Abstract
The clinical uses of cytoplasmic transfer and pronuclear transfer for infertility treatment have raised concerns, leading to restrictive regulatory responses in both the USA and China. In 2015, the UK legalized nuclear transfer from oocytes and zygotes to prevent the onset of serious mitochondrial disease in the children of affected mothers. A research team in the USA then performed egg nuclear transfer, with subsequent embryo transfer in Mexico, to prevent mitochondrial disease. A live birth resulted, but the cross-border activity attracted attention from regulatory authorities. In order to respond appropriately to the likelihood of the wider use of such mitochondrial manipulation techniques (MMT), the present study first surveyed countries where MMT have been clinically implemented or where such experimental procedures are advertised on the internet. Sixteen countries were selected for an analysis of the legal position regarding germline genetic modification and egg donation. It was found that regulation of the clinical use of MMT could be broken down into three categories: (i) largely prohibited (USA and China), (ii) not regulated (Northern Cyprus and Ukraine), and (iii) insufficiently regulated (the remaining 12 countries, including Mexico). The reasons for no or insufficient regulation included no intention to oversee experimental procedures, no consideration of the manipulation in eggs, unclear technical terms and ambiguous medical purposes. To protect future children, this study underscores the pressing need for regulatory frameworks with policies that cover MMT. Wider implications regarding the responsible implementation of procedures in experimental reproductive medicine are discussed.
Keywords: mitochondria, mitochondrial replacement, infertility treatment, mitochondrial disease, regulation, responsibility
Introduction
Mitochondria are cellular organelles characterized by having their own genome (termed ‘mtDNA’). Their functions are exerted through the coordinated gene expression of mtDNA and nuclear DNA (nDNA) (Ishii, 2016a). The most crucial function of mitochondria is in the respiratory chain, by which energy is produced as adenosine triphosphate while precisely regulating the generation of deleterious free radicals. Mitochondria are abundant in the human egg (oocyte), resulting in 200,000–300,000 copies of mtDNA per oocyte (Schatten et al., 2014). After fertilization, paternal mitochondria from the fertilizing spermatozoon are selectively degraded (Ishii, 2016a). Therefore, mtDNA is maternally inherited in offspring. There are approximately 30 mtDNA haplogroups in humans (van Oven and Kayser, 2009).
As some mtDNA mutations in oocytes have been considered to be associated with infertility and the onset of mitochondrial disease in offspring, several mitochondrial manipulation techniques (MMT) have been developed and used in fertility clinics. In 1997, the world’s first successful MMT case was reported from the USA (Cohen et al., 1997). Donor oocyte-derived cytoplasm (ooplasm) containing mtDNA was injected into a patient’s oocytes to treat an infertility case of insufficient embryonic development. Over the subsequent 4 years, this cytoplasmic transfer (CT) technique was repeated by the same group in other patients, leading to 17 live births in the USA. However, two pregnancies with Turner syndrome were identified following CT. These resulted in one miscarriage and one elective abortion. In addition, one child was diagnosed with borderline pervasive developmental disorder at 18 months of age (Barritt et al., 2001c); in a recent survey of parents, he was reported to have received special education for the pre-school year alone, and to have had episodes of depression. A family history of depression was also reported (Chen et al., 2016). Buccal smears from two of eight of the children checked after birth were found to contain donor mtDNA (Barritt et al., 2001b). Another MMT case was reported from China in 2003, this time using pronuclear transfer (PNT) – in which a karyoplast (a small bag of membrane-bound cytoplasm) harbouring nDNA and mtDNA is transferred to an enucleated zygote created using a donor oocyte – for an infertile woman who had suffered embryonic arrests (Zhang et al., 2016b). Although this attempt led to a triplet pregnancy, it ultimately resulted in no live births. A fetus was reduced to allow for better development of the other two fetuses. However, according to the case report, these fetuses died of respiratory distress and cord prolapse, respectively. CT and PNT have incurred regulatory interventions. The US Food and Drug Administration (FDA) exerted jurisdiction over CT technology by requiring that an Investigational New Drug application be filed in order to continue offering this procedure, as well as PNT, to patients (Castro, 2016, Ishii, 2015). The Chinese Ministry of Health established assisted reproductive technology guidelines and prohibited PNT in 2003 (Ishii, 2015).
An autologous type of MMT – autologous germline mitochondrial transfer (AUGMENT) – was reported in 2015 (Fakih et al., 2015). In the procedure, mitochondria are extracted from an infertile patient’s ‘egg precursor cells’ and injected into the patient’s oocyte. Canadian and United Arab Emirates (UAE) groups asserted that AUGMENT showed marked improvements in pregnancy rates; however, academic societies, such as the European Society of Human Reproduction and Embryology (ESHRE), expressed concerns over efficacy and safety due to undisclosed technical details regarding the mtDNA status, and the preparation and transfer of the mitochondria (British_Fertility_Society, 2017, Heindryckx et al., 2015).
On 24 February 2015, the UK became the first jurisdiction to permit the clinical use of two types of MMT to reduce mtDNA mutations that can cause serious mitochondrial diseases in offspring (UK Department of Health, 2015): PNT, and spindle nuclear transfer (SNT), in which a karyoplast carrying the second meiotic spindle from a patient’s oocyte is transferred to an enucleated donor oocyte (Kang et al., 2016, Yamada et al., 2016). In the same year, a group led by a US physician performed SNT in the USA, and shipped the resultant embryo for transfer to an affiliate clinic in Mexico to prevent the onset of a mitochondrial disease (Leigh syndrome) in offspring (Zhang et al., 2017a). Although the clinical implementation resulted in the live birth of a boy, the parents requested that no further genetic testing be undertaken, unless there was a clinical benefit for the child. This could be because the risk information was explained insufficiently during the process of obtaining informed consent (Alikani et al., 2017).
Thus, MMT can alter the mtDNA content of human oocytes or zygotes through CT, karyoplast transfer (which includes carryover mtDNA) or autologous mitochondrial transfer (which might undergo mutagenesis during preparation) to treat intractable infertility or prevent mitochondrial disease in offspring. Although MMT have the potential to address unmet reproductive needs, all of these techniques remain experimental with regard to human reproduction. Moreover, the cross-border use of SNT between the USA and Mexico suggests that the clinical use of MMT is likely to spread in an unregulated manner (Ishii, 2017b, Palacios-González and de Jesús Medina-Arellano, 2017). Some studies have analysed the legalization process of PNT and SNT in the UK and the regulatory discussions in the USA (Castro, 2016, Cohen and Adashi, 2016, Cohen et al., 2015, Ishii, 2014, Schandera and Mackey, 2016). However, the current state of MMT-relevant activity and regulation remains largely elusive in many countries. In order to respond appropriately to the likelihood of the wider use of experimental MMT, the present study first identified a selection of countries in which some MMT have already been clinically implemented or advertised. We then investigated how the clinical use of MMT is regulated in 16 selected countries. Clinical use is largely prohibited in the USA and China; however, it is not regulated in Northern Cyprus or Ukraine, and is insufficiently regulated in the remaining 12 countries. The wider implications of these findings are also discussed from regulatory and socio-ethical standpoints.
Survey methods
To analyse the regulation of MMT worldwide, we attempted to identify countries in which MMT have been clinically implemented or are advertised using three approaches: (i) literature search, (ii) clinical trial database search, and (iii) internet search to locate relevant advertisements.
Literature search
For the regulatory analysis, we surveyed and identified countries in which MMT have been clinically implemented by searching the literature and relevant clinical trials. The surveyed reports included clinical cases published to the end of March 2017 in English, Chinese or Japanese that were available through PubMed. In this survey, basic research reports on MMT were excluded from the scope. Only clinical uses of MMT were searched using keywords: ‘ooplasmic transfer, ‘cytoplasmic transfer’, ‘germinal vesicle transfer’ (in which a karyoplast from a premature oocyte is transferred to an enucleated donor oocyte; Takeuchi et al., 2001), ‘pronuclear transfer’, ‘spindle nuclear transfer’, ‘metaphase II (MII) spindle transfer’ (another name for SNT), ‘maternal spindle transfer’ (another name for SNT), and two types of ‘polar body transfer’ in which a polar body including nDNA and mtDNA is transferred to an enucleated donor oocyte (polar body 1 transfer) or a female pronucleus-removed zygote (polar body 2 transfer) (Ma et al., 2017, Zhang et al., 2017b). Such MMT involve oocyte donation from a third party, thereby rendering them allologous mitochondrial transfers. AUGMENT (‘autologous germline mitochondrial transfer’) was also included because there is no evidence that mitochondria for transfer are genetically equivalent to those of a patient’s oocyte, and because it increases the mtDNA copy number in an oocyte (British_Fertility_Society, 2017, Gosden and Johnson, 2016, Heindryckx et al., 2015). The results are shown as of 30 March 2017 (Supplemental Document 1).
Clinical trial database search
Relevant clinical trials were surveyed to the end of March 2017 using four registries: ClinicalTrials.gov, EU-CTR, ChiCTR (http://www.chictr.org.cn/) and UMIN-CTR (http://www.umin.ac.jp/ctr/). The same keywords were used as in the literature search in English. The results are shown as of 30 March 2017 (Supplemental Document 1).
Internet search for relevant advertisements
We further attempted to identify countries in which fertility clinics are presumed to offer MMT. Using the same keywords in English mentioned above in a Google search, relevant information was investigated on the websites of fertility clinics and two different websites offering cross-border reproductive care (also known more colloquially as 'reproductive tourism'). This internet survey was performed from November 2016 to March 2017. Regarding advertisements found on cross-border reproductive care websites, the accuracy of information was checked by sending an e-mail enquiry to the contact address provided on the website. Each clinic was only contacted once. In accordance with the Japan Sociological Society guidelines (http://www.gakkai.ne.jp/jss/about/researchpolicy.php), we did not attempt to contact a clinic again if there was no response as clinics have the right to refuse such an enquiry. The results shown are as of 30 March 2017 (Supplemental Document 1). Only countries where relevant advertisements were found on clinic websites and/or where advertisements on cross-border reproductive care websites were confirmed were selected for the regulatory analysis.
We performed the searches and survey to analyse the regulation of MMT in selected countries, not to systematically identify all countries in which MMT have been clinically implemented or are advertised. Thus, the survey results may not fully reflect the actual circumstances.
Analyses of regulation
Selected countries were investigated regarding the regulatory status of the clinical use of MMT. As many countries have government at both national and state levels, relevant regulation might be nationally inconsistent or vary by state, as shown by a recent analysis of the legality of MMT in Mexico (Palacios-González and de Jesús Medina-Arellano, 2017). In the present study, we focused our regulatory analysis on national (federal) and international policies to compare the relevant regulations in select countries. Specifically, we evaluated the regulations regarding human germline genetic modifications and oocyte donation in the national and international regulations pertinent to medically assisted reproduction in the countries. Using the latest available version of legal documents in the local language, relevant provisions were investigated to assess the legality of MMT in a given country (Supplemental Document 2).
Why does regulation matter?
One might consider that MMT are similar to the techniques of pre-implantation genetic diagnosis (PGD) because both techniques have the potential dual purpose of preventing disease and overcoming infertility (Sengupta et al., 2016). However, MMT are substantially different from PGD – which most European countries permit based on a specific regulation (Council of Europe, 2015) – as follows.
First, MMT require more invasive manipulations (CT or karyoplast transfer in oocytes or zygotes) than embryonic cell biopsy, which is applied in PGD and can potentially be performed at fertility clinics with a micromanipulator. Second, as mentioned in relation to the cases of CT in the USA and PNT in China, potential unfavourable consequences, such as unexplained chromosomal abnormalities in the resultant fetus and non-live birth, can occur following the application of MMT. The long-term consequences following their use are largely uncertain; however, potential risks exist due to induced heteroplasmy, random mtDNA segregation and nDNA–mtDNA mismatch, in addition to the potential side-effects caused by the manipulation technique itself (Alikani et al., 2017, Hyslop et al., 2016, Ishii, 2016a, Morrow et al., 2015, Reinhardt et al., 2013, Yamada et al., 2016). Third, MMT can result in human germline genetic modification that has been viewed as taboo in many countries. In particular, the transfer of female embryos created via MMT can impact future generations through the maternal transmission of mtDNA. A recent preliminary analysis revealed that 29 of 39 selected countries, legally or by guidelines, prohibit the reproductive use of germline genetic modification (Ishii, 2017a). Finally, some MMTs, including CT, PNT and SNT, involve oocyte donation, which is not required in PGD procedures. Oocyte donation has been controversial, especially in countries with no clear regulation, due to the possibility of exploitation and commodification, and the potential short- and long-term consequences of oocyte retrieval (Baylis, 2013, Kenney and Mcgowan, 2010, Schneider et al., 2017).
Therefore, MMTs are characterized by invasive manipulation, potential risks to resultant fetuses and children, taboo, and occasional involvement of third-party donors. Due to these characteristics, MMT should be subject to national regulations, separately from PGD. Whether a country considers the social introduction of MMT to be favourable or unfavourable, the ethical and social implications of MMT, as well as the likelihood of its wider use, underscore the need for in-depth reviews, and the amendment or establishment of appropriate regulations, in order to protect children who are born after the application of these experimental techniques.
MMT-relevant activities in the world
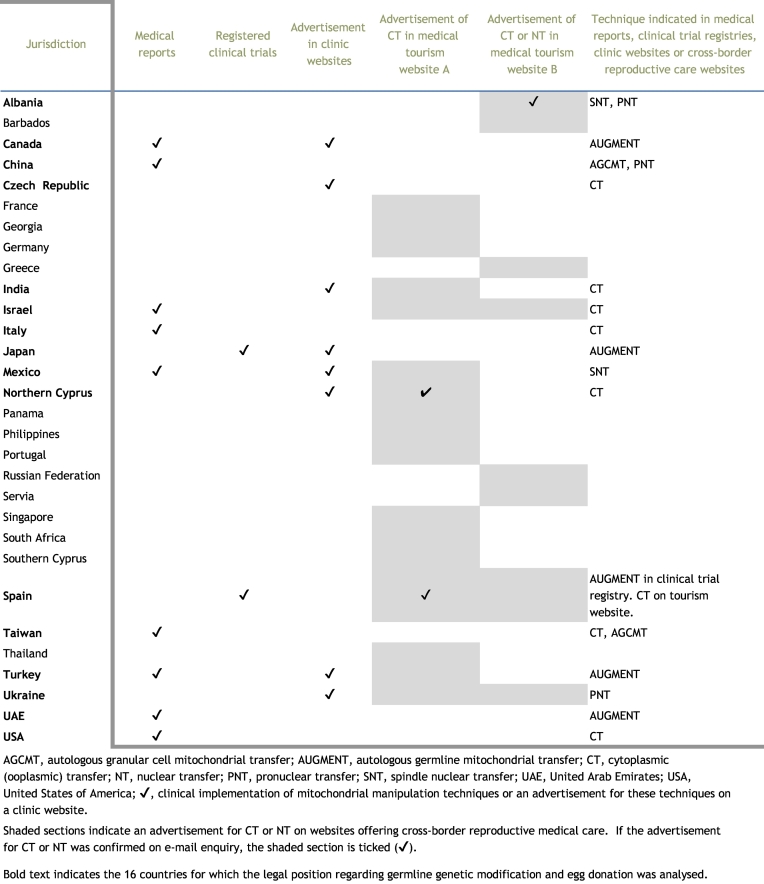
First, we identified the countries where any type of MMT has been performed or is currently being studied in clinical settings (Supplemental Document 1). A search of the literature revealed the clinical application of CT in Israel (Barritt et al., 2001a), Italy (Dale et al., 2001), Taiwan (Huang et al., 1999) (in which the cytoplasm of tripronucleate zygotes was transferred) and the USA (Barritt et al., 2001b, Barritt et al., 2001c, Cohen et al., 1997, Cohen et al., 1998, Lanzendorf et al., 1999); PNT (pregnancies were attained but resulted in no births) in China (Zhang et al., 2016b); SNT in Mexico/USA (Zhang et al., 2017a); AUGMENT in Canada, the UAE (Fakih et al., 2015) and Turkey (Oktay et al., 2015); and autologous granular cell mitochondrial transfer (AGCMT, in which mitochondria prepared from autologous granular cells are transferred into each oocyte) in China (Kong et al., 2003a, Kong et al., 2003b) and Taiwan (Tzeng et al., 2001). There were no reports showing any clinical data for germinal vesicle transfer or polar body transfer. Thus, a total of 15 case reports were found regarding CT, PNT, SNT, AUGMENT and AGCMT. In these reports, MMT were used for infertility treatment, except for one case for disease prevention in Mexico. The breakdown by country shows that the USA is the most MMT-active country (six reports), followed by China (three reports), Taiwan (two reports), and Israel, Italy, Mexico, Canada, UAE and Turkey (one report each). The USA, Mexico, Canada and UAE include a double-count.
The clinical trial search revealed that two clinics in Japan (UMIN000021387) and Spain (NCT02586298) are conducting clinical trials using AUGMENT (Supplemental Document 1). The AUGMENT trial in Japan is a non-randomized, open-label, uncontrolled study. Meanwhile, the AUGMENT trial in Spain is a randomized, double-blinded study. However, the endpoint of the Spanish trial is the rate of ongoing pregnancy (12 weeks), not the livebirth rate. More importantly, we found only two MMT-relevant trials, potentially implying that other clinics might practice experimental MMT without performing clinical trials.
Thus, our search of the literature and clinical trial registries revealed 11 countries in which MMT has been or is being used clinically (Canada, China, Israel, Italy, Japan, Mexico, Spain, Taiwan, Turkey, UAE and USA) (Table 1).
Table 1.
Results of a survey of reproductive medicine involving mitochondrial manipulation in 30 countries (as of 30 March 2017).
Clinical information on MMT was further investigated via the internet. Searching clinic websites identified advertisements for AUGMENT in Canada (four clinics), Japan (one clinic) and Turkey (one clinic); CT in Czech Republic (one clinic), India (two clinics) and Turkish Republic of Northern Cyprus (not Republic of Cyprus, one clinic); PNT in Ukraine (one clinic); and SNT in Mexico by a US clinic (one clinic) (Supplemental Document 1). AUGMENT is most actively advertised in Canada (four clinics). CT is advertised by two clinics in India. Although one of these clinics recently held a press conference on a successful CT case, no medical reports were found by our survey (Supplemental Document 1). Similar active advertisements were found in a Ukrainian clinic displaying a PNT case and a US clinic showing an SNT case in Mexico. Again, to our knowledge, the Ukrainian clinic has not reported the case in a medical journal.
We conducted deeper searches for relevant information on the internet, and found two different websites for cross-border reproductive care (Supplemental Document 1). On these tourism websites, CT was advertised at clinics in 20 countries (Barbados, France, Georgia, Germany, Greece, India, Israel, Mexico, Northern Cyprus, Panama, Philippines, Portugal, Russian Federation, Serbia, Singapore, South Africa, Spain, Thailand, Turkey and Ukraine), and ‘nuclear transfer’ was advertised at clinics in four countries (Albania, Israel, Russian Federation and Spain). E-mail enquiries were sent to such clinics to confirm the accuracy of the advertisement (shaded sections in Table 1). Although most of the enquiries to the clinics resulted in no response or no offer of MMT at that time, one clinic in Albania, one clinic in Northern Cyprus and one clinic in Spain replied that they offer ‘nuclear transfer’, CT and CT, respectively (shaded and checked sections in Table 1). Remarkably, ‘nuclear transfer’ advertised by the Albanian clinic turned out to be SNT and PNT (Supplemental Document 1).
Therefore, our survey of medical reports, clinical trial registries and the websites of clinics offering cross-border reproductive care identified 16 countries in which any type of MMT had been clinically implemented, is being studied in clinical trials, or is advertised (Albania, Canada, Czech Republic, China, India, Israel, Italy, Japan, Mexico, Northern Cyprus, Spain, Taiwan, Turkey, Ukraine, UAE and USA) (Table 1).
Legal analysis of MMT
We investigated the regulation of clinical use of MMT in the 16 countries identified above. First, we investigated the national and international policies regarding oocyte donation. The European Union (EU) Tissues and Cells Directives (2004/23/EC, 2006/17/EC and 2006/86/EC) are the only international regulatory framework for gamete and embryo donations. The EU Directives, which stipulate the quality and safety standards for donated human tissues and cells (including reproductive cells) across EU states, do not substantially restrict or ban reproductive donations (EUR Lex).
At the national level, oocyte donation is generally permitted in 14 of the 16 countries, but not in Turkey or the UAE (Table 2). Specifically, oocyte donation is not regulated (Albania, Japan, Mexico and the USA), not prohibited (Italy), or allowed with some limitations on anonymity, age limit or compensation (Canada, China, Czech Republic, India, Israel, Northern Cyprus, Spain, Taiwan, and Ukraine). Although Italian Law 2004 prohibited gamete donation, the Constitutional Court removed the prohibition in 2014 (Benagiano et al., 2014). The UAE prohibits oocyte donation for research as well as reproduction. Turkey explicitly prohibits Turkish citizens from participating in gamete donation (Gürtin, 2016). Such findings on the regulation of oocyte donation suggest the feasibility of MMT using allogeneic mitochondria in the 14 countries, whereas only MMT using autologous mitochondria can be performed legally in Turkey and the UAE.
Table 2.
Regulations relevant to reproductive medicine involving mitochondrial manipulation in 16 selected countries.
| Jurisdiction | Relevant regulations | Articles regarding germline genetic modification | Articles regarding egg donation | |
|---|---|---|---|---|
| The Americas | Canada | Assisted Human Reproduction Act S.C. 2004, c. 2 | Article 5 (1) No person shall knowingly: (c) for the purpose of creating a human being, create an embryo from a cell or part of a cell taken from an embryo or fetus or transplant an embryo so created into a human being. (f) alter the genome of a cell of a human being or in-vitro embryo such that the alteration is capable of being transmitted to descendants. |
Article 12 (1) No person shall, except in accordance with the regulations, reimburse a donor for an expenditure incurred in the course of donating sperm or an ovum. |
| Mexico | Regulation to the General Health Law on Health Research 1987 | No relevant provisions. Article 56 stipulates that research on assisted fertilization will only be admissible when it is applied to solve sterility problems that cannot be solved otherwise, respecting the couple’s moral, cultural and social point of view, even if these differ from those of the researcher. |
No specific constraints. | |
| USA | • Consolidated Appropriations Act 2016 Sec. 749 (still effective in Consolidated Appropriations Act 2017 Sec. 736) • NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules 2016 |
• Act 2016 prohibits the Food and Drug Administration from spending federal budget to review or approve an application for an exemption for investigational use of biologics in which a human embryo is intentionally created or modified to include a heritable genetic modification. • NIH Guidelines state that clinical trial proposals for germline alterations will not be, at present, accepted by the Recombinant DNA Advisory Committee of NIH (Appendix M. Points to consider). |
At federal level, egg donation is largely permitted whether it is anonymous or not. Egg donors may be compensated. A professional society, ASRM, has published opinion papers regarding the interests, obligations and rights in gamete donation. |
|
| Asia | China | The Ministry of Health: the Human Assisted Reproductive Technology Specifications and the Human Assisted Reproductive Technology and Human Sperm Bank Ethical Principles 2003 | In Specifications, Article 7 of Chapter 3 prohibits the implementation of human oocyte-cytoplasmic transplantation and nuclear transfer techniques for the purpose of infertility treatment and Article 9 of Chapter 3 prohibits genetically manipulating human gametes, zygotes and embryos for the purpose of reproduction. In Ethical Principles, 7, Article 3 of Chapter 1 also stipulates that medical personnel should not implement human oocyte-cytoplasmic transplantation and human oocyte-nuclear transplantation for the purpose of infertility treatment because the safety issues of human oocyte-cytoplasmic transplantation and human oocyte-nuclear transfer still remain unsolved. | In Specifications, Article 5 of Chapter 3 stipulates the fundamental conditions for egg donation as follows: (1) egg donation is a kind of humanitarian behaviour, prohibit any organization and individuals in any form to raise the eggs for commercialization; (2) the eggs are limited to the remaining eggs in the human assisted reproductive treatment cycle; (3) the recipients must undergo a health check; (4) the recipients of the eggs should be fully informed of the rights and obligations and sign informed consent; (5) each donor can only contribute to five pregnancies; (6) the clinical follow-up rate of eggs must be 100%. |
| India | • Department of Biotechnology: Ethical Policies on the Human Genome, Genetic Research and Services 2002 • Indian Council for Medical Research: Ethical Guidelines for Biomedical Research on Human Participants 2006 • National Guidelines for Accreditation, Supervision & Regulation of ART Clinics in India 2005 |
• Ethical Policies 2002 stipulate that germline therapy in humans shall be proscribed, considering the present state of knowledge, in 'Gene therapy & human cloning'. • Ethical Guidelines 2006 prohibit germline therapy, gene therapy for enhancement of genetic characteristics (so-called 'designer babies') and eugenic genetic engineering for selection against personality, character, formation of body organs, fertility, intelligence and physical, mental and emotional characteristics (p. 70). |
In ART Guidelines 2005, physical, educational and professional information of the egg donor must be recorded in addition to health history of her family (3.7.3.). The age of the donor must not be less than 21 or more than 35 years (3.7.4.). The oocyte donor may be compensated suitably (e.g. financially) by the law firm or semen bank (3.9.2.). |
|
| Japan | • Act on Regulation of Human Cloning Techniques 146/2000 • Guidelines on the Handling of Specified Embryos 2000 • Ministry of Health, Labour and Welfare: Guidelines for Clinical Research such as Gene Therapy 2015 |
• For the time being, specified embryos (including human embryonic nuclear transfer embryos) may not be transferred to human or animal uterus (Article 7, Guidelines 2000). • Clinical research that intentionally conducts or may conduct genetic modification of human germ cells or embryos is prohibited (Article 7, Guidelines 2015). |
No legal constraints. Since 2001, the Ministry of Health, Labour and Welfare has demanded that egg donation should not be conducted until relevant legislation is established. A professional society, JISART, established a voluntary guideline on egg donation. |
|
| Taiwan | Artificial Reproduction Act 2007 | No relevant provisions found. Item 1, Article 16 prohibits using reproductive cells or embryos provided exclusively for research purposes in artificial reproduction. Meanwhile, Article 17 allows artificial reproduction which constitutes a human subject research in compliance with the regulations of the Medical Care Act. |
Items 1 and 3 of Article 8 permit egg donation if it is gratuitous and the donors are aged 20-40 years. Item 4 of Article 8 allows egg donors to receive a financial compensation, termed 'nutrition cost'. |
|
| Europe | Albania | • Ratification of Council of Europe Treaty No. 164; Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine 1997 • Law 8876/2002 on Reproductive Health |
• Article 13 of Treaty No. 164 stipulates that: an intervention seeking to modify the human genome may only be undertaken for preventive, diagnostic or therapeutic purposes and only if its aim is not to introduce any modification in the genome of any descendants. • Article 33 of Law 2002: The techniques of reproduction are used only in cases when: a) other methods of treatment of male or female infertility are not productive or appropriate and do not guarantee the desired result; b) they prevent the transmission of genetic diseases to offspring, or other diseases that would cause premature death, mental retardation or serious invalidity; c) they are considered as an alternative to a natural birth. |
Article 40 of Law 2002: participation in artificial fecundation is conditioned by the written permission of the woman who donates the eggs and the man who donates the sperm. |
| Czech Republic | • Ratification of Council of Europe Treaty No. 164 • Act on Research on Human Embryonic Stem Cells and Related Activities and on Amendment to Some Related Acts 227/2006 • Act on Specific Health Services 373/2011 |
• Article 13 of Treaty No. 164 stipulates that: an intervention seeking to modify the human genome may only be undertaken for preventive, diagnostic or therapeutic purposes and only if its aim is not to introduce any modification in the genome of any descendants. • 1(a), Section 209b of Act 2006 prohibits medical interventions leading to creation of a human embryo for purposes other than implantation into a woman’s body. |
In Act 2011, C), (4), § 3 stipulates that egg donors must be anonymous, and aged 18-35 years. § 3 allows egg donors to receive reimbursement for expenses associated with the donation. |
|
| Italy | Law 40/2004 Rules in the Field of Medically Assisted Reproduction | 3(b), Article 13 of Chapter VI of Law 2004 prohibits any forms of selection of gametes or embryos for eugenic purposes, or interventions that, through technical selection, handling or otherwise using artificial processes, are intended to alter the genetic heritage of the embryo or gamete, or to predetermine genetic characteristics, except assistance with diagnostic and therapeutic purposes. | Although 1, Article 12 of Law 2004 had prohibited gamete donation, the Constitutional Court removed the prohibition on gamete donation by judgement 162/2014. | |
| Northern Cyprus | • Law Regulating Human Cell, Tissue and Organ Transplantation Rules 57/2014 • Assisted Reproductive Treatment Centers and Assisted Reproductive Treatment Procedures Regulation 381/2016 |
No relevant provisions found in Law 2014 as well as Regulation 2016. | (2) Article 19 of Regulation 2016 stipulates that: (A) enrolment shall be made on the electronic system which the competent authority shall prepare; (B) a donor can donate eggs up to three stimulations per year; and (C) stimulation protocols should be preferred to reduce the risk of ovarian hyperstimulation syndrome in the person who is donating the eggs. It is warned that an average of 10 eggs will be collected each time. |
|
| Spain | • Ratification of Council of Europe Treaty No. 164 • Law 14/2007 on Biomedical Research • Law 14/2006 on Assisted Human Reproduction Techniques |
• Article 13 of Treaty No. 164 stipulates that: an intervention seeking to modify the human genome may only be undertaken for preventive, diagnostic or therapeutic purposes and only if its aim is not to introduce any modification in the genome of any descendants. • Item 1, Article 33 of Law 2007 prohibits the creation of human pre-embryos and embryos exclusively for experimentation purposes. • Item 1, Article 13 of Law 2006 stipulates that any intervention for therapeutic purposes on the viable pre-embryo created in vitro may only have the purpose of treating a disease or preventing its transmission, with reasonable and proven guarantees. • Item 2, Article 13 stipulates that therapy in pre-embryos created in vitro will only be authorized if the requirements below are met: informed consent, diagnostic or therapeutic purposes, no modification of non-pathological hereditary characters, carried out in authorized health centres. • Item 3, Article 13 stipulates that the implementation of in-vitro pre-embryo therapy requires the authorization of the corresponding health authority, following a favourable report from the National Commission for Assisted Human Reproduction. |
Item 5, Article 5 of Law 2006 permits egg donation if the donors are anonymous. Egg donors may be compensated only for physical discomfort, travel and wage loss (Item 3), and must be aged 18-35 years (Item 6). Item 7 stipulates that the maximum number of ovarian stimulations per donor is limited to six, and the maximum number of newborns from one donor is also limited to six. |
|
| Ukraine | Ministry of Health Order No. 771, Instruction on Procedures for Assisted Reproductive Technologies 2008 | No relevant provisions found. | In Section 5 of Order 2008, Item 5 allows anonymous egg donors. Item 6 stipulates that oocyte donors are women aged 20-32 years. Item 7 shows the indications for IVF using donor oocytes, which include: - lack of oocytes, caused by natural menopause; - a syndrome of premature ovarian failure; - state after ovariectomy, radiation therapy or chemotherapy; - abnormalities of the genital organs; the risk of transmission of inherited diseases associated with sex; and - failed repeated IVF attempts (four or more) in low ovarian response to induction of superovulation repeatedly receiving poor quality embryos. |
|
| UKa | Human Embryology and Fertilisation Act 1990, amended by Act 2008 Human Fertilisation and Embryology (Mitochondrial Donation) Regulations (2015) Human Fertilisation and Embryology Authority (HFEA) Code of Practice |
3, Part 1, Act 2008 stipulates 'permitted eggs', 'permitted sperm' and 'permitted embryos', whose nuclear DNA and/or mtDNA have not been altered. 26, 26, Part 1, Act 2008 stipulates the conditions to permit mitochondrial donation (SNT and PNT). Part 1 of Regulations 2015 stipulates the details of 'permitted eggs' and 'permitted embryos' for mitochondrial donation, by defining the process to make 'permitted eggs' (SNT) and 'permitted embryos' (PNT), the circumstances to perform SNT and PNT (there is a significant risk of maternal transmission of serious mitochondrial diseases), and its licence conditions. |
11.2 HFEA Code of Practice requests that eggs should not be taken from egg donors aged 36 years or over according to professional guidelines. Gametes for the treatment of others should not be taken from anyone under the age of 18 years (11.6). Egg donors are registered as identifiable donors (11.7, 8). Egg donors can receive compensation of up to £750 per cycle of donation (13A). The number of families created using donor eggs is limited to 10 or less (11.52). |
|
| Middle East | Israel | • Minister of Health: Public Health (In Vitro Fertilization) Regulations 5747/1987 • Prohibition of Genetic Intervention (Human Cloning and Genetic Change in Reproductive Cells) Law 5759-1999 • Amendment Law No. 3 Prohibition on Genetic Intervention (Human Cloning and Genetic Change in Reproductive Cells), 5776/2016 (valid until 23 May 2020) • Egg Donation Law 5770/2010 |
Item 2, Article 3 of Law 1999 stipulates that 'no person shall use reproductive cells that have undergone a permanent intentional genetic modification (germline gene therapy) in order to cause the creation of a person'. Item 3, Article 4 stipulates that the advisory committee shall advise the Minister on the matter of genetic experimentation on human beings and shall provide him with its recommendations concerning the prohibitions. |
Sections 6 and 29 of Law 2010 allow the research use of donor eggs when: (1) An approval was given under any law for conducting the research. (2) The donor or patient has signed with the attending physician consent to egg allocation and the purpose of research. (3) The number of eggs allocated for research purposes shall not exceed the number of eggs equal to 20% of the number of eggs extracted from the body of the donor or patient or two eggs, whichever is lower. Section 12 stipulates egg donors who have reached the age of 21 years and have not yet reached the age of 35 years. Section 13 stipulates that an egg donor may not be a married woman if she is a Jew, and that the donation is anonymous in principle unless a special permission is obtained. Section 14 allows the donors to undergo two egg retrievals at intervals of at least 180 days. Section 43 declares that the state pays donors compensation, although Section 8 prohibits direct or indirect compensation for egg donation. |
| Turkey | • Ratification of Council of Europe Treaty No. 164 • Penal Code of Turkey • Legislation Concerning Assisted Reproductive Treatment Practices and Centres 27513/2010 • Regulation on Assisted Reproduction Treatment and Assisted Reproduction Treatment Centres 29135/2014 |
• Article 13 of Treaty No. 164 stipulates that: An intervention seeking to modify the human genome may only be undertaken for preventive, diagnostic or therapeutic purposes and only if its aim is not to introduce any modification in the genome of any descendants. • Article 231 of Penal Code stipulates that it is illegal to change or obscure a child’s ancestry. |
Item 5, Article 18 of Legislation 2010 prohibits any use of gamete donors to obtain embryos using donors and to use embryos created using other eggs and sperm obtained from other candidates or those who are not candidates. | |
| United Arab Emirates | • Federal Law No.11 of 2008 concerning Licensing of Fertilization Centres in the State. | Article 14 of Law 2008 prohibits that unfertilized or fertilized ova or sperms are used for commercial purposes, or conducting research, or executing genetic modifications of the characteristics of neonates, or disposing of them for the benefit of others. | Item 1, Article 10 of Law 2008 prohibits the centre from performing fertilization using the sperm of the husband and the ovum of an alien woman, and then implanting the fertilized ovum into the wife’s uterus. |
ART, assisted reproductive technology; ASRM, American Society for Reproductive Medicine; JISART, Japanese Institution for Standardizing Assisted Reproductive Technology; NIH, National Institutes of Health; PNT, pronuclear transfer; SNT, spindle nuclear transfer; UK, United Kingdom; USA, United States of America; UAE, United Arab Emirates.
Although the UK was not included in the 16 selected countries, it is shown here for comparison.
The regulatory status of MMT in the 16 countries was further analysed based on national and international policies pertinent to human germline genetic modification, in consideration of the regulations on oocyte donation (Table 2). We focused our regulatory analysis on the confirmed MMT (autologous types: AGCMT, AUGMENT; allogeneic types: CT, PNT and SNT) (Table 1).
The Americas
In Canada, the Assisted Human Reproduction Act 2004 prohibits the alteration of the genome of in-vitro embryos in order to create a human being [Article 5 (1) (f), Table 2]. It appears that the clinical use of MMT is legally banned in Canada. However, the provision can be viewed as ambiguous regarding the regulation of MMT because the term ‘genome’ may specifically address the nDNA (Alberts et al., 2008), and because mitochondrial manipulation may not be ‘the alternation of the genome’. However, Article 5 (1) (c) explicitly prohibits the clinical use of PNT because the reconstituting of embryos using karyoplast transfer can be interpreted as the act to ‘create an embryo from a part of a cell taken from an embryo’ (Table 2). To our interpretation, the regulation of MMT in human oocytes is insufficient in Canada, except for PNT.
Mexico has no laws directly pertaining to reproductive medicine (Palacios-González and de Jesús Medina-Arellano, 2017) or human germline genetic modification (Ishii, 2017a). However, the Regulation to the General Health Law on Health Research 1987 states that ‘research on assisted fertilisation will only be admissible when it is applied to solve sterility problems that cannot be solved otherwise’ (Table 2). A legal study judged that the first SNT case in Mexico was illegal because the MMT was performed to prevent the onset of a mitochondrial disease (Leigh syndrome) in offspring, not to ‘solve sterility problems’ (Palacios-González and de Jesús Medina-Arellano, 2017). However, this judgement can be rebutted because women with an mtDNA mutation responsible for Leigh syndrome have undergone ‘sterility problems’ of being unable to conceive as well as carry pregnancy to full term (Ishii, 2017b). Furthermore, Regulation 1987 suggests that MMT research to treat ‘sterility problems’ may be admissible if there are no other options available after the failure of in-vitro fertilization (IVF) and pre-implantation genetic screening (Ishii, 2017b). More recently, a further legal article considered that SNT research for dealing with all types of ‘sterility’ would be legal under Mexican law if Article 56 of Regulations 1987 was followed (Palacios-González and Medina-Arellano, 2017). Again, the legally ambiguous definition of ‘sterility’ is problematic in this case. Moreover, Regulation 1987 is insufficient due to the lack of technical details, such as references to germline genetic modification or mitochondrial manipulation, in clinical research on reproductive medicine.
The USA also has no legislation to regulate the clinical use of human germline genetic modification, although the 2016 guidelines of the National Institutes of Health (NIH) state that NIH will not accept clinical trial proposals involving germline alternations (Table 2). However, as addressed in the Introduction, the FDA decided to review a clinical trial involving mitochondrial manipulation after the CT case, conducted from the mid-1990s to 2001. In 2015, an appropriation bill rider, the Consolidated Appropriations Act 2016 Sec. 749, was passed, which prohibits the FDA from spending federal budget to review an application for an exemption for investigational use of biologics in which ‘a human embryo is intentionally created or modified to include a heritable genetic modification (Adashi and Cohen, 2017). Of note, a human embryo is viewed as biologics in the USA. More importantly, this law implies no possibility to obtain regulatory approval to perform MMT that can cause a heritable alternation of mtDNA content (Castro, 2016), although the rider is ambiguous about whether it is permissible to clinically use male embryos created via SNT. As the rider was again incorporated into an appropriation bill for fiscal year 2017 (Kadakia, 2016), the prohibition will likely be renewed annually to maintain the prohibition in the USA, as illustrated by the Dickey-Wicker amendment 1995 (Rodriguez et al., 2011). Indeed, the Consolidated Appropriations Act 2017 Sec. 736 was passed (Table 2). Of note, we found no US clinics that advertised MMT, except for the clinic that performed SNT recently (Supplemental Document 1).
In Canada, Mexico and the USA, the current regulatory situation regarding MMT differs substantially. Canada prohibits PNT but regulates other MMT procedures insufficiently. Mexico also regulates MMT insufficiently due to the lack of technical details in the provision regarding ‘research on assisted fertilization’. The USA assumes a prohibitive policy regarding human germline genetic modifications, including MMT.
Asia
As addressed in the Introduction, the 2003 guidelines of the Chinese Ministry of Health largely prohibit infertility treatment using MMT by clearly mentioning certain MMT procedures, mentioning ‘oocyte-cytoplasmic transplantation, nuclear transfer techniques’ in the Specifications, and ‘oocyte-cytoplasmic transplantation and oocyte-nuclear transplantation’ in the Ethical Principles (Table 2). The 2003 guidelines prohibit the clinical use of AUGMENT because the mitochondria for transfer are derived from the cytoplasm of ‘egg precursor cells’. Of note, two cases of AGCMT were published from China in 2003 (Kong et al., 2003a, Kong et al., 2003b). AGCMT involves the transfer of mitochondria derived from autologous granular cells (not oocytes), which might fall outside of the scope of the 2003 guidelines. Nonetheless, the Chinese guidelines stipulate explicitly that most MMT are prohibited in the clinical setting, despite no specific mention of the possible purpose of preventing mitochondrial diseases. Of note, our survey found no clinics in China that advertised MMT (Supplemental Document 1).
Indian guidelines stipulate that procedures such as ‘germline gene therapy’ and ‘germline therapy’ are banned (Table 2). However, the terms are too ambiguous to determine whether or not human reproduction using MMT is included in the prohibitions. Our survey showed that at least two clinics advertised CT on their clinic sites (Supplemental Document 1).
In Japan, Guidelines on Specified Embryos 2000 prohibit reproduction using PNT by stipulating ‘human embryonic nuclear transfer embryo’ (Table 2). Meanwhile, Guidelines for Clinical Research such as Gene Therapy 2015 prohibit a clinical trial involving genetic modification of human germ cells and embryos, suggesting that MMT research is not permitted in Japan. However, the prohibitive policy is applied only to the case of ‘gene therapy’ (defined as ‘therapeutic intervention involving gene transfer’; cellular organelles, such as mitochondria, are not included in this definition), not to MMT (Ishii, 2016b). Therefore, Japanese guidelines regulate the clinical use of MMT in human oocytes insufficiently.
In Taiwan, the Artificial Reproduction Act 2007 allows oocyte donation for which the donor is financially compensated. In contrast, the law does not include any articles regarding human germline genetic modification (Table 2). Act 2007 prohibits ‘artificial reproduction’ (the use of ‘artificial’ means not involving sexual intercourse to achieve conception and birth with assistance from reproductive medicine) using ‘reproductive cells or embryos provided exclusively for research purposes’. However, Act 2007 allows ‘artificial reproduction’ which constitutes human subject research in compliance with the regulations of the Medical Care Act. It is unclear whether or not the prohibition of ‘artificial reproduction’ using ‘reproductive cells or embryos provided exclusively for research purposes’ is applied to human subject research involving MMT using reproductive cells or embryos provided for both research and ‘artificial reproduction’ purposes. In addition, Act 2007 does not regulate AGCMT in which mitochondria derived from granular cells (not ‘reproductive cells’) are transferred to each oocyte. Therefore, Taiwan law regulates the clinical use of MMT insufficiently.
In Asia, China prohibits most MMT. However, the regulation of MMT is insufficient in India, Japan (except for PNT) and Taiwan because of the lack of due consideration regarding the technical details, particularly with regard to newer techniques such as SNT.
Europe
The Council of Europe Treaty No. 164: the Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine 1997 (so-called ‘Oviedo Convention’) was the first international binding policy pertaining to biomedicine (Council of Europe, 1997). Article 13 of the Oviedo Convention stipulates that, ‘An intervention seeking to modify the human genome may only be undertaken for preventive, diagnostic or therapeutic purposes and only if its aim is not to introduce any modification in the genome of any descendants.’ Among the 16 selected countries, Albania, Czech Republic, Spain and Turkey ratified the Oviedo Convention (Council of Europe, 1997) (Table 2). However, with regard to the clinical use of MMT, the provision can be viewed as ambiguous because the term ‘genome’ may address nDNA specifically, and because mitochondrial manipulation may not ‘introduce any modification in the genome’, as mentioned in the Canadian law.
In Albania, in addition to the Oviedo Convention, Law 8876/2002 on Reproductive Health declares that a reproductive technique can be performed if there are no effective infertility treatments, or if it intends to prevent the inheritance of a serious disease in offspring, suggesting that MMT can be performed for the treatment of intractable infertility or for the prevention of inheritance of serious mitochondrial diseases in offspring (Table 2). Law 2002 also includes the permissible condition that a reproductive technique is regarded as an alternative to a natural birth (Table 2). It is unclear whether or not a birth following MMT is considered as a natural birth, in particular in the case of MMT using autologous mitochondria. Our analysis indicates that the reproductive use of MMT is regulated insufficiently in Albania.
Czech Republic is also a ratifier of the Oviedo Convention. Furthermore, Act 2006 prohibits medical procedures that require the creation of a human embryo for purposes other than embryo transfer (Table 2). Although the law suggests that PNT, which requires the creation of a human embryo to provide enucleated zygotes, is banned, the regulation of other MMT, such as SNT, is insufficient in Czech Republic.
Italian Law 2004 prohibits medical interventions that intend ‘to alter the genetic heritage of the embryo or gamete’, potentially banning the clinical use of MMT. However, the phrase of ‘the genetic heritage of the embryo or gamete’ is ambiguous. As such, it is unclear whether or not the prohibition can be truly applied to MMT that manipulate mtDNA in oocytes or zygotes. Therefore, Italian regulation is insufficient regarding the clinical use of MMT.
With regard to Northern Cyprus, no provisions relevant to human germline genetic modification were found in Law 2014 or Regulation 2016 pertaining to reproductive medicine (Table 2). We therefore concluded that this country does not regulate the clinical use of MMT. Indeed, CT was advertised on clinic and tourism websites for this country.
Spain is a ratifier of the Oviedo Convention. Moreover, Spanish Law 2006 permits medical interventions in ‘pre-embryos’ (IVF embryos) only for ‘treating a disease or preventing its transmission’ in authorized health centres if such interventions cause ‘no modification of non-pathological hereditary characters’ (Table 2). However, Spanish Law 2007 prohibits the creation of human embryos exclusively for experimental purposes, suggesting the prohibition of PNT clinical research (Table 2). Of note, such laws do not address medical interventions in human oocytes, suggesting that MMT, except PNT, are not regulated in Spain. Therefore, the regulation of MMT in human oocytes is insufficient in Spain.
The 2008 Ukrainian guidelines (Ministry of Health Order No. 771, Instruction on Procedures for Assisted Reproductive Technologies 2008) include no provisions relevant to human germline genetic modification (Table 2). It was judged that Ukraine does not regulate the clinical use of MMT, similar to Northern Cyprus. Indeed, a Ukrainian clinic advertised that PNT is a source of hope for helping women suffering from embryonic arrest who wish to have a genetically related child, as illustrated by a news report on a live birth following PNT (despite the absence of a medical report) (Supplemental Document 1).
In Europe, the regulation of MMT (except PNT in Czech Republic and Spain) was deemed to be insufficient in Albania, Czech Republic and Spain, despite the ratification of the Oviedo Convention. Italy also regulates MMT insufficiently. However, Northern Cyprus and Ukraine do not regulate experimental MMT.
Middle East
Regarding the Middle East, we must again address oocyte donation. The Israeli Egg Donation Law 2010 permits the use of donor oocytes in research under certain conditions (Table 2). The Israeli policy does not prohibit oocyte donation for research, despite its restrictiveness. In contrast, Turkey and the UAE prohibit oocyte donation, which limits MMT to autologous mitochondrial transfers, such as AGCMT and AUGMENT (Table 2).
With regard to germline genetic modification for reproduction, Israeli Law 2016, which is effective until 2020, prohibits the clinical use of ‘reproductive cells that have undergone a permanent intentional genetic modification’ (Table 2). However, male embryo transfer following MMT does not fall under ‘a permanent intentional genetic modification’ because mtDNA is only maternally inherited in children. The regulation of MMT was therefore deemed to be insufficient in Israel.
In Turkey, only autologous types of MMT can be considered for human reproduction due to the prohibition against oocyte donation. Although Turkey is a ratifier of the Oviedo Convention, the international policy may be insufficient regarding the regulation of autologous MMT, as mentioned in Europe. The Penal Code stipulates that it is illegal to change or obscure a child’s ancestry (Table 2). However, it is unclear whether the article is truly applicable to autologous types of MMT, such as AGCMT and AUGMENT, which only increase the mtDNA copy number while maintaining the nDNA in the resultant children. Likewise, legal questions could arise if mitochondria of a haplotype identical to the mother's are used in SNT and PNT. Additionally, given that the mother’s mitochondria are carried over in SNT and PNT, heteroplasmy could be viewed favourably due to the traceable mother’s genealogical line (Palacios-González, 2016). Therefore, the regulation of autologous MMT is insufficient in Turkey.
Similarly, only autologous types of MMT can be considered for clinical use in the UAE. Law 2008 prohibits implementation of genetic modifications regarding the characteristics of neonates. However, it is difficult to simply apply this prohibition to autologous types of MMT, because AGCMT and AUGMENT just increase the copy number of mtDNA encoding only 13 respiratory chain proteins that are not closely associated with a child’s characteristics (Anderson et al., 1981). Therefore, the regulation of autologous MMT is insufficient in the UAE.
In the Middle East, the regulation of MMT is insufficient in Israel due to the use of an inappropriate technical term, whereas the regulation of autologous MMT in Turkey and the UAE is insufficient due to the prohibition of oocyte donation.
Taken together, these findings indicate that the USA and China largely prohibit the clinical use of MMT by different regulatory approaches; however, Northern Cyprus and Ukraine do not regulate the experimental procedures involving mitochondrial manipulation (Fig. 1). The remaining 12 countries (Albania, Canada, Czech Republic, India, Israel, Italy, Japan, Mexico, Spain, Taiwan, Turkey and UAE) could be considered to regulate the clinical use of MMT insufficiently, although Canada, Japan, Czech Republic and Spain prohibit PNT, and Turkey and the UAE do not allow allogeneic MMT due to prohibition of oocyte donation.
Fig. 1.
Regulation of the clinical use of mitochondrial manipulation techniques (MMT) in 16 selected countries. Not regulated (red): Northern Cyprus and Ukraine; insufficiently regulated (striped): Albania, India, Israel, Italy, Mexico and Taiwan; pronuclear transfer prohibited but other MMT regulated insufficiently (pink): Canada, Czech Republic, Japan and Spain; allogeneic MMT not allowed but autologous MMT regulated insufficiently (yellow): Turkey and the United Arab Emirates; and MMT largely prohibited (blue): China and the USA. Other countries were not analysed.
Discussion
This study does not intend to encourage cross-border reproductive travel for MMT. These results depend, in part, on the accuracy of the information available on the internet. However, our survey shows that some MMT have been clinically implemented, are being studied in clinical trials, or are advertised in 16 countries (Albania, Canada, Czech Republic, China, India, Israel, Italy, Japan, Mexico, Northern Cyprus, Spain, Taiwan, Turkey, Ukraine, UAE and USA) (Table 1). Considering the unconfirmed advertisements, our survey also suggests the possibility that MMT are advertised and used clinically outside of these 16 countries.
The UK’s relevant regulation explicitly prohibits the use of human eggs, spermatozoa or embryos where the nDNA or mtDNA has been altered for reproduction, whereas it allows SNT and PNT to be performed for the prevention of maternal inheritance of ‘serious mitochondrial diseases’ in offspring at licensed clinics that can demonstrate expertise in these techniques and can carry out follow-up of the resultant children (Table 2) (HFEA, 2016). Thus, the clinical practice of SNT and PNT is limited to such rare cases, regulated sufficiently, and the resultant children must be, in principle, followed-up in a relatively responsible manner in the UK. However, our study revealed the possibility that Northern Cyprus and Ukraine do not regulate MMT at all, and Albania, Canada, Czech Republic, India, Israel, Italy, Japan, Mexico, Spain, Taiwan, Turkey and the UAE regulate some MMT insufficiently, particularly mitochondrial manipulation in oocytes (AUGMENT, CT and SNT).
MMT procedures that do not require any special tools are feasible worldwide at many clinics that can perform intracytoplasmic sperm injection. Allogeneic types of MMT can be performed if donor oocytes are available domestically or can be imported from abroad. However, all MMT are still experimental in human reproduction. As suggested by the CT case in the USA and the PNT case in China, the risks to resultant fetuses or children may be substantial whether due to induced heteroplasmy, random mtDNA segregation, or mismatching between nDNA and mtDNA by the different mtDNA haplogroups, as well as procedures such as aspiration and fusion of cytoplasm or karyoplast (Alikani et al., 2017, Hyslop et al., 2016, Ishii, 2016a, Morrow et al., 2015, Reinhardt et al., 2013, Yamada et al., 2016). While MMT could be considered as an acceptable germline intervention, there have been considerable controversies over the therapeutic implications of SNT and PNT (Palacios-GonzáLez, 2017, Rulli, 2016, Rulli, 2017, Wrigley et al., 2015). However, the authors who published the first SNT case confessed that the mtDNA haplogroup of the oocyte donor (L2c) was different from that of the patient (I) in the SNT (Zhang et al., 2017a), suggesting that there was insufficient consideration of the need to match between nDNA and mtDNA. Moreover, they did not present clear evidence regarding the safety of the ‘modified electrofusion’ technique (Alikani et al., 2017).
Meanwhile, the use of SNT and PNT to prevent maternal transmission of mitochondrial disease to offspring is based on evidence that mutations in 13 mtDNA genes are linked to the onset of the genetic disease (Koopman et al., 2012). The SNT case in Mexico intended to reduce the MTATP6 mutation load in the oocytes, which is responsible for the onset of a mitochondrial disease (Leigh syndrome) in offspring (Zhang et al., 2017a). With regard to the use of MMT for infertility treatment, AUGMENT is currently advertised and offered in Canada, Japan and Turkey (Supplemental Document 1). Moreover, PNT is also used for infertility treatment in Ukraine (Coghlan, 2017). Although the PNT case in Ukraine has not been published in a peer-reviewed journal, PNT is advertised on the website of a Ukrainian clinic (Supplemental Document 1). Furthermore, the US physician who performed the first SNT has established a company to provide SNT to rejuvenate the oocytes of infertile women aged 42–47 years outside the USA (Mullin, 2017). Although it has been suggested that POLG in nDNA is the only candidate gene to show an association with both male and female infertility, there is no clear evidence to suggest that any mtDNA mutations are causative of infertility (Demain et al., 2017). However, MMT may be viewed as a last resort for treating intractable female infertility in those infertile women who suffer from repeated embryonic arrests, and value genetic relatedness with prospective children (Ishii, 2017b). When some MMT begin to be used for infertility treatment in a country, their widespread use will likely follow rapidly. Thus, from a social standpoint, should countries (including Northern Cyprus, Mexico and Ukraine) prohibit the use of experimental MMT for infertility treatment for the time being due to the risks to the prospective children and parents?
Based on the results and aforementioned discussions, how should policy-making surrounding MMT be carried out? At present, there are no countries that will follow the UK in legalizing PNT and SNT for disease prevention. Rather, Israel decided to maintain a 5-year legal prohibition regarding the reproductive use of human germline genetic modification in 2016, as mentioned above. More importantly, the US Congress passed the Consolidated Appropriations Act 2016 Sec. 749 at the end of 2015, which is still effective in Consolidated Appropriations Act 2017 Sec. 736. Although this law does not prohibit MMT itself, it prevented the FDA from approving its clinical use. The legal events in Israel and the USA display the legal difficulties in breaking the taboo of human germline genetic modification. However, such prohibitive legal actions without deliberation about MMT may lead to social problems. As mentioned in the Introduction, the US physician who implemented the cross-border use of SNT between the USA and Mexico (Zhang et al., 2017a) has stated that his group went to Mexico because ‘there are no rules’ (Hamzelou, 2016). As noted above, this physician plans to market oocytes reconstituted via SNT to treat infertile women in their 40s. On 4 August 2017, the FDA sent a formal letter to order him to comply with federal regulations, informing him of his violations in a non-inclusive list, including marketing with no valid biologics licence and no supporting data that should be obtained after approved clinical research (FDA, 2017). Nevertheless, the FDA cannot approve such clinical research applications due to the appropriation bill rider from 2016. Although our legal analysis concluded that the USA and China largely prohibit the clinical use of MMT, the USA currently requires a clear regulation that is established after reaching a broad social consensus (Cohen and Adashi, 2016). In so doing, the cross-border use of MMT by shipping the modified oocytes and embryos should be considered in order to prevent international problems.
In the near future, some countries might consider the use of MMT to prevent the onset of serious mitochondrial diseases in offspring as a reproductive option for prospective parents who wish to have a genetically related child and who have experienced PGD failure due to the high load of mtDNA mutations in the oocytes. In so doing, such countries must deliberate the potential harm and benefit of MMT from the standpoints of prospective children, as well as oocyte donors. Mexico and Japan will face difficulties in this deliberation because the two countries have no regulation regarding oocyte donation (Table 2). Moreover, available alternatives, such as the straightforward use of donor oocytes for IVF, should be recognized as other reproductive options. Again, Japan will face difficulties; there have been only 77 oocyte donations since 2007 under a professional guideline by the Japanese Institution for Standardizing Assisted Reproductive Technology (JISART, 2017). If such problems are solved, the countries must establish the regulation of MMT by defining the legal technical term that clearly addresses the state of mtDNA or the details of procedures using donor oocytes and embryos, as illustrated by UK and Chinese regulations. However, newer MMT may pose certain legal questions for policy makers. For instance, polar body transfer is difficult to legally interpret as CT or nuclear transfer that is prohibited in the 2003 Chinese guidelines (Table 2). Although China may amend the 2003 guidelines to include this new technique, it may also amend the guidelines so as to regulate MMT consistently, by clearly addressing mtDNA content in the technical terms, and the prevention of mitochondrial diseases other than infertility treatment in the medical purpose.
Ideally, an internationally binding regulation regarding MMT should be established (Schandera and Mackey, 2016), although this may take many years to attain. It usually takes years to establish or amend a medical regulation. Professional societies, such as ESHRE and the American Society for Reproductive Medicine (ASRM), will likely be asked to consider a clinical report on MMT for presentation or publication, as illustrated by the presentation of the first SNT at an ASRM meeting (Zhang et al., 2016a). Since such professional societies should confirm whether each MMT case was performed in a responsible way, some recommendations are proposed below.
First, professional societies should review the legality of a given MMT case in the country where the procedure was performed. Second, if the legality can be confirmed, the process of ethical review and informed consent should be reviewed carefully (Alikani et al., 2017). Although the protocol should have been approved by a research ethics committee, the reviewers for professional societies should investigate whether or not alternatives were explained carefully to a patient; whether or not predictable risks, such as miscarriages, congenital anomalies and late-onset diseases, were explained; and whether or not efforts to minimize the risks, such as the implementation of PGD prior to embryo transfer and matching the mtDNA haplotype between a patient and an oocyte donor, were made in the prior explanation document. Third, the reviewers should also confirm whether a follow-up survey plan for the resultant child was proposed in the prior explanation, whether or not the prospective parents accepted the plan, and whether or not appropriate reasons for refusal were included in the consent form if they did not accept it. To our knowledge, only one article has reported the results of a follow-up survey of children born following CT (Chen et al., 2016). The parents of a boy born following SNT requested that no further follow-up examinations be performed, unless there were clinical benefits (Alikani et al., 2017). Finally, we recommend that professional societies should establish policies regarding MMT in order to prevent society members from practising MMT at their clinics in an irresponsible manner.
Conclusion
Although the interpretation of legal documents may have influenced our analysis regarding the regulation of MMT, our study found a possibility that Northern Cyprus and Ukraine do not regulate MMT, and Albania, Canada, Czech Republic, India, Israel, Italy, Japan, Mexico, Spain, Taiwan, Turkey and the UAE regulate some MMT insufficiently, particularly those in oocytes, demanding wider and in-depth legal investigations regarding the clinical use of MMT. In addition, our findings underscore the pressing need for regulatory consideration of MMT in order to protect children born following such experimental techniques, and ameliorate the potential harm to society.
The following are the supplementary data related to this article.
Survey results regarding the implementation and advertisement of reproductive medicine involving mitochondrial manipulation.
List of the legal documents regarding egg donation and germline genetic modification for reproduction in 16 selected countries.
Authors’ roles
TI primarily designed the study, and YH participated in the process. Both authors conducted investigations. TI analysed the result of investigations and wrote the manuscript, and YH confirmed the findings.
Acknowledgements
We acknowledge the contribution of physicians who we asked to consult regulators in their own countries about the regulatory status of each MMT. This research was supported by JSPS KAKENHI Grant Number 26460586.
Biography

Tetsuya Ishii is Professor of Office of Health and Safety, Hokkaido University, Japan. He obtained his PhD in Bioscience at Hokkaido University in 2003. He subsequently joined Japan Science and Technology Agency and worked as a programme officer. In 2005, he completed the international program officer training program at the US National Institutes of Health John E. Fogarty International Center. Subsequently, he worked at the Center for iPS Cell Research and Application (CiRA), Kyoto University (Director, Shinya Yamanaka). At CiRA, he was in charge of clinical research application. Currently, he is studying bioethics, focusing on the relationship between society and biomedicine. He is a member of the European Society of Human Reproduction and Embryology, the International Society for Stem Cell Research, and the American Association for the Advancement of Science.
Declaration: The authors report no financial or commercial conflicts of interest.
References
- Adashi E.Y., Cohen I. Mitochondrial replacement therapy: Unmade in the usa. JAMA. 2017;317:574–575. doi: 10.1001/jama.2016.20935. [DOI] [PubMed] [Google Scholar]
- Alberts B., Johnson A., Lewis J., Raff M., K., R, Walter P. Molecular Biology of The Cell. 5 ed. Garland Science; New York: 2008. Genetic information in eucaryotes. [Google Scholar]
- Alikani M., Fauser B.C.J., Garcia-Valesco J.A., Simpson J.L., Johnson M.H. First birth following spindle transfer for mitochondrial replacement therapy: hope and trepidation. Reprod. BioMed. Online. 2017;34:333–336. doi: 10.1016/j.rbmo.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A.T., Barrel B.G. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:57–65. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Barritt J., Willadsen S., Brenner C., Cohen J. Cytoplasmic transfer in assisted reproduction. Hum. Reprod. Update. 2001;7:428–435. doi: 10.1093/humupd/7.4.428. [DOI] [PubMed] [Google Scholar]
- Barritt J.A., Brenner C.A., Malter H.E., Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum. Reprod. 2001;16:513–516. doi: 10.1093/humrep/16.3.513. [DOI] [PubMed] [Google Scholar]
- Barritt J.A., Brenner C.A., Malter H.E., Cohen J. Rebuttal: interooplasmic transfers in humans. Reprod. BioMed. Online. 2001;3:47–48. doi: 10.1016/s1472-6483(10)61966-9. [DOI] [PubMed] [Google Scholar]
- Baylis F. The ethics of creating children with three genetic parents. Reprod. BioMed. Online. 2013;26:531–534. doi: 10.1016/j.rbmo.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Benagiano G., Filippi V., Sgargi S., Gianaroli L. Italian Constitutional Court removes the prohibition on gamete donation in Italy. Reprod. BioMed. Online. 2014;29:662–664. doi: 10.1016/j.rbmo.2014.08.013. [DOI] [PubMed] [Google Scholar]
- British_Fertility_Society British Fertility Society response to OvaScience’s “stem cell baby” claim. 2017. https://britishfertilitysociety.org.uk/press-release/british-fertility-society-response-to-ovasciences-stem-cell-baby-claim/
- Castro R. Mitochondrial replacement therapy: the UK and US regulatory landscapes. J. Law Biosci. 2016;3:726–735. doi: 10.1093/jlb/lsw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.H., Pascale C., Jackson M., Szvetecz M.A., Cohen J. A limited survey-based uncontrolled follow-up study of children born after ooplasmic transplantation in a single centre. Reprod. BioMed. Online. 2016;33:737–744. doi: 10.1016/j.rbmo.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Coghlan A. First baby born using 3-parent technique to treat infertility. 2017. https://www.newscientist.com/article/2118334-first-baby-born-using-3-parent-technique-to-treat-infertility/
- Cohen I.G., Adashi E.Y. Science and regulation. The FDA is prohibited from going germline. Science. 2016;353:545–546. doi: 10.1126/science.aag2960. [DOI] [PubMed] [Google Scholar]
- Cohen J., Scott R., Schimmel T., Levron J., Willadsen S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350:186–187. doi: 10.1016/S0140-6736(05)62353-7. [DOI] [PubMed] [Google Scholar]
- Cohen J., Scott R., Alikani M., Schimmel T., Munne S., Levron J., Wu L., Brenner C., Warner C., Willadsen S. Ooplasmic transfer in mature human oocytes. Mol. Hum. Reprod. 1998;4:269–280. doi: 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- Cohen I.G., Savulescu J., Adashi E.Y. Medicine. Transatlantic lessons in regulation of mitochondrial replacement therapy. Science. 2015;348:178–180. doi: 10.1126/science.aaa8153. [DOI] [PubMed] [Google Scholar]
- Council of Europe Treaty No.164. Convention for the protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine. 1997. https://www.coe.int/en/web/conventions/full-list/-/conventions/treaty/164
- Council of Europe Background document on preimplantation and prenatal genetic testing: Clinical Situation and Legal situation. 2015. https://rm.coe.int/16804583d8 (accessed August 9, 2017) [Online]
- Dale B., Wilding M., Botta G., Rasile M., Marino M., Di Matteo L., De Placido G., Izzo A. Pregnancy after cytoplasmic transfer in a couple suffering from idiopathic infertility: case report. Hum. Reprod. 2001;16:1469–1472. doi: 10.1093/humrep/16.7.1469. [DOI] [PubMed] [Google Scholar]
- Demain L.A., Conway G.S., Newman W.G. Genetics of mitochondrial dysfunction and infertility. Clin. Genet. 2017;91:199–207. doi: 10.1111/cge.12896. [DOI] [PubMed] [Google Scholar]
- EUR_Lex SUMMARY OF: Directive 2004/23/EC – quality and safety standards for donated human tissues and cells. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=URISERV%3Ac11573
- Fakih M.H., El Shmoury M., Szeptycki J., Dela Cruz D.B., Lux C., Verjee S., Burgess C.M., Cohn G.M., Casper R.F. The AUGMENT SM Treatment: Physician Reported Outcomes of the Initial Global Patient Experience. JFIV Reprod. Med. Genet. 2015:3. [Google Scholar]
- FDA August 4, 2017 - Letter to Darwin Life Inc. 2017. https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/UCM570225.pdf? [Online]
- Gosden R.G., Johnson M.H. Can oocyte quality be augmented? Reprod. BioMed. Online. 2016;32:551–555. doi: 10.1016/j.rbmo.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Gürtin Z.B. Patriarchal pronatalism: Islam, secularism and the conjugal confines of Turkey’s IVF boom. Reprod. Biomed. Soc. Online. 2016;2:39–46. doi: 10.1016/j.rbms.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzelou J. World’s First Baby Born With New ‘3 Parent’ Technique New Scientist (2016) 2016. https://www.newscientist.com/article/2107219-exclusive-worlds-first-baby-born-with-new-3-parent-technique/
- Heindryckx B., Cristina Eguizabal C., Chuva De Sousa Lopes S., Geens M., Vassena R. ESHRE SIG Stem Cells statement: THE USE OF MITOCHONDRIAL TRANSFER TO IMPROVE ART OUTCOME. 2015. https://www.eshre.eu/Press-Room/ESHRE-News/2015.aspx (Available: (accessed May 11, 2017))
- HFEA Human Fertilisation and Embryology Authority Code of Practice: 33. Mitochondrial donation. 2016. http://www.hfea.gov.uk/9931.html#guidanceSection10063http://www.hfea.gov.uk/9931.html#guidanceSection10063 Available:
- Huang C.C., Cheng T.C., Chang H.H., Chang C.C., Chen C.I., Liu J., Lee M.S. Birth after the injection of sperm and the cytoplasm of tripronucleate zygotes into metaphase II oocytes in patients with repeated implantation failure after assisted fertilization procedures. Fertil. Steril. 1999;72:702–706. doi: 10.1016/s0015-0282(99)00309-x. [DOI] [PubMed] [Google Scholar]
- Hyslop L.A., Blakeley P., Craven L., Richardson J., Fogarty N.M., Fragouli E., Lamb M., Wamaitha S.E., Prathalingam N., Zhang Q., O'keefe H., Takeda Y., Arizzi L., Alfarawati S., Tuppen H.A., Irving L., Kalleas D., Choudhary M., Wells D., Murdoch A.P., Turnbull D.M., Niakan K.K., Herbert M. Towards clinical application of pronuclear transfer to prevent mitochondrial DNA disease. Nature. 2016;534:383–386. doi: 10.1038/nature18303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T. Potential impact of human mitochondrial replacement on global policy regarding germline gene modification. Reprod. BioMed. Online. 2014;29:150–155. doi: 10.1016/j.rbmo.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Ishii T. Germline genome-editing research and its socioethical implications. Trends Mol. Med. 2015;21:473–481. doi: 10.1016/j.molmed.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Ishii T. Mitochondrial Manipulation for Infertility Treatment and Disease Prevention. In: Schatten H., editor. Human Reproduction: Updates and New Horizons. Wiley Blackwell; New Jersey: 2016. [Google Scholar]
- Ishii T. Science Council of Japan: Document No.3; the summary of 3rd committee for consideration of gene editing in biomeidicne: from regulatory aspect. 2016. http://www.scj.go.jp/ja/member/iinkai/genome/pdf23/siryo4-3.pdf
- Ishii T. Germ line genome editing in clinics: the approaches, objectives and global society. Brief. Funct. Genomics. 2017;16:46–56. doi: 10.1093/bfgp/elv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T. Peer Commentary: Mitochondrial replacement techniques and Mexico's rule of law: on the legality of the first maternal spindle transfer case. J. Law Biosci. 2017;2017 doi: 10.1093/jlb/lsw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JISART Past result of egg donation under Japanese Institution for Standardizing Assisted Reproductive Technology (JISART) guideline. 2017. https://jisart.jp/about/external/proven/ [Online]
- Kadakia K. Duke SciPol, “Consolidated Appropriations Act, 2016 (Public Law 114-113)”. 2016. http://scipol.duke.edu/content/consolidated-appropriations-act-2016-public-law-114-113 Available:
- Kang E., Wu J., Gutierrez N.M., Koski A., Tippner-Hedges R., Agaronyan K., Platero-Luengo A., Martinez-Redondo P., Ma H., Lee Y., Hayama T., Van Dyken C., Wang X., Luo S., Ahmed R., Li Y., Ji D., Kayali R., Cinnioglu C., Olson S., Jensen J., Battaglia D., Lee D., Wu D., Huang T., Wolf D.P., Temiakov D., Belmonte J.C., Amato P., Mitalipov S. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature. 2016;540:270–275. doi: 10.1038/nature20592. [DOI] [PubMed] [Google Scholar]
- Kenney N.J., Mcgowan M.L. Looking back: egg donors' retrospective evaluations of their motivations, expectations, and experiences during their first donation cycle. Fertil. Steril. 2010;93:455–466. doi: 10.1016/j.fertnstert.2008.09.081. [DOI] [PubMed] [Google Scholar]
- Kong L.H., Liu Z., Li H., Zhu L., Chen S.L., Xing F.Q. First twins born in Mainland China by autologous granular cell mitochondria transfer. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:990–991. [PubMed] [Google Scholar]
- Kong L.H., Liu Z., Li H., Zhu L., Xing F.Q. Pregnancy in a 46-year-old woman after autologous granular cell mitochondria transfer. Di Yi Jun Yi Da Xue Xue Bao. 2003;23(743):747. [PubMed] [Google Scholar]
- Koopman W.J., Willems P.H., Smeitink J.A. Monogenic mitochondrial disorders. N. Engl. J. Med. 2012;366:1132–1141. doi: 10.1056/NEJMra1012478. [DOI] [PubMed] [Google Scholar]
- Lanzendorf S.E., Mayer J.F., Toner J., Oehninger S., Saffan D.S., Muasher S. Pregnancy following transfer of ooplasm from cryopreserved-thawed donor oocytes into recipient oocytes. Fertil. Steril. 1999;71:575–577. doi: 10.1016/s0015-0282(98)00504-4. [DOI] [PubMed] [Google Scholar]
- Ma H., O'neil R.C., Marti Gutierrez N., Hariharan M., Zhang Z.Z., He Y., Cinnioglu C., Kayali R., Kang E., Lee Y., Hayama T., Koski A., Nery J., Castanon R., Tippner-Hedges R., Ahmed R., Van Dyken C., Li Y., Olson S., Battaglia D., Lee D.M., Wu D.H., Amato P., Wolf D.P., Ecker J.R., Mitalipov S. Functional Human Oocytes Generated by Transfer of Polar Body Genomes. Cell Stem Cell. 2017;20:112–119. doi: 10.1016/j.stem.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow E.H., Reinhardt K., Wolff J.N., Dowling D.K. Risks inherent to mitochondrial replacement. EMBO Rep. 2015;16:541–544. doi: 10.15252/embr.201439110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin, E. 2017. The Fertility Doctor Trying to Commercialize Three-Parent Babies. MIT Technology Review (June 13, 2017)https://www.technologyreview.com/s/608033/the-fertility-doctor-trying-to-commercialize-three-parent-babies/ [Online].
- Oktay K., Baltaci V., Sonmezer M., Turan V., Unsal E., Baltaci A., Aktuna S., Moy F. Oogonial Precursor Cell-Derived Autologous Mitochondria Injection to Improve Outcomes in Women With Multiple IVF Failures Due to Low Oocyte Quality: A Clinical Translation. Reprod. Sci. 2015;22:1612–1617. doi: 10.1177/1933719115612137. [DOI] [PubMed] [Google Scholar]
- Palacios-GonzáLez C. Mitochondrial replacement techniques: egg donation, genealogy and eugenics. Monash Bioeth. Rev. 2016;34:37–51. doi: 10.1007/s40592-016-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-GonzáLez C. Are there moral differences between maternal spindle transfer and pronuclear transfer? Med. Health Care Philos. 2017;20:503–511. doi: 10.1007/s11019-017-9772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-González C., de Jesús Medina-Arellano M. Mitochondrial replacement techniques and Mexico's rule of law: on the legality of the first maternal spindle transfer case. J. Law Biosci. 2017;2017 doi: 10.1093/jlb/lsw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-González C., Medina-Arellano M.D.J. Author's Response to Peer Commentaries: Mexico's rule of law and MRTs. J. Law Biosci. 2017;4:623–629. doi: 10.1093/jlb/lsx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt K., Dowling D.K., Morrow E.H. Medicine. Mitochondrial replacement, evolution, and the clinic. Science. 2013;341:1345–1346. doi: 10.1126/science.1237146. [DOI] [PubMed] [Google Scholar]
- Rodriguez S., Campo-Engelstein L., Tingen C., Woodruff T. An obscure rider obstructing science: the conflation of parthenotes with embryos in the Dickey-Wicker amendment. Am. J. Bioeth. 2011;11:20–28. doi: 10.1080/15265161.2010.546472. [DOI] [PubMed] [Google Scholar]
- Rulli T. What Is the Value of Three-Parent IVF? Hastings Cent. Rep. 2016;46:38–47. doi: 10.1002/hast.594. [DOI] [PubMed] [Google Scholar]
- Rulli T. The Mitochondrial Replacement ‘Therapy’ Myth. Bioethics. 2017;31:368–374. doi: 10.1111/bioe.12332. [DOI] [PubMed] [Google Scholar]
- Schandera J., Mackey T.K. Mitochondrial Replacement Techniques: Divergence in Global Policy. Trends Genet. 2016;32:385–390. doi: 10.1016/j.tig.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Schatten H., Sun Q.Y., Prather R. The impact of mitochondrial function/dysfunction on IVF and new treatment possibilities for infertility. Reprod. Biol. Endocrinol. 2014;12:111. doi: 10.1186/1477-7827-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Lahl J., Kramer W. Long-term breast cancer risk following ovarian stimulation in young egg donors: a call for follow-up, research and informed consent. Reprod. BioMed. Online. 2017;34:480–485. doi: 10.1016/j.rbmo.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Sengupta S.B., Dhanjal S., Harper J.C. Quality control standards in PGD and PGS. Reprod. BioMed. Online. 2016;32:263–270. doi: 10.1016/j.rbmo.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Gong J., Veeck L.L., Rosenwaks Z., Palermo G.D. Preliminary findings in germinal vesicle transplantation of immature human oocytes. Hum. Reprod. 2001;16:730–736. doi: 10.1093/humrep/16.4.730. [DOI] [PubMed] [Google Scholar]
- Tzeng C., Hsieh R., Chang S., Tsai N., Cheng Y., Wei Y. Pregnancy derived from mitochondria transfer (MIT) into oocyte from patient’s own cumulus granulosa cells (cGCs) Fertil. Steril. 2001;76:S67–S68. [Google Scholar]
- UK_Department_of_Health The Human Fertilisation and Embryology (Mitochondrial Donation) Regulations 2015. 2015. http://www.legislation.gov.uk/ukdsi/2015/9780111125816/contents
- van Oven M., Kayser M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum. Mutat. 2009;30:E386–94. doi: 10.1002/humu.20921. [DOI] [PubMed] [Google Scholar]
- Wrigley A., Wilkinson S., Appleby J.B. Mitochondrial Replacement: Ethics and Identity. Bioethics. 2015;29:631–638. doi: 10.1111/bioe.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Emmanuele V., Sanchez-Quintero M.J., Sun B., Lallos G., Paull D., Zimmer M., Pagett S., Prosser R.W., Sauer M.V., Hirano M., Egli D. Genetic Drift Can Compromise Mitochondrial Replacement by Nuclear Transfer in Human Oocytes. Cell Stem Cell. 2016;18:749–754. doi: 10.1016/j.stem.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Liu H., Luo S., Chavez-Badiola A., Liu Z., Yang M., Munne S., Konstantinidis M., Wells D., Huang T. First live birth using human oocytes reconstituted by spindle nuclear transfer for mitochondrial DNA mutation causing leigh syndrome. Fertil. Steril. 2016;106:e375–e376. [Google Scholar]
- Zhang J., Zhuang G., Zeng Y., Grifo J., Acosta C., Shu Y., Liu H. Pregnancy derived from human zygote pronuclear transfer in a patient who had arrested embryos after IVF. Reprod. BioMed. Online. 2016;33:529–533. doi: 10.1016/j.rbmo.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu H., Luo S., Lu Z., Chavez-Badiola A., Liu Z., Yang M., Merhi Z., Silber S.J., Munne S., Konstandinidis M., Wells D., Huang T. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reprod. BioMed. Online. 2017;34:361–368. doi: 10.1016/j.rbmo.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Zhang S.P., Lu C.F., Gong F., Xie P.Y., Hu L., Zhang S.J., Lu G.X., Lin G. Polar body transfer restores the developmental potential of oocytes to blastocyst stage in a case of repeated embryo fragmentation. J. Assist. Reprod. Genet. 2017;34:563–571. doi: 10.1007/s10815-017-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey results regarding the implementation and advertisement of reproductive medicine involving mitochondrial manipulation.
List of the legal documents regarding egg donation and germline genetic modification for reproduction in 16 selected countries.