Abstract
The nucleolus is a ubiquitous, mostly spheroidal nuclear structure of all protein-synthesizing cells, with a well-defined functional compartmentalization. Although a number of nonribosomal proteins involved in ribosome formation have been identified, the elements responsible for the shape and internal architecture of nucleoli are still largely unknown. Here, we report the molecular characterization of a novel protein, NO145, which is a major and specific component of a nucleolar cortical skeleton resistant to high salt buffers. The amino acid sequence of this polypeptide with a SDS-PAGE mobility corresponding to Mr 145,000 has been deduced from a cDNA clone isolated from a Xenopus laevis ovary expression library and defines a polypeptide of 977 amino acids with a calculated mass of 111 kDa, with partial sequence homology to a synaptonemal complex protein, SCP2. Antibodies specific for this protein have allowed its recognition in immunoblots of karyoskeleton-containing fractions of oocytes from different Xenopus species and have revealed its presence in all stages of oogenesis, followed by a specific and rapid degradation during egg formation. Immunolocalization studies at the light and electron microscopic level have shown that protein NO145 is exclusively located in a cage-like cortical structure around the entire nucleolus, consisting of a meshwork of patches and filaments that dissociates upon reduction of divalent cations. We propose that protein NO145 contributes to the assembly of a karyoskeletal structure specific for the nucleolar cortex of the extrachromosomal nucleoli of Xenopus oocytes, and we discuss the possibility that a similar structure is present in other cells and species.
INTRODUCTION
Ever since the discovery (1835–1838) by R. Wagner, G. Valentin, and M. Schleiden of the nucleolus as a large and constitutive nuclear organelle common to all biosynthetically active animal and plant cells (reviewed by Franke, 1988; Gerbi, 1997; Pederson, 1998), this distinct, mostly spheroidal structure has attracted the special interest of cell biologists. It has also been noted early on that the number of nucleoli per nucleus can vary greatly, from one or a few located in chromosomal loci, termed nucleolar organizers, to more than a thousand amplified extrachromosomal nucleoli in certain amphibian oocytes (Hadjiolov, 1985). Although the nucleoli have been known for some decades as the sites of rRNA genes and their expression, followed by the assembly of ribosomal precursor structures (Hadjiolov, 1985; Reeder, 1990; Scheer and Weisenberger, 1994), more recent evidence has indicated additional functions such as the assembly, modification, storage, and transport of a series of non-rRNA ribonucleoprotein particles, the buildup of locally enriched enzyme pools, and as a compartment for intranuclear sequestration and regulated inactivation of proteins (Pederson, 1998; Carmo-Fonseca et al., 2000; Olson et al., 2000; Pederson and Politz, 2000; Visintin and Amon, 2000). Therefore, it is not surprising that the nucleolus contains, in addition to proteins involved in ribosome formation, numerous other proteins engaged in any of the other functions mentioned (Shaw and Jordan, 1995; Busch, 1997).
Morphologically, the nucleolus displays three major structural components and this is true for both nucleoli on chromosomal nucleolar organizer and extrachromosomal amplified rDNA copies: 1) the fibrillar center (FC), surrounded by 2) the dense fibrillar component (DFC) and 3) the granular component (GC). Localization studies with the use of antibodies and hybridization probes have also indicated that the biosynthesis and assembly of ribosomal particles is a vectorial process, in which nascent preribosomes move from the DFC region to the more peripherally located GC (Scheer and Hock, 1999; Thiry et al., 2000).
Although the initial formation of a nucleolus appears to require the transcription of rDNA by RNA polymerase I, it is still controversial whether continued transcriptional activity is needed to maintain the near-spheroidal shape and the dense and complex three-component organization (Oakes et al., 1993; Dousset et al., 2000; Verheggen et al., 2000; for the “pseudonucleoli” in embryos of the 0-nu mutant of the clawed toad, Xenopus laevis, lacking functional rRNA genes, see Hay and Gurdon, 1967; Steele et al., 1984). Obviously, the specific architecture is dependent on some intrinsic nucleolar activities or factor(s) because several inhibitors of transcription result in dramatic rearrangements, the best studied of which is the actinomycin D-induced condensation and hemisphere segregation of FC, DFC, and GC (Hadjiolov, 1985). Moreover, the dense-packed arrangement of the nucleolar components into a spheroidal structure is by no means a trivial consequence of rDNA transcription as is perhaps best illustrated by the effect of the RNA polymerase II inhibitor 5,6,-dichloro-β-d-ribofuranosylbenzimidazole, resulting in a spectacular unraveling of the transcribed rDNA chromatin and the distribution of the nucleolar components over the nucleoplasm so that the nucleolus as a distinct body is no longer seen (“necklace formation”; Granick, 1975a,b; Scheer et al., 1984; Desnoyers et al., 1996; Le Panse et al., 1999).
The extrachromosomal nucleoli formed by amplified rDNA copies in oocytes of various species provide an especially “pure” form of nucleolar material. In particular, the nucleoli present in amphibian oocyte nuclei (“germinal vesicles”, GVs) present an excellent model system for studies of the biochemical composition and structural organization of the nucleoli and the regulation of nucleolar activities, due to their enormous size, high rDNA copy content, and high transcriptional activity, the massive accumulation of primary and secondary gene products, and show a structural organization remarkably similar to that of somatic nucleoli (Gall, 1968; Buongiorno-Nardelli et al., 1972; Mais and Scheer, 2001).
Several years ago, in studies of amplified nucleoli of advanced stages of oogenesis in X. laevis, a further structural component has been described as a layer of tangles of filaments and knot-like aggregates confined to the very nucleolar cortex (Franke et al., 1981; Krohne et al., 1982; Benavente et al., 1984). In the present study we report on the identification and molecular characterization of the major protein of this cortical nucleolar structure.
MATERIALS AND METHODS
Biological Material
Clawed toads (X. laevis) were purchased from the South African Snake Farm (Krysna, Republic of South Africa). Toads (X. borealis, X. tropicalis, Bombina orientalis), newts (Triturus cristatus), and salamanders (Pleurodeles waltl) were reared in our laboratory.
Procedures for snap-freezing of tissue samples as well as culture conditions for X. laevis kidney epithelium (XLKE, line A6) and mammalian cells have been described (Krohne and Franke, 1980; Zirwes et al., 2000).
Large-Scale Isolation and Fractionation of X. laevis Oocyte Nuclei
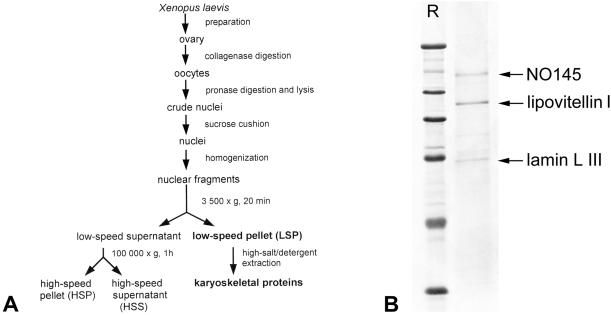
Large numbers of mature X. laevis oocyte nuclei (stages IV–VI; Dumont, 1972) were obtained by mass isolation, a large-scale procedure described by Scalenghe et al. (1978) and modified by Kleinschmidt and Franke (1982). Subsequent fractionation of nuclear contents by differential centrifugation was performed as described in detail by Hügle et al. (1985), resulting in fractions (Figure 1A) termed low-speed pellet (LSP), high-speed pellet (HSP), and high-speed supernatant (HSS). For enrichment of karyoskeletal proteins isolated LSPs were extracted as described (Krohne et al., 1982).
Figure 1.
Isolation of karyoskeletal proteins from X. laevis oocyte nuclei. (A) Schematic representation of the experimental procedure for the isolation and fractionation of mass-isolated X. laevis oocyte nuclei (see MATERIALS AND METHODS). The essential protein fractions are shown in bold letters. (B) Coomassie Blue staining of SDS-PAGE-separated karyoskeletal proteins present in the LSP fraction after high salt/detergent extraction. The three major polypeptides identified by MALDI mass spectrometry are marked by arrows. R, reference proteins: 205, 116, 97.4, 66, 45, and 29 kDa (from top to bottom).
Small-Scale Oocyte Isolation, Microinjections, Spreads of GV Contents, and Preparation of Cell Lysates
Small ovary pieces were removed from anesthetized animals, defolliculated by collagenase treatment, and the individual oocytes were kept at 18°C for several days. Stage I–VI oocytes were grouped based on size, rinsed with OR2 buffer (Wallace et al., 1973), and stored at −80°C until use.
Microinjection of in vitro translation products as well as the manual isolation of nuclei, ooplasms, nuclear contents, and nuclear envelopes from oocytes were as described (Krohne et al., 1989; Cordes et al., 1991).
Stage VI oocytes were induced to mature in vitro by addition of 40 μM progesterone (Sigma, Munich, Germany). For some experiments oocytes were incubated in the presence of actinomycin D (Serva, Heidelberg, Germany) at a final concentration of 10 μg/ml for 4 h.
Spreadings of GV contents were performed according the experimental procedure described in detail before (Gall et al., 1991, 1999; Gall, 1998). Total cellular lysates of X. laevis cultured cells (XLKE, line A6) were prepared as described (Schmidt-Zachmann et al., 1998).
Mass Spectrometry and Amino Acid Sequence Analysis
Protein bands of interest were excised from the gel and digested with sequencing grade modified trypsin in 40 mM NH4CO2 overnight at 37°C. The reaction was stopped by freezing. Matrix-assisted laser desorption ionization (MALDI), spectrometric analysis, database searches, and amino acid sequence analysis were performed as described (Kuhn et al., 2001).
Isolation of cDNA Clones and Polymerase Chain Reaction (PCR) Products
Total DNA from a λ Unizap cDNA expression library from X. laevis ovary (Stratagene, Heidelberg, Germany) was used for PCR with the library-specific T7 primer as antisense primer and a degenerated sense primer deduced from the amino acid sequence DFWEDQY. Subsequently, an amplified 329-nucleotide (nt) cDNA fragment was used as random-primed, 32P-labeled fragment for screening the same cDNA library. One of 10 isolated positive clones, termed pBT-NO145-211, contained a full mRNA-length cDNA.
Because clone pBT-NO145-211 did not contain an upstream stop-codon in frame with the putative start-codon (nt 116–118), the 5′ end was also verified by the rapid amplification of cDNA ends (RACE) procedure (Frohman et al., 1988), starting from X. laevis ovary poly(A)+ RNA and with the use of the SMART RACE cDNA Amplification kit (CLONTECH, Heidelberg, Germany). The resulting PCR products of ∼150 base pairs were subcloned into the pCRII-TOPO-Vector (Invitrogen, Groningen, The Netherlands).
RNA Isolation, Northern Blot Hybridization, and Coupled In Vitro Transcription-Translation
Total RNA from ovaries of X. laevis and the other amphibian species mentioned in MATERIALS AND METHODS or from A6 cell cultures was prepared as described by Chomczynski and Sacchi (1987). Poly(A)+ RNA was obtained with the use of an mRNA purification kit (Pharmacia, Freiburg, Germany). Total RNA from other tissues was prepared with the use of the TriPure Isolation Reagent (Roche Molecular Biochemicals, Mannheim, Germany), and total RNA from staged X. laevis oocytes and eggs was extracted (Krieg and Melton, 1984). RNAs were separated on 1% agarose gels containing 0.6% formaldehyde, and transferred to Biodyne A filters (Pall, Dreieich, Germany), hybridized with a random-primed, 32P-labeled DNA fragment derived from clone pBT-NO145-211 after EcoRI digestion. Hybridization was carried out at 65°C in 0.5 M Na2HPO4 buffer, pH 7.2, containing 7% SDS and 10 mM EDTA. Blots were washed twice for 15 min in 0.1% SDS, 1× SSC and twice for 30 min in 0.1% SDS, 0.3× SSC, and then processed by autoradiography.
For in vitro synthesis of [35S]methionine-labeled protein, we used the transcription/translation-coupled reticulocyte lysate system (Promega, Heidelberg, Germany) programmed by the construct pBT-NO145-211. In vitro translation reactions used for microinjection into Xenopus oocytes were dialyzed against injection buffer [10 mM piperazine-N,N′-bis(2-ethanesulfonic acid), pH 7.4; 40 mM KCl; 10 mM NaCl] and stored at −20°C until use.
Xenopus Protein NO145-specific Antibodies
Guinea pig antibodies for protein NO145 were obtained by immunization with synthetic peptides (Schnölzer et al., 1992) representing various parts of the amino acid (aa) sequence deduced from the cDNA sequence pBT-NO145-211 (Figure 2A). In the experiments reported here, antibodies NO145-E, NO145-H, and NO145-M directed against the peptide sequences QTSEHSSTTKTSSANRSV (aa 450–467), FTSRKEMHRPEDINPKSPH (aa 672–690), and VDGNNIYHAADTLQ (aa 764–777), respectively, were routinely used after affinity purification on iodoacetyl-immobilized peptide (Mertens et al., 1996). All antibodies reacted equally well with protein NO145.
Figure 2.
Amino acid sequence of Xenopus protein NO145 and comparison with the rat SCP2. (A) Amino acid sequence of protein NO145 of X. laevis deduced from the cDNA clone pBT-NO145-211 (EMBL accession no. AJ249963). The peptide sequences determined by protein sequencing are shaded gray. The putative nuclear localization signal is indicated by a box and sequences used for generating antibodies are underlined. (B) Partial sequence comparison between X. laevis protein NO145 (aa 90–319) with the rat protein SCP2 (aa 95–323; cf. Offenberg et al., 1998). The symbols between the two primary sequences are defined as follows: vertical bar, identical amino acids; 1–5, conservative exchanges (1, aliphatic, nonpolar; 2, aliphatic, polar; 3, aromatic; 4, basic; 5, acidic).
Monoclonal antibodies (mAbs) were raised essentially according to the method of Köhler and Milstein (1975) following standard protocols, with the use of recombinant His6-tagged NO145 for immunization. The two mAbs obtained in this preparation were named NO145-12 and NO145-26, respectively.
Other Antibodies
mAbs No-185 and No-63, respectively, against the major nucleolar protein NO38/B23 as well as mAb No-114 reacting with the nucleolar protein xNopp180 have been described (Schmidt-Zachmann et al., 1984, 1987). Xenopus coilin was detected with the use of mAb H1 (Tuma et al., 1993), and the oocyte-specific lamin LIII (Krohne and Benavente, 1986) was recognized by mAb Nuc-195 (our unpublished data). The mAb 9E10 (ATCC CRL 1729) specifically recognizes an epitope in the decapeptide EQKLISEEDL of the human c-myc protein (Evan et al., 1985).
Secondary antibodies used for immunofluorescence microscopy were Texas Red-, Cy3-, and Alexa 488-conjugated goat antibodies to murine or guinea pig. For immunoblotting, horseradish peroxidase-conjugated antibodies were used (Dianova, Hamburg, Germany).
Expression and Purification of His-tagged Protein NO145
To express an amino-terminal His6-tagged version of protein NO145, the blunt-ended XbaI-XhoI fragment derived from clone pBT-NO145-211 was subcloned into the vector pQE-31 (QIAGEN, Hilden, Germany), previously cut with SmaI. The recombinant protein was purified under denaturing conditions following the manufacturer's protocol.
Gel Electrophoresis and Immunoblotting
Protein fractions were analyzed by SDS-PAGE (cf. Kleinschmidt and Franke, 1982). The polypeptides were transferred to nitrocellulose membranes and visualized by Ponceau S staining. The nitrocellulose membranes were blocked in Tris-buffered saline containing 0.05% Tween 20 (TBST) and 5% nonfat dry milk for 1 h at room temperature and then incubated at room temperature with affinity-purified or monoclonal antibodies for 1 h in TBST and 5% nonfat dry milk. Bound antibodies were detected by chemiluminescence with the use of the ECL system (NEN, Dreieich, Germany) after incubation with horseradish peroxidase-coupled secondary antibodies (Dianova) diluted 1:10 000 in TBST and 5% nonfat dry milk for 1 h.
Immunofluorescence Microscopy
For immunofluorescence microscopy, cells grown on coverslips were fixed (Zirwes et al., 2000), washed twice in phosphate-buffered saline (PBS), and incubated with purified guinea pig antibodies (1:200 diluted in PBS) or mAbs (culture supernatant, undiluted) for 30 min at room temperature. After several washes in PBS, cells were incubated for 30 min with the appropriate secondary antibodies (1:100–1:500 in PBS), washed in PBS, dehydrated in ethanol, air-dried, and mounted in Fluoromount (Biozol, Eching, Germany).
Cryosections (∼5 μm) of frozen tissues were either fixed with acetone (10 min, −20°C) or in PBS containing 2% formaldehyde (15 min, room temperature). Formaldehyde-fixed samples were washed once in PBS containing 50 mM NH4Cl for 5 min and then twice for 5 min in PBS before incubation with the antibodies.
GV spreads were fixed after centrifugation in 2% formaldehyde/1 mM MgCl2 in PBS for 1 h. After fixation, preparations were rinsed in PBS, blocked with 10% horse serum, in PBS, and incubated with the appropriate antibodies as outlined above. The preparations were examined with the use of a Zeiss Axiophot, a Zeiss confocal laser scanning microscope (LSM 510; Zeiss, Oberkochen, Germany), or a Leica TCS NT (Leica, Nussloch, Germany).
Immunoelectron Microscopy
Cyrosections of 5 μm of Xenopus ovaries were fixed for 10 min in PBS containing 2% formaldehyde, 2% sucrose, 1 mM MgCl2, washed in NH4Cl and PBS, and finally blocked in 5% goat serum in PBS. Incubation with the primary antibody (affinity-purified serum NO145-H, diluted 1:100) was performed in a wet chamber for 1 h at room temperature. After three washes with PBS for 5 min each, bound antibodies were reacted for 1 h at room temperature with anti-guinea pig IgG-conjugated nanogold (Nanoprobes, Stony Brook, NY) diluted 1:50 in PBS. After several washes with PBS for 5 min each, the tissue was fixed with 2.5% glutaraldehyde in 0.05 cacodylate buffer for 15 min at 4°C. Silver enhancement was according to Uchida et al. (1996). Subsequently, the tissue was postfixed with 2% osmium tetroxide solution and processed for flat embedding in Epon (Franke et al., 1978).
RESULTS
Isolation and Analysis of a cDNA Clone Encoding a Novel Type of Structural Nucleolar Protein
When X. laevis oocyte nuclei were isolated (Scalenghe et al., 1978), homogenized, and further fractionated by differential centrifugation (Figure 1A), some well-characterized protein fractions were obtained (cf. Hügle et al., 1985). LSP fractions, known to be highly enriched in nucleoli, nuclear envelopes, and chromosomes, were subsequently extracted with a high salt/detergent-containing buffer to enrich for karyoskeletal structures. The proteins of the resulting residual pellet were subjected to SDS-PAGE and revealed only three major polypeptides of mobilities corresponding to 145, 100, and 68 kDa (Figure 1B). To identify these proteins, the bands were excised and subjected to “in-gel” tryptic digestion. The eluted fragments were then analyzed by MALDI mass spectrometry, and the peptide mass fingerprints were compared with predicted peptides of the NCBInr database by the ProFound search algorithm. Although the digestion profile of the 100-kDa polypeptide matched the peptide fingerprints of lipovitellin I (Wiley and Wallace, 1981), a yolk component and known contaminant of isolated oocyte nuclei, and the 68-kDa protein was identified as the major oocyte-specific lamin LIII (Krohne and Benavente, 1986; Stick, 1988), the mass information obtained for the 145-kDa protein could not be assigned to any known protein in the database. This prompted us to prepare enough material allowing the microsequencing of the polypeptide. Thirteen amino acid sequences were obtained (Figure 2A) and again database searches were negative for all of them.
With the use of this partial amino acid sequence information we set out to start cDNA cloning the 145-kDa polypeptide. Degenerated sense primers deduced from the peptide sequence DFWEDQY and the library-specific T7 primer as antisense primer were used to amplify a 329-nt cDNA fragment from a cDNA library from X. laevis ovary in PCR reactions. This cDNA fragment was then used as a probe for screening the same cDNA library. Ten positive recombinants, all containing cDNA inserts of ∼1.5–3 kb, were obtained, and sequencing revealed that they overlapped extensively. One clone, denoted pBT-NO145-211, was further analyzed in detail (see EMBL database, accession no. AJ249963).
Clone pBT-NO145-211 (3206 base pairs) contains an initiation codon at position 116, an open reading frame of 2931 base pairs, and a 3′-untranslated region of 159 base pairs with a poly(A) tail of 28 base pairs. The open reading frame encodes a polypeptide of 977 aa, with a calculated molecular mass of 111 kDa and an isoelectric point of 5.9, containing all 13 oligopeptides initially determined by amino acid sequencing (Figure 2A). Although the open reading frame continues to the 5′ end of the cDNA, i.e., does not contain an in-frame stop-codon, the presumptive start-codon at nt positions 116–118 is likely to be the authentic initiation codon because the surrounding sequence (ACAATGAGT) perfectly matches the optimal sequence for eukaryotic initiation of translation (Kozak, 1989). Moreover, the authenticity of the 5′ end of the isolated cDNA clone was further verified by several independent 5′-RACE experiments, with the use of poly(A)+ mRNA isolated from Xenopus ovary as template. The sequence information obtained from the resulting PCR fragments did not extend beyond the 5′ end of the isolated cDNA clone (our unpublished data).
The most conspicuous feature of the encoded protein is the extraordinarily high content of potential phosphorylation sites, including 26 for protein kinase C, 15 for casein kinase II, and 2 for tyrosine kinase. Presently, we do not know the actual degree of phosphorylation. Thus, we cannot exclude that post-translational modifications might account for the observed difference in molecular mass estimated for the polypeptide from the cDNA-derived sequence (111 kDa) or from its SDS-PAGE mobility (145 kDa; see below). This protein has been designated protein NO145.
We have also noted a putative nuclear localization signal (NLS) between aa positions 782–799 [KRK(x)11KPRK, denoted by a box in Figure 2A; Dingwall and Laskey, 1991]. Other notable features, e.g., sequence elements involved in nucleic acid binding or protein–protein interactions, have not been detected.
In X. laevis database searches we have noticed two expressed sequence tags of 656 and 555 nt in length, which correspond to the 5′ and 3′ end, respectively, of clone pBT-NO145-211 (accession no. BE680607 and BE678123). Although NO145 is a novel protein, it displays a striking homology to SCP2, a rat synaptonemal complex (SC) protein of 173 kDa (EMBL accession no. Y08981; Offenberg et al., 1998), with an overall amino acid sequence identity of 26% and a similarity, including conservative exchanges, of 38%. Notably, the N-terminal region of both proteins contains a domain of 229 aa with a remarkably high sequence homology (43% identity and 59% similarity; Figure 2B).
Molecular Characterization of the cDNA Encoding the Xenopus Protein NO145
The completeness of the isolated cDNA clone was demonstrated by three different types of experiments illustrated in Figure 3.
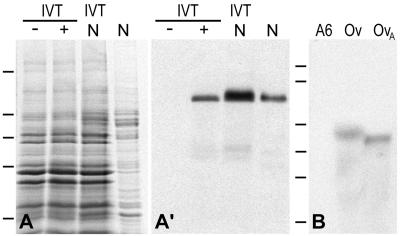
Figure 3.
Molecular characterization of the cDNA clone encoding Xenopus protein NO145. (A) Coomassie Blue staining of SDS-PAGE–separated proteins present in a rabbit reticulocyte lysate after in vitro transcription/translation (IVT) in the absence (−) or presence (+) of the pBT-NO145-211 template, total proteins prepared from manually isolated oocyte nuclei (N), and coelectrophoresis of the two protein fractions (IVT/N). Bars on the left indicate the position of reference proteins (same as in Figure 1). (A′) Corresponding immunoblot probed with mAb NO145-12, which specifically reacts with the 145-kDa protein synthesized in vitro, which shows the same SDS-PAGE mobility as native protein NO145 present in oocyte nuclei. (B) Identification of the NO145 mRNA by Northern blot analysis. Total RNA from Xenopus A6 cells (A6), Xenopus ovary (Ov), and poly(A)+-RNA from Xenopus ovary (OvA) separated by agarose gel electrophoresis was hybridized with a random prime-labeled NO145-specific probe. Note the reaction of a single band corresponding to a ∼3.2-kb RNA present in the fractions from the ovary tissue. RNA-size markers of 9.5, 7.4, 4.4, 2.4, 1.4, and 0.24 kb are indicated on the left (from top to bottom).
Experiment 1
In vitro transcription and translation of pBT-NO145-211 in a reticulocyte lysate yielded a polypeptide with a SDS-PAGE mobility corresponding to ∼145 kDa, clearly different from the predicted Mr of 111,000. Similar deviations of estimates based on SDS-PAGE from predicted Mr values have also been reported for several other proteins, in particular some carrying a very high negative charge, be it due to a very low isoelectric point (e.g., N1/N2, nucleoplasmin, NO38, NO29) or to a high degree of phosphorylation (Nopp140; Kleinschmidt et al., 1986; Dingwall et al., 1987; Schmidt-Zachmann et al., 1987; Meier and Blobel, 1992; Zirwes et al., 1997). The protein synthesized in vitro could be detected with NO145-specific antibodies, confirming both the identity of the translation product and the specificity of the antibodies. Moreover, it showed the same SDS-PAGE mobility as the native protein detected in manually isolated oocyte nuclei, as ultimately confirmed by coelectrophoresis of both polypeptides (Figure 3, A and A′).
Experiment 2
Total RNA and poly(A)+ RNA from X. laevis ovary tissue as well as from X. laevis A6 cells were probed in Northern blot experiments with a 0.95-kb random prime-labeled cDNA fragment derived from clone pBT-NO145-211. A strong signal corresponding to a mRNA of ∼3.2 kb was detected in the ovary, indicating that the pBT-NO145-211 clone was of full or nearly full length. Due to the large amount of rRNAs present in the total RNA sample, the mRNA coding for NO145 shows a slightly decreased electrophoretic mobility. No signal was obtained on mRNAs isolated from X. laevis kidney epithelial cells (XLKE, line A6), suggesting that expression of the gene encoding NO145 is cell type specific (Figure 3B). However, an mRNA of similar size could be demonstrated in ovaries from other Xenopus species (X. borealis and X. tropicalis), whereas we did not detect NO145-specific mRNA in ovaries from P. waltl and T. cristatus (our unpublished data).
Experiment 3
When protein NO145 synthesized in vitro was microinjected into Xenopus ooplasms, it was subsequently recovered exclusively in the manually isolated nuclei by autoradiography as well as by immunoblotting with the use of NO145 antibodies. The antibodies reacted with one single polypeptide band, indicating that the injected and the endogenous protein have the same size (our unpublished data). This result suggests that NO145 is a nuclear protein that is accumulated in oocyte nuclei, presumably by an active process.
Biochemical Characterization of Protein NO145 and Its Synthesis
To study the intracellular distribution and location of endogenous protein NO145, a panel of polyclonal antibodies against peptides deduced from the cDNA sequence of pBT-NO145-211 (Figure 2A) as well as several mAbs against the recombinant protein expressed in Escherichia coli were generated. The presence of NO145 in different nuclear fractions of X. laevis oocyte nuclei (total nuclei, LSP, HSP, and HSS) and in X. laevis cells of line A6 cells was analyzed by immunoblotting. Antibody NO145-H recognized its antigen in total oocyte nuclei as well as in the LSP and HSP fractions, indicating that NO145 is a nuclear protein associated with relatively large structures. The protein was detectable neither in the HSS fraction containing soluble nuclear proteins nor in cultured cells (Figure 4, A and A′). Moreover, we did not detect NO145 in immunoblots of total proteins from cultured cells of different species (human, bovine, rat, mouse, rat kangaroo) or in different tissues of X. laevis (heart, muscle, testis, kidney). This indicated that protein NO145 was either exclusively synthesized in Xenopus oocytes or that the antibodies used were very restricted in their cross-reactivities. Antibodies against other peptides of NO145 as well as the different mAbs gave essentially the same results.
Figure 4.
Identification of protein NO145 in different nuclear fractions from Xenopus oocytes. (A) Coomassie Blue staining of various nuclear fractions of Xenopus oocytes and somatic cells, respectively, separated by SDS-PAGE. Whole mass-isolated oocyte nuclei (N); proteins of the LSP, HSP, and HSS fractions of fractionated oocyte nuclei; and total cellular proteins of Xenopus XLKE-A6 cells (A6) are shown. (A′) Corresponding immunoblot probed with antibody NO145-H. Protein NO145 is present in mass-isolated oocyte nuclei, in the LSP and HSP fraction, but not detectable in the HSS fraction and Xenopus XLKE-A6 cells. (B) Nuclear (N) and cytoplasmic (C) fraction, respectively, of manually dissected Xenopus oocytes was analyzed by immunoblotting. Protein NO145 is exclusively detectable in the nuclear fraction. (C) Immunoblot of SDS-PAGE–separated, hand-isolated nuclear envelopes (NE) and nuclear contents (NC) protein fractions. Protein NO145 is specifically enriched in the protein fraction representing the nuclear content. (C′) To confirm the fractionation procedure, the immunoblot shown in C was reprobed with mAb Nuc-195 directed against Xenopus lamin LIII, the major constituent of the nuclear lamina in oocyte nuclei. Reference proteins are indicated by bars on the left (same as in Figure 1).
The appearance of protein NO145 exclusively in the nuclear fraction of Xenopus oocytes was confirmed in analyses of manually dissected oocytes (Figure 4B). Moreover, on further fractionation of GVs into nuclear contents and nuclear envelopes, protein NO145 was recovered only in the nuclear interior and was not detected in the nuclear envelope fraction (Figure 4, C and C′). The identity and integrity of the fractions was ascertained by reprobing the nitrocellulose filters containing the blotted proteins with mAb Nuc-195 directed against lamin LIII.
All our attempts to solubilize protein NO145 from nuclear fractions such as LSP and HSP failed. On treatments of these fractions with buffers containing high salt (up to 1.5 M), nonionic detergent (1% Triton X-100), and Benzonase to digest nucleic acids, protein NO145 always remained in the residual pellet, classifying this protein as a bona fide karyoskeletal protein.
Because protein NO145 had been detected in the HSP fraction of oocyte nuclei known to be highly enriched in preribosomal particles, HSP components were further separated by centrifugation in 10–40% sucrose gradients, and the resulting fractions were analyzed by immunoblotting. Interestingly, NO145 was enriched in fractions containing the precursors for the large ribosomal subunit, recognized as 65S particle. In contrast, the protein synthesized in vitro sedimented with ∼5.5S, indicative of a monomer (our unpublished data). However, characterization of the native state of protein NO145 and its possible association with other nucleolar molecules requires further experiments.
Protein NO145 and Its mRNA during Oogenesis and Maturation
We had to recognize that NO145 is an oocyte-specific protein. When protein NO145 and its mRNA was studied during oogenesis and oocyte maturation, the protein was found only in trace amounts in stage I and II oocytes, in which it became detectable only upon loading of large amounts (our unpublished data). Its concentration per oocyte markedly increased in stages III, IV, and V and then appeared to decrease slightly in stage VI (Figure 5A). After maturation in vitro, i.e., at the time of GV breakdown, protein NO145 decreased drastically in the egg at the “white spot stage” and was no longer detectable by the antibodies. This rapid decrease in the level of NO145 was also apparent when NO145 was compared with the major nucleolar protein NO38/B23, which remained at a high concentration level throughout oogenesis and on maturation where it appeared in a hyperphosphorylated form (Figure 5A; cf. Schmidt-Zachmann et al., 1987, 1998).
Figure 5.
Synthesis of protein NO145 and its mRNA during Xenopus oogenesis and maturation (egg formation). (A) Immunoblot of SDS-PAGE–separated total proteins from one oocyte stages I–VI and from an unfertilized egg, respectively, per lane, probed with mAb NO145-26 (top) and mAb No-185 directed against the nucleolar protein NO38/B23 (bottom). (B) Total RNA equivalents of two oocytes (each lane) of stages I–VI and from unfertilized eggs, respectively, were separated by agarose gel electrophoresis. Northern blots were hybridized with a random prime-labeled NO145-specific (top) or NO38/B23-specific (bottom) probe.
We also determined the synthesis of protein NO145 at the mRNA level (Figure 5B). NO145 mRNA accumulated during oogenesis and even appeared to increase on oocyte maturation and in eggs. In comparison, the mRNA level of NO38/B23 accumulated during early oogenesis until stage IV then decreased somewhat in stages V and VI but remained high in the egg. These results indicate that protein NO145 is stockpiled during oogenesis, with an increased protein synthetic activity in stages III and VI, and that the rapid disappearance of protein NO145 on oocyte maturation does not correlate with, and hence is not due to, mRNA instability.
Immunolocalization Studies at Light Microscopic Level
Tissue Sections
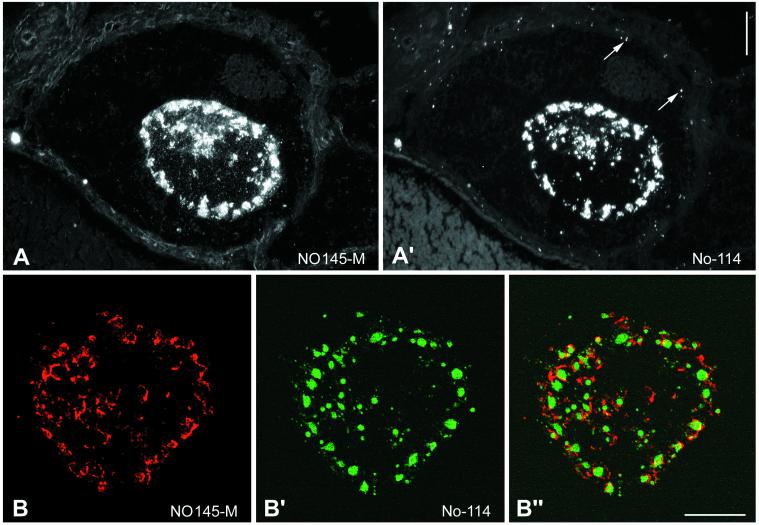
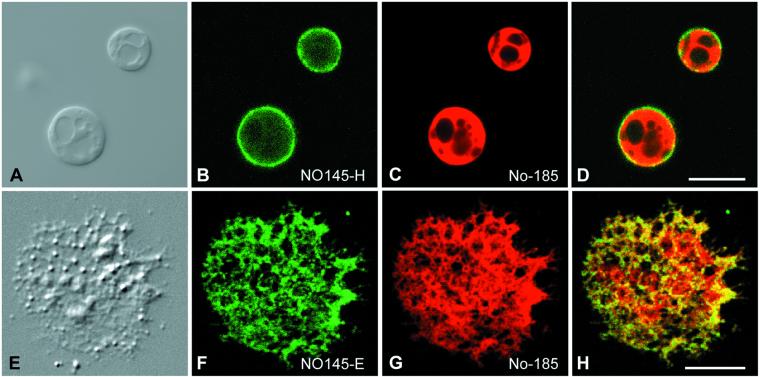
Immunofluorescence microscopy with the use of protein NO145-specific antibodies on cryostat sections through ovaries of X. laevis (Figure 6) and X. borealis (our unpublished data) showed a bright staining of the large nucleoli most of which were located in the GV periphery (Figure 6A). The exclusively nucleolar localization of protein NO145 was confirmed by double staining with mAb No-114 against the 180-kDa nucleolar protein (Schmidt-Zachmann et al., 1984), also termed xNopp180 (Cairns and McStay, 1995). Like protein NO145, xNopp180, a marker for the DFC, was found in the large amplified nucleoli, but in contrast to NO145, it was also present in the much smaller nucleoli of the surrounding follicle epithelial cells (Figure 6A′). Comparison of NO145 with xNopp180 disclosed that both proteins occurred in the nucleolus, but did not colocalize: although protein NO145 was enriched in the nucleolar cortex (Figure 6B), xNopp180 appeared mostly in the nucleolar interior (Figure 6B′). Moreover, closer inspection suggested that NO145 might also occur in some internucleolar filamentous structures (Figure 6B").
Figure 6.
Double-label immunolocalization of protein NO145 on frozen sections through a X. laevis ovary. (A and A′) Double-label immunofluorescence staining with NO145-specific antibodies NO145-M (A) and mAb No-114 antibody (A′) directed against the nucleolar protein xNopp180 (Schmidt-Zachmann et al., 1984). Both antibodies show a bright immunostaining of the amplified nucleoli. In addition, mAb No-114 specifically decorates the smaller nucleoli of the surrounding follicle cells (some are denoted by arrows in A′). (B–B") Laser scanning confocal microscopy. The intracellular distribution of protein NO145 (B) is compared with that of protein xNopp180 (B′). The corresponding merged picture is shown in (B"). Bars, 40 μm (A and A′) and 20 μm (B–B").
We also compared the localization of protein NO145 with those of other nucleolar proteins known to be enriched in the GC of the nucleolus. Neither protein NO38/B23 (Schmidt-Zachmann et al., 1987) nor protein NOH61 (Zirwes et al., 2000) showed significant colocalization (our unpublished data). In summary, these results indicated that NO145 was a nucleolar protein that did not colocalize with markers for the DFC and GC but was strictly cortical, thus defining a novel nucleolar subcompartment.
Spreads of Nuclear Contents
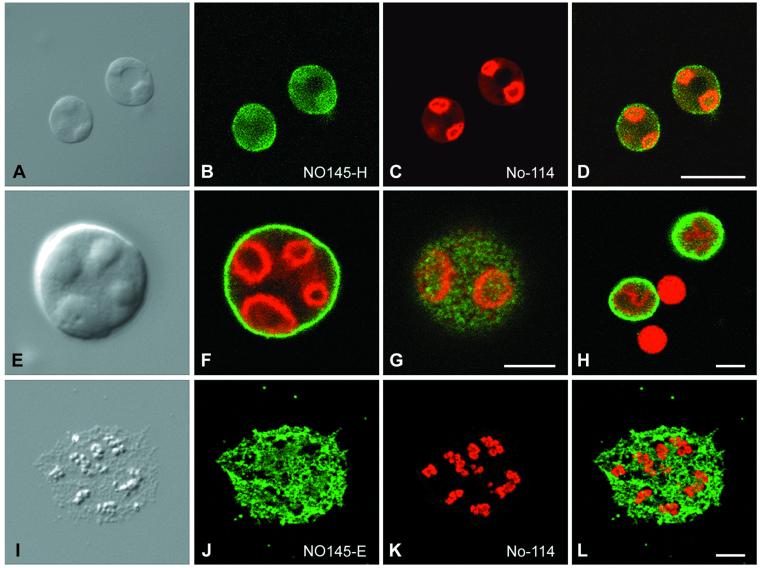
To analyze in more detail the topological relationship between protein NO145 and other nucleolar proteins in the amplified nucleoli of stage VI oocytes, we performed double-label immunolocalization experiments on spread nuclear structures. When GV contents were spread on a microscope slide, the lampbrush chromosomes and other nuclear organelles such as nucleoli, Cajal bodies, and fibrillar elements were well separated from each other, thus allowing good resolution and accessibility of nuclear elements (Gall et al., 1999; Narayanan et al., 1999; Lange and Gerbi, 2000; Morgan et al., 2000). Immunofluorescence colocalization studies performed with this technique and analyzed by confocal laser scanning microscopy are presented in Figure 7, showing the intranucleolar distribution of proteins NO145 and xNopp180. The extrachromosomal nucleoli, varying in size from 1 to 15 μm, were brightly decorated by both antibodies. However, although protein NO145 was localized exclusively to the nucleolar periphery, xNopp180 was restricted to the nucleolar interior, specifically to the nucleolar cores representing mainly DFCs (Figure 7, A–D). Depending on the specific confocal plane, one could get the impression that protein NO145 formed a shell- or cage-like structure around the nucleolus (Figure 7, E and F). When focused on the nucleolar surface, however, protein NO145 appeared in a patchy arrangement, suggestive of a cortical net surrounding the entire nucleolus (Figure 7G). The exclusively nucleolar localization of protein NO145 is also demonstrated in Figure 7H in which xNopp180 is found in the DFC of the nucleoli as well as in the matrix of the Cajal bodies, whereas protein NO145 was exclusive for the cortex of the nucleoli.
Figure 7.
Laser scanning confocal microscopy showing double-label immunolocalizations on spread preparations of GV contents of X. laevis oocytes. (A–D) Differential interference contrast image of single nucleoli (A), corresponding immunofluorescence micrographs with polyclonal antibodies NO145-H (B), and mAb No-114 antibody (C), respectively. The resulting merged picture is shown in (D). (E–G) Differential interference contrast image of a single nucleolus at higher magnification (E) and the respective double-label immunofluorescence localization of proteins NO145 (green) and xNopp180 (red). The surface view (G) of the nucleolus discloses a characteristic meshwork arrangement of NO145 in distinct patches surrounding the entire nucleolus. (H) Protein NO145 (green) is restricted to the nucleolus, i.e., does not accumulate in Cajal bodies, which by contrast are specifically decorated by the antibody reacting with xNopp180 (red). Note, that protein xNopp180 is present in both nuclear structures, i.e., nucleoli and Cajal bodies (cf. Gall, 2000). (I–L) Immunolocalization studies on GV spreads performed in Mg2+-free medium. Differential interference contrast image of a single, partly dispersed nucleolus (I) and corresponding immunofluorescence with antibodies NO145-H (J, green) and mAb No-114 antibody against protein xNopp180 (K, red), respectively. The resulting merged image is shown in (L). Bars, 20 μm (A–D) and 5 μm (E–L).
When the spreading was performed in the absence of Mg2+ ions the cortical structure of the nucleolus was disrupted and dispersed to varying degrees, and protein NO145 localized to loose fibrillar tangles surrounding the core structures (Figure 7, I–L). This core material also expanded, often appearing as chains of beads positive for xNopp180 (Wu and Gall, 1997).
The specific arrangement of protein NO145 in the cortical layer of the nucleoli was also shown in GV spreads in which it was compared with another major nucleolar protein, NO38/B23, a GC marker (Figure 8). This latter staining pattern was clearly different from that observed for the DFC protein xNopp180 (Figure 8, A–D; cf. Figure 7). Again, no significant colocalization of proteins NO38/B23 and NO145 was seen, indicating that protein NO145 defines a truly novel nucleolar substructure, a cortical meshwork representing the outermost part of the GC. GV spreads performed in the absence of Mg2+ again resulted in a far-reaching dispersal of the GC, which then seemed to overlap partly with the filamentous meshwork stained by NO145 antibodies (Figure 8, E–H).
Figure 8.
Double-label immunolocalization studies on nucleoli present in GV spreads analyzed by laser scanning confocal microscopy. The distribution of protein NO145 (B and F; antibody NO145-H) is compared with that of protein NO38/B23, a major nucleolar shuttling protein mostly located in the GC (C and G; antibody No-185). The corresponding merged images are shown in D and H, and the differential interference contrast images in A and E. The GV spreads shown in E–H were prepared in the absence of Mg2+ ions in the spreading buffer. Bars, 15 μm (A) and 5 μm (E).
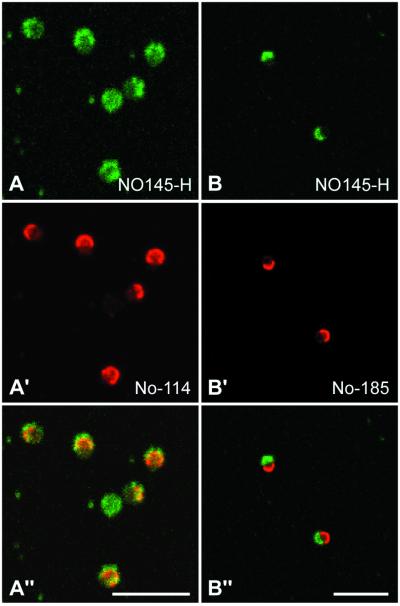
In cell cultures, the inactivation of nucleoli, whether naturally occurring or experimentally induced, such as by actinomycin D treatment, leads to a typical segregation of the fibrillar components (FC and DFC) from the GC material, usually resulting in the formation of distinct nucleolar hemispheres (Simard et al., 1974). Recently, this phenomenon has also been described for the amplified nucleoli of X. laevis oocytes (Mais and Scheer, 2001). Colocalization studies with the use of antibodies against proteins NO145, xNopp180, or NO38/B23 on nucleoli of actinomycin D-treated oocytes has led to an unexpected observation. Under these conditions, proteins NO145 and xNopp180 display an extensive, although not complete colocalization (Figure 9, A–A"), whereas proteins NO145 and NO38/B23 accumulate at opposite poles (Figure 9, B–B"). Obviously, during this inhibitory treatment and the extensive rearrangements, protein NO145 becomes, at least in part, associated with the DFC material.
Figure 9.
Laser scanning confocal microscopy showing double-label immunolocalizations on nucleoli from X. laevis in GV spread preparations after actinomycin D treatment. The distribution of protein NO145 (A and B) is compared with that of protein xNopp180 (A′), which is a component of the DFC and NO38/B23 (B′), which specifically decorates the GC. The corresponding merged pictures are shown in A" and B", respectively. Bars, 10 μm.
Electron Microscopic Immunolocalization of Protein NO145
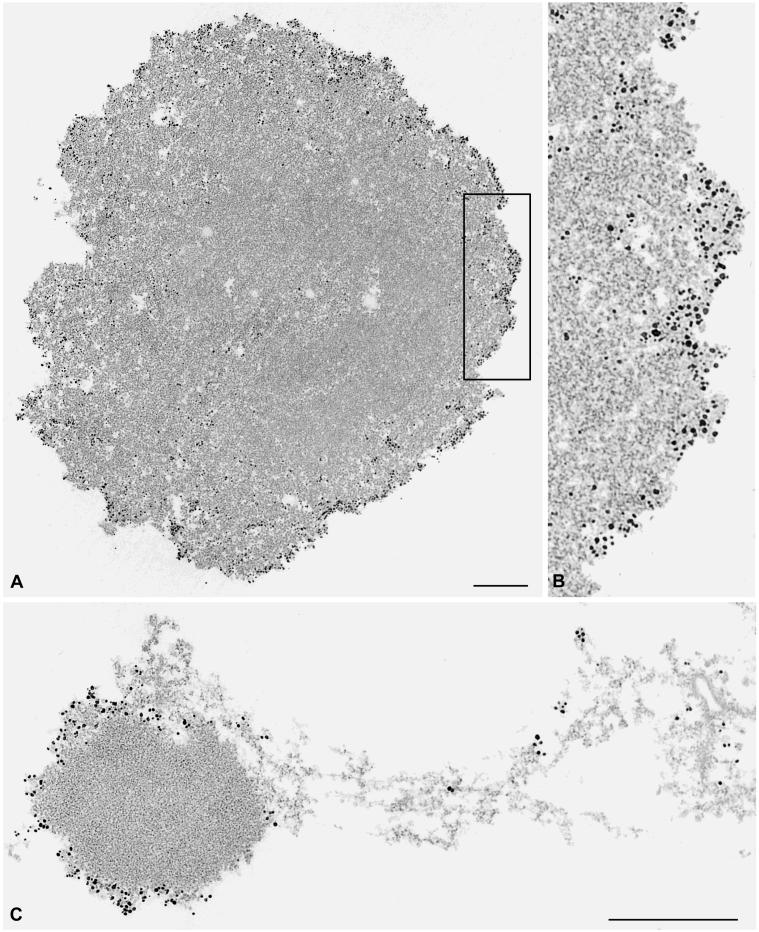
In the electron microscope, we examined the distribution of protein NO145 with the use of secondary antibodies coupled to colloidal gold particles on cryostat sections of frozen Xenopus ovaries. An intense and specific labeling of the outermost cortical layer, up to 0.1 μm in thickness, of the nucleolus was observed, whereas the nucleolar interior was practically devoid of gold particles (Figure 10, A and A′). Essentially the same result was obtained by immunoelectron localization of protein NO145 on GV spreads (our unpublished data). These analyses confirmed our immunolocalizations at the light microscopic level and allowed a better resolution of the reactive structure, demonstrating directly that protein NO145 was a specific marker for a cortical entity, different from the three major nucleolar subcompartments, i.e., FC, DFC, and GC. Occasionally we also noted, in addition, a specific labeling of certain filaments extending from the nucleolus into the nucleoplasm (Figure 10B).
Figure 10.
Immunoelectron microscopic localization of protein NO145 on frozen sections through X. laevis ovary. Antibody NO145-E directed against protein NO145 was detected by secondary antibodies coupled to nanogold particles. The periphery of the nucleolus is specifically labeled (A), whereas the main body of the nucleolus, i.e., all other nucleolar subcompartments, is practically free of gold particles. The box indicated in A demarcates the nucleolar region that is presented at higher magnification in B. Occasionally, strongly labeled nucleolar filaments extending from the nucleolar cortex into the nucleoplasm can be observed (C). Bars, 0.5 μm.
DISCUSSION
Our molecular characterization of nucleolar protein NO145 has identified a novel type of karyoskeletal protein and also a molecular marker for a specific nucleolar substructure. Besides the nuclear envelope-associated lamin LIII, protein NO145 represents a major component of the residual fraction from X. laevis oocyte nuclei obtained after extraction in high salt buffers, nonionic detergents, and nucleases, and it is by far the predominant protein of the high salt- and nuclease-resistant nucleolar material (cf. Franke et al., 1981). Moreover, our immunolocalization studies at the light and electron microscopic level have revealed a very specific nucleolar location of the protein NO145-containing structures: Whereas all other nucleolar proteins known so far have been localized to one of the subnucleolar compartments, i.e., FC, DFC, or GC, protein NO145 is exclusive for a relatively thin cortical structure, forming a cage-like perinucleolar structure. The constitutive cortical cage containing the NO145 protein is a relatively stable, truly karyoskeletal structure, but is depending on a critical concentration of divalent cations, notably Mg2+. Thus, the combination of our biochemical and structural (see also Franke et al., 1981; Moreno Diaz de la Espina et al., 1982) observations has led us to the conclusion that the extrachromosomal nucleoli of Xenopus contain an exoskeletal meshwork formed by patches of dense nodular aggregates interconnected by tangles of filaments with diameters varying between ∼4 and 12 nm, sometimes displaying a beads-on-a-string appearance (cf. Benavente et al., 1984). We have begun to examine the self-assembly potential of protein NO145 in vitro.
Analysis of the amino acid sequence of protein NO145 has disclosed a remarkable homology to the rat SC protein 2 (SCP2), which is particularly striking (43%) in a domain of ∼200 aa located in the N-terminal part of the two molecules. Whereas the functional significance of the homology between these two architectonic proteins and their karyoskeletal roles remains to be elucidated, it seems worth mentioning that some relationships between certain nucleolar and SC proteins have been reported previously. For example, nucleolar protein No55 has been reported to be almost identical to the rat SC protein SC65 (Ochs et al., 1996). Moreover, it has been described that some antibodies to SC proteins also label nucleoli and vice versa (Dresser, 1987; Moens et al., 1987), and the nucleolar protein Pch2 identified in yeast has also been detected in a punctate pattern along synapsed chromosomes (San-Segundo and Roeder, 1999). These observations may be taken as an indication that certain, although yet unknown amino acid sequence motifs have been conserved in proteins of both nuclear structures, SC and nucleoli, serving similar structure-defining functions.
Although our immunolocalization results in Xenopus oocytes are in line with those previously reported, they are at variance with some observations of a few tiny, nucleoli-associated fluorescent “dots” made with the same murine antiserum on some somatic Xenopus cells such as hepatocytes, Sertoli cells, spermatogonia, and A6 cells (Krohne et al., 1982; Benavente et al., 1984). In contrast, the various well-characterized antibodies to protein NO145 used in the present study have shown an exclusive occurrence in amplified oocyte nucleoli, and we have failed to detect NO145 immunostaining and immunoblotting reactions in any other cell type than oocytes. Although we have no definitive explanation for this difference of reaction we cannot exclude that these tiny dots detected in the previous studies might be due to a component cross-reactive with an as yet unknown, apparently minor nucleolar protein present in somatic cells. In this context it is perhaps also worth stating that the Xenopus protein NO145 has no relationship to the mammalian nucleolar 120–145-kDa proteins reported in the literature (Freeman et al., 1986; Busch, 1997).
However, we have to mention that we have isolated a partial cDNA clone coding for NO145 from a X. laevis kidney expression library by DNA screening (our unpublished data). Possibly, the cDNA clone identified in the kidney cDNA library results from very low levels of transcription of the NO145 gene in these somatic cells. We are currently performing reverse transcription-PCR experiments to identify possible mRNAs encoding NO145-related proteins in somatic cells of Xenopus and other species. At present, we cannot decide whether similarly looking cortical nucleolar structures in other cells and outside the genus Xenopus are formed by more distantly related homologous proteins or represent analog structures.
A most remarkable phenomenon is the rapid and complete degradation of nucleolar protein NO145 during meiotic maturation and egg formation, which experimentally can be followed in detail upon addition of progesterone to oocytes (Wasserman and Smith, 1978). Closely correlated with nuclear envelope breakdown and disassembly of the nuclear lamina, the multiple amplified nucleoli also disassemble rapidly, all processes being accompanied by extensive phosphorylation events (Belenguer et al., 1990; Heald and McKeon, 1990; Peter et al., 1990a,b). Already minutes upon the appearance of the “white spot” at the pigmented animal pole, most nucleolar proteins are dispersed throughout the ooplasm (Bell et al., 1992; Messmer and Dreyer, 1993; Bauer et al. 1994; some residual rDNA units detectable by DNA staining might correspond to FCs; cf. Shah et al., 1996). Interestingly, the disappearance of protein NO145 correlates perfectly with this rapid dispersion of nucleolar material, a behavior that distinguishes NO145 from all other nucleolar proteins so far studied in this system. Moreover, we have shown that the rapid decrease in NO145 protein concentration does not correspond to lowered levels of NO145 mRNA, which remains stable through oocyte maturation and in unfertilized eggs. We conclude that a special mechanism for the selective degradation of protein NO145 must exist. It would be interesting to know whether this degradation occurs via the proteasome pathway, with ubiquitin-conjugated intermediates, as recently described for the cytoplasmic polyadenylation element-binding protein (CPEB; Reverte et al., 2001), or whether it is regulated by rapid changes in the phosphorylation state of protein NO145 (for a high density of potential phosphorylation sites and a high degree of phosphorylation, see this study; Benavente et al., 1984).
Regardless, the nucleolar cortex protein NO145 presents a remarkable dual character: Although it is a major component of a rather stable structure in the nucleolar periphery, where it may be associated with other, yet unknown minor components, it is obviously also very sensitive to regulated proteolysis. Future studies will have to elucidate the specific mechanisms involved in both the formation and maintenance of the stable cage structure and in the rapid disassembly and degradation.
ACKNOWLEDGMENTS
We gratefully acknowledge Astrid Hofmann, Susanne Franz, and Cilly Kuhn for expert technical assistance; Hans-Richard Rackwitz for preparing and KLH-coupling of synthetic peptides; Andreas Hunziker for competent sequencing work; Peter Eichhorn for thoughtful care of the amphibians; Jutta Osterholt for preparing the photographs; and Eva Ouis for arranging the typescript. We also thank Christof Niehrs for providing X. tropicalis and Andreas Köhler for stimulating discussions and continuous interest in the project. This study was supported by the Deutsche Forschungsgemeinschaft (grant Schm 862/3-2 to M.S.S.-Z.).
Abbreviations used:
- aa
amino acid(s)
- DFG
dense fibrillar component
- FC
fibrillar center
- GC
granular component
- GV
germinal vesicle
- HSP
high-speed pellet
- HSS
high-speed supernatant
- LSP
low-speed pellet
- MALDI
matrix-assisted laser desorption ionization
- SC
synaptonemal complex
REFERENCES
- Bauer DW, Murphy C, Wu CH, Gall JG. In vitro assembly of coiled bodies in Xenopus egg extract. Mol Biol Cell. 1994;5:633–644. doi: 10.1091/mbc.5.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenguer P, Caizergues-Ferrer M, Labbe JC, Doree M, Amalric F. Mitosis-specific phosphorylation of nucleolin by p34cdc2 protein kinase. Mol Cell Biol. 1990;10:3607–3618. doi: 10.1128/mcb.10.7.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P, Dabauvalle MC, Scheer U. In vitro assembly of prenucleolar bodies in Xenopus egg extract. J Cell Biol. 1992;118:1297–1304. doi: 10.1083/jcb.118.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente R, Krohne G, Stick R, Franke WW. Electron microscopic immunolocalization of a karyoskeletal protein of molecular weight 145 000 in nucleoli and perinucleolar bodies of Xenopus laevis. Exp Cell Res. 1984;151:224–235. doi: 10.1016/0014-4827(84)90370-7. [DOI] [PubMed] [Google Scholar]
- Buongiorno-Nardelli M, Amaldi F, Lava-Sanchez PA. Amplification as a rectification mechanism for the redundant rRNA genes. Nat New Biol. 1972;238:134–137. doi: 10.1038/newbio238134a0. [DOI] [PubMed] [Google Scholar]
- Busch H. Nucleolar and nucleolonemal proteins of cancer cells. J Tumor Marker Oncol. 1997;12:4–68. [Google Scholar]
- Cairns C, McStay B. Identification and cDNA cloning of a Xenopus nucleolar phosphoprotein, xNopp180, that is the homolog of the rat nucleolar protein Nopp140. J Cell Sci. 1995;108:3339–3347. doi: 10.1242/jcs.108.10.3339. [DOI] [PubMed] [Google Scholar]
- Carmo-Fonseca M, Mendes-Soares L, Campos I. To be or not to be in the nucleolus. Nat Cell Biol. 2000;2:E107–E112. doi: 10.1038/35014078. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cordes V, Waizenegger I, Krohne G. Nuclear pore complex glycoprotein p62 of Xenopus laevis and mouse: cDNA cloning and identification of ist glycosylated region. Eur J Cell Biol. 1991;55:31–47. [PubMed] [Google Scholar]
- Desnoyers S, Kaufmann SH, Piorier GG. Alteration and the nucleolar localization of poly(ADP-ribose) polymerase upon treatment with transcription inhibitors. Exp Cell Res. 1996;227:146–153. doi: 10.1006/excr.1996.0259. [DOI] [PubMed] [Google Scholar]
- Dingwall C, Dilworth SM, Black SJ, Kearsey SE, Cox LS, Laskey RA. Nucleoplasmin cDNA reveals polyglutamic acid tracts and a cluster of sequences homologous to putative nuclear localization signals. EMBO J. 1987;6:69–74. doi: 10.1002/j.1460-2075.1987.tb04720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C, Laskey RA. Nuclear targeting sequences - a consensus? Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Dousset T, Wang C, Verheggen C, Chen D, Hernandez-Verdun D, Huang S. Initiation of nucleolar assembly is independent of RNA polymerase I transcription. Mol Biol Cell. 2000;11:2705–2717. doi: 10.1091/mbc.11.8.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser ME. The synaptonemal complex and meiosis: an immunocytochemical approach. In: Moens PB, editor. Meiosis. New York: Academic Press; 1987. pp. 245–274. [Google Scholar]
- Dumont JN. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory-maintained animals. J Morphol. 1972;136:153–164. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW. Matthias Jacob Schleiden and the definition of the cell nucleus. Eur J Cell Biol. 1988;47:145–156. [PubMed] [Google Scholar]
- Franke WW, Grund C, Osborn M, Weber K. The intermediate-sized filaments in rat kangaroo PtK2 cells. I. Morphology in situ. Cytobiologie. 1978;17:365–391. [PubMed] [Google Scholar]
- Franke WW, Kleinschmidt JA, Spring H, Krohne G, Grund C, Trendelenburg MF, Stoehr M, Scheer U. A nucleolar skeleton of protein filaments demonstrated in amplified nucleoli of Xenopus laevis. J Cell Biol. 1981;90:289–299. doi: 10.1083/jcb.90.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JW, McRorie DK, Busch RK, Gyorkey F, Gyorkey P, Ross BE, Spohn WH, Busch H. Identification and partial characterization of a nucleolar antigen with a molecular weight of 145,000 found in a broad range of human cancers. Cancer Res. 1986;46:3593–3598. [PubMed] [Google Scholar]
- Frohman MA, Dush MK, Martin GR. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG. Differential synthesis of the genes for ribosomal RNA during amphibian oogenesis. Proc Natl Acad Sci USA. 1968;60:553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG. Spread preparation of Xenopus germinal vesicle contents. In: Spector D, Goldman R, Leinwand L, editors. Cells: A Laboratory Manual. Vol. 1. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1998. pp. 52.1–52.4. [Google Scholar]
- Gall JG. Cajal bodies: the first 100 years. Annu Rev Cell Dev Biol. 2000;16:273–300. doi: 10.1146/annurev.cellbio.16.1.273. [DOI] [PubMed] [Google Scholar]
- Gall JG, Bellini M, Wu Z, Murphy C. Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes. Mol Biol Cell. 1999;10:4385–4402. doi: 10.1091/mbc.10.12.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG, Callan HG, Wu Z, Murphy C. Lampbrush chromosomes. In: Kay BK, Peng HB, editors. Xenopus laevis: Practical Uses in Cell and Molecular Biology. Vol. 36. San Diego: Academic Press; 1991. pp. 149–166. [PubMed] [Google Scholar]
- Gerbi SA. The nucleolus: then and now. Chromosoma. 1997;105:385–387. doi: 10.1007/BF02510476. [DOI] [PubMed] [Google Scholar]
- Granick D. Nucleolar necklaces in chick embryo fibroblast cells. I. Formation of necklaces by dichlororibobenzimidazole and other adenosine analogues that decrease RNA synthesis and degrade preribosomes. J Cell Biol. 1975a;65:398–417. doi: 10.1083/jcb.65.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick D. Nucleolar necklaces in chick embryo fibroblast cells. II. Microscope observations of the effect of adenosine analogues on nucleolar necklace formation. J Cell Biol. 1975b;65:418–427. doi: 10.1083/jcb.65.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiolov AA. The Nucleolus and Ribosome Biogenesis, Cell Biology Monographs. Vol. 12. New York: Springer Verlag; 1985. pp. 1–268. [Google Scholar]
- Hay ED, Gurdon JB. Fine structure of the nucleolus in normal and mutant Xenopus embryos. J Cell Sci. 1967;2:151–162. doi: 10.1242/jcs.2.2.151. [DOI] [PubMed] [Google Scholar]
- Heald R, McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990;61:579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- Hügle B, Scheer U, Franke WW. Ribocharin: a nuclear Mr 40,000 protein specific to precursor particles of the large ribosomal subunit. Cell. 1985;41:615–627. doi: 10.1016/s0092-8674(85)80034-9. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt JA, Dingwall C, Maier G, Franke WW. Molecular characterization of a karyophilic, histone-binding protein: cDNA cloning, amino acid sequence and expression of nuclear protein N1/N2 of Xenopus laevis. EMBO J. 1986;5:3547–3552. doi: 10.1002/j.1460-2075.1986.tb04681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt JA, Franke WW. Soluble acidic complexes containing histones H3 and H4 in nuclei of Xenopus laevis oocytes. Cell. 1982;29:799–809. doi: 10.1016/0092-8674(82)90442-1. [DOI] [PubMed] [Google Scholar]
- Köhler G, Milstein C. Continuos cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg PA, Melton DA. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984;12:7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G, Benavente R. The nuclear lamins. A multigene family of proteins in evolution and differentiation. Exp Cell Res. 1986;162:1–10. doi: 10.1016/0014-4827(86)90421-0. [DOI] [PubMed] [Google Scholar]
- Krohne G, Franke WW. A major soluble acidic protein located in nuclei of diverse vertebrate species. Exp Cell Res. 1980;129:167–189. doi: 10.1016/0014-4827(80)90341-9. [DOI] [PubMed] [Google Scholar]
- Krohne G, Stick R, Kleinschmidt JA, Moll R, Franke WW, Hausen P. Immunological localization of a major karyoskeletal protein in nucleoli of oocytes and somatic cells of Xenopus laevis. J Cell Biol. 1982;94:749–754. doi: 10.1083/jcb.94.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G, Waizenegger I, Höger TH. The conserved carboxy-terminal cysteine of nuclear lamins is essential for lamin association with the nuclear envelope. J Cell Biol. 1989;109:2003–2011. doi: 10.1083/jcb.109.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J, Götting C, Schnölzer M, Kempf T, Brinkmann T, Kleesiek K. First isolation of human UDP-D-xylose: proteoglycan core protein β-D-xylosyltransferase secreted from cultured JAR choriocarcinoma cells. J Biol Chem. 2001;276:4940–4947. doi: 10.1074/jbc.M005111200. [DOI] [PubMed] [Google Scholar]
- Lange TS, Gerbi SA. Transient nucleolar localization of U6 small nuclear RNA in Xenopus laevis oocytes. Mol Biol Cell. 2000;11:2419–2428. doi: 10.1091/mbc.11.7.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Panse S, Masson C, Héliot L, Chassery J-M, Junéra HR, Herandez-Verdun D. 3-D organization of ribosomal transcription units after DRB inhibition of RNA polymerase II transcription. J Cell Sci. 1999;112:2145–2154. doi: 10.1242/jcs.112.13.2145. [DOI] [PubMed] [Google Scholar]
- Mais C, Scheer U. Molecular architecture of the amplified nucleoli of Xenopus oocytes. J Cell Sci. 2001;114:709–714. doi: 10.1242/jcs.114.4.709. [DOI] [PubMed] [Google Scholar]
- Meier UT, Blobel G. Nopp140 shuttles on tracks between nucleolus and cytoplasm. Cell. 1992;70:127–138. doi: 10.1016/0092-8674(92)90539-o. [DOI] [PubMed] [Google Scholar]
- Mertens C, Kuhn C, Franke WW. Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol. 1996;135:1009–1025. doi: 10.1083/jcb.135.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer B, Dreyer C. Requirements for nuclear translocation and nucleolar accumulation of nucleolin of Xenopus laevis. Eur J Cell Biol. 1993;61:369–382. [PubMed] [Google Scholar]
- Moens PB, Heyting C, Dietrich AJ, van Raamsdonk W, Chen Q. Synaptonemal complex antigen location and conservation. J Cell Sci. 1987;105:93–103. doi: 10.1083/jcb.105.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno Diaz de la Espina S, Franke WW, Krohne G, Trendelenburg MF, Grund C, Scheer U. Medusoid fibril bodies: a novel type of nuclear filament of diameter 8 to 12 nm with periodic ultrastructure demonstrated in oocytes of Xenopus laevis. Eur J Cell Biol. 1982;27:141–150. [PubMed] [Google Scholar]
- Morgan GT, Doyle O, Murphy C, Gall JG. RNA polymerase II in Cajal bodies of amphibian oocytes. J Struct Biol. 2000;129:258–268. doi: 10.1006/jsbi.2000.4231. [DOI] [PubMed] [Google Scholar]
- Narayanan A, Lukowiak A, Jády BE, Dragon F, Kiss T, Terns RM, Terns MP. Nucleolar localization signals of box H/ACA small nucleolar RNAs. EMBO J. 1999;18:5120–5130. doi: 10.1093/emboj/18.18.5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes M, Nogi Y, Clark MW, Nomura M. Structural alterations of the nucleolus in mutants of Saccharomyces cerevisiae defective in RNA polymerase I. Mol Cell Biol. 1993;13:2441–2455. doi: 10.1128/mcb.13.4.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs RL, Stein TW, Chan EKL, Ruutu M, Tan EM. cDNA cloning and characterization of a novel nucleolar protein. Mol Biol Cell. 1996;7:1015–1024. doi: 10.1091/mbc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenberg HH, Schalk JA, Meuwissen RLJ, van Aalderen M, Kester HA, Dietrich AJJ, Heyting C. SCP2: a major protein component of the axial elements of synaptonemal complexes of the rat. Nucleic Acids Res. 1998;26:2572–2579. doi: 10.1093/nar/26.11.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MO, Dundr M, Szebeni A. The nucleolus: an old factory with unexpected capabilities. Trends Cell Biol. 2000;10:189–196. doi: 10.1016/s0962-8924(00)01738-4. [DOI] [PubMed] [Google Scholar]
- Pederson T. The plurifunctional nucleolus. Nucleic Acids Res. 1998;26:3871–3876. doi: 10.1093/nar/26.17.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T, Politz JC. The nucleolus and the four ribonucleoproteins of translation. J Cell Biol. 2000;148:1091–1095. doi: 10.1083/jcb.148.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M, Nakagawa J, Dorée M, Labbé JC, Nigg EA. Identification of major nucleolar proteins as candidate mitotic substrates of cdc2 kinase. Cell. 1990a;60:791–801. doi: 10.1016/0092-8674(90)90093-t. [DOI] [PubMed] [Google Scholar]
- Peter M, Nakagawa J, Dorée M, Labbé JC, Nigg EA. In vitro disassembly of the nuclear lamina and M-phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990b;61:591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Reeder RH. rRNA synthesis in the nucleolus. Trends Genet. 1990;6:390–395. doi: 10.1016/0168-9525(90)90298-k. [DOI] [PubMed] [Google Scholar]
- Reverte CG, Ahearn MD, Hake LE. CPEB degradation during Xenopus oocyte maturation requires a PEST domain and the 26S proteasome. Dev Biol. 2001;231:447–458. doi: 10.1006/dbio.2001.0153. [DOI] [PubMed] [Google Scholar]
- San-Segundo PA, Roeder GS. Pch2 links chromatin silencing to meiotic checkpoint control. Cell. 1999;97:313–324. doi: 10.1016/s0092-8674(00)80741-2. [DOI] [PubMed] [Google Scholar]
- Scalenghe F, Buscaglia M, Steinheil C, Crippa M. Large scale isolation of nuclei and nucleoli from vitellogenic oocytes in Xenopus laevis. Chromosoma. 1978;66:299–308. doi: 10.1007/BF00328531. [DOI] [PubMed] [Google Scholar]
- Scheer U, Hock R. Structure and function of the nucleolus. Curr Opin Cell Biol. 1999;11:385–390. doi: 10.1016/S0955-0674(99)80054-4. [DOI] [PubMed] [Google Scholar]
- Scheer U, Hügle B, Hazan R, Rose KM. Drug-induced dispersal of transcribed rRNA genes and transcriptional products: immunolocalization and silver staining of different nucleolar components in rat cells treated with 5,6,-dichloro-β-D-ribofuranosylbenzimidazole. J Cell Biol. 1984;99:672–679. doi: 10.1083/jcb.99.2.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U, Weisenberger D. The nucleolus. Curr Opin Cell Biol. 1994;6:354–359. doi: 10.1016/0955-0674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Schmidt-Zachmann MS, Hügle-Dörr B, Franke WW. A constitutive nucleolar protein identified as a member of the nucleoplasmin family. EMBO J. 1987;6:1881–1890. doi: 10.1002/j.1460-2075.1987.tb02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Zachmann MS, Hügle B, Scheer U, Franke WW. Identification and localization of a novel nucleolar protein of high molecular weight by a monoclonal antibody. Exp Cell Res. 1984;153:327–346. doi: 10.1016/0014-4827(84)90604-9. [DOI] [PubMed] [Google Scholar]
- Schmidt-Zachmann MS, Knecht S, Krämer A. Molecular characterization of a novel, widespread nuclear protein that colocalizes with spliceosome components. Mol Biol Cell. 1998;9:143–160. doi: 10.1091/mbc.9.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnölzer M, Alewood P, Jones A, Alewood D, Kent SB. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Rapid, high yield assembly of difficult sequences. Int J Pept Protein Res. 1992;40:180–193. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Shah SB, Terry CD, Wells DA, DiMario PJ. Structural changes in oocyte nucleoli of Xenopus laevis during oogenesis and meitotic maturation. Chromosoma. 1996;105:111–123. doi: 10.1007/BF02509521. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Jordan EG. The nucleolus. Annu Rev Cell Dev Biol. 1995;11:93–121. doi: 10.1146/annurev.cb.11.110195.000521. [DOI] [PubMed] [Google Scholar]
- Simard R, Langelier Y, Mandeville R, Maestracci N, Royal A. Inhibitors as tools in elucidating the structure and function of the nucleus. In: Busch H, editor. The Cell Nucleus. Vol. 3. New York: Academic Press; 1974. pp. 447–487. [Google Scholar]
- Steele RE, Thomas PS, Reeder RH. Anucleolate frog embryos contain ribosomal DNA sequences and a nucleolar antigen. Dev Biol. 1984;102:409–416. doi: 10.1016/0012-1606(84)90205-7. [DOI] [PubMed] [Google Scholar]
- Stick R. cDNA cloning of the developmentally regulated lamin LIII of Xenopus laevis. EMBO J. 1988;7:3189–3197. doi: 10.1002/j.1460-2075.1988.tb03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiry M, Cheutin T, O'Donohue MF, Kaplan H, Ploton D. Dynamics and three-dimensional localization of ribosomal RNA within the nucleolus. RNA. 2000;6:1750–1761. doi: 10.1017/s1355838200001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma RS, Stolk JA, Roth MB. Identification and characterization of sphere organelle protein. J Cell Biol. 1993;122:767–773. doi: 10.1083/jcb.122.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheggen C, Almouzni G, Hernandez-Verdun D. The ribosomal RNA processing machinery is recruited to the nucleolar domain before RNA polymerase I during Xenopus laevis development. J Cell Biol. 2000;149:293–306. doi: 10.1083/jcb.149.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R, Amon A. The nucleolus: the magician's hat for the cell cycle. Curr Opin Cell Biol. 2000;12:372–377. doi: 10.1016/s0955-0674(00)00102-2. [DOI] [PubMed] [Google Scholar]
- Wallace RA, Ho T, Salter DW, Jared DW. Protein incorporations by isolated amphibian oocytes. IV. The role of follicle cells and calcium during protein uptake. Exp Cell Res. 1973;82:287–295. doi: 10.1016/0014-4827(73)90343-1. [DOI] [PubMed] [Google Scholar]
- Wasserman WJ, Smith LD. Oocyte maturation: non-mammalian vertebrates. In: Jones RE, editor. The Vertebrate Ovary. New York: Plenum; 1978. pp. 443–468. [Google Scholar]
- Wiley HJ, Wallace RA. The structure of vitellogenin. Multiple vitellogenins in Xenopus laevis give rise to multiple forms of the yolk proteins. J Biol Chem. 1981;256:8626–8634. [PubMed] [Google Scholar]
- Wu Z, Gall JG. “Micronucleoli” in the Xenopus germinal vesicle. Chromosoma. 1997;105:438–443. doi: 10.1007/BF02510480. [DOI] [PubMed] [Google Scholar]
- Zirwes RF, Eilbracht J, Kneissel S, Schmidt-Zachmann MS. A novel helicase-type protein in the nucleolus: protein NOH61. Mol Biol Cell. 2000;11:1153–1167. doi: 10.1091/mbc.11.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirwes RF, Schmidt-Zachmann MS, Franke WW. Identification of a small, very acidic constitutive nucleolar protein (NO29) as a member of the nucleoplasmin family. Proc Natl Acad Sci USA. 1997;94:11387–11392. doi: 10.1073/pnas.94.21.11387. [DOI] [PMC free article] [PubMed] [Google Scholar]