Abstract
Summary
The present study, drawn from a sample of the Icelandic population, quantified high immediate risk and utility loss of subsequent fracture after a sentinel fracture (at the hip, spine, distal forearm and humerus) that attenuated with time.
Introduction
The risk of a subsequent osteoporotic fracture is particularly acute immediately after an index fracture and wanes progressively with time. The aim of this study was to quantify the risk and utility consequences of subsequent fracture after a sentinel fracture (at the hip, spine, distal forearm and humerus) with an emphasis on the time course of recurrent fracture.
Methods
The Reykjavik Study fracture registration, drawn from a sample of the Icelandic population (n=18,872), recorded all fractures of the participants from their entry into the study until December 31, 2012. Medical records for the participants, were manually examined and verified. First sentinel fractures were identified. Subsequent fractures, deaths, ten-year probability of fracture and cumulative disutility using multipliers derived from the International Costs and Utilities Related to Osteoporotic fractures Study (ICUROS) were examined as a function of time after fracture, age and sex.
Results
Over 10 years subsequent fractures were sustained in 28 % of 1498 individuals with a sentinel hip fracture. For other sentinel fractures the proportion ranged from 35-38 %. After each sentinel fracture, the risk of subsequent fracture was highest in the immediate post fracture interval and decreased markedly with time. Thus, amongst individuals who sustained a recurrent fracture, 31-45 % did so within one year of the sentinel fracture. Hazard ratios for fracture recurrence (population relative risks) were accordingly highest immediately after the sentinel fracture (2.6-5.3, depending on the site of fracture) and fell progressively over 10 years (1.5-2.2). Population relative risks also decreased progressively with age. The utility loss during the first 10 years after a sentinel fracture varied by age (less with age) and sex (greater in women). In women at the age of 70 years, the mean utility loss due to fractures in the whole cohort was 0.081 whereas this was 12-fold greater in women with a sentinel hip fracture, and was increased 15-fold for spine fracture, 4-fold for forearm fracture and 8-fold for humeral fracture.
Conclusion
High fracture risks and utility loss immediately after fracture suggests that treatment given as soon as possible after fracture would avoid a higher number of new fractures compared with treatment given later. This provides the rationale for very early intervention immediately after a sentinel fracture.
Keywords: Fracture probability, Utility loss, Imminent risk, Sentinel fracture
Introduction
It is now well established that a prior fragility fracture is an important risk factor for a future fracture [1–5]. The relative risk of having a hip fracture or a vertebral fracture is approximately 2-fold higher for most types of prior fracture. However, the increase in risk may not be constant with time or age. A large meta-analysis showed that a prior fracture history was a significant risk factor for hip fracture at all ages but was highest at younger ages and decreased progressively with age [3]. There is also a growing body of evidence that the risk of a subsequent osteoporotic fracture is particularly acute immediately after the index fracture and wanes progressively with time [1, 6–12]. A recent population-based study examined the age-dependency of this immediate increase in fracture risk [13] and showed that the phenomenon of immediate risk was also age-dependent, the transient effect being more evident at older ages.
This transiency suggests that treatment given to such patients immediately after fracture might avoid a higher number of new fractures compared with treatment given at a later date. This would provide the rationale for very early intervention immediately after fractures to avoid recurrent fractures. Such a strategy would have important health economic implications, not only for the number of fractures avoided, but also for the loss of quality of life that, for some fractures, have on average lifelong consequences.
The aim of the present study was to characterise the natural history and consequences of recurrent fractures, particularly the loss of utility. In clinical practice, when physicians are faced with patients with a recent fracture, prognostic guidance may be helpful in taking treatment decisions. Against this background, few studies have examined the immediate and long-term consequences of a sentinel fracture. The sentinel fractures in the present study are those at the hip, spine, forearm and humerus.
Methods
The study cohort consisted of 30,795 men and women, comprising all residents in the greater Reykjavik Area on December 1, 1967, born between 1907 and 1935 (inclusive), which represented 55 % of the total Icelandic population in this age range. The current analysis was based on 18,872 participants who attended during the recruitment period in 1967–1991, with 9,116 men and 9,756 women, with a 71.8 % recruitment rate.
Assessment of fractures
The Reykjavik Study fracture registration recorded all fractures of the participants from their entry into the study until December 31, 2012. All medical records for the participants, including referral letters if needed, were manually examined and verified. Fracture avulsions less than 5×6 mm, fractures secondary to malignancy and stress fractures were excluded by manual examination of medical records and radiographs. All fractures were registered according to the International Classification of Diseases (ICD version 10 or ICD version 9). The Reykjavik Study fracture registration has been shown to have a capture rate of about 97 % for hip, forearm, and clinical vertebral fractures [14]. In the present study, we describe the natural history following a humeral fracture (ICD 10: S42.2-S42.3), a vertebral fracture (ICD 10: S12.0-S12.2, S12.7, S22.0-S22.1, S32.0), a forearm fracture (ICD 10: S52.5-S52.6) or a hip fracture (ICD 10: S72.0-S72.2).
The cohorts selected for study comprised men and women with a first sentinel fracture, i.e. a hip, spine (clinical vertebral), forearm or humeral fracture. The date and sites of subsequent fractures were documented over a ten-year interval or for the duration of follow up (including death). Fractures at sites not considered to be associated with osteoporosis were excluded in the follow up. Fractures at the ankle, face, foot, hand, patella and skull were regarded as non-osteoporotic fracture. Fractures at the tibia were considered as osteoporotic fracture in women but not in men [15]. To minimise double counting [13], subsequent consecutive fractures that occurred at the same site were excluded where the interval between fractures was less than 30 days.
Outcomes
For each sentinel fracture the following outcomes were studied at fixed time intervals from the first fracture:
% fractured
% multiple fractures
Deaths
Ten-year probability of fracture
Cumulative disutility
The time intervals of interest were from baseline to 1, 2, 3, 4, 5, and 10 years’ duration of follow up.
Utilities
Cumulative loss of utilities was calculated using utility multipliers derived from the EQ-5D 3 Levels descriptive system (EQ-5D 3L). The instrument has shown good responsiveness to osteoporotic fracture [16, 17] and is widely used in health economic assessments [18]. Accumulated quality of life (QoL) loss and QoL multipliers were those derived where possible from the International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS), which is the largest prospective observational study on QoL consequences of osteoporotic fracture conducted to date with an analysis that included more than 3,000 fracture cases [19, 20]. Empirical data were available for most fracture sites. For ‘other femoral fracture’, it was assumed that these had the same utility loss as fractures at the hip. For ‘other fractures’ (clavicle, scapula, sternum), we used previously published utility values [15] (supplementary Table s1).
Utility loss
The multipliers in Table S1 were applied to health state values (HSVs) published for the UK general population [21], since Iceland-specific value sets were not available. The UK value set is considered to be the most robust and is recommended by the EUROQoL group in the absence of country-specific value sets [18]. At the start of follow up the HSV was set taking account of the sentinel fracture. For example, an individual with a humeral fracture entered the analysis with a utility loss of the age-specific HSV value X 0.19 over the first year, X 0.05 in the second year, etc. The inclusion of disutility of the sentinel fracture was considered more realistic than ignoring the utility loss of the sentinel fracture.
Thereafter, multipliers were applied for each subsequent eligible fracture that occurred within each subsequent period of 1/10th of a year for the duration of follow up. In the case of death, no utility loss was ascribed, since the aim of the study was to determine the consequences of the fracture in individuals who survived. Notwithstanding, deaths were recorded and incorporated in estimates of fracture probability that include competing mortality. Utility losses from non-osteoporotic fractures or co-morbidity were not included, though some utility loss captured in the ICUROS study would be related to co-morbidities.
Analysis
An extension of the Poisson regression model was used to study the relationship between age and time since baseline on the one hand and on the other hand, the risk of fracture and death with only one endpoint being counted per patient [22] expressed in person years. Age was piecewise linear with knots at 65 and 80 years for the groups with sentinel fractures and 50, 70 and 80 years for the entire population studied based on the distribution and range of age. In order to study the effect of time since sentinel fracture in detail a spline Poisson regression model was fitted using knots at 0.5, 2.5 and 15 years after the sentinel fracture. Outcomes of fracture and death were expressed as hazard ratios (HR) compared to the entire population denoted here as the ‘whole cohort’.
Ten-year probabilities of fracture at different sites for the whole cohort and for the cohorts with a sentinel fracture were calculated from the hazards of fracture and death [23]. Note that this was not a FRAX estimate but a calculation based on the hazards of death and fracture in the sentinel cohorts and the whole cohort.
The cumulative loss of utility was calculated for the whole cohort and for the population having a sentinel fracture at any of the four designated fracture sites. Since the age and sex distribution differed for the five populations, linear regression was applied within each cohort for utility loss according age and sex. Thus, utility losses were calculated for a man or woman of the same age in each population.
Results
The population (whole cohort) included 18,872 individuals aged on average 52.8 years (range 33-81 years) at baseline and 52% were women. The whole population was followed for 27 years. Sentinel hip fractures arose in 2074 men and women; for clinical spine, forearm and humerus fractures, the numbers were 1365, 2364 and 1092, respectively Subsequent fractures were sustained in 35 % of 2074 individuals with a sentinel hip fracture. For other sentinel fractures the proportion was 37-49 %. The lower refracture rate following a sentinel hip fracture was attributable to the higher death rate (74 %) compared with other sentinel sites of fracture (34-52 %). The mean duration of follow up was 5.7 years after a hip fracture and 9.8, 15.1 and 10.7 years after a clinical spine, forearm and humeral fracture, respectively. The mean age (range) for the first hip fracture was 79.6 (45-104) years. For sentinel spine, forearm and humeral fractures, the respective ages were 74.5 (39-97), 69.7 (34-99), and 73.8 (39-96) years. Characteristics by sex are given in supplementary Table S2.
Site of subsequent fracture
For sentinel fractures at the hip, spine and forearm, subsequent fractures were most commonly noted at the same site (supplementary Table S3). Site specificity was not observed following a humeral fracture. The most common subsequent fracture after a humeral fracture was a hip fracture followed by distal forearm fracture.
Time course of fracture
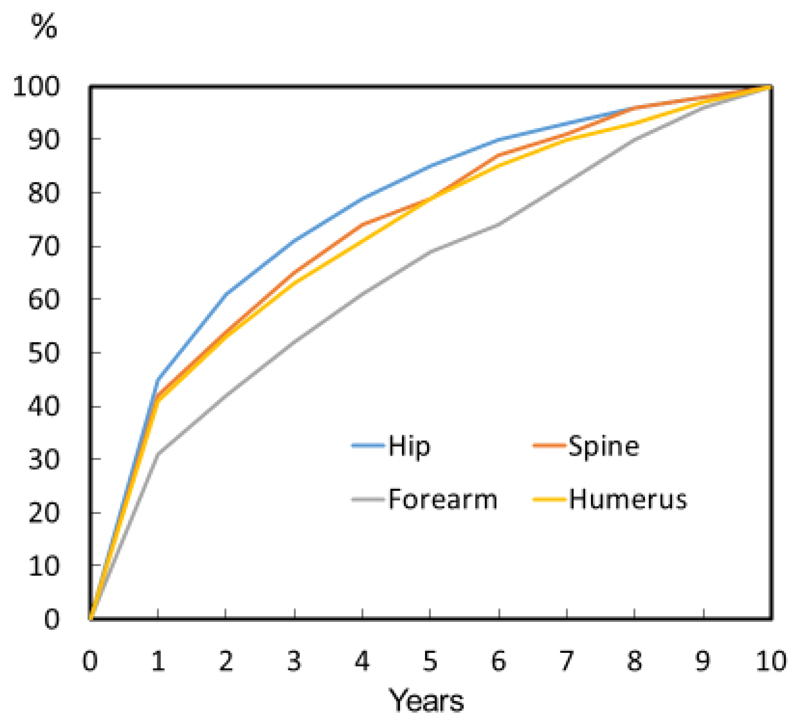
For all fracture sites, there was a time-dependent effect in that the recurrent fracture numbers waned with time (Figure 1). For example, in individuals with a further fracture following a sentinel hip fracture, 45 % sustained the first further fracture within one year of the sentinel fracture. For spine, forearm and humeral fractures, the proportion was 42, 31 and 41 %, respectively. In contrast, recurrence rates were less than 5 % between year 9 and 10.
Figure 1.
Time course of recurrent fractures in men and women following individual sentinel fractures, expressed as a percentage of all those experiencing recurrent fractures.
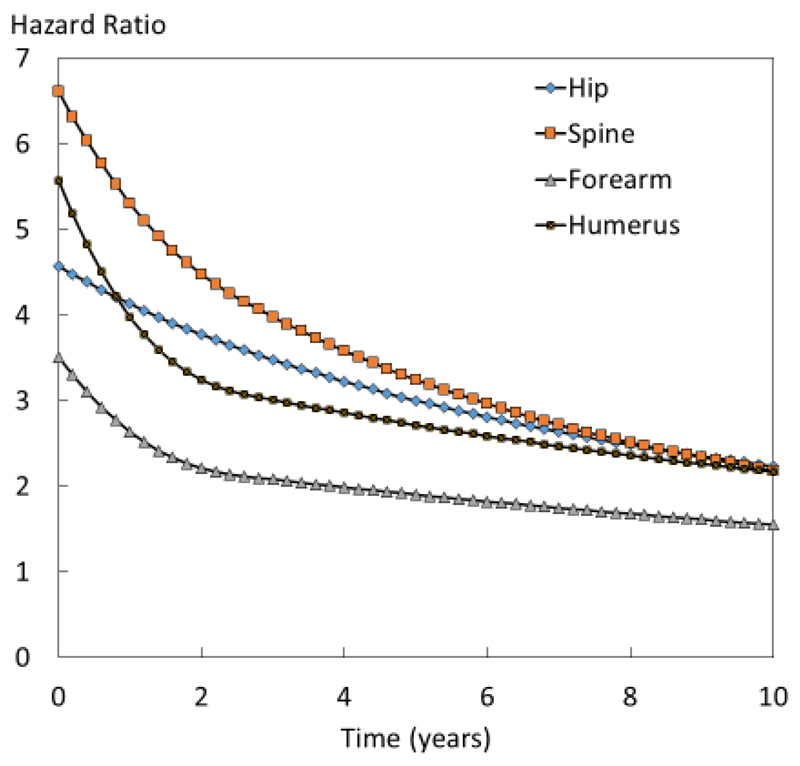
For all sentinel factures, the risk of a subsequent fracture compared with the whole cohort was highest immediately after the sentinel fracture and decreased with time. An example for women at the age of 70 years is shown in Figure 2. Hazard ratios (i.e. population relative risks) immediately after fracture were highest for spine fractures (HR=6.6 (95% CI: 5.1-8.6)), followed by fractures at the humerus (HR=5.6 (95% CI: 4.1-7.5), hip (HR=4.6 (95% CI: 3.5-6.0) and forearm (HR=3.5 (95% CI: 2.7-4.5). Hazard ratios fell progressively with time and at 10 years were 1.5 for forearm fractures and 2.2 for the other sentinel fracture sites.
Figure 2.
Hazard ratio (sentinel fracture compared with the whole cohort) of osteoporotic fracture in women at the age of 70 years by time according to the site of sentinel fracture
For all sentinel fractures, hazard ratios were generally lower at advanced age though there was no statistical interaction with age. Hazard ratios for men and women are shown by time and age in Table 1.
Table 1.
Hazard ratio (HR) for women of an osteoporotic fracture and 95% confidence intervals (95% CI) following a sentinel fracture at the sites shown by age and time compared to the age-matched female whole cohort.
| Time (years) since fracture | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | |||||
| Sentinel fracture | Age (years) at baseline |
HR | 95% CI | HR | 95% CI | HR | 95% CI |
| WOMEN | |||||||
| Hip | 60 | 3.8 | 2.4-5.9 | 3.4 | 2.6-4.5 | 2.8 | 2.2-3.5 |
| 70 | 4.6 | 3.5-6.0 | 3.0 | 2.6-3.5 | 2.2 | 1.8-2.8 | |
| 80 | 3.6 | 2.8-4.7 | 2.1 | 1.8-2.4 | 1.4 | 1.1-1.7 | |
| Spine | 60 | 6.4 | 4.7-8.8 | 3.5 | 2.8-4.4 | 2.6 | 2.1-3.2 |
| 70 | 6.6 | 5.1-8.6 | 3.2 | 2.8-3.7 | 2.2 | 1.8-2.7 | |
| 80 | 5.5 | 4.2-7.2 | 2.6 | 2.2-3.0 | 1.6 | 1.3-2.1 | |
| Forearm | 60 | 3.7 | 2.8-4.8 | 2.3 | 2.0-2.7 | 2.1 | 1.8-2.4 |
| 70 | 3.5 | 2.7-4.5 | 1.9 | 1.7-2.1 | 1.5 | 1.3-1.8 | |
| 80 | 2.6 | 2.0-3.4 | 1.6 | 1.4-1.8 | 1.5 | 1.2-1.8 | |
| Humerus | 60 | 5.7 | 4.0-8.1 | 2.8 | 2.2-3.6 | 2.5 | 2.0-3.1 |
| 70 | 5.6 | 4.1-7.5 | 2.7 | 2.4-3.1 | 2.2 | 1.7-2.7 | |
| 80 | 4.9 | 3.6-6.6 | 2.3 | 1.9-2.7 | 1.8 | 1.4-2.3 | |
| MEN | |||||||
| Hip | 60 | 6.8 | 4.2-10.9 | 6.1 | 4.5-8.3 | 5.1 | 3.9-6.6 |
| 70 | 8.2 | 6.1-11.1 | 5.4 | 4.4-6.6 | 4.0 | 3.1-5.2 | |
| 80 | 6.5 | 4.9-8.8 | 3.8 | 3.1-4.7 | 2.5 | 1.9-3.3 | |
| Spine | 60 | 8.8 | 6.3-12.4 | 4.8 | 3.7-6.3 | 3.6 | 2.8-4.6 |
| 70 | 9.1 | 6.7-12.4 | 4.5 | 3.6-5.5 | 3.0 | 2.3-3.9 | |
| 80 | 7.6 | 5.5-10.4 | 3.5 | 2.8-4.4 | 2.2 | 1.7-3.0 | |
| Forearm | 60 | 6.9 | 5.1-9.3 | 4.3 | 3.5-5.3 | 3.9 | 3.2-4.8 |
| 70 | 6.6 | 4.9-8.9 | 3.6 | 3.0-4.3 | 2.9 | 2.4-3.6 | |
| 80 | 4.9 | 3.6-6.7 | 3.0 | 2.5-3.7 | 2.8 | 2.2-3.5 | |
| Humerus | 60 | 8.0 | 5.4-11.8 | 3.9 | 2.8-5.4 | 3.5 | 2.6-4.6 |
| 70 | 7.8 | 5.4-11.2 | 3.8 | 3.0-4.8 | 3.0 | 2.3-4.1 | |
| 80 | 6.8 | 4.7-9.8 | 3.2 | 2.4-4.2 | 2.5 | 1.8-3.4 | |
Deaths
In the whole cohort, 1768 deaths occurred within the 10 years of follow up. The risk of death was highest in the cohort with a sentinel hip fracture; 1335 deaths occurred within 10 years of follow up representing 64% of the original cohort (supplementary Table S4). At 10 years, the crude death rate was lowest following a forearm fracture (22%) and intermediate after humeral fracture (35%) or spine fracture (48%). The crude death rates (see supplementary Table S2) do not take account of the differing ages at the time of fracture.
As expected, there were higher age-specific death rates in men than in women (p<0.001, data not shown). The age- and sex adjusted hazard ratios for death rate are shown in Table 2. In general, mortality was higher than that of the whole cohort for hip and spine fractures. For forearm and humeral fractures, there was no excess mortality. Indeed, in the case of forearm fractures, death rates were generally lower than that of the whole cohort.
Table 2.
Hazard ratios (HR with 95% confidence intervals) of death associated with a sentinel fracture compared to the whole cohort of the same age and sex at 5 years after the sentinel fracture (and 5 years after baseline for the whole cohort). Bold type indicates HRs that are significantly different from the whole cohort.
| Sentinel fracture | ||||||||
|---|---|---|---|---|---|---|---|---|
| Hip | Spine | Forearm | Humerus | |||||
| Age | Men | Women | Men | Women | Men | Women | Men | Women |
| 60 | 0.57 (0.13, 2.53) |
3.00* (1.61, 5.60) |
0.68 (0.27, 1.70) |
2.36 * (1.39, 4.00) |
0.57 (0.26, 1.27) |
0.07 * (0.02, 0.26) |
0.92 (0.37, 2.30) |
0.94 (0.42, 2.13) |
| 70 |
2.69 (1.89, 3.83) |
2.76 (2.07, 3.69) |
1.21 (0.79, 1.87) |
1.85 (1.37, 2.49) |
0.66 (0.43, 1.02) |
0.37 * (027, 0.51) |
1.09 (0.68, 1.76) |
0.86 (0.57, 1.31) |
| 80 |
2.31 (1.91, 2.79) |
2.20 (1.91, 2.53) |
1.46 (1.15, 1.86) |
1.41 (1.19, 1.67) |
0.74 (0.56, 0.97) |
0.83 (0.71, 0.96) |
1.27 (0.95, 1.69) |
1.05 (0.87, 1.28) |
| 90 |
1.40 (1.18, 1.65) |
1.60 (1.42, 1.79) |
1.06 (0.86, 1.31) |
1.24 (1.07, 1.42) |
0.74 (0.59, 0.93) |
0.88 (0.77, 1.00) |
1.11 (0.84, 1.46) |
1.05 (0.89, 1.23) |
p<0.05 comparing men and women, ns otherwise
Fracture probability
The 10-year probability of major osteoporotic fracture was higher in the population with a sentinel fracture than in the whole cohort of the same age and sex. For example, in men, the probability at the age of 60 years after a spine fracture was 6-times higher than that of the whole cohort of the same age and sex. The probability ratio fell, however, with age from 6 to 5, 3 and 3 in men age 70, 80 and 90 years, respectively. For women, the same ratio at age 60 was 3 falling to 1.4 at the age of 90 years. Thus, the difference between 10-year probabilities between cases and the whole cohort was greater for men than for women for all ages. Similar effects were noted for other sentinel fractures (supplementary Table s5).
Utility loss
Utility loss over 10 years was much greater in fracture cases than in the whole cohort. Loss was greatest following a vertebral fracture (0.92; 95% CI: 0.89-0.95), followed by hip (0.63; 95% CI: 0.61-0.65), humeral (0.51 (95% CI: 0.49-0.53) and forearm fracture (0.32; 95% CI: 0.31-0.33).
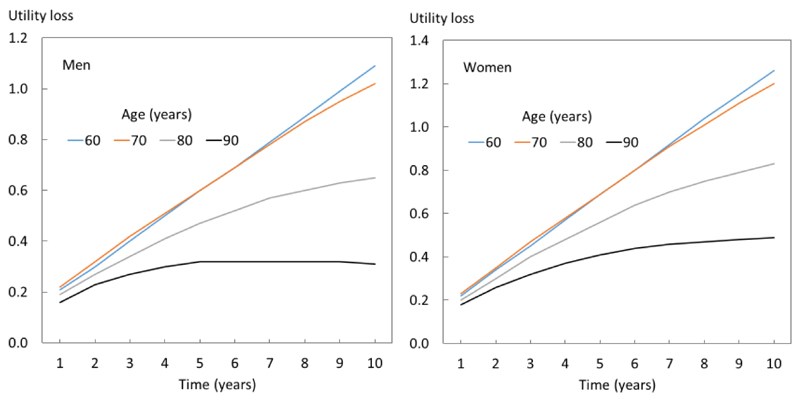
Since the whole cohort and each of the sentinel fracture cohorts differed in age and sex distribution, a linear regression model was applied to derive utility losses that were age- and sex-specific. The utility loss sustained during the subsequent 10 years for men and women with a sentinel fracture varied both by age and sex. An example is given in Figure 3 for men and women with a sentinel vertebral fracture (n=1365). The mean loss was 0.92 (95% CI: 0.89-0.95) but decreased with age, related to the attrition due to mortality and the lower HSVs on which the multipliers were applied. As expected, utility loss was higher in women than in men due to the higher risk of fractures in women.
Figure 3.
Cumulative utility loss for men and women at ages shown after vertebral fracture
Utility loss decreased with age for the cohorts with a sentinel fracture but increased with age for the whole cohort for both men and women. Thus, the excess utility loss (observed/whole cohort population) decreased with age for all sentinel fractures so that, after 10 years, it was 2 to 3 times higher than in the whole cohort. Data for men and women are given in Table 3. The utility loss was higher for women than for men.
Table 3.
Loss of utility for men and women at different ages after 10 years follow up with 95% confidence intervals.
| At the age (years) | Subgroup with sentinel fracture |
Whole cohort | Ratioa |
|---|---|---|---|
| Hip | |||
| Men | |||
| 60 | 1.00 (0.93-1.07) | 0.022 (0.018-0.027) | 45 |
| 70 | 0.82 (0.78-0.87) | 0.056 (0.050-0.062) | 15 |
| 80 | 0.45 (0.40-0.50) | 0.091 (0.079-0.10) | 5 |
| 90 | 0.21 (0.16-0.26) | 0.12 (0.11-0.14) | 2 |
| Women | |||
| 60 | 1.17 (1.11-1.24) | 0.047 (0.043-0.051) | 25 |
| 70 | 1.00 (0.96-1.04) | 0.081 (0.075-0.087) | 12 |
| 80 | 0.63 (0.59-0.67) | 0.12 (0.10-0.13) | 5 |
| 90 | 0.39 (0.35-0.43) | 0.15 (0.13-0.17) | 3 |
| Spine | |||
| Men | |||
| 60 | 1.09 (1.02-1.16) | 0.022 (0.018-0.027) | 50 |
| 70 | 1.02 (0.95-1.09) | 0.056 (0.050-0.062) | 18 |
| 80 | 0.65 (0.59-0.72) | 0.091 (0.079-0.10) | 7 |
| 90 | 0.31 (0.22-0.41) | 0.12 (0.11-0.14) | 3 |
| Women | |||
| 60 | 1.26 (1.21-1.32) | 0.047 (0.043-0.051) | 27 |
| 70 | 1.20 (1.14-1.25) | 0.081 (0.075-0.087) | 15 |
| 80 | 0.83 (0.78-0.88) | 0.12 (0.10-0.13) | 7 |
| 90 | 0.49 (0.21-0.36) | 0.15 (0.13-0.17) | 3 |
| Humerus | |||
| Men | |||
| 60 | 0.55 (0.49-0.61) | 0.022 (0.018-0.027) | 25 |
| 70 | 0.54 (0.49-0.50) | 0.056 (0.050-0.062) | 10 |
| 80 | 0.37 (0.31-0.44) | 0.091 (0.079-0.10) | 4 |
| 90 | 0.19 (0.10-0.27) | 0.12 (0.11-0.14) | 2 |
| Women | |||
| 60 | 0.64 (0.60-0.69) | 0.047 (0.043-0.051) | 14 |
| 70 | 0.64 (0.60-0.68) | 0.081 (0.075-0.087) | 8 |
| 80 | 0.47 (0.42-0.51) | 0.12 (0.10-0.13) | 4 |
| 90 | 0.28 (0.21-0.36) | 0.15 (0.13-0.17) | 2 |
| Forearm | |||
| Men | |||
| 60 | 0.34 (0.31-0.38) | 0.022 (0.018-0.027) | 15 |
| 70 | 0.35 (0.31-0.39) | 0.056 (0.050-0.062) | 6 |
| 80 | 0.31 (0.27-0.34) | 0.091 (0.079-0.10) | 4 |
| 90 | 0.25 (0.20-0.31) | 0.12 (0.11-0.14) | 2 |
| Women | |||
| 60 | 0.35 (0.32-0.37) | 0.047 (0.043-0.051) | 7 |
| 70 | 0.35 (0.31-0.38) | 0.081 (0.075-0.087) | 7 |
| 80 | 0.31 (0.29-0.33) | 0.12 (0.10-0.13) | 3 |
| 90 | 0.25 (0.21-0.30) | 0.15 (0.13-0.17) | 2 |
Ratios between utility loss for whole cohort and the cohorts with sentinel fracture
Discussion
It is well established that a fragility fracture increases the risk of a further fracture. This paper outlines the temporal consequences of the 4 fracture sites that comprise major osteoporotic fractures, namely the hip, spine, forearm and humerus. The common finding is that the increased risk of subsequent fracture is not constant with time or age. In the present study, 45 % of individuals who refractured did so within one year of a sentinel hip fracture. For spine, forearm and humeral fractures, the proportion was 42, 31 and 41 %, respectively. Thus, the one-year period following a sentinel fracture represents an interval of very high risk for fracture that can be termed imminent risk. At 10 years, the risk had decreased to values consistent with meta-analyses that did not include time since fracture [1, 5].
The waning effect on refracture risk after a sentinel fracture has implications for the assessment of fracture risk with FRAX. Whereas a prior fragility fracture (without knowledge of its date) confers a 1.5-2-fold increase in fracture probability, the present study suggests that, in the presence of a recent fracture, 10-year probability is underestimated in many cases by more than two-fold. Thus, the recency of prior fracture, at least for major osteoporotic fractures, may influence the interpretation of FRAX if the phenomenon is replicated in independent cohorts.
The phenomenon of imminent risk has other consequences of clinical and economic significance. Each fracture contributes to a loss in quality of life. The present report has characterised the disutility incurred by fractures over 10 years which is substantial particularly after hip and spine fractures – but not inconsequential following a forearm or humeral fracture. In the present analysis, the utility loss was much greater in an individual with a sentinel fracture than in the whole cohort. The difference became less with increasing age or longer follow up. The utility loss was higher for women than for men for both sentinel fractures and in the whole cohort. Note, however, that the utility loss in individuals with a sentinel fracture includes the losses due to the sentinel fracture itself which would not generally be avoidable following a therapeutic intervention. When these are excluded (data not shown), the cumulative loss of utility decreased, as expected, but remained significantly higher than that of the general population at the ages of 60-80 years. At older ages for men, the utility loss for hip, vertebral and humeral fractures was less than that in the whole population, most probably due to the high mortality which is not accounted for in this analysis. It is also important to note that the utility losses do not include deaths which were increased after hip and spine fractures. Thus, the overall QALYs lost are underestimated.
An increased risk immediately after fracture that subsequently wanes over time is now well established [1, 6, 7, 10, 11, 12, 13]. This transiency suggests that treatment given to patients immediately after fracture might avoid a higher number of new fractures compared with treatment given some time later. This provides a rationale for very early intervention immediately after fractures to avoid recurrent fractures. Such a strategy, consistent with the aims of fracture liaison services [24], has important implications for public health and health economic analysis. The present study, drawn from a large population sample, quantifies the risk over time for each major osteoporotic fracture and will permit the population of future fracture-specific health economic models with fracture-specific scenarios. The strategy of early intervention depends on the assumption that the immediate increment in risk is amenable to therapeutic intervention. Many randomised studies have shown the early onset of effectiveness of pharmaceutical intervention for spine fractures and in some cases for appendicular fractures [25]. Although early intervention at the time of fracture has not been widely tested, the risk of a clinical fracture has been shown to decrease by 35% when zoledronic acid was given to patients very shortly after a sentinel hip fracture [26], suggesting that the assumption is sound.
The reason for the transient marked increase in risk is not known, but immobilisation and impaired coordination are potential factors [27, 28, 29]. Indeed, a recent study of US claim databases identified fall-related factors such as age, poor mobility neurological comorbidity and psychoactive medication use as associated with increased risk of first fracture over 12–24 months [29].
Strengths in this study was the random sampling of a large population and the detail placed on fracture ascertainment and the long duration of observation. Participants were identified from nationwide registers representing 34% of the midlife Icelandic population born between 1907 and 1935 [30, 31], so that major selection bias is unlikely. However, there were also, some limitations to this study. First, the validity of fracture ascertainment was collected retrospectively but was based on all available records and X-rays from the main hospitals in Iceland. Although the accuracy of the acquisition of hip, clinical spine and forearm fractures is high (>97 %) [14], the accuracy for other fracture sites has not been determined. Second, there are known to be substantial differences in age and sex-specific fracture incidence in different regions of the world [32]. Although the absolute incidence values we observed may not be representative of other populations, there is no reason to suppose that there would be differences in the hazard ratios with time. Third, radiographically defined vertebral fractures were not included. This would have increased the rates, but we aimed to assess clinical fractures, and temporal evaluation of radiographic vertebral fractures would have required multiple sequential radiographs. Fourth, as with all such studies, the possibility of under-ascertainment and misclassification exists, but as both capture and classification of several fractures have been shown to be highly reliable in this cohort [14], it is unlikely that this would alter the results materially. Finally, health state values and utility losses were necessarily derived from data not derived in Iceland.
A problem that potentially confounds most long-term studies of incident fractures is the risk of double counting, and this can be of major relevance in studies examining rates of re-fracture within short timeframes. This is particularly problematic for vertebral fractures since the diagnosis is confirmed by radiography and the deformities are persistent over time, at least in adults. In a previous study, varying the interval where a second fracture at the same site was counted, had little effect on the pattern with time. The most robust sensitivity analysis was to only count the second fracture when the site of the second fracture differed from the site of the first. The imminent fracture risk was still higher than after 5–10 years [13]. These findings indicate that the concept of imminent fracture risk is a reality rather than an artefact of double counting.
The attrition of the rate of utility loss with age after sentinel fracture occurred for two reasons. First, the starting HSV was lower with advancing age so that the multipliers associated with incident fractures had less overall impact. Second, and more important, was the appreciable mortality following each sentinel cohort. Indeed, in the case of hip and clinical vertebral fracture, mortality was significantly higher than that of the age-matched whole population. Since death was not incorporated into the utility loss, progressively fewer patients were at risk – a phenomenon that increased with age. Utility losses in those patients that survived 10 years indicated that, after a sentinel fracture, the rate of utility losses due to subsequent fractures decreased less with age (data not shown). It is not clear, however, whether this finding was due to a ‘healthy survivor bias’.
We conclude that the risk of additional fracture after a first major osteoporotic fracture is increased over at least 10 years, but the imminent fracture risk is even higher. Our findings suggest that treatment should be started immediately after a major osteoporotic fracture to reduce the high immediate risk of future fracture.
Supplementary Material
Acknowledgements
We thank the participants in the Reykjavik Study for their valuable contribution.
Footnotes
Compliance with ethical standards
The study was approved by the National Bioethics Committee and the Data Protection Authority in Iceland. All participants gave informed written consent.
Competing interest
Radius Health, Inc., was the sponsor for this study and provided financial support for its completion. LF is an employee of Radius Health. None of the other authors declare competing interests with regard to this paper.
References
- 1.Johnell O, Kanis JA, Oden A, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int. 2004;15:175–179. doi: 10.1007/s00198-003-1514-0. [DOI] [PubMed] [Google Scholar]
- 2.Hansen L, Petersen KD, Eriksen SA, et al. Subsequent fracture rates in a nationwide population-based cohort study with a 10-year perspective. Osteoporos Int. 2015;26:513–9. doi: 10.1007/s00198-014-2875-2. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Haentjens P, Johnell O, Kanis JA, et al. Gender-related differences in short and long-term absolute risk of hip fracture after Colles’ or spine fracture: Colles’ fracture as an early and sensitive marker of skeletal fragility in men. J Bone Miner Res. 2004;19:1933–1944. doi: 10.1359/JBMR.040917. [DOI] [PubMed] [Google Scholar]
- 5.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 6.Johnell O, Oden A, Caulin F, Kanis JA. Acute and long-term increase in fracture risk after hospitalization for vertebral fracture. Osteoporos Int. 2001;12:207–214. doi: 10.1007/s001980170131. [DOI] [PubMed] [Google Scholar]
- 7.Giangregorio LM, Leslie WD, Manitoba Bone Density Program Time since prior fracture is a risk modifier for 10-year osteoporotic fractures. J Bone Miner Res. 2010;25:1400–5. doi: 10.1002/jbmr.35. [DOI] [PubMed] [Google Scholar]
- 8.Dretakis KE, Dretakis EK, Papakitsou EF, Psarakis S, Steriopoulos K. Possible predisposing factors for the second hip fracture. Calcif Tissue Int. 1998;62:366–369. doi: 10.1007/s002239900446. [DOI] [PubMed] [Google Scholar]
- 9.Nymark T, Lauritsen JM, Ovesen O, Röck ND, Jeune B. Short time-frame from first to second hip fracture in the Funen County Hip Fracture Study. Osteoporos Int. 2006;17(9):1353–7. doi: 10.1007/s00198-006-0125-y. 2006. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 11.Ryg J, Rejnmark L, Overgaard S, Brixen K, Vestergaard P. Hip fracture patients at risk of second hip fracture: a nationwide population-based cohort study of 169,145 cases during 1977-2001. J Bone Miner Res. 2009;24:1299–307. doi: 10.1359/jbmr.090207. [DOI] [PubMed] [Google Scholar]
- 12.van Geel TACM, van Helden S, Geusens PP, Winkens B, Dinant G-J. Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis. 2016;68:99–102. doi: 10.1136/ard.2008.092775. [DOI] [PubMed] [Google Scholar]
- 13.Johansson H, Siggeirsdóttir K, Harvey NC, et al. Imminent risk of fracture after fracture. Osteoporos Int. 2017;28:775–780. doi: 10.1007/s00198-016-3868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siggeirsdottir K, Aspelund T, Sigurdsson G, et al. Inaccuracy in self-report of fractures may underestimate association with health outcomes when compared with medical record based fracture registry. Eur J Epidemiol. 2007;22:631–639. doi: 10.1007/s10654-007-9163-9. [DOI] [PubMed] [Google Scholar]
- 15.Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int. 2001;12:417–427. doi: 10.1007/s001980170112. [DOI] [PubMed] [Google Scholar]
- 16.Olerud P, Tidermark J, Ponzer S, Ahrengart L, Bergstrom G. Responsiveness of the EQ-5D in patients with proximal humeral fractures. J Shoulder Elbow Surg. 2011;20:1200–6. doi: 10.1016/j.jse.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Tidermark J, Bergstrom G. Responsiveness of the EuroQol (EQ-5D) and the Nottingham Health Profile (NHP) in elderly patients with femoral neck fractures. Qual Life Res. 2007;16:321–30. doi: 10.1007/s11136-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 18.Oppe M, Devlin NJ, Szende A. EQ-5D value sets: inventory, comparative review and user guide. Springer; 2007. [PubMed] [Google Scholar]
- 19.Borgstrom F, Zethraeus N, Johnell O, et al. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int. 2006;17:637–50. doi: 10.1007/s00198-005-0015-8. [DOI] [PubMed] [Google Scholar]
- 20.Svedbom A, Borgstöm B, Hernlund E, et al. Quality of life after hip, vertebral, and distal forearm fragility fractures measured using the EQ-5D-3L, EQ-VAS, and Time Trade-Off: Results from the ICUROS. Quality of Life Research. 2017 doi: 10.1007/s11136-017-1748-5. Osteoporos Int, in press. [DOI] [PubMed] [Google Scholar]
- 21.Dolan P. Modeling valuations for EuroQol health states. Medical Care. 1997;35:1095–108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Breslow NE, Day NE. Statistical Methods in Cancer Research. 32. II. IARC Scientific Publications; 1987. pp. 131–135. [PubMed] [Google Scholar]
- 23.Oden A, Dawson A, Dere W, Johnell O, Jonsson B, Kanis JA. Lifetime risk of hip fracture is underestimated. Osteoporos Int. 1998;8:599–603. doi: 10.1007/s001980050105. [DOI] [PubMed] [Google Scholar]
- 24.Javaid MK, Kyer C, Mitchell PJ, et al. Effective secondary fracture prevention: implementation of a global benchmarking of clinical quality using the IOF Capture the Fracture® Best Practice Framework tool. Osteoporos Int. 2015;26:2573–8. doi: 10.1007/s00198-015-3192-0. [DOI] [PubMed] [Google Scholar]
- 25.Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799–809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bischoff Ferrari HA, Dawson Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 28.Helden van S, Wyers CE, Dagnelie PC, et al. Risk of falling in patients with a recent fracture. BMC Musculoskelet Disord. 2007;8:55. doi: 10.1186/1471-2474-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonafede M, Shi N, Barron R, Li X, Crittenden DB, Chandler D. Predicting imminent risk for fracture in patients aged 50 or older with osteoporosis using US claims data. Arch Osteoporos. 2016;11:26. doi: 10.1007/s11657-016-0280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjornsson G, Bjornsson OJ, Davidsson D, et al. Report abc XXIV. Health survey in the Reykjavik area—women. Stages I-III, 1968–1969, 1971–1972 and 1976–1978. Participants, invitation, response etc. The Icelandic Heart Association; Reykjavík: 1982. [Google Scholar]
- 31.Bjornsson OJ, Davidsson D, Olafsson H, et al. Report XVIII. Health survey in the Reykjavik area—men. Stages I–III, 1967–1968, 1970–1971 and 1974–1975. Participants, invitation, response etc. The Icelandic Heart Association; Reykjavík: 1979. [Google Scholar]
- 32.Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl D, Cyrus Cooper C, on behalf of the IOF Working Group on Epidemiology and Quality of Life A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23:2239–2256. doi: 10.1007/s00198-012-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.