechanism in which mutations in the mitogen-activated protein kinase (MAPK) pathway result in uncontrolled cell survival and proliferation of histiocytes. This produces a xanthomatous infiltration into various organs, resulting in multi-systemic involvement leading to end-organ dysfunction.1
2. Case report
Our patient is a 52-year-old Vietnamese woman with history of hypothyroidism as well as multiple medical problems that lacked a unifying diagnosis. She initially presented at age 33 with anemia and leukocytosis and was diagnosed with JAK-2 negative, calreticulin positive, essential thrombocythemia (ET). She subsequently underwent numerous surgical procedures for pancreatitis, hepatomegaly, multiple portal, splenic, and mesenteric vein thromboses, recurrent chronic pleural and pericardial effusions, constrictive cardiomyopathy, and severe restrictive lung disease. Her work up was negative for infectious, rheumatologic, or malignant etiology and numerous biopsies showed multi-organ fibrosis. She suffered from severe chronic dyspnea and abdominal bloating. Her chronic and debilitating condition with oxygen requirements resulted in multiple hospital admissions for medical complications. She was noted to have depression with intermittent suicidal ideation.
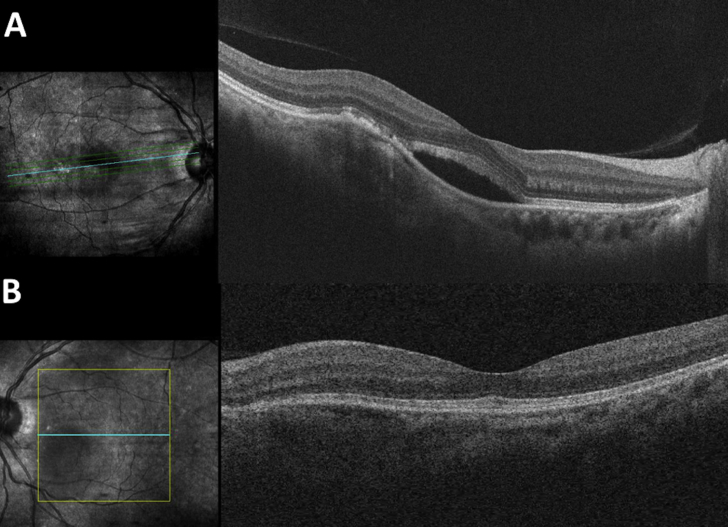
She presented to the ophthalmology clinic at age 51 with proptosis of the left eye. Ocular exam was significant for visual acuity 20/30 OD, 20/40 OS, normal intraocular pressure, no afferent pupillary defect, full extraocular movements with left upper eyelid retraction, resistance to retropulsion, and 3.5 mm of proptosis on Hertel. Dilated fundus exam revealed normal optic nerves and bilateral inferior placoid orange subretinal lesions with subretinal fluid (Fig. 1A–B). Humphrey visual field testing showed an enlarged blind spot with a superior arcuate defect (Fig. 2A). Spectral-domain optical coherence tomography (SD-OCT) of her macula demonstrated a sub-retinal pigment epithelium (RPE) hypolucent mass associated with foveal subretinal fluid in the right eye and a similar sub-RPE hypolucent mass with choroidal elevation without subretinal fluid in the left eye (Fig. 3A–B). Fluorescein angiography demonstrated associated hyperfluorescence of the placoid lesions with late staining consistent with non-exudative, multifocal choroidal infiltrates in both eyes (Fig. 4A–E). Magnetic resonance imaging (MRI) of the brain and orbits revealed a T1/T2 hypointense, mass with heterogeneous enhancement measuring 2.9 × 1.6 × 2.5 cm located in the left superomedial orbit which displaced the optic nerve and a right 4 mm superolateral orbital lesion (Fig. 5A–B). Brain imaging showed bulky symmetric enhancing bilateral lesions along the tentorial leaflets with heterogeneous enhancement.
Fig. 1.
(A–B): Title: Fundus photos on presentation. Caption: A, Color fundus photo of the right eye on presentation demonstrating a yellow placoid subretinal lesion along the inferior arcade extending towards the fovea and a smaller yellow lesion nasal to the optic disc. B, The left eye demonstrates similar yellow subretinal lesion along the inferior arcade with extension into the fovea and a smaller subretinal yellow lesion superotemporal to fovea. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
(A–B): Title: Humphrey visual field 30-2 on presentation and after treatment with vemurafenib. Caption: A, HVF 30-2 left eye on presentation with superior arcuate defects. B, HVF 30-2 left eye performed 6 weeks after initiation of treatment demonstrates improved reliability and resolution of prior defects.
Fig. 3.
(A–B): Title: OCT macula on presentation. Caption: A, OCT macula of the right eye demonstrates irregularity and disruption of the RPE with sub-RPE hyperlucent mass. There is subretinal fluid within the fovea. B, The left eye demonstrates a similar choroidal elevation with sub-RPE hyperlucent mass. There is no edema.
Fig. 4.
(A–E): Title: Fluorescein angiogram on presentation. Caption: A-C, Fluorescein angiogram of the right eye in laminar filling phase demonstrates a subtle hyperfluorescence along the inferior arcade that becomes more prominent with time. In later stages, there is staining along the inferior arcade associated with RPE irregularities temporal to the fovea. This is consistent with a choroidal vascular filling process. D-E, The left eye late stages demonstrate a focal area of hyperfluorescence and slight staining temporal to the nerve.
Fig. 5.
(A–B): Title: MRI orbits and brain. Caption: A, Coronal MRI T1 with contrast and fat suppression demonstrates a fairly homogenous lesion measuring 25.3mm in height that enhances with contrast and molds to the superomedial globe with displacement of the globe. B, Axial MRI T1 shows a right 4 mm superolateral orbital lesion and the left lesion displacing the optic nerve.
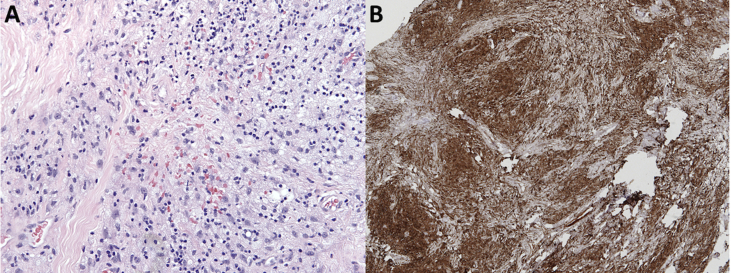
Transcaruncular orbitotomy with incisional biopsy revealed a yellow, rubbery mass consistent with a left orbital xanthogranuloma. Immunohistochemical testing showed that the lipid-laden cells expressed CD163, confirming histiocytic origin (Fig. 6A–B). Additionally, S100, CD1a, and langerin were negative, excluding Langerhans cell histiocytosis. Bone marrow biopsy revealed ECD involvement as manifested by hemophagocytosis and multinucleated Touton giant cells; megakaryocytic dysplasia and complex cytogenetics were also present, suspicious for myelodysplastic syndrome (MDS) secondary to ET. Molecular studies confirmed the presence of the BRAF V600E mutation in both the orbital xanthogranuloma and the bone marrow aspirate. Whole body fluorine-2-fluoro-2-Deoxy-d-glucose (18F-FDG) positron emission tomography/computerized tomography (PET/CT) scan demonstrated hypermetabolic areas within the bilateral tentorial leaflets, midline falx, and left superomedial orbit. There was diffuse FDG uptake in the mediastinum, pericardium, retroperitoneum, central mesentery, and bilateral iliopsoas muscles as well as patchy medullary sclerosis with a low-level FDG uptake in the proximal humeri and femurs. The constellation of pathology results, ocular exam, long bone sclerosis, and multi-organ fibrosis confirmed the diagnosis of ECD.
Fig. 6.
(A–B): Title: Histopathological specimen of left intraconal and extraconal mass. Caption: A, H&E stain demonstrates abundant foamy mononuclear cell infiltrate within a background of fibrosis and inflammation. B, CD163 stain is diffusely positive, confirming cells of histiocytic origin.
Vemurafenib therapy beginning at 480 mg by mouth daily tapered to 240 mg daily resulted in rapid visual and systemic improvement. After 4 weeks of treatment her visual acuity improved to 20/20 OU, left proptosis improved by 2 mm, and HVF testing showed nearly resolved superior defects with a mean deviation improvement from −5.08 to −0.98 (Fig. 2B). Her oxygen requirements decreased by over 50%. Notably, she had a dramatic improvement in energy, feeling of well-being, nutrition, leg edema, and ability to perform her activities of daily living without oxygen supplementation for brief periods. She experienced mild (grade I) skin rash and diarrhea as a side effect of the medication. After 6 weeks of treatment, there was no change in the bilateral choroidal infiltrates and the right subretinal fluid worsened slightly (Fig. 7A–B). Intravitreal bevacizumab injection was administered in the right eye. Approximately 12 weeks after starting vemurafenib treatment and 6 weeks after the bevacizumab injection, there was no improvement in the subretinal fluid; thereafter, the patient declined further bevacizumab. She declined to increase the vemurafenib dose.
Fig. 7.
(A–B): Title: OCT macula after initiation of vemurafenib therapy. Caption: A, OCT macula of the right eye shows worsening subretinal fluid seen 10 weeks after initiating vemurafenib therapy. At this point, the patient had received an intravitreal injection of bevacizumab 4 weeks earlier. B, OCT macula of the left eye shows no edema.
Despite substantial visual and quality of life improvements, anemia worsened due to MDS and probable transformation to acute myeloid leukemia (AML), based on 20% circulating blasts. Hemophagocytic lymphohistiocytosis (HLH) was not felt to be likely. Approximately 3 months after initiation of low dose vemurafenib, the patient was admitted to the hospital with worsening anemia and progressive respiratory dysfunction. She declined to undergo another marrow biopsy, increase the vemurafenib dose, or initiate therapy for AML. She did not permit invasive diagnostic testing of her lungs. Five months after her diagnosis, she died. An autopsy was declined.
3. Discussion
Erdheim-Chester disease (ECD) is a rare, multi-systemic, histiocytic disorder that should be recognized as early as possible to minimize morbidity and mortality. Intraocular involvement is uncommon with only 5 patients reported in the literature.2, 3, 4 We present the first reported case of bilateral intracranial, orbital, and intraocular involvement in a patient with biopsy-confirmed ECD treated with vemurafenib.
Clinical manifestations can involve the skeletal, cardiac, pulmonary, cutaneous, endocrine, and central nervous systems (CNS), resulting from histiocytic infiltration producing soft tissue thickening and chronic fibrotic disease. CNS disease, which can develop in up to 51–92% of patients5,6 has been demonstrated to be an independent predictor of death.7 Orbital involvement, which can occur in 25–37% of patients, presents with retrobulbar masses that can cause visual impairment, motility disturbance, proptosis, and optic nerve edema.1,6,8 Additional visual disturbances related to CNS lesions can occur due to involvement of the optic nerve or chiasm.1 Prompt orbital biopsy with immunostaining and molecular studies can diagnose this systemic disease and permit targeted therapy.9,10 Intraocular involvement, which is very rare compared to orbital involvement, can present as choroidal infiltrates with associated serous retinal detachment3 or choroidal neovascular membrane.2 The differential diagnosis for the chorioretinal lesions found in our patient includes primary or metastatic malignancy, autoimmune conditions, infectious choroiditis, or vascular tumors such as hemangioma. However, in the setting of biopsy-proven systemic ECD, whose funduscopic appearance, clinical course, and ancillary testing are consistent with the 5 prior reports of ECD chorioretinal involvement in the literature2, 3, 4 (of which 1 case underwent a chorioretinal biopsy with characteristic immunohistochemical testing3), a diagnosis of choroidal ECD is favored. Of note, evaluation of the bilateral orbital and intraocular involvement led to the diagnosis of an advanced systemic disease as well as undiagnosed CNS involvement, which portends a poorer prognosis.
The pathogenesis of ECD is increasingly understood today as part of a dysregulated mechanism in which mutations in the mitogen-activated protein kinase (MAPK) pathway result in uncontrolled cell survival, differentiation, and proliferation of histiocytes.11,12 Alteration of the RAS-RAF-MEK-ERK-MAPK (RAS-MAPK) pathway is commonly associated with uncontrolled activation of receptor tyrosine kinases or proto-oncogene “gain of function” mutations.13 Other myeloid neoplasms, such as MDS, are commonly seen in ECD patients with one group reporting an overlap in 10.1% (19/189) of patients.14 This is applicable to our patient who had a preexisting myeloid neoplasm that underwent transformation shortly after the diagnosis of ECD. Recent genetic and molecular advances have demonstrated that activating mutations in BRAF exist in greater than 50% of ECD patients, which can be detected in biopsies.15 Our patient had a positive BRAF V600E mutation, enabling her to receive vemurafenib, which is an oral therapy that is a targeted inhibitor of mutated BRAF16,17.
Vemurafenib has been reported to rapidly ameliorate systemic symptoms of ECD with reports of marked improvement in radiologic, clinical, and laboratory markers in as early as 4 weeks.16, 17, 18 Orbital manifestations have also been reported to improve with vemurafenib in 5 patients.16,17,19,20 To our knowledge, this is the first case report describing a patient with intraocular manifestations of ECD being treated with vemurafenib. Our patient had a similar rapid response to treatment, including improved overall well-being, energy, nutrition, oxygen requirement, vision, proptosis, and HVF testing. Conversely, the chorioretinal manifestations and subretinal fluid showed no response to a single anti-VEGF injection after 6 weeks of follow-up nor to vemurafenib after 12 weeks of follow-up, although the visual acuity improved. Additional commentary about the patient's subretinal fluid, however, warrants attention as its etiology remains unclear. The central serous chorioretinopathy-like picture has previously been reported in a 3 patient case series by Tan et al. with those authors suggesting it may be due to histiocyte-induced choroidal inflammation, or more rarely, secondary choroidal neovascularization.3 These authors generally noted a favorable, though variable, response to anti-VEGF injections. One patient demonstrated resolution of subretinal fluid in one eye and no response in their other eye. A second patient demonstrated short-term responses to anti-VEGF injections and required re-treatment until resolution of subretinal fluid and chorioretinal infiltrates only 10 months after initiation of definitive systemic therapy with MEK-inhibitor, sorafenib (prompted by chorioretinal biopsy that confirmed a positive ARAF mutation commonly associated with abnormalities in the MEK signaling pathway). The third patient suffered progressive vision loss over 1 year despite intravitreal anti-VEGF and methotrexate injections and photodynamic therapy. One possibility, suggested by the non-exudative appearance of the lesions on FA, peri-lesional RPE changes on OCT, advanced level of disease, and lack of response to intravitreal anti-VEGF and systemic chemotherapy is that the subretinal fluid simply represents impaired RPE pump dysfunction from ECD choroidal infiltration. Whether such RPE damage occurs by direct physical trauma or choriocapillaris ischemia (as have been postulated in the pachychoroid disease spectrum21) remains equivocal.
The subretinal fluid in this patient is not likely a side effect from systemic therapy (as has been described in MEK-inhibitors) since she demonstrated subretinal fluid before initiating vemurafenib. It is feasible, however, that worsening of subretinal fluid can be precipitated by BRAF inhibitors such as vemurafenib, however, with the only suggestion of this being a recent report citing a 0.41% incidence of subretinal fluid in melanoma patients placed on vemurafenib.22 Due to the rarity of chorioretinal manifestations associated with ECD, with only 5 reported cases in the literature to date,2, 3, 4 long-term visual outcomes with various intraocular and systemic treatments is limited. The optimal management of subretinal fluid associated with ECD remains unclear. Our case demonstrates novel evidence that vemurafenib treatment resulted in marked improvement of orbital and systemic symptoms of ECD, but did not produce equally efficacious results for subretinal fluid associated with chorioretinal infiltrates. The totality of this albeit limited data suggests that chorioretinal infiltrate and subretinal fluid resolution in ECD patients may require a longer duration of definitive systemic treatment or may not be responsive to treatment with vemurafenib.
After more than a decade of delayed diagnoses leading to disease progression, our patient had accumulated significant fibrosis and lipogranulomatous infiltration of multiple organs. By the time she presented to ophthalmology, she had simultaneous bilateral orbital and intraocular manifestations with rapid development of CNS infiltrates within 2 months. Although treatment with low dose vemurafenib enabled improvement in visual symptoms, comfort, and quality of life, her concurrent myelodysplastic syndrome and respiratory disease rapidly progressed. We suspect continued treatment with high dose vemurafenib could have significantly improved her outcome if ET had not transformed to MDS.
4. Conclusions
In conclusion, ECD is a rare, systemic, histiocytic proliferative disorder often associated with a delayed diagnosis and poor prognosis. Orbital and, rarely, chorioretinal manifestations of the disease enable the ophthalmologist to play a crucial role in initial diagnosis and management of these patients. Recent molecular and genetic advances enable testing for specific mutations in the mitogen-activated protein kinase (MAPK) pathway such as BRAF V600E that results in uncontrolled activation of receptor tyrosine kinases. If positive for a BRAF V600E mutation, the patient is a candidate for targeted therapy with vemurafenib, an inhibitor of mutated BRAF, which may yield rapid improvement in visual and systemic symptoms. Further investigation is necessary to determine the utility of vemurafenib in treatment of the chorioretinal features of this disease. Our case is the first reported case of simultaneous bilateral orbital and intraocular involvement in biopsy-confirmed ECD treated with vemurafenib.
Patient consent
Written consent from the patient and the patient's family has been obtained to allow details of this case to be published.
Acknowledgments and disclosures
Funding
Research to Prevent Blindness and NIHP30-EY026877 were used to fund this research.
Conflicts of interest
The following authors have no financial disclosures (LH, KT, DG, RB, BM, RW, AK).
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship. All authors have made significant contributions to the manuscript including literature search (Huang, Topping, Silva, Kossler), figure compilation (Huang, Topping, Brown, Silva, Kossler), data interpretation and analysis (Huang, Topping, Brown, Gratzinger, Martin, Silva, Kossler), and writing of manuscript (Huang, Topping, Gratzinger, Martin, Silva, Kossler).
Acknowledgments
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ajoc.2018.07.005.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Cives M. Erdheim-Chester disease: a systematic review. Crit Rev Oncol Hematol. 2015;95(1):1–11. doi: 10.1016/j.critrevonc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Biccas Neto L., Zanetti F. Intraocular involvement in Erdheim-Chester disease–first report in the literature: case report. Arq Bras Oftalmol. 2007;70(5) doi: 10.1590/s0004-27492007000500025. 862–7. [DOI] [PubMed] [Google Scholar]
- 3.Tan A.C. Three cases of Erdheim-Chester disease with intraocular manifestations: imaging and histopathology findings of a rare entity. Am J Ophthalmol. 2017;176:141–147. doi: 10.1016/j.ajo.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Abdellatief A. Choroidal involvement in Erdheim-Chester disease. Ophthalmic Surg Laser Imag Retina. 2015;46(6) doi: 10.3928/23258160-20150610-13. 674–6. [DOI] [PubMed] [Google Scholar]
- 5.Cavalli G. The multifaceted clinical presentations and manifestations of Erdheim-Chester disease: comprehensive review of the literature and of 10 new cases. Ann Rheum Dis. 2013;72(10) doi: 10.1136/annrheumdis-2012-202542. 1691–5. [DOI] [PubMed] [Google Scholar]
- 6.Estrada-Veras J.I. The clinical spectrum of Erdheim-Chester disease: an observational cohort study. Blood Adv. 2017;1(6):357–366. doi: 10.1182/bloodadvances.2016001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnaud L. CNS involvement and treatment with interferon-α are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood. 2011;117(10):2778–2782. doi: 10.1182/blood-2010-06-294108. [DOI] [PubMed] [Google Scholar]
- 8.Haroche J. Erdheim-Chester disease. Curr Rheumatol Rep. 2014;16(4):412. doi: 10.1007/s11926-014-0412-0. [DOI] [PubMed] [Google Scholar]
- 9.Lau W.W., Chan E., Chan C.W. Orbital involvement in Erdheim-Chester disease. Hong Kong Med J. 2007;13(3):238–240. [PubMed] [Google Scholar]
- 10.Shields J.A. Orbital and eyelid involvement with Erdheim-Chester disease. A report of two cases. Arch Ophthalmol. 1991;109(6):850–854. doi: 10.1001/archopht.1991.01080060114037. [DOI] [PubMed] [Google Scholar]
- 11.Vassallo R., Harari S., Tazi A. Current understanding and management of pulmonary Langerhans cell histiocytosis. Thorax. 2017;72(10):937–945. doi: 10.1136/thoraxjnl-2017-210125. [DOI] [PubMed] [Google Scholar]
- 12.Prince H.M. Identifying mutant pathways in the histiocytoses. Blood. 2014;124(19):2901–2903. doi: 10.1182/blood-2014-09-597765. [DOI] [PubMed] [Google Scholar]
- 13.Santarpia L., Lippman S.M., El-Naggar A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papo M. High prevalence of myeloid neoplasms in adults with non-Langerhans cell histiocytosis. Blood. 2017;130(8):1007–1013. doi: 10.1182/blood-2017-01-761718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haroche J. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700–2703. doi: 10.1182/blood-2012-05-430140. [DOI] [PubMed] [Google Scholar]
- 16.Haroche J. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121(9):1495–1500. doi: 10.1182/blood-2012-07-446286. [DOI] [PubMed] [Google Scholar]
- 17.Grumbine F.L. Orbital MRI pre- and post-vemurafenib therapy for Erdheim-Chester disease. Ophthalmic Plast Reconstr Surg. 2015;31(6) doi: 10.1097/IOP.0000000000000401. e169. [DOI] [PubMed] [Google Scholar]
- 18.Cohen-Aubart F. Marked efficacy of vemurafenib in suprasellar Erdheim-Chester disease. Neurology. 2014;83(14):1294–1296. doi: 10.1212/WNL.0000000000000832. [DOI] [PubMed] [Google Scholar]
- 19.Haroche J. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol. 2015;33(5):411–418. doi: 10.1200/JCO.2014.57.1950. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A. Vemurafenib (BRAF inhibitor) therapy for orbital Erdheim-Chester disease. Ophthalmic Plast Reconstr Surg. 2017;33(6):e138–e139. doi: 10.1097/IOP.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 21.Warrow D.J., Hoang Q.V., Freund K.B. Pachychoroid pigment epitheliopathy. Retina. 2013;33(8):1659–1672. doi: 10.1097/IAE.0b013e3182953df4. [DOI] [PubMed] [Google Scholar]
- 22.de la Cruz-Merino L. Clinical features of serous retinopathy observed with cobimetinib in patients with BRAF-mutated melanoma treated in the randomized coBRIM study. J Transl Med. 2017;15(1):146. doi: 10.1186/s12967-017-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.