Abstract
Backgrounds
G protein-coupled receptor 110 (GPR110) belongs to the subfamily of the adhesion G protein-coupled receptors (GPCRs). The potential role of GPR110 has been correlated with cancer cell invasion in some tumors such as glioma. However, its expression and role in human osteosarcoma has not been identified. This study aimed to examine the expression level of GPR110 and determine whether the expression of GPR110 was correlated with aggressive clinicopathological characteristics and prognosis of osteosarcoma.
Material/Methods
This retrospective study included 94 osteosarcoma patients. Immunohistochemistry staining and quantitative real-time polymerase chain reaction were performed to detect the expression level of GPR110 in osteosarcoma specimens. We then determined the correlation of the GPR110 expression with the clinicopathological characteristics and prognosis by univariate or multivariate analysis. Patient outcomes were evaluated using the Kaplan-Meier log-rank test and prognostic factors were detected by multivariate analysis. The function of GPR110 on cell proliferation, migration, and invasion were examined in this in vitro study.
Results
Overexpression of GPR110 was correlated with the advanced stage of osteosarcoma. Patients with high expression level of GPR110 had significantly poorer 5-year overall survival; the multivariate analysis found that GPR110 expression level can act as an independent prognosis factor. Knockdown of GPR110 can decrease the proliferation, migration, and invasion capacity of human osteosarcoma cell lines.
Conclusions
Our studies suggest a role of GPR110 in tumor progression and as a potential novel prognostic biomarker in osteosarcoma.
MeSH Keywords: Cell Proliferation, Osteosarcoma, Prognosis
Background
Osteosarcoma develops in the bone and is the most common type of primary malignant bone tumor and a contributor to the tumor-related mortality especially in children and young adults [1,2]. Osteosarcoma arises most often in the metaphysis of long bones and produces malignant osteoid. Treatment for osteosarcoma includes surgical resection, radiotherapy, and adjuvant chemotherapy [3]. Despite great improvements in treatment therapies in the past decades, patients diagnosed with high-grade metastatic osteosarcoma have very poor prognosis with the 5-year survival rate at only 4% to 16% [4,5]. Therefore, it is critical to study the progression mechanism of osteosarcoma and explore new prognostic biomarkers as well as therapeutic targets for more effective therapeutic strategies.
G protein-coupled receptors (GPCRs) are the largest family of cell membrane proteins and are involved extensively in various biological processes [6]. Upon ligand binding and activation, the conformation of GPCR changes, and then activates the heterotrimeric G protein, consequently transduce the extracellular signal via G protein [7]. This mechanism has been used in various drug designs to select activators or inhibitors of different biological pathways. GPCRs are currently the target of a wide range of prescription drugs on the market. Drugs targeting members of GPCRs represent the core in modern medicine [8].
The GPR110, also named adhesion G-protein coupled receptor F1 (ADGRF1) is an orphan GPCR that belongs to the subfamily of the adhesion GPCRs [9]. An orphan GPCR indicates a receptor without known information about its ligand, signaling pathway, or physiological function. However, the potential role of GPR110 has been recently reported to be correlated with cell malignant transformation and tumor metastasis in glioma [10]. In addition, GPR110 was confirmed as an oncogene in prostate and lung cancer [11]. However, the expression level and prognostic significance of GPR110 in human osteosarcoma has not been identified. This study aimed to determine whether GPR110 expression was correlated with aggressive clinicopathological characteristics and prognosis of osteosarcoma patients.
In the present study, we first tested the protein expression level and the RNA level of GPR110 in osteosarcoma tissues. Second, we found that high GPR110 expression was associated with advanced disease stages and poor prognosis by statistical analysis. Finally, we carried out cellular experiments to determine the oncogenic mechanisms of GPR110 in osteosarcoma cell lines, which demonstrated that the proliferation, migration, and invasion capacity of SAOS-2 and K7M2 cells could be enhanced by GPR110 overexpression.
Material and Methods
Patient and samples
Osteosarcoma tissue from 94 patients at the Department of Pathology in Yidu Central Hospital of Weifang between July 2008 and July 2014 were obtained. Patients who had radiotherapy or chemotherapy before curative tumor resection were excluded. All the tumors were confirmed based on pathology examination of the specimens obtained from surgery. All the osteosarcoma patients were followed for 38 months. The cohort included 52 male patients and 42 female patients.
Immunohistochemistry staining
Immunohistochemistry (IHC) staining was carried out by using the 2-step PV-9000 kit (ZSGB-BIO, China) as described previously [12]. To be briefly, paraffin-embedded tissues were cut into 8-μm serial sections and dried at 70°C for 2 hours. The sections were then washed 3 times with PBS (phosphate buffered saline) and deparaffinized with xylene and rehydrated with alcohol. The antigen retrieval was carried out in a microwave oven using sodium citrate buffer. Specimens were then incubated with 10% serum to block nonspecific binding after washing 3 times with PBS. The incubation with a rabbit anti-human GPR110 antibody (1: 500 dilution; ab150547; Abcam) was performed at 4°C overnight. Then the sections were washed and incubated with the Polymer Helper and using poly peroxidase-anti-rabbit IgG was used for detection and sections mounted with a neutral resin. Negative control staining was carried out with PBS.
Evaluation of GPR110 staining
The specimens were examined and scored by 2 independent pathologists who were blinded to the patients’ clinicopathological information. Six fields at 400× magnification were randomly selected. Tumor cell membrane and cytoplasm staining were considered to indicate positive expression. Each section was defined by a score standard according to a previous semiquantitative system. Staining intensity was defined as follows: 1 (no staining); 2 (weak staining); 3 (moderate staining); and 4 (strong staining). The proportion of positive cells was scored as follows: 1 (<2 5%); 2 (25–50%); 3 (51–75%); 4 (>75%). The final score was determined by multiplying the intensity score by the positive proportion score. In this study, the expression of GPR110 was defined as low expression when the score was <9, and high expression when the score was ≥9.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from the tissues using the TRIzol reagent (ThermoFisher, PA, USA) following the manufacturer’s instructions and reversed transcribed with the Primer-Script RT reagent kit. Quantitative real-time polymerase chain reaction (qRT-PCR) was carried out with SYBR Premix Ex Tag by 7500 PCR System (Applied Biosystems, CA, USA) following the manufacturer’s instructions [13]. Primers used for RT-PCR are as follows: GPR110-F: 5′-ACGCAACCTAGCAATACC-3′; GPR110-R: 5′-AGCAGCACCACAACGAA-3′; GAPDH-F: 5′-AGGGCTGCTTTTAACTCTGGT-3′; and GAPDH-R: 5′-CCCCACTT GATTTTGGAGGGA-3′. The expression of GPR110 was normalized to the gene GAPDH and calculated by 2−ΔΔCt method.
Proliferation, migration, and invasion assays
Cell proliferation was examined using the MTT assay. Briefly, 5×103 cells were added to 96-well plates and cultured at different time points. The MTT solution was added to each well and incubated for 4 hours at 37°C followed by measuring OD4590 nm absorbance using an automated plate reader. The migration was examined using the wound-healing assay. Transfected cells were added to the 6-well plates and cultured to confluence, and then “wounded” by using sterile pipette tips. The closing of wounds was measured after 24 hours and 48 hours, and then normalized with the control group [14]. The invasion was measured by the Transwell assay [15]. Briefly, 6×104 cells were added to the upper chamber and cultured for 48 hours. Invaded cells were fixed and stained. Cell counting was carried out in 6 random fields of view. All the experiments were performed in triplicate.
Statistical analysis
All statistical analyses were performed using SPSS 20.0 software. Difference between means was analyzed with Student’s t-test. The correlations between expression levels of GPR110 and clinicopathologic features were analyzed by chi-square test. The overall survival data of osteosarcoma patients were analyzed using the Kaplan-Meier test and log-rank test. The independent prognostic factors of overall survival were tested using Cox regression model analysis. Student’s t-test was applied to analyze the data of cellular experiments. A value of P<0.05 was considered statistically significant.
Results
The expression of GPR110 in human osteosarcoma tissues
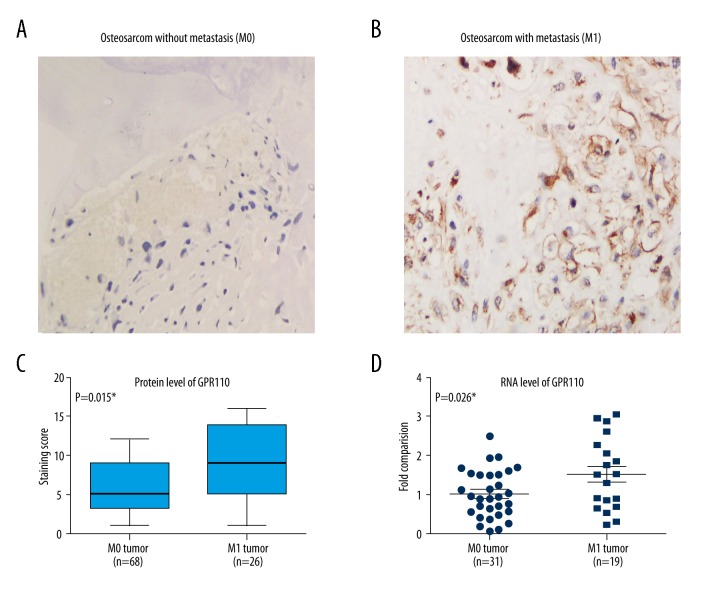
To investigate the effect of GPR110 in osteosarcoma, we first examined the expression of GPR110 using IHC staining. Staining for GPR110 was detected in the cytoplasm and membrane in human osteosarcoma specimens with (M1 tumors) or without (M0 tumors) metastasis (Figure 1A, 1B). We observed that the expression level of GPR110 was upregulated in M1 tumors compared with M0 tumors (Figure 1C). We next measured the expression of GPR110 in 50 fresh samples from osteosarcoma patients by qRT-PCR assays (Figure 1D). The GPR110 expression was also higher in patients with M1 tumors than in patients with M0 tumors (P<0.05). Taken together, these results suggested that the expression of GPR110 may be involved in osteosarcoma progression.
Figure 1.
Analysis of GPR110 expression in human osteosarcoma. (A, B) Immunohistochemistry staining of GPR110 in human osteosarcoma without or with metastasis. (C) Staining score of the protein expression level of GPR110 in A and B. (D) Relative mRNA level of GPR110 in M0 and M1 tumor of human osteosarcoma. The M1 means the results were derived from tumor tissues with distant metastasis, therefore representing high aggressive tumors. The M0 means the results were from tumor tissues without distant metastasis, therefore representing less aggressive tumors.
Correlation between GPR110 expression and clinicopathologic characteristics of osteosarcoma patients
For statistical analysis, we divided the osteosarcoma patients into the GPR110 low expression group (n=53) and the GPR110 high expression group (n=41) based on the mean value of GPR110 expression. Then, we determined the correlation of GPR110 expression with the clinicopathological features in osteosarcoma patients (Table 1). We found that the level of GPR110 expression was significantly correlated with tumor diameter (P<0.01) and distant metastasis (P<0.01). However, no associations were observed between GPR110 expression and age, gender, tumor location, and tumor grade (all P>0.05).
Table 1.
Correlation between GPR110 expression and clinicopathologic characteristics of osteosarcoma patients.
| Variables | Cases (N=94) | GPR110 expression | P# | |
|---|---|---|---|---|
| Low (N=53) | High (N=41) | |||
| Age (year) | 0.899 | |||
| ≥25 | 36 | 20 | 16 | |
| <25 | 58 | 33 | 25 | |
| Gender | 0.165 | |||
| Female | 42 | 27 | 15 | |
| Male | 52 | 26 | 26 | |
| Tumor diameter | 0.009* | |||
| <6.0 cm | 37 | 27 | 10 | |
| ≥6.0 cm | 57 | 26 | 31 | |
| Tumor location | 0.416 | |||
| Femur or Tibia | 66 | 39 | 27 | |
| Others | 28 | 14 | 14 | |
| Tumor grade | 0.194 | |||
| Low grade | 32 | 21 | 11 | |
| High grade | 62 | 32 | 30 | |
| Distant metastasis | 0.009* | |||
| Negative | 68 | 44 | 24 | |
| Positive | 26 | 9 | 17 | |
P value was analyzed by Chi-square test;
indicates P<0.05 with statistical significance.
Relationship between the expression level of GPR110 and overall survival in osteosarcoma patients
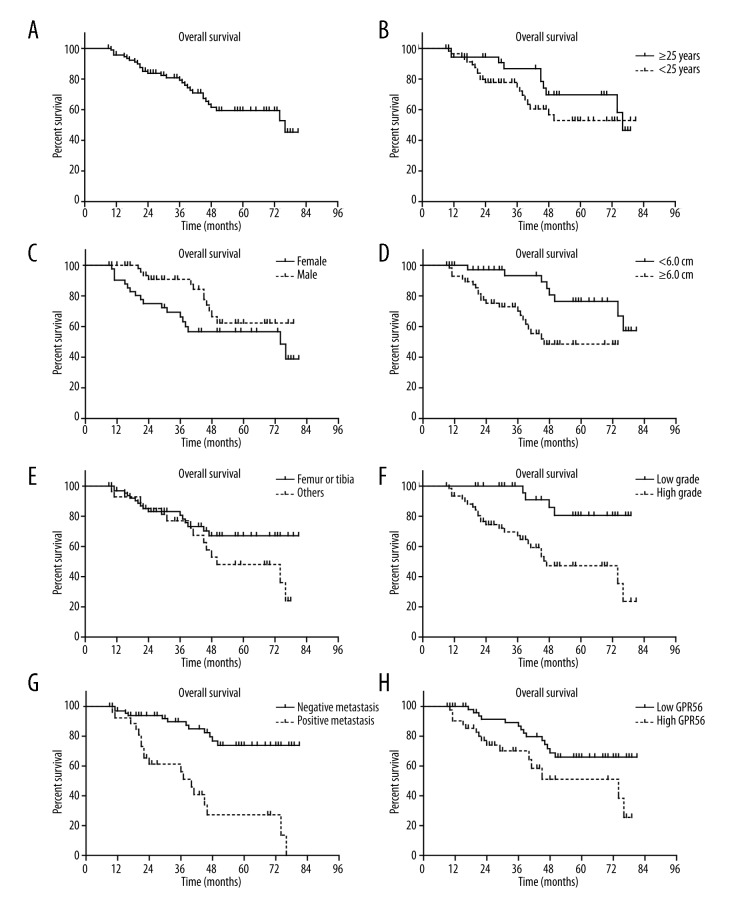
The role of GPR110 expression in overall survival of patients was tested by Kaplan-Meier analysis and the log-rank test. At the time of the final follow up, 30 patients had succumbed to osteosarcoma. Patients with osteosarcoma which expressed high levels of GPR110 had a poorer mean overall survival time (52.8±5.0 months) compared with patients with tumors which expressed low level of GPR110 (65.8±3.4; P<0.05; Figure 2, Table 2). Other prognostic factors were also determined to be associated with decreased overall survival time, including tumor diameter (P<0.01), tumor grade (P<0.005), and distant metastasis (P<0.001) (Figure 2, Table 2).
Figure 2.
The Kaplan-Meier curves of the overall survival of osteosarcoma patients. The overall survival time of osteosarcoma patients was evaluated by Kaplan-Meier and log-rank test, based on entire cohort (A), age (B), gender (C), tumor diameter (D), tumor location (E), tumor grade (F), distant metastasis (G), and GPR110 expression levels (H). * P<0.05 by log-rank test.
Table 2.
The overall survival analysis of osteosarcoma patients by Kaplan-Meier test.
| Variables | Cases (N) | 5-year OS rate (%) | Mean OS survival (months) | P# |
|---|---|---|---|---|
| Age (year) | 0.197 | |||
| ≥25 | 36 | 69.7% | 64.8 ± 4.0 | |
| <25 | 58 | 52.9% | 57.3±4.1 | |
| Gender | 0.070 | |||
| Female | 42 | 56.6% | 55.1±4.7 | |
| Male | 52 | 62.3% | 64.2±3.5 | |
| Tumor diameter | 0.005* | |||
| <6.0 cm | 37 | 76.4% | 70.4±3.4 | |
| ≥6.0 cm | 57 | 48.5% | 50.8±3.7 | |
| Tumor location | 0.135 | |||
| Femur or Tibia | 66 | 67.1% | 63.9±3.6 | |
| Others | 28 | 48.1% | 54.7±4.7 | |
| Tumor grade | 0.001* | |||
| Low grade | 32 | 80.5% | 72.2±3.1 | |
| High grade | 62 | 47.2% | 52.3±3.9 | |
| Distant metastasis | <0.001* | |||
| Negative | 68 | 73.8% | 69.0±3.1 | |
| Positive | 26 | 27.2% | 41.7±5.0 | |
| GPR110 expression | 0.019* | |||
| Low | 53 | 65.9% | 65.8±3.4 | |
| High | 41 | 51.1% | 52.8±5.0 |
P value was analyzed by log-rank test;
indicates P<0.05 with statistical significance.
In addition, GPR110 expression (P<0.05), tumor grade (P<0.05), and distant metastasis (P<0.05) were independent prognostic factors of poor overall survival in multivariate analysis of overall survival (Table 3).
Table 3.
Multivariate analysis of overall survival.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Tumor diameter | 1.541 | 0.665±3.570 | 0.313 |
| Tumor grade | 2.789 | 1.256±8.664 | 0.037* |
| Distant metastasis | 2.641 | 1.023±6.815 | 0.045* |
| GPR110 expression | 2.487 | 1.154±6.772 | 0.041* |
Indicates P<0.05 with statistical significance.
Overexpression of GPR110 promotes proliferation, migration, and invasion capacity of osteosarcoma cell lines
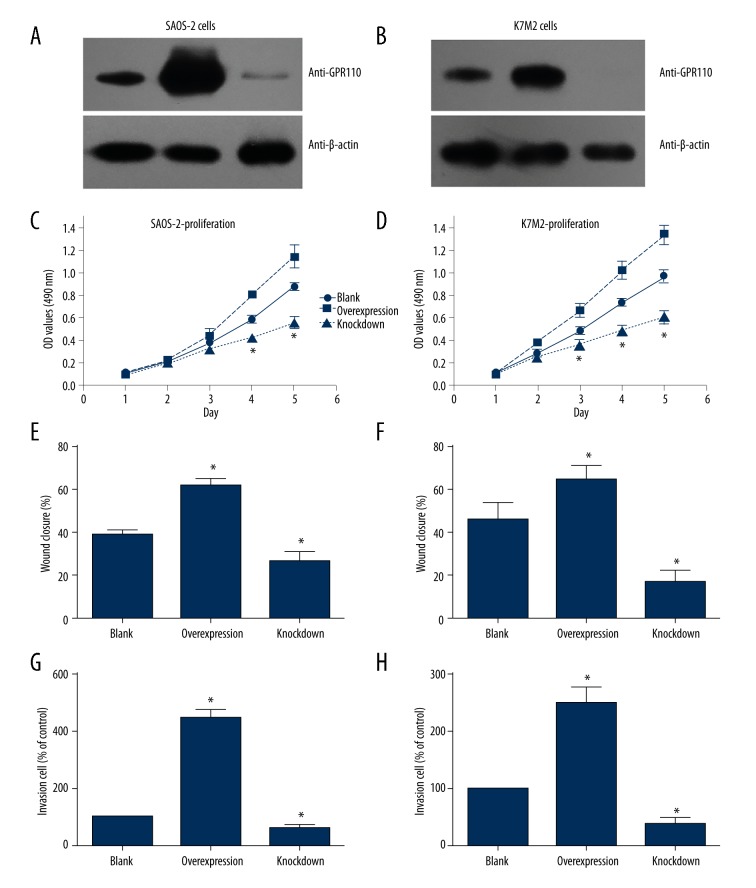
We performed cellular experiments to detect the function of GPR110 in osteosarcoma. The transfection efficiency of pcDNA3.1-GPR110 plasmids and GPR110-siRNA in SAOS-2 and K7M2 cells was tested by western blot (Figure 3A, 3B). Furthermore, we tested the cell characteristics to investigate whether GPR110 affected tumor progression. We found that the proliferation, migration, and invasion capacity of SAOS-2 and K7M2 cells were enhanced by GPR110-overexpression compared with knockdown cells (Figure 3C–3H). These results suggested that GPR110 might play a potential role in the progression of human osteosarcoma.
Figure 3.
GPR110 promotes proliferation, migration, and invasion capacity of osteosarcoma cell lines. (A, B) Western blot results showed transfection efficiency of pcDNA3.1-GPR110 plasmids and GPR110-siRNA in SAOS-2 and K7M2 cells. (C–H) Overexpression of GPR110 enhanced the proliferation, migration, and invasion capacity of SAOS-2 and K7M2 cell lines, whereas knockdown of GPR110 inhibited cell proliferation, migration, and invasion. Student’s t-test was used to compare the differences between tested groups with “Blank” group. * indicates statistical significance (P<0.05) between tested group with “Blank” group.
Discussion
Despite some improvements to treatments for osteosarcoma patients having been achieved in recent years, the 5-year survival rate of patients diagnosed with high-grade metastatic osteosarcoma is still unsatisfactory [5,16,5]. Therefore, it is important to find more effective prognostic biomarkers in osteosarcoma patients for diagnosis and treatment. GPCRs are involved in many diseases, including cancer, and are also the important targets for approximately 33% of all modern prescription drugs [17]. For example, various GPCRs for ligands, including endothelin, chemokines, prostaglandin, and lysophosphatidic acid, are involved in tumor progression [7]. Current research on orphan GPCRs will also likely result in significant clinical benefits; more and more efforts are being made to determine the associations between GPCR and cancer [18].
A recent study demonstrated that GPR110 shows high expression levels in human lung and prostate cancer and it has been confirmed as an oncogene, suggesting that GPR110 may play an important role in tumor pathology [11]. Analysis of the expression patterns of GPR110 in mice indicated that GPR110 was expressed highly in the liver, and GPR110 deficiency in mice decelerated carcinogen-induced hepato-carcinogenesis through the IL-6/STAT3 pathway [19]. It was also reported that GPR110 was highly expressed in some glioma patients and the overexpression of GPR110 can inhibit the phosphorylation and activation of STAT3 [20]. However, none of these studies, which describe the effects of GPR110 in several cancers, have elucidated the role of GPR110 in human osteosarcoma.
Here in our study, we found that GPR110 was highly expressed in some osteosarcoma patients by IHC staining and qRT-PCR. We also found that the high expression level of GPR110 was associated with the clinical stage of patients with osteosarcoma. Furthermore, GPR110 was identified as an independent prognostic biomarker by univariate and multivariate analyses; and higher expression of GPR110 indicated shorter overall survival time. Finally, we demonstrated that overexpression of GPR110 enhanced the proliferation, migration, and invasion capacity of osteosarcoma cell lines. Our findings suggest that the high expression of the orphan receptor GPR110 was correlated with unfavorable prognosis of osteosarcoma patients, possibly by promoting the progression of osteosarcoma cells.
However, we still have no idea about the effect of GPR110 endogenous ligand on the process although several studies have been carried out to screen for the receptor agonists. Furthermore, it has also been reported that STAT3 can be involved in GPR110-U87 cell invasion [20]. Recently, several studies reported the indispensable role of STAT3 in cancer inflammation and immunity, which may inhibit tumor metastasis [21–23]. It seems that GPR110 may inhibit the function of STAT3 in osteosarcoma and as a result, promote tumor progression. The in-depth mechanism exploring whether STAT3 is involved in GPR110-enhanced osteosarcoma cell progression is worth more investigation.
Conclusions
Our study detected the RNA and protein levels of GPR110 in patients with osteosarcoma for the first time and revealed its correlations with the clinical stages of osteosarcoma patients. Furthermore, we identified GPR110 as an independent prognostic biomarker for patients with osteosarcoma. Identification of GPR110 as a novel biomarker for osteosarcoma would be helpful for disease mechanism elucidation and clinical prognosis improvement.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422–41. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Li C, Zhang L, et al. Personalized identification of differentially expressed modules in osteosarcoma. Med Sci Monit. 2017;23:774–79. doi: 10.12659/MSM.899638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longhi A, Errani C, De Paolis M, et al. Primary bone osteosarcoma in the pediatric age: State of the art. Cancer Treat Rev. 2006;32(6):423–36. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Kovar H. Selective enhancer changes in osteosarcoma lung metastasis. Nat Med. 2018;24(2):126–27. doi: 10.1038/nm.4487. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Liu J, Zhang Y. Role of epithelial cell transforming sequence 2 (ECT2) in predicting prognosis of osteosarcoma. Med Sci Monit. 2017;23:3861–68. doi: 10.12659/MSM.905951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikkers H, Allen J, Knipscheer P, et al. High-throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nat Genet. 2002;32(1):153–59. doi: 10.1038/ng950. [DOI] [PubMed] [Google Scholar]
- 7.Lappano R, Maggiolini M. G protein-coupled receptors: Novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10(1):47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 8.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Ann Rev Pharmacol Toxicol. 2008;48:537–68. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 9.Lee JW, Huang BX, Kwon H, et al. Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function. Nat Commun. 2016;7:13123. doi: 10.1038/ncomms13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadras T, Heatley SL, Kok CH, et al. Differential expression of MUC4, GPR110 and IL2RA defines two groups of CRLF2-rearranged acute lymphoblastic leukemia patients with distinct secondary lesions. Cancer Lett. 2017;408:92–101. doi: 10.1016/j.canlet.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Lum AM, Wang BB, Beck-Engeser GB, et al. Orphan receptor GPR110, an oncogene overexpressed in lung and prostate cancer. BMC Cancer. 2010;10:40. doi: 10.1186/1471-2407-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H, Xu Y, Zhang Q, et al. Prognostic significance of TBL1XR1 in predicting liver metastasis for early stage colorectal cancer. Surg Oncol. 2017;26(1):13–20. doi: 10.1016/j.suronc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8(3):4888–90. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan W, Pan M, Liu H, et al. Ergosterol peroxide inhibits ovarian cancer cell growth through multiple pathways. Onco Targets Ther. 2017;10:3467–74. doi: 10.2147/OTT.S139009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Xu Y, Zhang Q, et al. Correlations between TBL1XR1 and recurrence of colorectal cancer. Sci Rep. 2017;7:44275. doi: 10.1038/srep44275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeitoun R, Shokry AM, Ahmed Khaleel S, Mogahed SM. Osteosarcoma subtypes: Magnetic resonance and quantitative diffusion weighted imaging criteria. J Egypt Natl Canc Inst. 2018;30(1):39–44. doi: 10.1016/j.jnci.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Stoveken HM, Hajduczok AG, Xu L, Tall GG. Adhesion G protein-coupled receptors are activated by exposure of a cryptic tethered agonist. Proc Natl Acad Sci USA. 2015;112(19):6194–99. doi: 10.1073/pnas.1421785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjarnadottir TK, Fredriksson R, Schioth HB. The adhesion GPCRs: A unique family of G protein-coupled receptors with important roles in both central and peripheral tissues. Cell Mol Life Sci. 2007;64(16):2104–19. doi: 10.1007/s00018-007-7067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma B, Zhu J, Tan J, et al. Gpr110 deficiency decelerates carcinogen-induced hepatocarcinogenesis via activation of the IL-6/STAT3 pathway. Am J Cancer Res. 2017;7(3):433–47. [PMC free article] [PubMed] [Google Scholar]
- 20.Shi H, Zhang S. Expression and prognostic role of orphan receptor GPR110 in glioma. Biochem Biophys Res Commun. 2017;491(2):349–54. doi: 10.1016/j.bbrc.2017.07.097. [DOI] [PubMed] [Google Scholar]
- 21.Darnell JE. Validating Stat3 in cancer therapy. Nat Med. 2005;11(6):595–96. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Lee HJ, Bae IJ, et al. Inhibition of STAT3/VEGF/CDK2 axis signaling is critically involved in the antiangiogenic and apoptotic effects of arsenic herbal mixture PROS in non-small lung cancer cells. Oncotarget. 2017;8(60):101771–83. doi: 10.18632/oncotarget.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]