Abstract
Introduction
Oral rifampicin has been shown to significantly reduce amyloid β (Aβ) and tau pathologies in mice. However, it shows occasional adverse effects such as liver injury in humans, making its use difficult for a long period.
Methods
To explore safer rifampicin treatment, APPOSK mice, a model of Alzheimer's disease, were treated with rifampicin for 1 month via oral, intranasal, and subcutaneous administration, and its therapeutic efficacy and safety were compared.
Results
Intranasal or subcutaneous administration of rifampicin improved memory more effectively than oral administration. The improvement of memory was accompanied with the reduction of neuropathologies, including Aβ oligomer accumulation, tau abnormal phosphorylation, and synapse loss. Serum levels of a liver enzyme significantly rose only by oral administration. Pharmacokinetic study revealed that the level of rifampicin in the brain was highest with intranasal administration.
Discussion
Considering its easiness and noninvasiveness, intranasal administration would be the best way for long-term dosing of rifampicin.
Keywords: Intranasal, Rifampicin, Aβ oligomers, Hepatotoxicity, Pharmacokinetics, Prevention, Alzheimer's disease
1. Background
Cerebral accumulation of amyloid oligomers is believed to be the initial step in the pathogenesis of neurodegenerative dementia such as Alzheimer's disease (AD) and tauopathy [1], [2]. Accordingly, reducing the content of amyloid oligomers has long been hypothesized as a rational strategy to treat these diseases. Meanwhile, clinical studies of amyloid β (Aβ)–targeting therapies in AD have revealed that treatment after disease onset has little effect on patient cognition [3], [4]. This finding suggests that the treatment of neurodegenerative dementia should be started before the onset of clinical symptoms [5]. This view prompted us to explore a preventive medicine which is orally available, has little adverse effects, and is effective at reducing neurotoxic oligomers with a broad spectrum. Based on these criteria, we demonstrated that a well-known antibiotic, rifampicin, is a good candidate [6]. Under cell-free conditions, rifampicin inhibited the oligomer formation of Aβ, tau, and α-synuclein, indicating its broad spectrum [6]. When orally administered to aged APPOSK mice (Aβ oligomer model) for 1 month, rifampicin reduced the accumulation of Aβ oligomers as well as tau abnormal phosphorylation, synapse loss, and microglial activation in a dose-dependent manner and improved memory to a level similar to that in non-Tg littermates at 1 mg/day [6]. Oral administration of rifampicin to aged tau609 mice (tauopathy model) for 1 month also reduced tau oligomer accumulation, tau hyperphosphorylation, synapse loss, and microglial activation in a dose-dependent fashion and improved memory almost completely at 1 mg/day [6].
Although these results indicate a therapeutic potential of rifampicin against dementia, rifampicin shows occasional, nonnegligible adverse effects such as liver injury (https://livertox.nih.gov/Rifampin.htm) and drug-drug interactions (https://www.drugs.com/drug-interactions/rifampin.html), which discourages the use of rifampicin for a long period. Orally administered rifampicin is absorbed from the intestine, carried to the liver via the portal vein, and largely metabolized in the liver before entering the blood circulation, resulting in a lowered bioavailability (i.e., first pass effect). The mechanism of rifampicin-induced hepatotoxicity is unknown and unpredictable, as there is no evidence for the presence of a toxic metabolite of rifampicin. Rifampicin-related drug-drug interactions are thought to occur via activation of the nuclear pregnane X receptor in hepatocytes that in turn upregulates the expression of cytochrome P450 and P-glycoprotein; the former metabolizes many drugs, and the latter mediates drug adsorption and efflux [7]. Thus, the delivery of rifampicin directly to the brain or the promotion of brain penetration of rifampicin through the blood-brain barrier by increasing rifampicin bioavailability to avoid the fast pass effect would permit a lower rifampicin dose and in turn reduce the risk of adverse effects.
To explore safer rifampicin treatment, we tested several routes of rifampicin application (oral, intranasal, and subcutaneous) in aged APPOSK mice and compared their therapeutic efficacy and safety. APPOSK mice are a transgenic (Tg) mouse model of AD that express Aβ oligomer-related pathologies without forming amyloid plaques by the presence of the Osaka mutation [8]. The mice display intraneuronal accumulation of Aβ oligomers and subsequent tau abnormal phosphorylation, synapse loss, and memory impairment at 8 months, microglial activation at 12 months, and neuronal loss at 24 months. Thus, APPOSK mice are an ideal model for investigating the effects of rifampicin on Aβ oligomers in vivo. The results in the present study show that intranasal and subcutaneous administration is safer and more effective than oral administration and that intranasal application achieves the highest brain delivery of rifampicin.
2. Methods
2.1. Rifampicin treatment to APPOSK mice
Rifampicin is usually prescribed to adult humans at 10 mg/kg orally once a day for tuberculosis (https://www.drugs.com/dosage/rifampin.html). Assuming a mean body weight of adult mice of 30 g, the mean dose for mice corresponds to 0.3 mg a day. We decided the daily dose of rifampicin for mice in our initial experiment as 0.25 mg. For oral and subcutaneous administration, rifampicin was dissolved to 0.83 mg/mL in 0.5% low-viscosity carboxymethylcellulose (CMC; Sigma). Three hundred micro liters of the solution (i.e., 0.25 mg) was administered orally using feeding needles or subcutaneously using injectors to 11-month-old male APPOSK mice 5 days a week (Monday through Friday) for 1 month. For intranasal administration, rifampicin was dissolved to 25 or 5 mg/mL in 0.5% CMC. Mice were held in an upright to supine position without anesthesia, and 10 μL of the rifampicin solution (i.e., 0.25 or 0.05 mg) was administered into the bilateral nasal cavity using 10 μL MiniFlex Round Tips (Sorenson BioScience, Inc., Salt Lake City, UT), utilizing capillary phenomenon. As controls, age-matched Tg and non-Tg littermates were treated with CMC solution intranasally and orally, respectively. All treatments were performed without prior habituation of mice to handling. All animal experiments were approved by the ethics committee of Osaka City University (Osaka, Japan) and performed in accordance with the Guide for Animal Experimentation, Osaka City University.
2.2. Behavioral test
Spatial reference memory in mice was assessed at 12 months of age using the Morris water maze, as described previously [6]. Mice were trained to swim to a hidden platform for four consecutive days, and the retention of spatial memory was assessed by a probe trial on day 5. Rifampicin treatment was continued during the behavioral test. To ensure that APPOSK mice have normal locomotor activity, their spontaneous locomotion in the light and dark was measured by an open field test at 18 months of age, as described previously [9]. In brief, mice were allowed to search freely in a square acrylic box (30 × 30 cm) for 20 min. The light attached to the ceiling of the enclosure was on during the first 10 min (light period) and off during the later 10 min. On each x and y bank of the open field, two infrared rays were attached 2 cm above the floor at 10 cm intervals, making a flip-flop circuit between the two beams. The number of beam crossing was counted every min as traveling behavior of mice.
2.3. Measurement of liver enzymes
After the water maze task, blood was collected under anesthesia, and sera was separated by centrifugation. The levels of liver enzymes aspartate transaminase (AST, also known as GOT) and alanine transaminase (ALT, also known as GPT) in the sera were measured using a 7180 Clinical Analyzer (Hitachi High-Technologies, Tokyo, Japan) with L-type Wako AST·J2 and ALT·J2 reagents (Wako Pure Chemical Industries, Osaka, Japan).
2.4. Immunohistochemical analysis
After collecting blood samples, mice were divided into two groups: one for immunohistochemical analysis and the other for biochemical analysis. Coronal brain sections were prepared every 5 μm from the position of interaural 2.0 mm toward the caudal end, and immunohistochemical staining was performed as described previously [6]. Aβ oligomers were stained with mouse monoclonal 11A1 antibody (IBL, Fujioka, Japan), and the staining intensity in a constant area of the pyramidal cell layer in the hippocampal CA3 region was quantified using National Institutes of Health ImageJ software, as described previously [6]. Phosphorylated tau was visualized with mouse monoclonal PHF-1 antibody (a kind gift from Dr. Peter Davies, Albert Einstein College of Medicine), and the staining intensity in a constant area of the mossy fibers in the hippocampal CA2-CA3 region was quantified. Synaptophysin was stained with mouse monoclonal antibody to synaptophysin (SVP-38; Sigma), and synapse levels in the apical dendritic-somata field in the hippocampal CA2-CA3 region were evaluated by quantifying the staining intensity in a constant area. Data were standardized with the background intensity in each section, and the mean values of staining intensity were calculated from randomly selected two sections per animal. We assume that synaptophysin intensity reflects synapse level, but other possibilities cannot be ruled out: lower synaptophysin intensity may simply represent its lesser expression in neurons, rather than fewer synapses, or its reduced binding to the antibody.
2.5. Biochemical analysis
Hippocampal tissues were dissected from mouse brains and homogenized by sonication in 4 volumes of 50 mM Tris-HCl, pH 7.6, and 150 mM NaCl (Tris-buffered saline [TBS]) containing 1/100 volume of protease inhibitor cocktail (P8340; Sigma-Aldrich). The homogenates were subjected to Western blot with SVP-38 antibody for synaptophysin and rabbit polyclonal antibody to actin (Sigma-Aldrich). Signals were visualized and quantified using an ImageQuant LAS 500 (GE Healthcare Bio-Sciences, Uppsala, Sweden).
The remaining brain tissues minus the cerebellum were homogenized in four volumes of TBS containing P8340 using a tissue grinder with Teflon pestle. The homogenates were fractionated by three-step ultracentrifugation, including TBS, 2% sodium dodecyl sulfate (SDS), and 70% formic acid (FA) extraction, essentially as described previously [8]. SDS and FA extracts were dialyzed against TBS at 4°C overnight using Slide-A-Lyzer Dialysis Cassette G2 with 2K cutoff membrane (Thermo Scientific). The levels of Aβ40, Aβ42, and Aβ oligomers in each fraction were measured using Human β Amyloid(1–40) enzyme-linked immunosorbent assay (ELISA) kit Wako II, Human β Amyloid(1–42) ELISA kit Wako, High-Sensitive (both from Wako Pure Chemical Industries), and Human Amyloid β Oligomers (81E1-specific) Assay kit-IBL (IBL), respectively. Aβ oligomers were also measured by direct ELISA with 11A1 antibody, as described previously [6].
2.6. Pharmacokinetic study
Radiolabeled rifampicin (rifampicin, [4-methylpiperazine-3H]-, specific activity; 1.57 TBq/mmol) was a product of Moravek (Brea, CA). Tissue solubilizer (Soluene-350®) and scintillation cocktail (Clear-sol I®) were purchased from PerkinElmer (Waltham, MA) and Nacalai Tesque (Kyoto, Japan), respectively. Unlabeled rifampicin was dissolved in phosphate buffered saline (PBS) at the concentration 250 μg/mL for intranasal application and 25 μg/mL for oral and subcutaneous application. To each solution, 3H-rifampicin was added at the dose 92.5 kBq/mice.
Male ddY mice weighing 25 g were purchased from Japan SLC (Shizuoka, Japan). All animal experiments were conducted according to the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication #85-23). The Animal Experiment Committee of Kyoto Pharmaceutical University approved all protocols of animal experiments. The dosing solution of rifampicin was applied into bilateral nasal cavity (10 μL), subcutaneously (100 μL), or orally (100 μL). At a predetermined time (15, 30, 60, 120, or 180 min) after application, blood was collected from the portacaval vein. Soon after blood sampling, saline including heparin was flushed by perfusion from the left cardioventricule to remove the blood from the cerebral blood vessel, and the whole brain was taken. The dissected brain was washed with ice-cold saline. Plasma was obtained from whole blood by centrifugation. For decoloration, the plasma (100 μL) was treated with 100 μL of 30% hydrogen peroxide at 0°C for 5 min. The brain tissue was minced, weighed, and solubilized with 1 mL of Soluene 350® in the counting vial. After complete solubilization, 200 μL of 10 N HCl was added to each sample for neutralization. After the addition of 5 mL scintillation cocktail (Clear-sol I®) to the treated plasma and brain tissue, radioactivity was determined with a liquid scintillation counter, LSC6100 (Hitachi Aloka Medical, Ltd, Mitaka, Tokyo, Japan).
2.7. Statistical analysis
All experiments and data analyses were performed under unblinded conditions. Comparisons of means among more than two groups were performed using analysis of variance or two-factor repeated measures analysis of variance (for the behavioral test), followed by Fisher's PLSD test (for immunohistochemistry and the behavioral test) or Tukey-Kramer test (for ELISA and Western blot). For results from the pharmacokinetic study, the difference was analyzed with Dunnett's test. Differences with a P value of <.05 were considered significant.
3. Results
3.1. Effects of differently administered rifampicin on memory in APPOSK mice
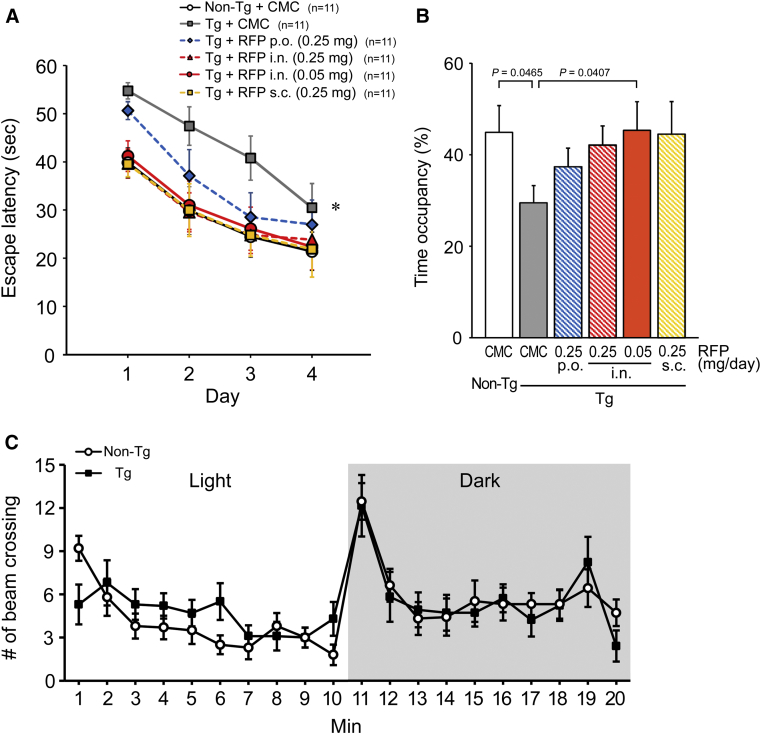
The spatial reference memory of mice was assessed by the Morris water maze after 1-month treatment with rifampicin. CMC-treated 12-month-old APPOSK mice showed significantly impaired memory compared with CMC-treated age-matched non-Tg littermates (Fig. 1A). Oral administration of rifampicin at 0.25 mg/day improved memory, but the effect was incomplete. Previously, we showed that 0.5 mg/day of oral rifampicin achieved almost complete recovery of mouse memory in 12-month-old APPOSK mice, but that in 18-month-old mice more than 1 mg/day of rifampicin was necessary to restore memory to the levels of age-matched non-Tg littermates [6]. These observations collectively indicate that the effect of rifampicin on memory is very sensitive to the age of the recipient and the daily dose of rifampicin. In contrast to oral administration, intranasal or subcutaneous treatment with the same dose (i.e., 0.25 mg/day) of rifampicin improved mouse memory almost completely. Notably, when administered intranasally, even 0.05 mg/day of rifampicin produced a sufficient improvement of mouse memory to a level similar to that of oral administration at 0.5 mg/day for age-matched mice [6]. In probe trials, all rifampicin treatments improved memory retention in mice (Fig. 1B). Intranasal and subcutaneous treatments appeared to be more effective than oral administration.
Fig. 1.
Effects of differently administered rifampicin on memory in APPOSK mice. (A and B) Rifampicin (RFP) was administered to APPOSK mice orally (p.o.), intranasally (i.n.) or subcutaneously (s.c.) at 0.25 or 0.05 mg/day for 1 month. As controls, carboxymethylcellulose (CMC) solution was administered to age-matched Tg and non-Tg littermates. (A) Spatial reference memory in mice was assessed at 12 months of age using the Morris water maze. Each point represents the mean of five trials per day ± SEM (sample number for each group is shown). The differences between groups were evaluated using two-factor repeated measures analysis of variance followed by Fisher's PLSD test. *P = .0027 vs. Non-Tg, P = .0038 vs. RFP (i.n.) (0.25 mg), P = .0059 vs. RFP (i.n.) (0.05 mg), P = .0029 vs. RFP s.c. (0.25 mg). (B) Retention of memory was assessed by a probe trial on day 5. Data are given as time occupancy in the target quadrant. (C) Locomotor activity of APPOSK mice in the light and dark was measured at 18 months of age by an open field test. Spontaneous locomotion of mice was estimated by the number of their beam crossing. Each point represents the mean ± SEM (n = 10 for each group). Abbreviation: SEM, standard error of the mean.
To ensure that APPOSK mice have normal locomotor activity, their exploration-motivated locomotion in a novel environment was measured in the light and dark phase by an open field test at 18 months of age. APPOSK mice displayed a pattern of spontaneous locomotion similar to that of age-matched non-Tg mice (Fig. 1C), implying that the Tg mice had no problem in locomotor activity.
3.2. Effects of differently administered rifampicin on neuropathology in APPOSK mice
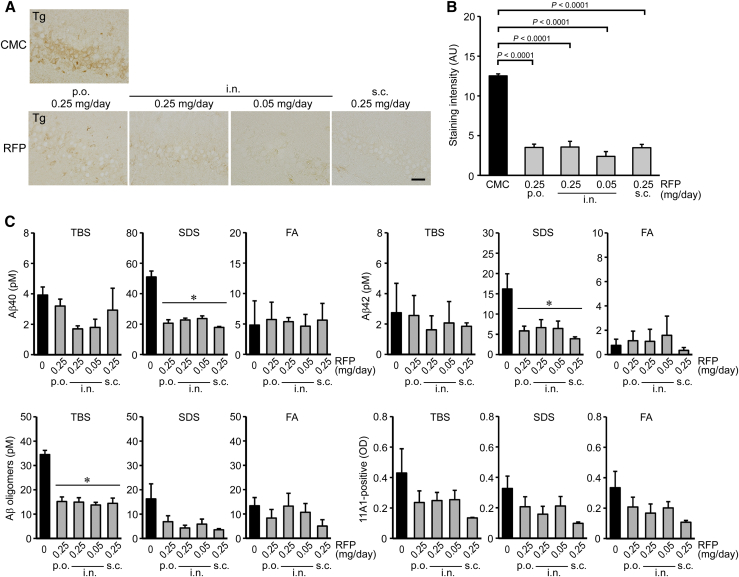
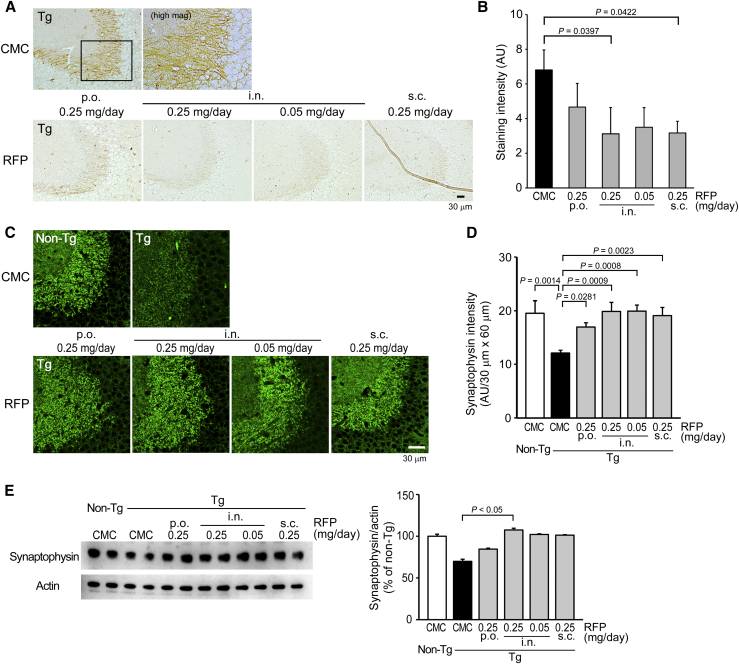
After the water maze task, mice were divided into two groups: one for immunohistochemical analysis and the other for biochemical analysis. Brain sections were stained with 11A1, PHF-1 (anti-pSer396/404-tau), and anti-synaptophysin antibodies for Aβ oligomers, tau phosphorylation, and synapse loss. Rifampicin reduced 11A1-positive staining in the hippocampal CA3 region regardless of the administration route (Fig. 2A). Intranasal rifampicin at 0.25 mg/day was most effective at reducing Aβ oligomers (Fig. 2B). Brain homogenates were separated into TBS-, SDS-, and FA-soluble fractions, and the levels of Aβ40, Aβ42, and Aβ oligomers were measured by ELISA. Rifampicin significantly reduced Aβ40 and Aβ42 in the SDS-soluble fraction and tended to decrease them in the TBS-soluble fraction but showed no discernible effect in the FA-soluble fraction (Fig. 2C). Aβ oligomers were significantly decreased in the TBS-soluble fractions and tended to decrease in the SDS- and FA-soluble fractions by rifampicin treatment. Rifampicin also decreased PHF-1-positive staining in the hippocampal mossy fibers regardless of the administration route (Fig. 3A). Intranasal and subcutaneous rifampicin at 0.25 mg/day displayed a significant effect, whereas oral rifampicin at the same dose showed a weaker effect than even 0.05 mg/day intranasal rifampicin (Fig. 3B). Furthermore, rifampicin restored synaptophysin levels in the hippocampal CA3 region regardless of the administration route (Fig. 3C). Again, oral rifampicin showed a weaker effect than the other administration routes, including intranasal rifampicin at 0.05 mg/day (Fig. 3D). The rifampicin-mediated recovery of synaptophysin levels was confirmed by Western blot with hippocampal homogenates. Intranasal rifampicin at 0.25 mg/day was most effective, whereas oral rifampicin at the same dose showed incomplete recovery (Fig. 3E).
Fig. 2.
Effects of differently administered rifampicin on Aβ accumulation in APPOSK mice. After the behavioral test, the mice were divided into two groups: one for immunohistochemical analysis and the other for biochemical analysis. (A) Brain sections were stained with Aβ oligomer-specific 11A1 antibody. The photographs were taken from the hippocampal CA3 region. Scale bar = 30 μm. (B) The staining intensity in a constant area was quantified. Each bar represents the mean ± SEM (n = 6 for each group). AU, arbitrary unit. (C) Brain homogenates were separated into TBS-, SDS-, and FA-soluble fractions and subjected to Aβ40, Aβ42, and Aβ oligomer sandwich ELISA. Aβ oligomers were also measured by direct ELISA with 11A1 antibody. Each bar represents the mean ± SEM (n = 5 for CMC and subcutaneous RFP groups, and n = 6 for oral and intranasal RFP groups). *P < .05 vs. CMC treatment by Tukey-Kramer test. Abbreviations: Aβ, amyloid β; CMC, carboxymethylcellulose; ELISA, enzyme-linked immunosorbent assay; FA, formic acid; SDS, sodium dodecyl sulfate; SEM, standard error of the mean; TBS, Tris-buffered saline.
Fig. 3.
Effects of differently administered rifampicin on tau phosphorylation and synapse loss in APPOSK mice. Rifampicin-treated mice were further analyzed. (A) Brain sections were stained with PHF-1 (anti-pSer396/404-tau) antibody. The photographs were taken from the hippocampal CA2/3 region. Scale bar = 30 μm. (B) The staining intensity in a constant area was quantified. Each bar represents the mean ± SEM (n = 6 for each group). (C) Brain sections were stained with anti-synaptophysin antibody. The photographs were taken from the hippocampal CA2/3 region. Scale bar = 30 μm. (D) The staining intensity in a constant area was quantified. Each bar represents the mean ± SEM (n = 6 for each group). (E) Hippocampal homogenates were subjected to Western blot for synaptophysin and actin. The intensity of each signal was quantified. Each bar represents the mean ± SEM (n = 6 for non-Tg, oral and intranasal RFP groups, and n = 5 for CMC and subcutaneous RFP groups). Abbreviations: CMC, carboxymethylcellulose; SEM, standard error of the mean.
3.3. Hepatotoxicity of differently administered rifampicin in APPOSK mice
The levels of AST and ALT in the sera were measured to estimate adverse effects of rifampicin. As shown in Table 1, the serum levels of AST remained normal even after 1-month subcutaneous administration, slightly and dose-dependently increased with intranasal administration, and markedly rose with oral administration. These results suggest that oral application of rifampicin risks liver dysfunction. In spite of the AST alteration, the levels of ALT did not change.
Table 1.
Serum levels of liver enzymes after 1-month rifampicin treatment
| Non-Tg |
APPOSK mice |
|||||
|---|---|---|---|---|---|---|
| CMC |
CMC |
Rifampicin (mg/day) |
||||
| 0.25 |
0.25 |
0.05 |
0.25 |
|||
| Oral |
Intranasal |
Oral |
Intranasal |
Intranasal |
Subcutaneous |
|
| n = 10 | n = 9 | n = 12 | n = 12 | n = 11 | n = 10 | |
| AST | 74 ± 5 | 104 ± 13 | 248 ± 56∗ | 189 ± 25 | 165 ± 32 | 72 ± 6 |
| ALT | 29 ± 1 | 35 ± 3 | 39 ± 5 | 45 ± 5 | 40 ± 10 | 29 ± 2 |
Abbreviations: AST, aspartate transaminase; ALT, alanine transaminase; CMC, carboxymethylcellulose; SEM, standard error of the mean.
Note. Values represent the mean ± SEM (IU/L).
P < .05 vs. Non-Tg mice, CMC-treated Tg mice, and subcutaneous rifampicin-treated Tg mice by Tukey-Kramer test.
3.4. Pharmacokinetics of differently administered rifampicin in normal mice
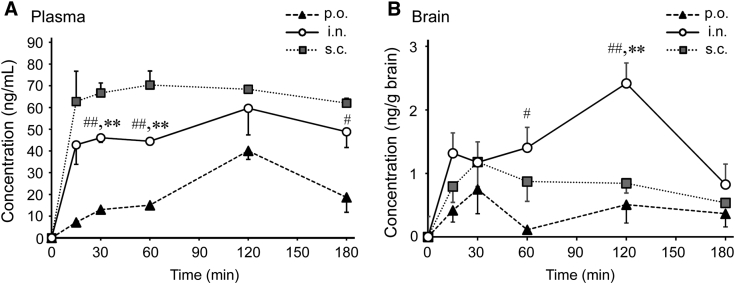
The pharmacokinetics of rifampicin was studied in normal mice. The concentrations of rifampicin in the plasma and the brain were measured after a single administration as a function of time. Regardless of the administration route, the concentration in plasma increased rapidly during the initial 15 min after administration and subsequently stabilized, but at different values, with subcutaneous administration giving the highest concentration and oral administration giving the lowest (Fig. 4A). On the contrary, in the brain, intranasal administration led to the highest concentration of rifampicin in the brain, and oral administration led to the lowest, indicating an enhanced delivery of rifampicin to the brain by intranasal application (Fig. 4B). For quantitative comparisons, the area under the curve of the concentrations in the plasma (AUCplasma) and the brain (AUCbrain) up to 180 min after administration were calculated (Table 2). AUCbrain after intranasal application was twice that after subcutaneous application. Since the target organ of rifampicin is the brain, high plasma concentration is a risk factor of peripheral side effects.
Fig. 4.
Pharmacokinetics of differently administered rifampicin in normal mice. A mixture of unlabeled and radiolabeled rifampicin was single administered to wild-type mice orally (p.o.), intranasally (i.n.), or subcutaneously (s.c.) at a dose of 2.5 μg/mouse. At the indicated time (15, 30, 60, 120, or 180 min) after application, blood and whole brains were collected. Plasma (A) and brain homogenates (B) were prepared, and radioactivity in the samples was determined with a liquid scintillation counter. Each bar represents the mean ± SEM (n = 3 for each point except for n = 4 at 120 min after intranasal application and at 15 min after subcutaneous application). #P < .05 vs. p.o., ##P < .01 vs. p.o., ∗∗P < .01 vs. s.c. by Dunnett's test. Abbreviation: SEM, standard error of the mean.
Table 2.
AUC values after single rifampicin administration (2.5 μg/mouse)
| AUCplasma | AUCbrain | |
|---|---|---|
| Oral | 4039.0 | 69.7 |
| Intranasal | 8724.7 | 279.2 |
| Subcutaneous | 11,573.6 | 144.5 |
Abbreviations: AUC, area under the curve; AUCplasma, area under the curve of the concentrations in the plasma; AUCbrain, area under the curve of the concentrations in the brain.
4. Discussion
Previously, we showed that oral rifampicin significantly reduces Aβ and tau pathologies in model mice of AD and tauopathy [6], indicating its potential in dementia treatment. However, rifampicin shows occasional adverse effects such as liver injury and drug-drug interaction, making it difficult to use for a long period in humans, particularly in elderly people who usually take various medicines. To explore safer rifampicin treatment, we compared oral, intranasal, and subcutaneous administrations in aged APPOSK mice for therapeutic efficacy and hepatotoxicity, the latter of which was evaluated by measuring the serum levels of liver enzymes. We found that intranasal and subcutaneous administrations were safer and more effective at improving memory and attenuating neuropathology than oral administration. Notably, intranasal rifampicin at 0.05 mg/day had more beneficial effects than 0.25 mg/day oral rifampicin.
The lower therapeutic efficacy of oral rifampicin is probably due to its lower bioavailability. P-glycoprotein is expressed on intestinal [10] and nasal epithelial cells [11] and mediates drug adsorption and efflux, including those of rifampicin [12]. While the transepithelial permeability of rifampicin is high, P-glycoprotein works as a barrier against rifampicin absorption. The efflux of rifampicin by P-glycoprotein in the intestine could explain the low bioavailability of oral rifampicin. Although nasal epithelial P-glycoprotein may also reduce the absorption of rifampicin from the nasal cavity, its efflux capacity may be easily saturated because the concentration of rifampicin in the nasal dosing solution is higher than that in the oral dosing solution. Thus, the inhibition of rifampicin adsorption by P-glycoprotein in nasal epithelial cells is likely small compared with that in intestinal epithelial cells. According to Hosagrahara et al. [13], repeated oral dosing of rifampicin induces the expression of P-glycoprotein in intestinal epithelial cells in mice. They found AUCplasma of rifampicin after 5-day oral application was decreased to 40% that after 1-day oral application due to the increased expression of P-glycoprotein in the intestine. This finding implies that in the present study, the actual brain distribution of rifampicin in mice after 1-month oral application might be lower than that after the single oral application shown in Fig. 4. Another reason for the low bioavailability of oral rifampicin would be that orally administered rifampicin undergoes the first pass effect. Rifampicin absorbed from the intestine is carried to the liver, where a large portion of rifampicin would be inactivated and/or degraded by the function of hepatic cytochrome P450 enzymes before entering the blood circulation. Intranasal and subcutaneous rifampicin can avoid this first pass metabolism.
Consistent with the previous explanations, rifampicin is known to upregulate the expression of cytochrome P450 and P-glycoprotein in hepatocytes, which could have an effect on the metabolism of other drugs [7]. The rifampicin-induced alteration of drug metabolism leads to a decreased efficacy of drugs concomitantly administered with rifampicin (i.e., drug-drug interaction). In addition, rifampicin occasionally causes liver injury by an unidentified mechanism. These adverse effects are likely elicited during the first pass metabolism of rifampicin in the liver, which is attributed to its oral application. Thus, oral administration of rifampicin has disadvantages in both therapeutic efficacy and safety compared with intranasal and subcutaneous administration. Our results indicate that intranasal rifampicin caused a slight, dose-dependent increase in serum AST, which is a measure of liver dysfunction. However, we assumed that this effect occurred because the applied volume (10 μL) was too high for mice and therefore a portion of rifampicin administered into the nasal cavity overflowed and was swallowed from the back of the nostril and into the stomach. An appropriate volume of rifampicin and an adequate spraying device and drug carrier for intranasal application could prevent this side effect in humans.
It is known that efficient brain drug delivery is feasible by nasal application [14]. Not only small-size drugs but also proteins [15], DNA [16], and even cells [17] can be delivered directly through the nose-to-brain pathway. However, P-glycoprotein is also expressed highly on cerebral vascular endothelial cells [18]. This expression would be an obstacle for subcutaneously administered rifampicin to enter the brain via the blood-brain barrier. In contrast, intranasal rifampicin avoids P-glycoprotein at the blood-brain barrier. The observed higher brain distribution and lower plasma concentration of rifampicin after intranasal application in this study is presumably a result of this bypass. Zhang et al. [19] proposed the direct transport percentage to evaluate the contribution of direct brain delivery. When calculating direct transport percentage, it is assumed that the brain uptake of rifampicin from systemic circulation is linearly correlated with AUCplasma. Using a slight modification of the direct transport percentage, we found direct delivery resulted in 75.1% of total brain uptake of rifampicin 120 min after intranasal application.
Effective drug treatments for the prevention and treatment of dementia require an easy and noninvasive drug application because regular administration is conducted for a long period. In this regard, oral and needle-free intranasal administrations have advantages over subcutaneous administration. In AD, long-term intranasal drug application has been pursued with insulin. A pilot clinical trial of intranasal insulin in patients with AD and mild cognitive impairment revealed that 4-month application improved delayed memory and cognitive dysfunction and preserved the functional ability of patients without severe adverse events [20], [21]. The same research group performed another clinical trial with insulin detemir, a long-acting insulin analog, and confirmed that intranasal treatment improved cognition in patients [22]. In these studies, the drug administration was carried out with a commercial nasal drug delivery device, which with minor modifications would also be applicable to the intranasal application of rifampicin.
Recently, growing attention has been paid to the possible role of gut microbiota in cognition and dementia [23], [24], [25], [26]. Similarly to other antibiotics [23], rifampicin may also affect gut microbiota to influence brain function. However, our results indicate that intranasal or subcutaneous application of rifampicin was more effective than oral application to improve memory, suggesting that rifampicin directly acts on the brain without involving gut microbiota.
In conclusion, the intranasal or subcutaneous administration of rifampicin was safer and more effective than oral administration to improve memory and reduce neuropathology in model mice of AD. In addition, intranasal application achieved the highest brain delivery of rifampicin. Considering its easiness and noninvasiveness, intranasal administration would be the best way for long-term dosing of rifampicin. Our findings provide a novel approach to the prevention of dementia.
Research in Context.
-
1.
Systematic review: Recent evidence indicates the significance of dementia prevention, but no effective and safe preventive medicines have been developed. Previously, we showed that oral administration of rifampicin significantly reduced amyloid β (Aβ) and tau pathologies in model mice, indicating its therapeutic potential. However, rifampicin shows occasional adverse effects such as liver injury and drug-drug interaction in humans, making its use difficult for a long period.
-
2.
Interpretation: The present study shows that intranasal and subcutaneous rifampicin administration was safer and more effective than oral administration. Considering its easiness and noninvasiveness, intranasal administration would be the best way for long-term dosing of rifampicin.
-
3.
Future direction: Rifampicin is a generic drug at present and can be supplied cheaply. Therefore, even long-term use of rifampicin would be inexpensive, which is quite important, particularly in emerging countries. We believe our findings provide a novel approach to the prevention of neurodegenerative dementia.
Acknowledgments
The authors thank Momoko Yoshida for technical assistance and Peter Karagiannis for reading the manuscript.
Funding sources: This study was supported by the grant from the Research Foundation for Dementia of Osaka and in part by the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 16K15132).
Footnotes
Conflict of interest: T.T. and T.U. have a Japanese patent pending on intranasal rifampicin.
Ethics approval and consent to participate: All procedures performed in studies involving animals were in accordance with the ethical standards of the institutions at which the studies were conducted.
References
- 1.Benilova I., Karran E., De Strooper B. The toxic Aβ oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 2.Viola K.L., Klein W.L. Amyloid β oligomers in Alzheimer's disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015;129:183–206. doi: 10.1007/s00401-015-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imbimbo B.P., Giardina G.A. γ-Secretase inhibitors and modulators for the treatment of Alzheimer's disease: disappointments and hopes. Curr Top Med Chem. 2011;11:1555–1570. doi: 10.2174/156802611795860942. [DOI] [PubMed] [Google Scholar]
- 4.Panza F., Logroscino G., Imbimbo B.P., Solfrizzi V. Is there still any hope for amyloid-based immunotherapy for Alzheimer's disease? Curr Opin Psychiatry. 2014;27:128–137. doi: 10.1097/YCO.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 5.Sperling R.A., Jack C.R., Jr., Aisen P.S. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umeda T., Ono K., Sakai A., Yamashita M., Mizuguchi M., Klein W.L. Rifampicin is a candidate preventive medicine against amyloid-beta and tau oligomers. Brain. 2016;139:1568–1586. doi: 10.1093/brain/aww042. [DOI] [PubMed] [Google Scholar]
- 7.Chen J., Raymond K. Roles of rifampicin in drug-drug interactions: underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann Clin Microbiol Antimicrob. 2006;5:3. doi: 10.1186/1476-0711-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomiyama T., Matsuyama S., Iso H., Umeda T., Takuma H., Ohnishi K. A mouse model of amyloid β oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30:4845–4856. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iso H., Simoda S., Matsuyama T. Environmental change during postnatal development alters behaviour, cognitions and neurogenesis of mice. Behav Brain Res. 2007;179:90–98. doi: 10.1016/j.bbr.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Chan L.M., Lowes S., Hirst B.H. The ABCs of drug transport in intestine and liver: efflux proteins limiting drug absorption and bioavailability. Eur J Pharm Sci. 2004;21:25–51. doi: 10.1016/j.ejps.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira P., Fortuna A., Alves G., Falcao A. Drug-metabolizing enzymes and efflux transporters in nasal epithelium: influence on the bioavailability of intranasally administered drugs. Curr Drug Metab. 2016;17:628–647. doi: 10.2174/1389200217666160406120509. [DOI] [PubMed] [Google Scholar]
- 12.Mariappan T.T., Singh S. Evidence of efflux-mediated and saturable absorption of rifampicin in rat intestine using the ligated loop and everted gut sac techniques. Mol Pharm. 2004;1:363–367. doi: 10.1021/mp049937n. [DOI] [PubMed] [Google Scholar]
- 13.Hosagrahara V., Reddy J., Ganguly S., Panduga V., Ahuja V., Parab M. Effect of repeated dosing on rifampin exposure in BALB/c mice. Eur J Pharm Sci. 2013;49:33–38. doi: 10.1016/j.ejps.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Dhuria S.V., Hanson L.R., Frey W.H., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 15.Alcalá-Barraza S.R., Lee M.S., Hanson L.R., McDonald A.A., Frey W.H., 2nd, McLoon L.K. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Target. 2010;18:179–190. doi: 10.3109/10611860903318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han I.K., Kim M.Y., Byun H.M., Hwang T.S., Kim J.M., Hwang K.W. Enhanced brain targeting efficiency of intranasally administered plasmid DNA: an alternativeroute for brain gene therapy. J Mol Med (Berl) 2007;85:75–83. doi: 10.1007/s00109-006-0114-9. [DOI] [PubMed] [Google Scholar]
- 17.Danielyan L., Schäfer R., von Ameln-Mayerhofer A., Buadze M., Geisler J., Klopfer T. Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88:315–324. doi: 10.1016/j.ejcb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Tamai I., Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J Pharm Sci. 2000;89:1371–1388. doi: 10.1002/1520-6017(200011)89:11<1371::aid-jps1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q., Jiang X., Jiang W., Lu W., Su L., Shi Z. Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain. Int J Pharm. 2004;275:85–96. doi: 10.1016/j.ijpharm.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 20.Craft S., Baker L.D., Montine T.J., Minoshima S., Watson G.S., Claxton A. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freiherr J., Hallschmid M., Frey W.H., 2nd, Brünner Y.F., Chapman C.D., Hölscher C. Intranasal insulin as a treatment for Alzheimer's disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27:505–514. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claxton A., Baker L.D., Hanson A., Trittschuh E.H., Cholerton B., Morgan A. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. J Alzheimers Dis. 2015;44:897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- 23.Minter M.R., Zhang C., Leone V., Ringus D.L., Zhang X., Oyler-Castrillo P. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harach T., Marungruang N., Duthilleul N., Cheatham V., Mc Coy K.D., Frisoni G. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonfili L., Cecarini V., Berardi S., Scarpona S., Suchodolski J.S., Nasuti C. Microbiota modulation counteracts Alzheimer's disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7:2426. doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S.C., Cao Z.S., Chang K.M., Juang J.L. Intestinal microbial dysbiosis aggravates the progression of Alzheimer's disease in Drosophila. Nat Commun. 2017;8:24. doi: 10.1038/s41467-017-00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]