Abstract
BENCHMRK-1 and -2 are ongoing double-blind phase III studies of raltegravir in patients experiencing failure of antiretroviral therapy with triple-class drug-resistant human immunodeficiency virus infection. At week 96 (combined data), raltegravir (400 mg twice daily) plus optimized background therapy was generally well tolerated, with superior and durable antiretroviral and immunological efficacy, compared with optimized background therapy alone.
Raltegravir is a human immunodeficiency virus type 1 (HIV-1) integrase strand-transfer inhibitor that is active against multidrug-resistant HIV-1 in vitro [1, 2]. In phase II clinical trials, raltegravir demonstrated potent and durable antiretroviral activity for up to 96 weeks when given with optimized background therapy (OBT) in treatment-experienced patients [3] or with tenofovir and lamivudine in treatment-naive patients [4]. Week 48 results of the BENCHMRK phase III studies confirmed the efficacy of raltegravir in treatment-experienced patients infected with multidrug-resistant HIV-1 [5, 6]. This report presents follow-up results through week 96 of the ongoing BENCHMRK studies.
Methods.
BENCHMRK-1 (protocol 018; NCT 00293267) and BENCHMRK-2 (protocol 019; NCT 00293254) are double-blind, randomized, phase III studies with identical study designs, as described elsewhere [5]. This report presents efficacy results through study week 96 and all available safety data from the double-blind phase through 29 August 2008 (week 96 database lock). From study weeks 16–48, virologic failure was defined as <1 log10 decrease in HIV RNA levels from baseline, confirmed >1 log10 increase in HIV RNA level from the nadir, or 2 consecutive HIV RNA level measurements ≥400 copies/mL after a prior result of <400 copies/mL. To be consistent with the recently updated recommended target for viral suppression [7], virologic failure after week 48 was defined as confirmed HIV RNA level ≥50 copies/mL (2 consecutive measurements at least 1 week apart). Patients with virologic failure at any time after week 16 could (1) exit the double-blind study and enter an open-label phase to receive raltegravir as part of a new regimen, (2) remain in the blinded study, or (3) withdraw from the study. The potential emergence of resistance to raltegravir was investigated in patients with virologic failure by genotyping the integrase coding sequence according to standard methods [6] and by comparison with pretreatment genotypes.
The following predefined end points were examined at week 96: proportion of patients with HIV RNA levels <50 copies/mL, proportion of patients with HIV RNA levels <400 copies/mL, change from baseline in log10 HIV RNA level (copies/mL), and change from baseline in CD4 cell count (cells/mm3). For the proportions over time analysis, a worst-case approach as used that counted all patients who did not complete the study as treatment failures; missing HIV RNA level measurements were imputed as failures unless the values flanking the missing value were both successes, in which case the absent value was left as missing. For the change from baseline analyses, an observed failure approach was used: patients who discontinued treatment for lack of efficacy were assumed to have returned to their baseline value at subsequent times, but no other missing values were imputed. Data from patients who discontinued treatment for other reasons were censored at discontinuation. The observed failure approach was also used for exploratory subgroup analyses by potential prognostic factors and for the assessment of treatment effect homogeneity across subpopulations. Suspected AIDS-defining events were reviewed by an independent adjudicator, and blinded safety and efficacy results were periodically reviewed by an independent Data and Safety Monitoring Board. Additional details of the statistical analyses and the adjudication of AIDS-defining events are described elsewhere [5].
Results.
Baseline characteristics were generally balanced between treatment groups [5]. Most enrolled patients were highly treatment-experienced white male individuals with AIDS. Duration of double-blind follow-up was longer in the raltegravir group (median, 110.4 weeks; range, 3.0–127.4 weeks) than in the placebo group (median, 37.6 weeks; range, 5.6–126.4 weeks) because of more frequent discontinuations for virologic failure among placebo recipients (Table 1).
Table 1.
Patient Disposition
| Variable | No. (%) of patients |
|
|---|---|---|
| Raltegravir and OBT | Placebo and OBT | |
| Double-blind phase | ||
| Patients entered | 466 | 237 |
| Treated | 462 (99.1) | 237 (100) |
| Continuing in double-blind phase | 302 (64.8) | 67 (28.3) |
| Discontinued study | 70 (15.0) | 41 (17.3) |
| Lack of efficacy | 10 (2.1) | 8 (3.4) |
| Clinical adverse event | 17 (3.6) | 10 (4.2) |
| Laboratory adverse event | 1 (0.2) | 0 (0.0) |
| Consent withdrawn | 15 (3.2) | 11 (4.6) |
| Lost to follow-up | 6 (1.3) | 5 (2.1) |
| Did not enter extensiona | 3 (0.6) | 1 (0.4) |
| Otherb | 18 (3.9) | 6 (2.5) |
| OLPVF | ||
| Patients entered | 90 (19.3) | 129 (54.4) |
| Discontinued from OLPVF | 38 (8.2) | 39 (16.5) |
| Continuing in OLPVF | 52 (11.2) | 90 (38.0) |
NOTE. OBT, optimized background therapy; OLPVF, open-label post-virologic failure.
Patients completed original 48-week protocol but did not continue into extension.
Including patients who moved or relocated, or the clinical trial was terminated at the site.
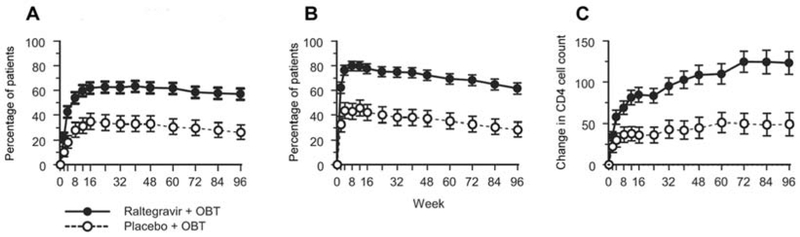
Virologic and immunologic responses were consistent between the 2 BENCHMRK studies at week 96 (homogeneity P > .1). In the combined studies, 57% of raltegravir recipients, compared with 26% of placebo recipients, sustained an HIV RNA level <50 copies/mL at week 96 (P < .001) (Figure 1A); 61% and 28%, respectively, had an HIV RNA level <400 copies/mL (P < .001) (Figure 1B). Both the mean changes in log10 HIV RNA level and CD4 cell counts from baseline to week 96 were significantly greater in the raltegravir group than in the placebo group (HIV RNA level: −1.5 log10 copies/mL [95% confidence interval, −1.6 to −1.4 log10 copies/mL] vs −0.6 log10 copies/mL [95% confidence interval, −0.7 to −0.5 log10 copies/mL]; P < .001; CD4 cell count: 123 cells/mm3 vs 49 cells/mm3; P < .001) (Figure 1C). There was a nonsignificant trend toward lower rates of AIDS-defining condition and mortality among raltegravir recipients (Table 2).
Figure 1.
A, Percentage of patients with a plasma HIV RNA level <50 copies/mL, over time, by treatment group. B, Percentage of patients with a plasma HIV RNA level <400 copies/mL, over time, by treatment group. The proportion of patients with an HIV RNA level below the limit of quantification for the ultrasensitive assay and the standard assay, respectively, are shown; patients who did not complete the study were counted as treatment failures. C, Change from baseline in CD4 cell count, over time, by treatment group. The mean change in CD4 cell count per mm3 from baseline are shown using the observed failure approach, carrying baseline values forward (thereby assigning a value of 0 to change from baseline) for all treatment failures. Vertical brackets represent the 95% confidence intervals. OBT, optimized background therapy.
Table 2.
Exposure-Adjusted Rates and Relative Risk of Confirmed AIDS-Defining Condition (ADC) and Death, Week 96 (Double-Blind Phase)
| No. of patients/PYR at risk (rate, cases per 100 PYR) |
|||
|---|---|---|---|
| Variable | Raltegravir and OBT (n = 462) |
Placebo and OBT (n = 237) |
Relative risk (95% CI) |
| New or recurrent ADCa | 18/807 (2.23) | 11/267 (4.13) | 0.54 (0.24–1.27) |
| New ADC | 8/817 (0.98) | 6/267(2.25) | 0.44 (0.13–1.52) |
| Death | 13/824 (1.58) | 7/269 (2.60) | 0.61 (0.23–1.80) |
| New or recurrent ADC or death | 26/806 (3.22) | 16/267 (6.00) | 0.54 (0.28–1.07) |
| New ADC or death | 18/817 (2.20) | 12/267 (4.49) | 0.49 (0.22–1.12) |
NOTE. The median time to the first new or recurrent AIDS-defining event was 9.3 weeks (interquartile range, 7.4–19.1 weeks) for raltegravir recipients and 15.0 weeks (interquartile range, 2.7–16.9 weeks) for placebo recipients. CI, confidence interval; PYR, person-years.
Prespecified analysis.
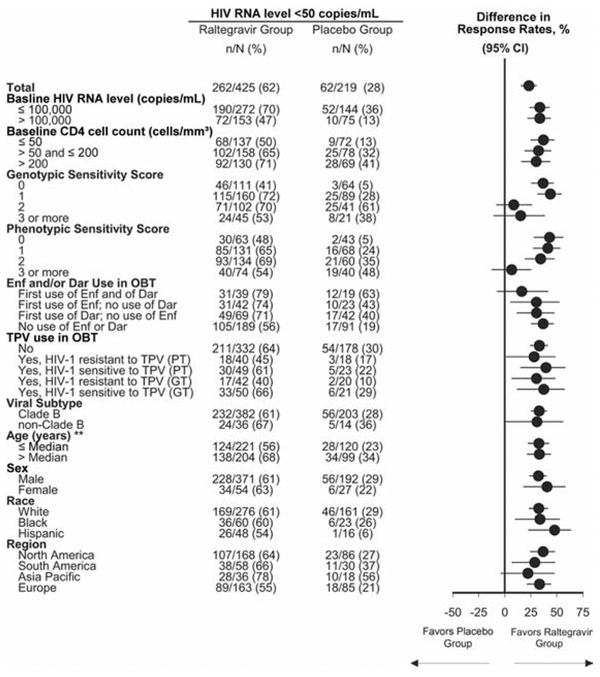
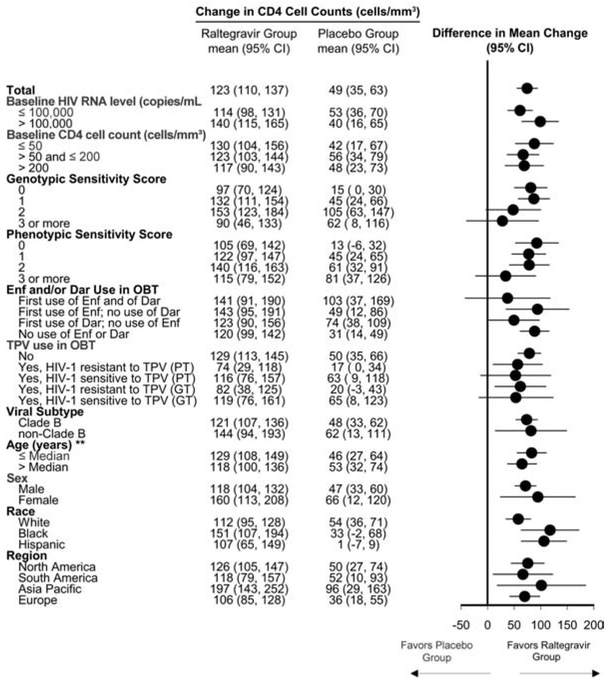
In subgroup analyses at week 96, efficacy, by prognostic factors, was generally consistent with the overall analysis, showing potent antiretroviral and immunological efficacy of raltegravir, compared with placebo (Figures 2 and 3), even in patients with poor prognostic factors at baseline, including high HIV RNA level, low CD4 cell count, and low genotypic and phenotypic sensitivity scores. For patients receiving multiple active drugs in their OBT, such as those with genotypic and phenotypic sensitivity scores of ≥2, there was a trend toward modestly higher numerical response rates for raltegravir, compared with placebo. Additional efficacy analyses, by viral subtype, age, sex, and race, demonstrated consistently greater response rates in the raltegravir group than in the placebo group.
Figure 2.
Proportion of patients with an HIV RNA level <50 copies/mL at week 96, by subgroup. Filled circles represent the point estimate of the between-group differences; horizontal bars represent the corresponding 95% confidence intervals (CIs). The vertical line at zero is provided as a reference indicating no treatment difference. Dar, darunavir; Enf, enfuvirtide; GT, genotypic test; OBT, optimized background therapy; PT, phenotypic test; TPV, tipranavir. **Median age, 45 years.
Figure 3.
Change in CD4 cell count from baseline to week 96, by subgroup. Filled circles represent the point estimate of the between-group differences; horizontal bars represent the corresponding 95% confidence intervals (CIs). The vertical line at zero is provided as a reference indicating no treatment difference. Dar, darunavir; Enf, enfuvirtide; GT, genotypic test; OBT, optimized background therapy; PT, phenotypic test; TPV, tipranavir. **Median age, 45 years.
Virologic failure occurred by week 96 in 150 (33%) of 462 raltegravir recipients and in 148 (62%) of 237 placebo recipients. Resistance test results were available for 112 of the raltegravir recipients who experienced virologic failure. Virus isolates from 73 (65%) of these patients had integrase mutations at 1 of 3 residues (Y143, Q148 or N155), usually in combination with at least 1 other mutation. Most drug-resistance mutations (77%) were observed by 24 weeks of therapy. Other known raltegravir-resistance mutations (E92E/Q and L74M+E92Q) were found in 2 of the 39 patients who did not have a primary raltegravir-resistance mutation. Available phenotypic data revealed no raltegravir resistance in virus isolates from the remaining 37 patients.
Outcomes were also available for 192 patients who entered the open-label post–virologic failure phase after documented virologic failure during the double-blind phase; these patients were permitted to reoptimize OBT, if possible, at entry to the open-label–post virologic failure phase. The mean duration of double-blind therapy at entry to the open-label post–virologic failure phase was 41 weeks for raltegravir recipients and 28 weeks for placebo recipients. At week 48 after entry to the open-label post–virologic failure phase, an HIV RNA level <50 copies/mL was achieved in 11 (15%) of 73 patients originally randomized to receive raltegravir therapy and in 51 (43%) of 119 patients originally randomized to receive placebo therapy; for this analysis, patients who did not complete the open-label post–virologic failure phase were counted as treatment failures. In both groups, only 18% of patients (13 of 73 and 22 of 119, respectively) had changed OBT to include new active antiretroviral agents.
Frequencies and exposure-adjusted rates of clinical adverse events (Table 3) and grade 3 and 4 laboratory abnormalities (Table 4) were similar in the raltegravir and placebo groups. Creatine kinase elevations were slightly more common in the raltegravir group but were not associated with clinical myopathy, myositis, or rhabdomyolysis and did not lead to treatment interruption or discontinuation. As of the cutoff date for this analysis, 13 patients (3%) in the raltegravir group and 7 patients (3%) in the placebo group had died during the double-blind phase of the study. Fatal adverse events in the double-blind phase since the previous report [5] were hypovolemic shock, cardiac failure, anal cancer, and head injury in the raltegravir group and lymphoma in the placebo group. Exposure-adjusted rates for new, recurrent, or progressive cancer during the double-blind phase were 3.0 cases per 100 person-years in the raltegravir group and 2.6 cases per 100 person-years in the placebo group (relative risk, 1.1; 95% confidence interval, 0.5–3.1).
Table 3.
Clinical Adverse Events (AEs).
| Variable | Raltegravir and OBT (n = 462) |
Placebo and OBT (n = 237) |
|---|---|---|
| Duration on therapy, mean weeks | 93.0 | 59.3 |
| PYR at risk | 823.8 | 269.4 |
| AEs | ||
| Any | 92.9 (52.1) | 88.6 (78.0) |
| Drug-relateda | 58.4 (32.8) | 58.6 (51.6) |
| Serious | 25.3 (14.2) | 22.4 (19.7) |
| Serious drug-related | 2.8 (1.6) | 3.8 (3.3) |
| Deaths | 2.8 (1.6) | 3.0 (2.6) |
| Requiring discontinuation of treatment | 3.7 (2.1) | 5.1 (4.5) |
| Most common drug-relatedb | ||
| Abdominal distension | 2.2 (1.2) | 1.7 (1.5) |
| Diarrhea | 3.2 (1.8) | 5.1 (4.5) |
| Nausea | 4.1 (2.3) | 4.6 (4.1) |
| Vomiting | 1.5 (0.8) | 2.1 (1.9) |
| Fatigue | 3.2 (1.8) | 0.8 (0.7) |
| Pyrexia | 0.9 (0.5) | 2.5 (2.2) |
| Headache | 4.8 (2.7) | 5.1 (4.5) |
NOTE. Data are percentage of patients with AEs (rate of AEs, cases per 100 person-years [PYR]), unless otherwise indicated. OBT, optimized background therapy.
Determined by the investigator to be possibly, probably, or definitely related to raltegravir or placebo (alone or in combination with OBT).
Incidence ≥2%, any intensity.
Table 4.
Grade 3 or 4 Laboratory Abnormalities
| Percentage of patients (rate, cases per 100 PYR) |
|||
|---|---|---|---|
| Laboratory test, toxicity criteria | Grade | Raltegravir and OBT (n = 462) |
Placebo and OBT (n = 237) |
| Hemoglobin level, g/dL | |||
| 6.5–7.4 | 3 | 0.9 (0.5) | 0.8 (0.7) |
| <6.5 | 4 | 0.2 (0.1) | 0 (0.0) |
| Absolute neutrophil count, 103 cells/μL | |||
| 0.50–0.749 | 3 | 3.0 (1.7) | 3.4 (3.0) |
| <0.50 | 4 | 1.3 (0.7) | 1.3 (1.1) |
| Platelet count, 103 cells/μL | |||
| 25–49.999 | 3 | 0.7 (0.4) | 0.4 (0.4) |
| <25 | 4 | 0.9 (0.5) | 0.4 (0.4) |
| Fasting LDL cholesterol level ≥190 mg/dL | 3 | 7.4 (3.2) | 6.1 (4.1) |
| Fasting cholesterol level >300 mg/dL | 3 | 11.0 (6.0) | 6.6 (5.6) |
| Fasting triglyceride level, mg/dL | |||
| 751–1200 | 3 | 6.7 (3.6) | 4.4 (3.7) |
| >1200 | 4 | 4.3 (2.3) | 2.2 (1.9) |
| Fasting glucose level, mg/dL | |||
| 251–500 | 3 | 2.7 (1.5) | 2.2 (1.9) |
| >500 | 4 | 0 (0.0) | 0 (0.0) |
| Creatinine level, mg/dL | |||
| 1.9–3.4 × ULN | 3 | 1.5 (0.8) | 0.8 (0.7) |
| ≥3.5 × ULN | 4 | 0.2 (0.1) | 0.4 (0.4) |
| Total bilirubin level, mg/dL | |||
| 2.6–5.0 × ULN | 3 | 3.0 (1.7) | 3.0 (2.6) |
| >5.0 × ULN | 4 | 0.9 (0.5) | 0 (0.0) |
| Aspartate aminotransferase level, IU/L | |||
| 5.1–10.0 × ULN | 3 | 4.3 (2.4) | 3.0 (2.6) |
| >10.0 × ULN | 4 | 0.7 (0.4) | 1.3 (1.1) |
| Alanine aminotransferase level, IU/L | |||
| 5.1–10.0 × ULN | 3 | 4.1 (2.3) | 2.5 (2.2) |
| >10.0 × ULN | 4 | 1.3 (0.7) | 1.7 (1.5) |
| Alkaline phosphatase level, IU/L | |||
| 5.1–10.0 × ULN | 3 | 0.4 (0.2) | 1.3 (1.1) |
| >10.0 × ULN | 4 | 0.7 (0.4) | 0.4 (0.4) |
| Pancreatic amylase level, IU/La | |||
| 2.1–5.0 × ULN | 3 | 5.0 (2.8) | 3.0 (2.6) |
| >5.0 × ULN | 4 | 0.2 (0.1) | 0.4 (0.4) |
| Lipase level, IU/L | |||
| 3.1–5.0 × ULN | 3 | 2.0 (1.1) | 0.8 (0.7) |
| >5.0 × ULN | 4 | 0 (0.0) | 0 (0.0) |
| Creatine kinase level, IU/L | |||
| 10.0–19.9 × ULN | 3 | 3.9 (2.2) | 2.5 (2.2) |
| ≥20.0 × ULN | 4 | 3.0 (1.7) | 0.8 (0.7) |
NOTE. Grades according to Division of AIDS criteria [8]. Both a baseline value and at least 1 on-treatment laboratory value had to be present. A patient was included as having a grade X event if his or her highest grade during treatment was X and the laboratory value was worse than that at baseline. LDL, low-density lipoprotein; OBT, optimized background therapy; ULN, upper limit of normal range.
Defined as no. of patients meeting specific pancreatic amylase criteria/no. of patients with amylase test result.
Discussion.
The ongoing BENCHMRK studies represent the longest placebo-controlled experience with raltegravir in treatment-experienced patients to date. More than 90% of patients in both studies had a history of AIDS. All patients had experienced failure of multiple prior antiretroviral regimens and had documented resistance to at least 1 nucleotide reverse-transcriptase inhibitor, 1 nonnucleotide reverse-transcriptase inhibitor, and 1 protease inhibitor. In these heavily pretreated patients with highly drug-resistant virus, raltegravir (400 mg twice daily) with OBT demonstrated a potent and superior antiretroviral effect, compared with OBT alone; 57% of patients in the raltegravir group achieved viral suppression to <50 copies/mL at week 96, compared with 26% of patients in the placebo group. These response rates are comparable to those observed at week 48 [5], showing durability of the superior efficacy of raltegravir.
Although the BENCHMRK studies were not powered to show statistically significant effects within subgroups [9], efficacy analyses by baseline prognostic factors continued to demonstrate a consistent treatment advantage of raltegravir over placebo, even in patients with high baseline HIV RNA levels or low baseline CD4 cell counts. Raltegravir also demonstrated superior efficacy, compared with placebo, in patients who received OBT and had genotypic and/or phenotypic sensitivity scores of 0—generally regarded as the most challenging treatment scenario. Among patients who received new, active antiretroviral therapy in their OBT, up to 79% of raltegravir recipients had undetectable HIV RNA levels at week 96. Overall, these data demonstrate that raltegravir has a potent antiviral effect in most patients with few or no remaining treatment options and has even greater efficacy when used in combination with other active antiretroviral agents.
Raltegravir was well tolerated in these trials despite a study population with advanced HIV infection and frequent comorbidities. After 96 weeks of treatment, adverse event profiles and laboratory abnormalities were generally comparable for raltegravir- and placebo-containing regimens, with few discontinuations of treatment because of adverse events. In addition, the development of cancer was comparable between the raltegravir and placebo groups.
In summary, in highly treatment-experienced HIV-infected patients, raltegravir combined with OBT provided continued superior viral suppression, compared with OBT alone, despite the presence of triple-class drug-resistant virus. The potent suppression of viremia observed in raltegravir recipients at week 16 and week 48 [5] was sustained through week 96. Raltegravir continued to demonstrate a consistently favorable treatment effect regardless of baseline viral load, CD4 cell count, genotypic and phenotypic sensitivity scores, or inclusion of enfuvirtide and/or darunavir in the OBT. The favorable safety profile of raltegravir after at least 96 weeks of treatment is consistent with previous reports [3–5]. These long-term data confirm that raltegravir offers a valuable treatment option for patients infected with multidrug-resistant HIV.
Acknowledgments
We thank all the patients and their caregivers who participated in this study; the investigators who enrolled their patients in this study, for their important contributions; and Bernard Akyena and Anthony Rodgers, for their expert assistance.
Financial support. Merck.
Footnotes
Principal investigators. BENCHMRK-1: A. Allworth, J. Anderson, M. Bloch, D. A. Cooper, J. Hoy, and C. Workman (Australia); N. Clumeck, R. Colebunders, and M. Moutschen (Belgium); J. Gerstoft, C. Larsen, L. Mathiesen, and C. Pedersen (Denmark); J. F. Delfraissy, P. Dellamonica, C. Katlama, J. M. Molina, F. Raffi, J. Reynes, D. Vittecoq, and P. Yeni (France); K. Arasteh, G. Fatkenheuer, H. Jaeger, J. Rockstroh, and A. Stoehr (Germany); F. Aiuti, G. Carosi, R. Cauda, F. Chiodo, G. Di Perri, G. Filice, M. Galli, A. Lazzarin, and V. Vullo (Italy); M. Castaneda, A. Florez, F. Mendo, A. Paredes, R. Salazar, and E. Ticona (Peru); R. Antunes, A. Diniz, K. Mansinho, J. Saraiva da Cunha, R. Sarmento, E. Teofilo, and J. Vera (Portugal); J. Arrizabalaga, B. Clotet, P. Domingo Pedrol, J. Gatell Artigas, S. Moreno Guillen, and V. Soriano Vazquez (Spain); B. Hirschel and M. Opravil (Switzerland); H.-H. Lin, W.-H. Sheng, and J.-H. Wang (Taiwan); S. Sungkanuparph and S. Suwanagool (Thailand). BENCHMRK-2: B. Grinsztejn, J. V. Madruga, and M. Schecter (Brazil); J.-G. Baril, M. R. Loutfy, J. S. Montaner, C. Tremblay, C. M. Tsoukas, and S. Vezina (Canada); J. A. Cortes, H. Mendoza, and J. Velez (Colombia); N. Quintero Perez, J. Ramos, and E. Rodriguez (Mexico); J. O. Morales-Ramirez and G. E. Sepulveda (Puerto Rico); J. Aberg, G. W. Beatty, P. Benson, R. K. Bolon, U. F. Bredeek, C. Bruno, T. Campbell, R. Campo, G. O. Coodley, R. B. Corales, E. DeJesus, J. J. Eron, W. J. Fessel, R. J. Fetchick, C. J. Gonzalez, C. Hicks, M. A. Horberg, D. B. Klein, M. J. Kozal, P. N. Kumar, A. LaMarca, J. L. Lennox, K. A. Lichtenstein, R. Liporace, S. J. Little, A. Luetkemeyer, F. Mariuz, M. Markowitz, D. K. McMahon, G. Perez, G. Pierone, R. C. Reichman, F. Rhame, P. Shalit, P. Sklar, W. Short, P. R. Skolnik, R. T. Steigbigel, E. M. Tedaldi, D. J. Ward, A. A. Wiznia, and D. P. Wright (United States).
Potential conflicts of interest. R.T.S. has received research support, speaker fees, and/or consulting fees from Merck. D.A.C. has received research support, speaker fees, and/or consulting fees from Merck. J.J.E. has received research support from Merck, Abbott, and Panacos and speaker fees and/or consulting fees from Merck, BMS, GSK, Gilead, Tibotec, Roche, and Pfizer. J.M.G. has received research support, speaker fees, and consulting fees from Merck. P.N.K. has been an investigator for Merck and GSK; has been a paid consultant to Boehringer-Ingelheim, BMS, GSK, and Tibotec; and has received speaker fees from GSK, Abbott, Tibotec, Pfizer, and Boehringer-Ingelheim. J.K.R. has received honoraria for lectures or advisory boards from Merck, Roche, GSK, BMS, Tibotec, Pfizer, Gilead, Abbott, and Boehringer Ingelheim. C.K. has received honoraria for advisory boards or lectures from Merck, Gilead, Roche, GSK, Tibotec, BMS, and Boehringer Ingelheim. M.M. has received research support, speaker fees, and/or consulting fees from Merck. P.Y. has received consulting fees from Merck. M.R.L. has received research support from Merck. A.L. has received honoraria for lectures or advisory board meetings and/or research support from Merck, GSK, BMS, Gilead, Roche, Tibotec, Pfizer, Boehringer-Ingelheim, Abbott, Monogram, and Schering-Plough. J.L.L. has received research support and speaker’s fees from Merck and serves on Merck’s antiretroviral scientific advisory board. B.C. has been a consultant on advisory boards, has participated in speakers’ bureaus, or has conducted clinical trials with Roche, Boehringer-Ingelheim, Abbott, BMS, GSK, Gilead, Tibotec, Janssen, Merck, Pfizer, Siemens, Monogram Biosciences, and Panacos. H.T., M.S., H.W., R.R.R., K.M.S., R.J.B., R.D.I., and B.-Y.T.N.are employees of Merck Research Laboratories and may own stock and/or stock options in the company.
References
- 1.Hazuda DJ, Felock P, Witmer M, et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 2000; 287:646–650. [DOI] [PubMed] [Google Scholar]
- 2.Miller M, Witmer M, Stillmock K, et al. Biochemical and antiviral activity of MK-0518, a potent HIV integrase inhibitor. In: Program and abstracts of the 16th International AIDS Conference (Toronto, Canada). 2006. Abstract THAA0302. [Google Scholar]
- 3.Gatell JM, Katlama C, Grinsztejn B, et al. Long-term efficacy and safety of the HIV integrase inhibitor raltegravir in patients with limited treatment options in a phase II study. J Acquir Immune Defic Syndr (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markowitz M, Nguyen B-Y, Gotuzzo E, et al. ; Protocol 004 Part II Study Team. Sustained antiretroviral effect of raltegravir after 96 weeks of combination therapy in treatment-naive patients with HIV-1 infection. J Acquir Immune Defic Syndr 2009; 52:350–356. [DOI] [PubMed] [Google Scholar]
- 5.Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med 2008; 359:339–354. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med 2008;359:355–365. [DOI] [PubMed] [Google Scholar]
- 7.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents.Department of Health and Human Services, 2008. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 29 September 2009.
- 8.Division of AIDS table for grading the severity of adult and pediatric adverse events, version 1.0, December 2004; clarification August 2009. http://rcc.tech-res.com/document/safetyandpharmacovigilance/DAIDS_AE_GradingTable_Clarification_August2009_Final.pdf. Accessed 13 January 2010.
- 9.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med 2007;357:2189–2194. [DOI] [PubMed] [Google Scholar]