Abstract
Small bowel cancers account for 3% of all gastrointestinal malignancies. Small bowel adenocarcinomas represent a third of all small bowel cancers. Rarity of small bowel adenocarcinomas restricts molecular understanding and presents unique diagnostic and therapeutic challenges. Better cross-sectional imaging techniques and development of enteroscopy and capsule endoscopy have facilitated earlier and more accurate diagnosis. Surgical resection remains the mainstay of therapy for locoregional disease. In metastatic setting, fluoropyrimidine and oxaliplatin based chemotherapy has shown clinical benefit in prospective non-randomized trials. Although frequently grouped under the same therapeutic umbrella as large bowel adenocarcinomas, small bowel adenocarcinomas are distinct clinical and molecular entities. Recent progress in molecular characterization has aided our understanding of the pathogenesis of these tumors and holds potential for prospective development of novel targeted therapies. Multi-institutional collaborative efforts directed towards cogent understanding of tumor biology and designing sensible clinical trials are essential for developing improved therapeutic strategies. In this Review, we endeavor to outline an evidence based approach to present-day management of small bowel adenocarcinoma, describe contemporary challenges and uncover evolving paradigms in management of these rare “orphan” neoplasias.
Keywords: Small bowel cancer, small bowel adenocarcinoma, small intestinal cancer, small intestinal adenocarcinoma
Introduction
Small bowel cancers represent a group of histologically diverse tumours. Carcinoids, adenocarcinomas, lymphomas and sarcomas represent the common histological small bowel types, which have a varied distribution across the three anatomical segments of the small intestine: duodenum, jejunum and ileum (Figure 1).1–3Albeit rare, these small bowel cancers, with an estimated 8,810 new cases diagnosed in the USA in 2013, have an incidence rate comparable to chronic myeloid leukaemia, testicular cancer, Hodgkin disease and anal cancer.4 However, small bowel cancers have received relatively little attention, both in terms of clinical cognizance and research efforts. The incidence of small bowel cancers is rising and their epidemiological landscape is changing.1,3,5,6 Carcinoids, that comprise 44% of all small bowel cancers, currently constitute the dominant histology whereas adenocarcinomas represent approximately one-third of all small bowel cancers.5 Discussion of all histological subtypes is beyond the scope of this Review. This Review will focus exclusively on small bowel adenocarcinomas. We summarize the existing knowledge of this ‘orphan’ malignancy and highlight the recent advances in our molecular understanding of the disease, together with diagnostic approaches and therapeutic options.
Figure 1 |.

Epidemiology of small bowel tumours from the National Cancer Data Base (NCDB) (1985–2005) and U.S. Surveillance, Epidemiology and End Results (SEER) (1973–2005) cohorts and Connecticut Tumor Registry 1980–2000.2,3,6 a | Majority of adenocarcinomas and carcinoids are seen in the duodenum and the ileum, respectively. b | Incidence of small bowel tumours, especially carcinoids, adenocarcinomas and lymphomas has increased in the past few years. c | The proportion of histological tumour subtypes found in the small bowel varies depending on the anatomic location of the small bowel.
Small bowel adenocarcinomas
Epidemiology
Small bowel adenocarcinomas represent the second most common small bowel cancer with an annual incidence of about 7.3 cases per million worldwide.5,6 The incidence rates vary with geographic regions, with higher rates in North America and Western Europe and lower rates in Asian countries.7 Overall, both small bowel adenocarcinoma and colorectal cancer (CRC) are more common in developed countries compared with developing countries.7 In the USA, it is estimated that approximately 3,250 new cases of small bowel adenocarcinomas will be diagnosed in 2013.4,5Although small bowel adenocarcinomas are found throughout the length of the small intestine, more than half (56%) are located in the duodenum (Figure 1).5 There is a slightly higher proportion of small bowel adenocarcinomas in males compared with females.8–10 The age-adjusted incidence rates of small bowel adenocarcinomas is highest among blacks (14.1/1,000,000) followed by whites (7.7) and Hispanics (6.2), and is lowest among Asians/Pacific Islanders (5.5).11
Small bowel adenocarcinoma and colorectal adenocarcinoma
Owing to their rarity and anatomic proximity to the large bowel, small bowel adenocarcinomas have frequently been grouped with large bowel adenocarcinomas. A number of interesting differences and similarities exist between these two cancers (Table 1).12–17 The incidence rates for these cancers are diverging in the US, with a rising rate for small bowel adenocarcinomas and a declining rate for CRC.5,18 However, these trends vary globally.6,7,18 Furthermore, outcomes for small bowel adenocarcinomas have been shown to be worse than for CRC.9,19 Earlier comparisons were deficient because they did not account for the advanced stage of small bowel adenocarcinomas, owing to inadequate lymph-node sampling in resected small bowel adenocarcinomas and anatomical difference due to partial retroperitoneal location of duodenum.9,19 However, recent work using the Surveillance, Epidemiology and End Results (SEER) database demonstrated that even after corrections to minimize the effect of stage migration and inadequate lymph-node evaluation, small bowel adenocarcinomas have a poorer stage-stratified cancer specific survival than colon cancer.12 Evidently, the 5-year stage-specific survival, even in stage I adenocarcinomas of jejunum and ileum with adequate lymph-node sampling is worse compared to stage I colon cancer, 81.6% versus 93.3%, P< 0.01, respectively.12
Table 1 |.
Comparison between small bowel and large bowel adenocarcinoma
| Factors | Small bowel adenocarcinomas | Large bowel adenocarcinomas |
|---|---|---|
| Estimated new cases in 2013 (USA) | 3,250 | 142,820 |
| Age-standardized incidence | Rising (+ 1.47% per year) | Falling (− 1.24% per year) |
| Age-standardized mortality | Stable | Decreasing (−2.31% per year) |
| Median age at diagnosis | 67 years | 71 years |
| Gender distribution | Male > female | Male > Female |
| Race distribution | Blacks > whites | Blacks > whites |
| Stage IV presentation | 32% | 20% |
| High-grade tumours | 33% | 21% |
| APC mutation rate | 7–13% | 60–68% |
| Lifetime cancer risk with Lynch Syndrome | 2–8% | 39–70% |
| 65-year cumulative risk with PJS | 13% | 39% |
| Lifetime cancer risk with FAP | 3–5% | 100% |
| IBD most associated | Crohn’s disease | Ulcerative colitis |
Abbreviations: FAP, Familial adenomatous polyposis; IBD, inflammatory bowel disease; PJS, Peutz-Jeghers syndrome.
The available data suggest that small bowel adenocarcinomas arise from a similar phenotypic adenoma to carcinoma transformation as seen in CRC (Figure 2).20,21 In a similar fashion to CRC, the risk of progression to a carcinoma is associated with size (8.3% for <1 cm versus 30% for >1cm) and histology (14.3% for tubular, 23.1% for tubulovillous and 36% for villous) of the adenoma.20 Although the genetic alterations that underline the development of small bowel adenocarcinomas have not been as clearly delineated as in CRC, a number of notable molecular similarities and differences between the two cancers exist.20,22 In a recent study using genomic hybridization, comparison of overall DNA copy number changes between adenocarcinomas of the colorectum, stomach and small bowel demonstrated that small bowel adenocarcinomas are more similar to CRC than gastric cancer.23 Also, this finding held true while evaluating the duodenal samples, as nine of 10 duodenal samples were shown to cluster with CRC.23 Studies evaluating the HER2 oncogene have found extremely low rates of HER2 amplification or overexpression in small bowel adenocarcinomas, a pattern that is more similar to CRC than gastric cancer.24–26
Figure 2 |.
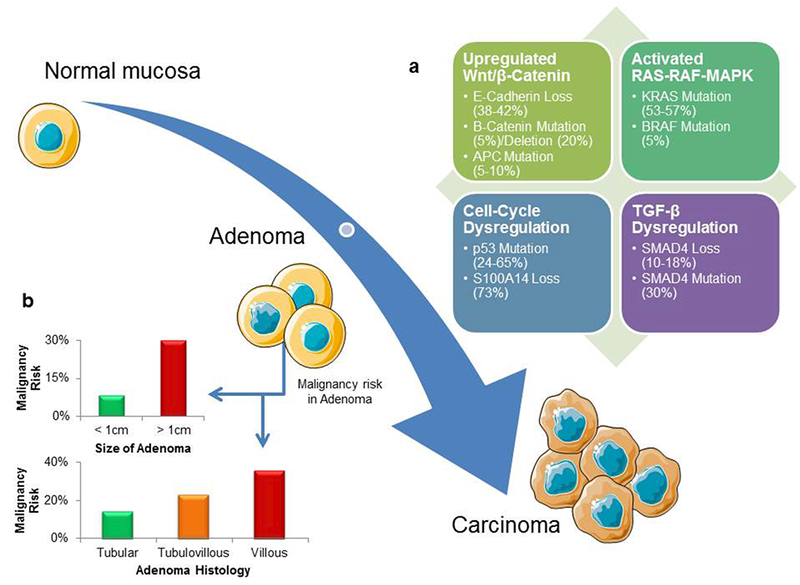
The adenoma–carcinoma sequence in small bowel adenocarcinomas. a | A number of molecular alterations are implicated in small bowel carcinogenesis. c | Risk of progression of adenoma to malignancy depends on the tumour size and histology.
One of the most obvious and dramatic differences between small bowel adenocarcinomas and CRC is the approximately 50-fold lower incidence of small bowel adenocarcinomas.4,7 This difference occurs despite the fact that the small intestine encompasses 80% of the anatomical length and 99% of the absorptive surface of the gastrointestinal tract.27 Such a dramatic difference raises a perplexing query about tissue-specific carcinogenesis and a number of theories have been postulated, although limited experimental evidence exists to support any one explanation. This significant discrepancy probably results from the interplay between dissimilar oncogenic mechanisms, such as the markedly lower rate of mutations in the adenomatous polyposis coli (APC) gene in sporadic small bowel adenocarcinomas in comparison to sporadic CRC, as well as the unique microenvironment of the small intestine that protects against carcinogenic stimuli.28–31 The low bacterial load, dilute liquid contents and relatively rapid transit time decreases the amount and duration of exposure to carcinogens in the small intestine. Additionally, higher levels of lymphoid aggregates and IgA levels in the small intestine compared to the large intestine might confer better tumour immunity and surveillance.29
Aetiology
Though the aetiology of most small bowel adenocarcinomas remains unclear, both familial cancer syndromes and conditions associated with increased small bowel inflammation, such as coeliac and Crohn’s disease, are responsible for a subset of patients who develop small bowel adenocarcinomas.2,9,32,33 Crohn’s disease has been reported to result in a 27-fold to 60-fold increase in the risk of small bowel adenocarcinomas, and this risk correlates with duration of the disease.34,35 Celiac disease also confers an increased risk, with one study reporting a 34-fold increase in risk for small bowel adenocarcinomas.36 Despite the suggestion of possible common risk factors for both small bowel adenocarcinomas and CRC, based upon the strong geographical correlation of incidence rates for both these tumours, the low number of small bowel adenocarcinomas in epidemiological studies has limited the ability to make any definitive conclusions.7 Multiple retrospective studies and two prospective studies investigating the role of alcohol, tobacco use and dietary habits as risk factors for small bowel adenocarcinomas were unable to identify consistent strong relationships between these factors and the development of these tumours.37–44 However, many studies have demonstrated an association between obesity and an increased risk of small bowel adenocarcinomas.45–47
Familial cancer syndromes
Multiple inherited syndromes such as Lynch syndrome, familial adenomatous polyposis (FAP) and Peutz-Jeghers syndrome (PJS) are associated with an increased risk of small bowel adenocarcinomas.13,14,48 The estimated lifetime risk of development of small bowel adenocarcinomas is 2–8% and 3–5% with Lynch syndrome and FAP, respectively.15,17 In patients with PJS, the cumulative risk of small bowel adenocarcinomas at age 65 years is 13%.14 In patients with FAP, duodenal adenomas are present in approximately 80% of patients, and regular endoscopic screening is required in these patients, with the frequency of screening based upon the number of polyps, polyp size, polyp histology and presence of dysplasia.49 Since a significant proportion of small bowel adenocarcinomas occur in patients with predisposing conditions, we recommend that patients presenting with a small bowel adenocarcinoma should be evaluated for a possible occult underlying condition, such Lynch syndrome and coeliac disease.
Molecular Biology
Recent efforts have improved the characterization of both the genetic and epigenetic changes that occur in small bowel adenocarcinoma (Figure 2).23,50 Limited molecular data indicates that accumulation of genetic alterations plays a key role in the adenoma–dysplasia–carcinoma sequence in the development of small bowel adenocarcinomas (Figure 2).51
Wnt-APC-β-catenin pathway
The most remarkable molecular finding in small bowel adenocarcinoma is that loss-of-function mutations in the APC tumour suppressor gene, which is the most common event in the early development of CRC, do not play a pivotal role in the development of small bowel adenocarcinomas.31,52 Although somatic mutations are found in 80% of sporadic CRC, only about 5% of sporadic small bowel adenocarcinomas harbour this defect.30,31,53 In one recent study of 48 patients with small bowel adenocarcinoma, no case was found to have an APC nonsense mutation.54
Despite the absence of APC gene mutations, upregulation of the Wnt–β-catenin pathway as indicated by aberrant protein expression of β-catenin is still seen in 40–48% of small bowel adenocarcinomas.30,52,55 Mutations in CTNNB1 (β-catenin gene), have been reported in 14% of patients with small bowel adenocarcinoma (six out of 42 tested cases).56–58 This mutation rate is still lower than the rate seen in CRC (26%).59 Interestingly, the mutation spectrum is also different, with gain-of-function missense point mutations being common in CRC, but only large insertions or deletions reported in small bowel adenocarcinomas.58,59 In a large study of 194 patients with small bowel adenocarcinomas, the presence of abnormal Wnt signalling has been correlated with a worse outcome. In 25% of cases, that had combined loss of E-cadherin and aberrant β-catenin expression, a significantly worse overall survival (13.9 months versus 49.9 months, P<0.001) was seen compared to cases without aberrant expression of both proteins.55 These cases had an increased rate of both higher grade and advanced T stage tumours.55
Chromosome 18q loss
Chromosome 18q harbours the ‘deleted in colon cancer’ (DCC) gene and SMAD4 gene and its loss is seen in 73% of sporadic CRC and 47% of patients with small bowel adenocarcinomas.22,57 The DCC protein seems to have a central role in cellular and extracellular matrix interactions.60 The SMAD4 protein is integrally involved in TGF-β signaling pathway and suppresses tumour growth.61 Although SMAD4 plays a role in a small subset of CRCs, DCC is the dominant player in tumour progression mediated by 18q loss in CRC.62 By contrast, mutations in DCC are uncommon in small bowel adenocarcinomas.51,63 Conversely, SMAD4 mutations are seen more commonly in small bowel adenocarcinomas (30%) compared to CRC (5–16%).64
KRAS and P53
Mutations in KRAS (codon 12 and 13) have been observed in 40–60% of all sporadic small bowel adenocarcinomas and this rate is comparable to that seen for CRC.51,65BRAFV600E mutations are rare, with no mutations seen in a study of 99 cases.30,50 Based on frequent KRAS mutations that occur in small bowel cancers, the RAS-RAF-MAPK pathway seems to have a role in small bowel carcinogenesis.30 p53 overexpression and mutations are seen in 40% of small bowel adenocarcinomas indicating the pivotal role of p53 in this disease.51 Both KRAS mutations and altered p53 are seen to progressively accumulate during the adenoma–carcinoma sequence.51
Microsatellite instability and methylator phenotype
Microsatellite instability (MSI) and loss of mismatch-repair proteins are seen in 18–35% of small bowel adenocarcinomas compared to approximately15% of CRCs.66–68 In small bowel adenocarcinomas, approximately 50% of cases reflect sporadic methylation of the MLH1 gene, with one study showing MLH1 methylation in 10 out of 20 MSI cases.50,68 Interestingly, the majority of cases with coeliac-related small bowel adenocarcinomas demonstrate MSI with two studies reporting similar rates of 73% and 67%.54,69 Determination of MLH1 methylation status was conducted in one of these studies, with all 10 cases of coeliac-related MSI small bowel adenocarcinomas demonstrating MLH1 methylation.54 In a study aimed at evaluating CpG island methylator phenotype (CIMP) status in duodenal adenocarcinomas, CIMP was seen in 27.3% of cases.50 CIMP-positive cases demonstrated a worse overall survival (33.9 months) than CIMP-negative cases (90.8 months), P=0.047, although this finding primarily reflected the dramatically worse outcomes seen for patients with CIMP-positive and MLH1 unmethylated tumours.50
Clinical presentation and diagnosis
Small bowel adenocarcinomas have a varied and non-specific presentation and a high index of suspicion is required to make an accurate and timely diagnosis.70 Median age of presentation of small bowel adenocarcinomas ranges from 55 to 65 years, with most cases diagnosed in the seventh or eighth decade.8,19 Age of onset tends to be lower in patients with predisposing conditions and familial cancer syndromes.13,14,33,34,48 Older people (age ≥ 60 years) tend to have a higher frequency of duodenal tumours.9,19 Owing to this non-specific presentation, the majority of patients are diagnosed with advanced-stage disease (32% stage IV, 27% stage III, 30% stage II and 10% stage I).12
The most common presenting symptom is abdominal pain (45–76%).9,19,71 Other common symptoms include nausea and vomiting (16–52%), weight loss (28%), fatigue and anaemia (15–30%) and gastrointestinal bleeding (7–23%).9,71 The most common presenting sign is anaemia and pallor, which is found in 40% of patients, with the next most common presenting symptoms being obstruction and bleeding.9 Because of this non-specific presentation, patients usually have symptoms for a long time before diagnosis, and the mean duration of symptoms before presentation in one study was 10 months.71
Laboratory testing reveals nonspecific results suggestive of gastrointestinal tract bleeding (overt or occult) leading to iron deficiency anaemia. There are no specific tumour markers for the diagnosis of small bowel adenocarcinomas because of inadequate sensitivity and specificity. Both carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA 19–9) are elevated in approximately 30% and 40% patients with small bowel adenocarcinomas, and these biomarkers can be used for monitoring disease status.72
Immunophenotyping
Immunophenotyping of small bowel adenocarcinomas shows CDX2, CK20 and CK7 expression in 70%, 57%, and 31% cases, respectively.67 While the most common combined cytokeratin profile is CK20+/CK7− (43%), small bowel adenocarcinomas demonstrate a much greater variability with a CK20−/CK7−, CK20+/CK7+ and CK20−/CK7+ representing 28%, 15% and 13% of tumours, respectively.67
Imaging
Preoperative diagnosis of small bowel adenocarcinomas is primarily made via radiographic studies and endoscopy. Although symptoms at presentation are non-specific, the major delay in diagnosis is due to the inability to order appropriate diagnostic tests or failure to make the diagnosis.73 In one study, the average delay in diagnosis attributable to patients failing to report symptoms, physicians not ordering the appropriate diagnostic tests and radiologists failing to make the diagnosis was less than 2 months, 8.2 months and 12 months, respectively.73 This indicates the importance of prompt and pertinent small bowel evaluation in patients with subtle, but persistent symptoms.
Conventional abdominal radiography can reveal obstruction, but otherwise has limited utility in the diagnostic work-up of small bowel adenocarcinomas. Series of studies of the upper gastrointestinal tract with either conventional small bowel follow-through or enteroclysis (contrast material infused directly into the small intestine through a nasogastric tube) can visualize primary tumours in 33% and 90% cases, respectively.74 The major drawback of these techniques is the inability to visualize extra-luminal disease, a limitation circumvented by use of cross-sectional imaging.75 CT and MRI based enteroclysis have an improved sensitivity and specificity for detection of small bowel tumours compared with conventional enteroclysis.76,77 The use of CT enterography (negative oral contrast agents such as water, polyethylene glycol or mannitol) is better tolerated than CT enteroclysis, and is able to achieve adequate small bowel distention with a similar sensitivity (93% versus 94%), but slightly lower specificity (94% versus 100%) than CT enteroclysis.78 The role of PET–CT in diagnosing small bowel adenocarcinomas is still investigational, but could offer better detection of occult metastatic disease.79,80
Endoscopy
Upper endoscopy, push enteroscopy and double-balloon enteroscopy can visualize the duodenum and proximal jejunum and the entire small-bowel, respectively. However, specialized techniques such as push and double-balloon enteroscopy are time consuming, technically challenging and limited in availability.81 Wireless video capsule endoscopy (VCE) allows non-invasive visualization of the entire small bowel lumen, has a low false-positive rate (2%) and in one pooled analysis of 24 prospective trials (n = 530 patients) comparing VCE to alternative diagnostic modalities, was found to have the lowest false-negative rate (19%).82,83 In a large retrospective study of 562 patients who underwent VCE for a variety of reasons, small bowel tumours were identified in 8.9% of patients.83 Based on these data, VCE has become the standard first choice endoscopic approach for patients with suspected non-duodenal small bowel cancers. The major drawback of VCE is the potential for capsule retention due to stenotic malignant and Crohn’s lesions.84 In some studies that evaluated obscure gastrointestinal bleeding, CT enterography and double-balloon enteroscopy have been shown to detect more lesions compared with VCE, and can be used in patients with stenotic lesions or patients with negative findings on VCE.85,86
Prognosis
Tumour stage is the single most important prognostic factor in small bowel adenocarcinomas.9,19 Other factors associated with poor prognosis include poor differentiation, positive margins, duodenal location, male gender, black ethnicity and older age.5 The presence of negative resection margins in curatively resected patients represents one of the strongest favourable predictors of long-term survival.9,87–89 High lymph-node ratio (> 50–75%) and a low number of assessed lymph nodes have been significantly associated with decreased survival.9,19,90 Two recent studies that used the SEER database have identified either ≥8 or ≥10 lymph nodes as the optimal number of assessed lymph nodes.90,91 For patients with stage II small bowel adenocarcinomas, the 5-year disease-specific survival rates vary markedly depending upon the number of assessed lymph nodes: 44% for 0 lymph nodes, 69% for 1–7 lymph nodes and 83% for > 7 lymph nodes.90
Treatment strategy
Treatment of small bowel adenocarcinomas is affected by site of disease, stage at presentation, available expertise, patient comorbidities and performance status. Small bowel adenocarcinomas are staged using the combined American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC) TNM staging system.92 For therapeutic purposes, small bowel adenocarcinomas can be divided broadly into two groups, locoregional disease and metastatic disease (Figure 3).
Figure 3|.
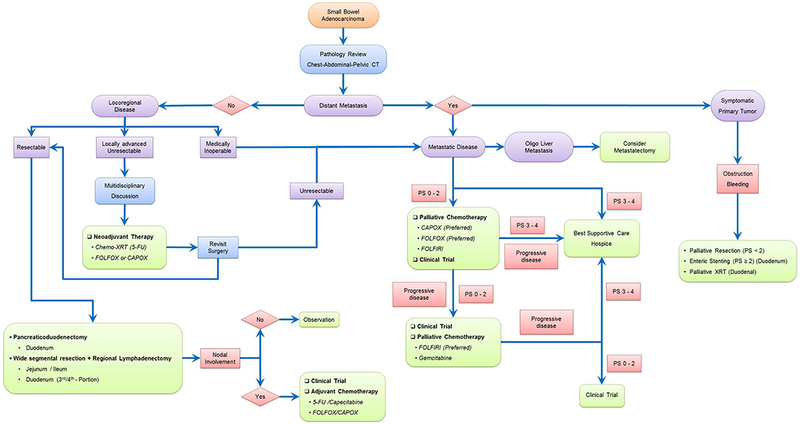
Schematic management of patients with small bowel adenocarcinomas. Treatment strategy depends on disease stage and involves en-bloc resection for locoregional disease and systemic chemotherapy for metastatic disease. All current recommendations are based on case series, retrospective reviews or non-randomized prospective trials because of an absence of any randomized data. Abbreviations: PS, performance status; 5-FU, 5-fluorouracil; FOLFOX, 5-FU, leucovorin and oxaliplatin; CAPOX, capecitabine plus oxaliplatin; FOLFIRI, 5-FU, leucovorin and irinotecan; KRAS-WT, KRAS wild-type; XRT, radiation therapy.
Surgery for locoregional disease
Surgery is the mainstay of therapy for small bowel adenocarcinomas presenting as locoregional disease.19 The 5-year survival of resected and unresected patients is 54% and 0%, respectively.93 Duodenal adenocarcinomas are managed with either pancreaticoduodenectomy, performed for tumours in the first and second portion of the duodenum, or wide local excision with regional lymphadenectomy for tumours in the third and fourth portion of duodenum provided that negative margins and an adequate lymph-node evaluation can be performed. Studies have shown that an optimal wide local excision is at least equivalent to pancreaticoduodenectomy in terms of long-term survival, and that wide excision is associated with lesser post-operative morbidity and duration of hospitalization.87,93–95 Although one study showed a survival benefit for pancreaticoduodenectomy over limited resection, this conclusion was driven by the high margin positive rate (23%) in the limited resection group.89 Jejunum or ileal adenocarcinomas should be treated by wide local excision and regional lymph node dissection. A right colectomy is indicated for tumors involving the distal/terminal ileum.
Adjuvant therapy
The recurrence pattern after potentially curative resection of small bowel adenocarcinoma is predominantly at distant sites. In the largest study of small bowel adenocarcinomas with reported recurrence pattern, 56 of 146 patients had distant and locoregional recurrences accounting for 86% and 18% of all recurrences, respectively.19 Even though the rates of locoregional failure are greater for duodenal adenocarcinomas, distant recurrence still predominates. A recent study of 122 patients with duodenal adenocarcinoma who underwent pancreaticoduodenectomy showed that the first site of recurrence is distant in 59% of cases, locoregional in 19% and both distant and locoregional in 22% of cases.96
Despite the absence of prospective randomized data elucidating the role of adjuvant therapy in small bowel adenocarcinomas, the use of adjuvant therapy has increased. Data from the National Cancer Database shows an increased use of adjuvant chemotherapy in small bowel adenocarcinomas from 8.1% in 1985 to 22.2% in 2005 (P<0.0001).5,97 In all likelihood this trend reflects the poor outcome of high-risk resected small bowel adenocarcinomas, the known efficacy of systemic chemotherapy in the metastatic setting and the significant survival benefit of adjuvant chemotherapy in patients with CRC.98 Retrospective studies have shown mixed results with regards to the benefit of adjuvant chemotherapy in treating small bowel adenocarcinomas.9,19,89,99 There exists a need for randomized control trials to investigate the benefit of adjuvant therapy in the management of these tumours.100 Currently the International Rare Cancers Initiative (IRCI) is planning to open a large prospective randomized trial evaluating the impact of adjuvant chemotherapy in resected small bowel adenocarcinoma termed the BALLAD study (A global study to evaluate the potential benefit of adjuvant chemotherapy for small bowel adenocarcinoma).100,101 Despite the limited data, it is reasonable to consider the role of adjuvant fluoropyrimidine-based chemotherapy for patients with high-risk disease, such as those positive lymph nodes.
Owing to the increased risk of locoregional failure for duodenal adenocarcinomas, adjuvant fluoropyrimidine-based chemoradiation has been used for high-risk patients.102 In a retrospective series of 26 duodenal adenocarcinoma patients who underwent a margin negative resection, use of adjuvant or neoadjuvant fluoropyrimidine-based radiotherapy demonstrated a trend toward improved 5-year overall survival in comparison to those patients treated with surgery alone (83% versus 53%, P=0.07).103 Of the 11 patients who underwent neoadjuvant fluoropyrimidine-based radiation therapy, a pathological complete response was seen in 2 (18%) patients.103 In a more recent series of six patients with locally advanced unresectable duodenal adenocarcinomas, neoadjuvant therapy enabled the completion of margin negative resections in all six patients.104 Given these results, further investigation of the use of neoadjuvant therapy for high-risk duodenal adenocarcinomas is warranted.
Metastatic disease
Systemic chemotherapy
Systemic chemotherapy has been regarded as the mainstay of treatment for patients with metastatic small bowel adenocarcinomas. Although no randomized trials have compared the use of palliative chemotherapy against best-supportive care in patients with small bowel adenocarcinomas, multiple retrospective comparisons have demonstrated the survival advantage of palliative chemotherapy (Table 2).9,19,105–108 A combined analysis of reported outcomes from six retrospective studies showed a median overall survival of 13 months for patients receiving systemic chemotherapy compared to 4 months for those treated with best-supportive care alone (P = 0.02; Figure 4).9,19,105–108 However, these studies are retrospective in nature, have a heterogeneous patient population and suffer from strong selection bias. Multiple agents have demonstrated activity in patients with metastatic small bowel adenocarcinomas, including 5-fluorouracil, capecitabine, oxaliplatin, cisplatin, gemcitabine and irinotecan with varying response rates (Table 2).72,105,109–119 A weighted Spearman correlation analysis of reported outcomes in these studies reveals a moderate-to-strong positive correlation between response rate and median overall survival (Spearman r 0.62, P = 0.002) and between median progression-free survival (PFS) and median overall survival (Spearman r 0.72, P< 0.001; Figure 4).This analysis indicates that PFS is a stronger predictor of patient survival compared to response rate, although further validation of such end points as surrogates for overall survival is needed.
Table 2 |.
Studies evaluating role of palliative chemotherapy in metastatic small bowel adenocarcinoma
| Reference | Year | Trial type | n | Chemotherapy regimen | RR (%) | TTP PFS (months) | Median OS versus BSC (months) |
|---|---|---|---|---|---|---|---|
| McWilliams | 2012 | Phase II (NCCTG) | 23 | CAPOXIRI | 39.0 | 8.7 | 12.7 |
| Xiang | 2012 | Phase II (China) | 33 | FOLFOX | 48.5 | 7.8 | 15.2 |
| Overman | 2009 | Phase II (MDACC) | 25 | CAPOX | 52.0 | 11.3 | 20.4 |
| Gibson | 2005 | Phase II (ECOG) | 38 | FAM | 18.4 | 5.0 | 8.0 |
| Tsushima | 2012 | Retrospective | 60 | 5-FU alone | 20.0 | 5.4 | 13.9 |
| 17 | 5-FU + cisplatin | 38.0 | 3.8 | 12.6 | |||
| 22 | FOLFOX | 42.0 | 8.2 | 22.2 | |||
| 11 | FOLFIRI | 25.0 | 5.6 | 9.4 | |||
| 22 | Various Agents | 21.0 | 3.4 | 8.1 | |||
| Koo | 2011 | Retrospective | 81 | 5-FU based | 11.1 | 5.7 | 11.8 vs 4.1; P<0.001 |
| Zhang | 2011 | Retrospective | 34 | FOLFOX/CAPOX | 32.3 | 6.3 | 14.2 |
| Zaanan | 2010 | Retrospective | 28 | FOLFIRI | 20.0 | 3.2 | 10.5 |
| Zaanan | 2010 | Retrospective | 38 | FOLFOX | 34.0 | 6.9 | 17.8 |
| 11 | FOLFIRI | 9.0 | 6.0 | 10.6 | |||
| 13 | 5-FU + cisplatin | 31.0 | 4.8 | 9.3 | |||
| Halfdanarson | 2009 | Retrospective | 165 | Various agents | NR | NA | 15.5 vs 3.3; P<0.001) |
| Overman | 2008 | Retrospective | 29 | 5-FU + platinum | 41.0 | 8.7 | 14.8 |
| 51 | Various agents | 16.0 | 3.9 | 12.0 | |||
| Czaykowski | 2007 | Retrospective | 37 | Various agents | 12.5 | NA | 15.6 vs 7.7 P=0.08) |
| Fishman | 2006 | Retrospective | 105 | Various agents | 36.0 | NA | 18.6 vs 13.4 P= 0.03) |
| Locher | 2005 | Retrospective | 20 | 5-FU + platinum | 21.0 | 8.0 | 14.0 |
| Dabaja | 2004 | Retrospective | 49 | NR | NR | NA | 12.0 vs 2.0 P=0.02) |
| Crawley | 1998 | Retrospective | 8 | 5-FU based | 37.5 | 7.8 | 13.0 |
| Ouriel | 1983 | Retrospective | 14 | 5-FU based | NR | NA | 10.7 vs 4.0; NR |
| Jigyasu | 1984 | Retrospective | 14 | Various agents | 7.0 | NA | 9.0 |
Abbreviations: N, total number of patients; RR, response rate; OS, overall survival; BSC, best supportive care; NR, not reported; NA, not applicable; 5-FU, 5-fluorouracil; FAM, 5-FU + doxorubicin + cisplatin; CAPOX, capecitabine + oxaliplatin; CAPOXIRI, CAPOX + irinotecan; FOLFOX, 5-FU + leucovorin + oxaliplatin.
Figure 4 |.
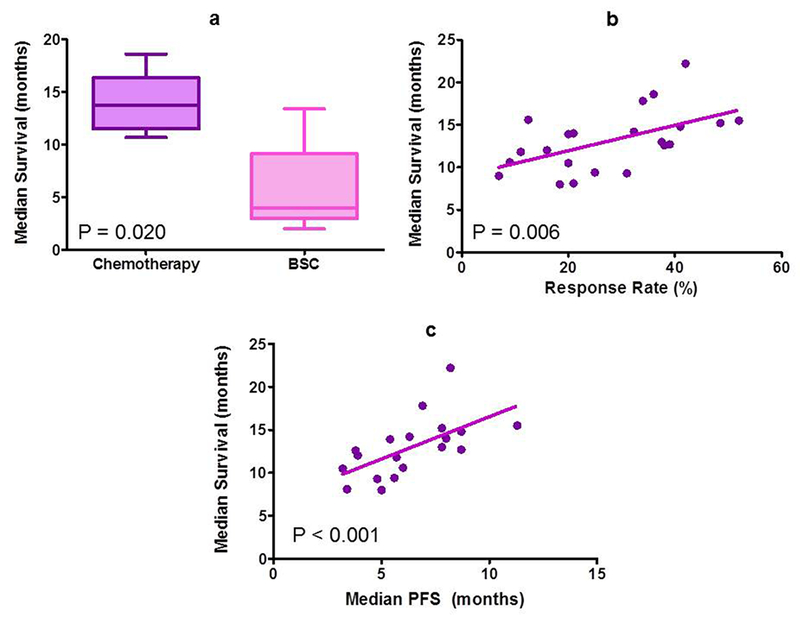
Role of chemotherapy in metastatic small bowel adenocarcinoma. a | Median overall survival is significantly more in patients receiving palliative chemotherapy (13.7 months) as compared to best-supportive care (4.0 months) alone. b | Response rate to palliative chemotherapy show moderate correlation with median overall survival in metastatic small bowel adenocarcinomas. c | Median progression-free survival on palliative chemotherapy correlates strongly with median survival in metastatic small bowel adenocarcinomas.
Although no randomized trials have compared the efficacy of different chemotherapy regimens in patients with small bowel adenocarcinomas, four prospective studies have been conducted with three using fluoropyrimidine and oxaliplatin as the backbone chemotherapy (Table 2). These studies demonstrate similar activity with response rates of 39–52% and median PFS of 7.8 to 11.3 months. Based on these findings, the standard frontline therapy for small bowel adenocarcinomas should consist of either CAPOX (capecitabine and oxaliplatin) or FOLFOX (5-fluorouracil, leucovorin and oxaliplatin).110,112 As second-line therapy, FOLFIRI (5-fluorouracil, leucovorin and irinotecan) has been evaluated after failure of 5-fluorouracil and platinum-based chemotherapy in two studies, and showed a disease control rate of approximately 50% and a median PFS of 3–5 months.113,114 At present, the role of targeted agents, such as bevacizumab, regorafenib or anti-EGFR monoclonal antibodies, which are commonly used in CRC, has not been established in small bowel adenocarcinomas. Notably both VEGF (91%) and EGFR (71%) are highly expressed in small bowel adenocarcinomas and KRAS mutations are similar for small bowel adenocarcinomas and CRCs.65,67 Anecdotal case reports have described the activity of anti-EGFR therapy (such as cetuximab), in patients with KRAS wild-type small bowel adenocarcinomas.120,121 A number of ongoing studies are exploring anti-EGFR agents in small bowel adenocarcinomas (Table 3).
Table 3 |.
Current clinical trials for advanced small bowel adenocarcinoma
| Identifier | Phase | Tumour type | n | Therapy line | Agent |
|---|---|---|---|---|---|
| NCT00354887 | II | SBAC + ampullary | 30 | 1st | CAPOX + bevacizumab |
| NCT00433550 | II | SBAC | 33 | 1st | Capecitabine/oxaliplatin/irinotecan |
| NCT01202409 | II | SBAC + ampullary | 20 | 1st | CAPOX + panitumumab (KRAS wildtype) |
| NCT00987766 | 1b | Duodenal + ampullary | 22 | 1st | GEMOX + erlotinib |
| NCT01730586 | II | SBAC | 10 | ≥ 2nd | Nab-paclitaxel |
Abbreviations: N, total number of patients; SBAC, small bowel adenocarcinoma; CAPOX, capecitabine + oxaliplatin; GEMOX, gemcitabine + oxaliplatin.
Surgery
The primary tumour site in small bowel adenocarcinomas can cause significant morbidity from obstruction (nausea, vomiting and poor nutrition) and bleeding. Palliative surgery (segmental resection or bypass) or radiation therapy (especially for duodenal primaries) might be necessary in cases with unresectable or metastatic disease.8 For patients with duodenal adenocarcinoma who are not surgical candidates, self-expandable metal stents can be considered for relief of bowel obstruction.122 Data concerning metastatectomy in small bowel adenocarcinomas are limited. Although two studies evaluating hepatic resection in patients with oligometastatic liver disease have demonstrated improved survival, the number of patients with small bowel adenocarcinomas (n = 30) were too few to draw any robust conclusions.123,124 Patient selection based on clinical behaviour of the disease and available surgical expertise plays a critical role in this decision.
Conclusions
Small bowel adenocarcinomas are rare malignancies. Even though small bowel adenocarcinomas are morphologically similar to CRC adenocarcinomas, they represent a distinct clinical, pathological and molecular entity. Dysregulation of the Wnt/β-catenin pathway, TGF-β signalling and cell-cycle regulation is integrally involved in molecular pathogenesis of these tumours. Inadequate evidence, non-specific symptoms and lack of clinical awareness and experience hamper deliver of optimum care to patients with this rare cancer. Surgery and systemic chemotherapy is the mainstay of therapy for locoregional and metastatic disease, respectively. Surgical resection with adequate lymph-node sampling is critical for long-term survival in resectable disease. Despite limited data on efficacy of adjuvant chemotherapy, its use in patients with high-risk disease should be discussed. Participation in clinical trials should be strongly encouraged.
With an increased understanding of the molecular biology of small bowel adenocarcinomas, the prospect of using targeted therapy in these tumours has become a distinct possibility. However, further work to establish valid preclinical model systems to enable the exploration of various therapeutic interventions is needed. As large-scale phase III randomized trials are problematic owing to the rare incidence of these tumours, multi-institutional collaborative initiatives and innovative clinical trial designs are vital to improve care for patients with this orphan malignancy.
Key points.
Small bowel tumours are rare cancers and their incidence worldwide is increasing
Clinical presentation is non-specific and specialized diagnostic modalities, such as enteroclysis, enterography, enteroscopy and video-capsule endoscopy are needed for early diagnosis
Surgery is the mainstay treatment for locoregional disease and the benefit from adjuvant chemotherapy is unclear
Systemic fluoropyrimidine and oxaliplatin-based chemotherapy has shown clinical benefit in metastatic disease
Recent molecular characterization efforts have revealed distinct molecular biology and pathogenesis compared to colorectal cancers
Large-scale collaborative research efforts are necessary to improving our knowledge regarding the management of these uncommon tumours
Footnotes
Note: Articles published only in English were considered. No results of experiments conveyed to the authors by personal communication were included.
References
- 1.Hatzaras I et al. Small-bowel tumors: epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch Surg 142, 229–235, doi:142/3/229 [pii] 10.1001/archsurg.142.3.229 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Schottenfeld D, Beebe-Dimmer JL & Vigneau FD The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol 19, 58–69, doi:S1047-2797(08)00320-7 [pii] 10.1016/j.annepidem.2008.10.004 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow JS, Chen CC, Ahsan H & Neugut AI A population-based study of the incidence of malignant small bowel tumours: SEER, 1973–1990. Int J Epidemiol 25, 722–728 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D & Jemal A Cancer statistics, 2013. CA Cancer J Clin 63, 11–30, doi: 10.3322/caac.21166 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Bilimoria KY et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 249, 63–71, doi: 10.1097/SLA.0b013e31818e464100000658-200901000-00011 [pii] (2009). [DOI] [PubMed] [Google Scholar]
- 6.Lu Y, Frobom R & Lagergren J Incidence patterns of small bowel cancer in a population-based study in Sweden: increase in duodenal adenocarcinoma. Cancer Epidemiol 36, e158–163, doi: 10.1016/j.canep.2012.01.008S1877-7821(12)00011-2 [pii] (2012). [DOI] [PubMed] [Google Scholar]
- 7.Haselkorn T, Whittemore AS & Lilienfeld DE Incidence of small bowel cancer in the United States and worldwide: geographic, temporal, and racial differences. Cancer Causes Control 16, 781–787, doi: 10.1007/s10552-005-3635-6 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Howe JR, Karnell LH, Menck HR & Scott-Conner C The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985–1995. Cancer 86, 2693–2706, doi: [pii] (1999). [DOI] [PubMed] [Google Scholar]
- 9.Halfdanarson TR, McWilliams RR, Donohue JH & Quevedo JF A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg 199, 797–803, doi: 10.1016/j.amjsurg.2009.05.037S0002-9610(09)00626-6 [pii] (2010). [DOI] [PubMed] [Google Scholar]
- 10.Chang HK et al. Adenocarcinoma of the small intestine: a multi-institutional study of 197 surgically resected cases. Hum Pathol 41, 1087–1096, doi: 10.1016/j.humpath.2010.01.006S0046-8177(10)00017-1 [pii] (2010). [DOI] [PubMed] [Google Scholar]
- 11.Goodman MT, Matsuno RK & Shvetsov YB Racial and ethnic variation in the incidence of small-bowel cancer subtypes in the United States, 1995–2008. Dis Colon Rectum 56, 441–448, doi: 10.1097/DCR.0b013e31826b9d0a00003453-201304000-00007 [pii] (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overman MJ et al. A population-based comparison of adenocarcinoma of the large and small intestine: insights into a rare disease. Ann Surg Oncol 19, 1439–1445, doi: 10.1245/s10434-011-2173-6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Bigas MA et al. Characteristics of small bowel carcinoma in hereditary nonpolyposis colorectal carcinoma. International Collaborative Group on HNPCC. Cancer 83, 240–244, doi: [pii] (1998). [DOI] [PubMed] [Google Scholar]
- 14.Giardiello FM et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 119, 1447–1453, doi:S001650850078005X [pii] (2000). [DOI] [PubMed] [Google Scholar]
- 15.Koornstra JJ et al. Management of extracolonic tumours in patients with Lynch syndrome. Lancet Oncol 10, 400–408, doi: 10.1016/S1470-2045(09)70041-5S1470-2045(09)70041-5 [pii] (2009). [DOI] [PubMed] [Google Scholar]
- 16.ten Kate GL et al. Is surveillance of the small bowel indicated for Lynch syndrome families? Gut 56, 1198–1201, doi:gut.2006.118299 [pii] 10.1136/gut.2006.118299 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galiatsatos P & Foulkes WD Familial adenomatous polyposis. Am J Gastroenterol 101, 385–398, doi:AJG375 [pii]10.1111/j.1572-0241.2006.00375.x (2006). [DOI] [PubMed] [Google Scholar]
- 18.Center MM, Jemal A & Ward E International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev 18, 1688–1694, doi: 10.1158/1055-9965.EPI-09-009018/6/1688 [pii] (2009). [DOI] [PubMed] [Google Scholar]
- 19.Dabaja BS, Suki D, Pro B, Bonnen M & Ajani J Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 101, 518–526 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Sellner F Investigations on the significance of the adenoma-carcinoma sequence in the small bowel. Cancer 66, 702–715 (1990). [DOI] [PubMed] [Google Scholar]
- 21.Perzin KH & Bridge MF Adenomas of the small intestine: a clinicopathologic review of 51 cases and a study of their relationship to carcinoma. Cancer 48, 799–819 (1981). [DOI] [PubMed] [Google Scholar]
- 22.Vogelstein B et al. Genetic alterations during colorectal-tumor development. N Engl J Med 319, 525–532, doi: 10.1056/NEJM198809013190901 (1988). [DOI] [PubMed] [Google Scholar]
- 23.Haan JC et al. Small bowel adenocarcinoma copy number profiles are more closely related to colorectal than to gastric cancers. Ann Oncol 23, 367–374, doi: 10.1093/annonc/mdr122mdr122 [pii] (2012). [DOI] [PubMed] [Google Scholar]
- 24.Chan OT et al. Lack of HER2 overexpression and amplification in small intestinal adenocarcinoma. Am J Clin Pathol 134, 880–885, doi: 10.1309/AJCPK6QHNNOEMJIM134/6/880 [pii] (2010). [DOI] [PubMed] [Google Scholar]
- 25.Kountourakis P et al. Clinicopathologic significance of EGFR and Her-2/neu in colorectal adenocarcinomas. Cancer J 12, 229–236 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Shitara K et al. Prognosis of patients with advanced gastric cancer by HER2 status and trastuzumab treatment. Gastric Cancer, doi: 10.1007/s10120-012-0179-9 (2012). [DOI] [PubMed] [Google Scholar]
- 27.DeSesso JM & Jacobson CF Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem Toxicol 39, 209–228, doi:S0278691500001368 [pii] (2001). [DOI] [PubMed] [Google Scholar]
- 28.Lowenfels AB Why are small-bowel tumours so rare? Lancet 1, 24–26 (1973). [DOI] [PubMed] [Google Scholar]
- 29.Calman KC Why are small bowel tumours rare? An experimental model. Gut 15, 552–554 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaker H et al. Mutational activation of the RAS-RAF-MAPK and the Wnt pathway in small intestinal adenocarcinomas. Scand J Gastroenterol 39, 748–753, doi:LT7DXF72KN07TJH3 [pii]10.1080/00365520410005847 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Miyaki M et al. Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res 54, 3011–3020 (1994). [PubMed] [Google Scholar]
- 32.Pan SY & Morrison H Epidemiology of cancer of the small intestine. World J Gastrointest Oncol 3, 33–42, doi: 10.4251/wjgo.v3.i3.33 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green PH & Cellier C Celiac disease. N Engl J Med 357, 1731–1743, doi:357/17/1731 [pii]10.1056/NEJMra071600 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Jess T, Winther KV, Munkholm P, Langholz E & Binder V Intestinal and extra-intestinal cancer in Crohn’s disease: follow-up of a population-based cohort in Copenhagen County, Denmark. Aliment Pharmacol Ther 19, 287–293, doi:1858 [pii] (2004). [DOI] [PubMed] [Google Scholar]
- 35.Jess T, Gamborg M, Matzen P, Munkholm P & Sorensen TI Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol 100, 2724–2729, doi:AJG287 [pii]10.1111/j.1572-0241.2005.00287.x (2005). [DOI] [PubMed] [Google Scholar]
- 36.Green PH et al. Risk of malignancy in patients with celiac disease. Am J Med 115, 191–195, doi:S0002934303003024 [pii] (2003). [DOI] [PubMed] [Google Scholar]
- 37.Wu AH, Yu MC & Mack TM Smoking, alcohol use, dietary factors and risk of small intestinal adenocarcinoma. Int J Cancer 70, 512–517, doi: [pii] (1997). [DOI] [PubMed] [Google Scholar]
- 38.Negri E et al. Risk factors for adenocarcinoma of the small intestine. Int J Cancer 82, 171–174, doi: [pii] (1999). [DOI] [PubMed] [Google Scholar]
- 39.Kaerlev L et al. Is there an association between alcohol intake or smoking and small bowel adenocarcinoma? Results from a European multi-center case-control study. Cancer Causes Control 11, 791–797 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Boffetta P et al. Body mass, tobacco smoking, alcohol drinking and risk of cancer of the small intestine--a pooled analysis of over 500,000 subjects in the Asia Cohort Consortium. Ann Oncol 23, 1894–1898, doi: 10.1093/annonc/mdr562mdr562 [pii] (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CC, Neugut AI & Rotterdam H Risk factors for adenocarcinomas and malignant carcinoids of the small intestine: preliminary findings. Cancer Epidemiol Biomarkers Prev 3, 205–207 (1994). [PubMed] [Google Scholar]
- 42.Chow WH et al. Risk factors for small intestine cancer. Cancer Causes Control 4, 163–169 (1993). [DOI] [PubMed] [Google Scholar]
- 43.Cross AJ et al. A prospective study of meat and fat intake in relation to small intestinal cancer. Cancer Res 68, 9274–9279, doi: 10.1158/0008-5472.CAN-08-201568/22/9274 [pii] (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schatzkin A, Park Y, Leitzmann MF, Hollenbeck AR & Cross AJ Prospective study of dietary fiber, whole grain foods, and small intestinal cancer. Gastroenterology 135, 1163–1167, doi: 10.1053/j.gastro.2008.07.015S0016-5085(08)01323-1 [pii] (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolk A et al. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 12, 13–21 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Samanic C et al. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control 15, 35–43, doi: 10.1023/B:CACO.0000016573.79453.ba5252184 [pii] (2004). [DOI] [PubMed] [Google Scholar]
- 47.Bjorge T, Tretli S & Engeland A Height and body mass index in relation to cancer of the small intestine in two million Norwegian men and women. Br J Cancer 93, 807–810, doi:6602789 [pii]10.1038/sj.bjc.6602789 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jagelman DG, DeCosse JJ & Bussey HJ Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet 1, 1149–1151 (1988). [DOI] [PubMed] [Google Scholar]
- 49.Saurin JC et al. Surveillance of duodenal adenomas in familial adenomatous polyposis reveals high cumulative risk of advanced disease. J Clin Oncol 22, 493–498, doi: 10.1200/JCO.2004.06.028JCO.2004.06.028 [pii] (2004). [DOI] [PubMed] [Google Scholar]
- 50.Fu T et al. CpG island methylator phenotype-positive tumors in the absence of MLH1 methylation constitute a distinct subset of duodenal adenocarcinomas and are associated with poor prognosis. Clin Cancer Res 18, 4743–4752, doi: 10.1158/1078-0432.CCR-12-07071078-0432.CCR-12-0707 [pii] (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rashid A & Hamilton SR Genetic alterations in sporadic and Crohn’s-associated adenocarcinomas of the small intestine. Gastroenterology 113, 127–135, doi:S0016508597003193 [pii] (1997). [DOI] [PubMed] [Google Scholar]
- 52.Wheeler JM et al. An insight into the genetic pathway of adenocarcinoma of the small intestine. Gut 50, 218–223 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyoshi Y et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet 1, 229–233 (1992). [DOI] [PubMed] [Google Scholar]
- 54.Diosdado B et al. High-resolution array comparative genomic hybridization in sporadic and celiac disease-related small bowel adenocarcinomas. Clin Cancer Res 16, 1391–1401, doi: 10.1158/1078-0432.CCR-09-17731078-0432.CCR-09-1773 [pii] (2010). [DOI] [PubMed] [Google Scholar]
- 55.Lee HJ et al. Combined loss of E-cadherin and aberrant beta-catenin protein expression correlates with a poor prognosis for small intestinal adenocarcinomas. Am J Clin Pathol 139, 167–176, doi: 10.1309/AJCPS54RTFCTHGWX139/2/167 [pii] (2013). [DOI] [PubMed] [Google Scholar]
- 56.Murata M et al. Molecular and biological analysis of carcinoma of the small intestine: beta-catenin gene mutation by interstitial deletion involving exon 3 and replication error phenotype. Am J Gastroenterol 95, 1576–1580, doi:S0002-9270(00)00915-1 [pii]10.1111/j.1572-0241.2000.02123.x (2000). [DOI] [PubMed] [Google Scholar]
- 57.Blaker H, von Herbay A, Penzel R, Gross S & Otto HF Genetics of adenocarcinomas of the small intestine: frequent deletions at chromosome 18q and mutations of the SMAD4 gene. Oncogene 21, 158–164, doi: 10.1038/sj.onc.1205041 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Breuhahn K, Singh S, Schirmacher P & Blaker H Large-scale N-terminal deletions but not point mutations stabilize beta-catenin in small bowel carcinomas, suggesting divergent molecular pathways of small and large intestinal carcinogenesis. J Pathol 215, 300–307, doi: 10.1002/path.2362 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Ilyas M, Tomlinson IP, Rowan A, Pignatelli M & Bodmer WF Beta-catenin mutations in cell lines established from human colorectal cancers. Proc Natl Acad Sci U S A 94, 10330–10334 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho KR et al. The DCC gene: structural analysis and mutations in colorectal carcinomas. Genomics 19, 525–531, doi:S0888-7543(84)71102-5 [pii] 10.1006/geno.1994.1102 (1994). [DOI] [PubMed] [Google Scholar]
- 61.Reiss M, Santoro V, de Jonge RR & Vellucci VF Transfer of chromosome 18 into human head and neck squamous carcinoma cells: evidence for tumor suppression by Smad4/DPC4. Cell Growth Differ 8, 407–415 (1997). [PubMed] [Google Scholar]
- 62.Thiagalingam S et al. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet 13, 343–346, doi: 10.1038/ng0796-343 (1996). [DOI] [PubMed] [Google Scholar]
- 63.Svrcek M et al. Immunohistochemical analysis of adenocarcinoma of the small intestine: a tissue microarray study. J Clin Pathol 56, 898–903 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blaker H et al. Loss of SMAD4 function in small intestinal adenocarcinomas: comparison of genetic and immunohistochemical findings. Pathol Res Pract 200, 1–7, doi:S0344-0338(04)00002-0 [pii] 10.1016/j.prp.2003.12.001 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Nishiyama K et al. Overexpression of p53 protein and point mutation of K-ras genes in primary carcinoma of the small intestine. Oncol Rep 9, 293–300 (2002). [PubMed] [Google Scholar]
- 66.Boland CR et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58, 5248–5257 (1998). [PubMed] [Google Scholar]
- 67.Overman MJ et al. Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine. Br J Cancer 102, 144–150, doi: 10.1038/sj.bjc.66054496605449 [pii] (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Planck M et al. Microsatellite instability and expression of MLH1 and MSH2 in carcinomas of the small intestine. Cancer 97, 1551–1557, doi: 10.1002/cncr.11197 (2003). [DOI] [PubMed] [Google Scholar]
- 69.Potter DD et al. The role of defective mismatch repair in small bowel adenocarcinoma in celiac disease. Cancer Res 64, 7073–7077, doi:64/19/7073 [pii]10.1158/0008-5472.CAN-04-1096 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Minardi AJ Jr., Zibari GB, Aultman DF, McMillan RW & McDonald JC Small-bowel tumors. J Am Coll Surg 186, 664–668, doi:S1072-7515(98)00092-1 [pii] (1998). [DOI] [PubMed] [Google Scholar]
- 71.Talamonti MS, Goetz LH, Rao S & Joehl RJ Primary cancers of the small bowel: analysis of prognostic factors and results of surgical management. Arch Surg 137, 564–570; discussion 570–561 (2002). [DOI] [PubMed] [Google Scholar]
- 72.Zaanan A et al. Chemotherapy of advanced small-bowel adenocarcinoma: a multicenter AGEO study. Ann Oncol 21, 1786–1793, doi:mdq038 [pii]10.1093/annonc/mdq038. [DOI] [PubMed] [Google Scholar]
- 73.Maglinte DD, O’Connor K, Bessette J, Chernish SM & Kelvin FM The role of the physician in the late diagnosis of primary malignant tumors of the small intestine. Am J Gastroenterol 86, 304–308 (1991). [PubMed] [Google Scholar]
- 74.Bessette JR, Maglinte DD, Kelvin FM & Chernish SM Primary malignant tumors in the small bowel: a comparison of the small-bowel enema and conventional follow-through examination. Ajr 153, 741–744 (1989). [DOI] [PubMed] [Google Scholar]
- 75.Dudiak KM, Johnson CD & Stephens DH Primary tumors of the small intestine: CT evaluation. AJR Am J Roentgenol 152, 995–998, doi: 10.2214/ajr.152.5.995 (1989). [DOI] [PubMed] [Google Scholar]
- 76.Pilleul F et al. Possible small-bowel neoplasms: contrast-enhanced and water-enhanced multidetector CT enteroclysis. Radiology 241, 796–801, doi:2413051429 [pii]10.1148/radiol.2413051429 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Van Weyenberg SJ et al. MR enteroclysis in the diagnosis of small-bowel neoplasms. Radiology 254, 765–773, doi: 10.1148/radiol.09090828254/3/765 [pii] (2010). [DOI] [PubMed] [Google Scholar]
- 78.Minordi LM, Vecchioli A, Mirk P & Bonomo L CT enterography with polyethylene glycol solution vs CT enteroclysis in small bowel disease. Br J Radiol 84, 112–119, doi: 10.1259/bjr/7164988871649888 [pii] (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laurent F et al. Diagnosis and categorization of small bowel neoplasms: role of computed tomography. Gastrointest Radiol 16, 115–119 (1991). [DOI] [PubMed] [Google Scholar]
- 80.Cronin CG et al. Utility of positron emission tomography/CT in the evaluation of small bowel pathology. Br J Radiol 85, 1211–1221, doi: 10.1259/bjr/6453457385/1017/1211 [pii] (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fry LC, Bellutti M, Neumann H, Malfertheiner P & Monkemuller K Incidence of bleeding lesions within reach of conventional upper and lower endoscopes in patients undergoing double-balloon enteroscopy for obscure gastrointestinal bleeding. Aliment Pharmacol Ther 29, 342–349, doi:APT3888 [pii]10.1111/j.1365-2036.2008.03888.x (2009). [DOI] [PubMed] [Google Scholar]
- 82.Lewis BS, Eisen GM & Friedman S A pooled analysis to evaluate results of capsule endoscopy trials. Endoscopy 37, 960–965, doi: 10.1055/s-2005-870353 (2005). [DOI] [PubMed] [Google Scholar]
- 83.Cobrin GM, Pittman RH & Lewis BS Increased diagnostic yield of small bowel tumors with capsule endoscopy. Cancer 107, 22–27, doi: 10.1002/cncr.21975 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Wiarda BM et al. Small bowel Crohn’s disease: MR enteroclysis and capsule endoscopy compared to balloon-assisted enteroscopy. Abdom Imaging 37, 397–403, doi: 10.1007/s00261-011-9816-8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huprich JE et al. Prospective blinded comparison of wireless capsule endoscopy and multiphase CT enterography in obscure gastrointestinal bleeding. Radiology 260, 744–751, doi: 10.1148/radiol.11110143radiol.11110143 [pii] (2011). [DOI] [PubMed] [Google Scholar]
- 86.Ross A et al. Double balloon enteroscopy detects small bowel mass lesions missed by capsule endoscopy. Dig Dis Sci 53, 2140–2143, doi: 10.1007/s10620-007-0110-0 (2008). [DOI] [PubMed] [Google Scholar]
- 87.Bakaeen FG et al. What prognostic factors are important in duodenal adenocarcinoma? Arch Surg 135, 635–641; discussion 641–632 (2000). [DOI] [PubMed] [Google Scholar]
- 88.Abrahams NA, Halverson A, Fazio VW, Rybicki LA & Goldblum JR Adenocarcinoma of the small bowel: a study of 37 cases with emphasis on histologic prognostic factors. Dis Colon Rectum 45, 1496–1502 (2002). [DOI] [PubMed] [Google Scholar]
- 89.Sohn TA et al. Adenocarcinoma of the duodenum: factors influencing long-term survival. J Gastrointest Surg 2, 79–87, doi:S1091-255X(98)80107-8 [pii] (1998). [DOI] [PubMed] [Google Scholar]
- 90.Overman MJ, Hu CY, Wolff RA & Chang GJ Prognostic value of lymph node evaluation in small bowel adenocarcinoma: analysis of the surveillance, epidemiology, and end results database. Cancer 116, 5374–5382, doi: 10.1002/cncr.25324. [DOI] [PubMed] [Google Scholar]
- 91.Nicholl MB et al. Small bowel adenocarcinoma: understaged and undertreated? Ann Surg Oncol 17, 2728–2732, doi: 10.1245/s10434-010-1109-x (2010). [DOI] [PubMed] [Google Scholar]
- 92.Edge S, Byrd DR, Compton CC. AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 7th edn, 181 (Springer, 2010). [DOI] [PubMed] [Google Scholar]
- 93.Barnes G Jr., Romero L, Hess KR & Curley SA Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol 1, 73–78 (1994). [DOI] [PubMed] [Google Scholar]
- 94.Kaklamanos IG et al. Extent of resection in the management of duodenal adenocarcinoma. Am J Surg 179, 37–41, doi:S0002-9610(99)00269-X [pii] (2000). [DOI] [PubMed] [Google Scholar]
- 95.Joesting DR, Beart RW Jr., van Heerden JA & Weiland LH Improving survival in adenocarcinoma of the duodenum. Am J Surg 141, 228–231, doi:0002-9610(81)90163-X [pii] (1981). [DOI] [PubMed] [Google Scholar]
- 96.Poultsides GA et al. Duodenal adenocarcinoma: clinicopathologic analysis and implications for treatment. Ann Surg Oncol 19, 1928–1935, doi: 10.1245/s10434-011-2168-3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lepage C, Bouvier AM, Manfredi S, Dancourt V & Faivre J Incidence and management of primary malignant small bowel cancers: a well-defined French population study. Am J Gastroenterol 101, 2826–2832, doi:AJG854 [pii]10.1111/j.1572-0241.2006.00854.x (2006). [DOI] [PubMed] [Google Scholar]
- 98.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 345, 939–944, doi:S0140-6736(95)90696-7 [pii] (1995). [PubMed] [Google Scholar]
- 99.Overman MJ, Kopetz S, Lin E, Abbruzzese JL & Wolff RA Is there a role for adjuvant therapy in resected adenocarcinoma of the small intestine. Acta Oncol 49, 474–479, doi: 10.3109/02841860903490051. [DOI] [PubMed] [Google Scholar]
- 100.Singhal N & Singhal D Adjuvant chemotherapy for small intestine adenocarcinoma. Cochrane Database Syst Rev, CD005202, doi: 10.1002/14651858.CD005202.pub2 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keat N et al. International rare cancers initiative. Lancet Oncol 14, 109–110, doi: 10.1016/S1470-2045(12)70570-3S1470-2045(12)70570-3 [pii] (2013). [DOI] [PubMed] [Google Scholar]
- 102.Swartz MJ et al. Adjuvant concurrent chemoradiation for node-positive adenocarcinoma of the duodenum. Arch Surg 142, 285–288, doi:142/3/285 [pii]10.1001/archsurg.142.3.285 (2007). [DOI] [PubMed] [Google Scholar]
- 103.Kelsey CR et al. Duodenal adenocarcinoma: patterns of failure after resection and the role of chemoradiotherapy. International journal of radiation oncology, biology, physics 69, 1436–1441, doi:S0360-3016(07)00827-9 [pii]10.1016/j.ijrobp.2007.05.006 (2007). [DOI] [PubMed] [Google Scholar]
- 104.Onkendi EO et al. Neoadjuvant treatment of duodenal adenocarcinoma: a rescue strategy. J Gastrointest Surg 16, 320–324, doi: 10.1007/s11605-011-1667-7 (2012). [DOI] [PubMed] [Google Scholar]
- 105.Fishman PN et al. Natural history and chemotherapy effectiveness for advanced adenocarcinoma of the small bowel: a retrospective review of 113 cases. American journal of clinical oncology 29, 225–231 (2006). [PubMed] [Google Scholar]
- 106.Koo DH et al. Systemic chemotherapy for treatment of advanced small bowel adenocarcinoma with prognostic factor analysis: retrospective study. BMC Cancer 11, 205, doi: 10.1186/1471-2407-11-2051471-2407-11-205 [pii] (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Czaykowski P & Hui D Chemotherapy in small bowel adenocarcinoma: 10-year experience of the British Columbia Cancer Agency. Clin Oncol (R Coll Radiol) 19, 143–149 (2007). [DOI] [PubMed] [Google Scholar]
- 108.Ouriel K & Adams JT Adenocarcinoma of the small intestine. Am J Surg 147, 66–71, doi:0002-9610(84)90036-9 [pii] (1984). [DOI] [PubMed] [Google Scholar]
- 109.Gibson MK, Holcroft CA, Kvols LK & Haller D Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist 10, 132–137 (2005). [DOI] [PubMed] [Google Scholar]
- 110.Overman MJ et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol 27, 2598–2603, doi:JCO.2008.19.7145 [pii]10.1200/JCO.2008.19.7145 (2009). [DOI] [PubMed] [Google Scholar]
- 111.McWilliams RR et al. Pharmacogenetic dosing by UGT1A1 genotype as first-line therapy for advanced small-bowel adenocarcinoma: A North Central Cancer Treatment Group (NCCTG) trial. ASCO Meeting Abstracts 30, 314 (2012). [Google Scholar]
- 112.Xiang XJ et al. A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs 23, 561–566, doi: 10.1097/CAD.0b013e328350dd0d00001813-201206000-00010 [pii] (2012). [DOI] [PubMed] [Google Scholar]
- 113.Locher C et al. Combination chemotherapy in advanced small bowel adenocarcinoma. Oncology 69, 290–294 (2005). [DOI] [PubMed] [Google Scholar]
- 114.Zaanan A et al. Second-line chemotherapy with fluorouracil, leucovorin, and irinotecan (FOLFIRI regimen) in patients with advanced small bowel adenocarcinoma after failure of first-line platinum-based chemotherapy: a multicenter AGEO study. Cancer 117, 1422–1428, doi: 10.1002/cncr.25614 (2011). [DOI] [PubMed] [Google Scholar]
- 115.Zhang L et al. Efficacy of the FOLFOX/CAPOX regimen for advanced small bowel adenocarcinoma: a three-center study from China. J BUON 16, 689–696 (2011). [PubMed] [Google Scholar]
- 116.Jigyasu D, Bedikian AY & Stroehlein JR Chemotherapy for primary adenocarcinoma of the small bowel. Cancer 53, 23–25 (1984). [DOI] [PubMed] [Google Scholar]
- 117.Crawley C, Ross P, Norman A, Hill A & Cunningham D The Royal Marsden experience of a small bowel adenocarcinoma treated with protracted venous infusion 5-fluorouracil. Br J Cancer 78, 508–510 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsushima T et al. Multicenter retrospective study of 132 patients with unresectable small bowel adenocarcinoma treated with chemotherapy. Oncologist 17, 1163–1170, doi:theoncologist.2012-0079 [pii]10.1634/theoncologist.2012-0079 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Overman MJ et al. Chemotherapy with 5-fluorouracil and a platinum compound improves outcomes in metastatic small bowel adenocarcinoma. Cancer 113, 2038–2045 (2008). [DOI] [PubMed] [Google Scholar]
- 120.Santini D et al. Cetuximab in small bowel adenocarcinoma: a new friend? Br J Cancer 103, 1305; author reply 1306, doi: 10.1038/sj.bjc.66058986605898 [pii] (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Dosso S, Molinari F, Martin V, Frattini M & Saletti P Molecular characterisation and cetuximab-based treatment in a patient with refractory small bowel adenocarcinoma. Gut 59, 1587–1588, doi: 10.1136/gut.2009.196428gut.2009.196428 [pii] (2010). [DOI] [PubMed] [Google Scholar]
- 122.Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ & Siersema PD Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol 7, 18, doi:1471-230X-7-18 [pii]10.1186/1471-230X-7-18 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ercolani G et al. The role of liver resections for noncolorectal, nonneuroendocrine metastases: experience with 142 observed cases. Ann Surg Oncol 12, 459–466, doi: 10.1245/ASO.2005.06.034 (2005). [DOI] [PubMed] [Google Scholar]
- 124.Adam R et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 244, 524–535, doi: 10.1097/01.sla.0000239036.46827.5f00000658-200610000-00007 [pii] (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]


