Abstract
By acoustically detecting the optical absorption contrast, photoacoustic (PA) tomography (PAT) has broken the penetration limits of traditional high-resolution optical imaging. Through spectroscopic analysis of the target’s optical absorption, PAT can identify a wealth of endogenous and exogenous molecules and thus is inherently capable of molecular imaging with high sensitivity. PAT’s molecular sensitivity is uniquely accompanied by non-ionizing radiation, high spatial resolution, and deep penetration in biological tissues, which other optical imaging modalities cannot achieve yet. In this concise review, we summarize the most recent technological advancements in PA molecular imaging and highlight the novel molecular probes specifically made for PAT in deep tissues. We conclude with a brief discussion of the opportunities for future advancements.
Keywords: Photoacoustic tomography, Molecular imaging, Signal unmixing, Near-infrared dye, Nanoparticle, Gene reporter
Introduction
Photoacoustic tomography (PAT, also referred to as optoacoustic or thermoacoustic tomography) is based on the photoacoustic effect [1], in which ultrasonic waves generated by optical excitation are detected to map the original optical energy deposition [2–6]. PAT naturally utilizes rich optical absorption contrast and weak acoustic scattering inherent in biological tissue, lending it a clear advantage over traditional high-resolution optical imaging in retrieving anatomical, functional, molecular, metabolic, and histologic information at large depths.
One of the strengths of PAT is its inherent molecular sensitivity. Unlike fluorescent imaging which relies on the fluorescent molecule’s radiative relaxation, PAT depends on the molecule’s thermoelastic expansion through nonradiative relaxation. PAT has a 100% relative sensitivity to small optical absorption variations, meaning that a given percentage change in the optical absorption coefficient yields the same percentage change in the PA signal amplitude [7]. All molecules have unique optical absorption features that can serve as their ‘fingerprints’ for PA identification. The spatial distribution and optical properties of molecules are often closely related to their microenvironment (e.g., hypoxia in tumors), allowing PAT to probe physiological functions and pathological conditions. Moreover, because acoustic scattering in tissue is much weaker than optical scattering, PAT can harness scattered photons to explore molecular information with high spatial resolution at depths far beyond the optical diffusion limit (~1 mm).
PA molecular imaging has advanced rapidly since the first reports on PAT of the tumor microenvironment in the 2000s [8–12]. Almost every technical aspect in PA molecular imaging has progressed, including the detection sensitivity and penetration depth of the imaging system [5, 13–22], the quantification accuracy of the signal unmixing [3, 13, 23–25], and the design and application of molecular probes in deep tissues [10, 26–39]. In this concise review, we focus on the major advancements in PA molecular imaging reported in the last several years (2014–2017) including novel imaging systems, signal unmixing methods, and molecular probes. We also overview the opportunities that may lead to future advances. Readers are referred to recent review articles to gain a more comprehensive knowledge of the principles of PAT [40, 41], the molecular contrast agents [34, 37, 42–44], and the biomedical applications [6, 16, 45–47].
Basic principles of PAT
A typical PAT system includes a short-pulsed laser for efficient wideband PA signal generation, an ultrasonic transducer (or transducer array) for signal detection, a signal amplification and digitization system, and a computer for image formation. PAT has been implemented with two major image formation methods [2]. The first method, direct image formation, is based on mechanical scanning of a focused or unfocused excitation light beam and a focused single-element ultrasonic transducer. The second method, reconstruction image formation, is based on wide-field light illumination and parallel acoustic detection by a multi-element ultrasonic transducer array. Direct image formation is commonly used in photoacoustic microscopy (PAM), whereas reconstruction image formation is the basis for photoacoustic computed tomography (PACT). Compared to PAM, PACT typically has a higher imaging speed and greater penetration depth but lower spatial resolutions [41]. Depending on the image formation method, PAT may require mechanical or electronic scanning to form two-dimensional (2D) and three-dimensional (3D) images.
PAT complements other imaging methods in contrast mechanism, spatial-temporal resolution, and penetration depth, and has found broad applications in the biomedical research, especially in functional brain mapping [48], cancer diagnosis and staging [45, 49], tissue engineering and regenerative medicine [50], developmental biology [51], and molecular cell biology [52], as comprehensively reviewed elsewhere [6, 53, 54]. In particular, PAT has been widely used for various cancer studies [45], including fundamental research of cancerogenesis [55], cancer detection and staging [56], and navigation and evaluation in cancer treatment [57]. Using either endogenous contrast (e.g., melanin in melanoma cells) or exogenous contrast (e.g., targeted nanoparticles or organic dyes), PAT has become increasingly popular in providing accurate and early diagnosis of cancers [45].
Advances in PAT implementations
Continuous developments in laser technology, ultrasonic detection, digitization electronic systems, and parallel computation have driven technical breakthroughs in PAT technologies. Notably, inspired by PAT’s rapid development and its increasingly important role in biomedical research, more and more manufacturers are developing commercial products specifically designed for PAT, including high-energy, high-speed pulsed lasers (e.g., Pulsed laser diode illuminator, Quantel-Laser, Inc.), ultra-wideband ultrasonic transducers (e.g., 225 MHz bandwidth transducer, Olympus, Inc.), and high-speed, multi-channel data acquisition systems (e.g., 128 channel DAQ, Ultrasonix, Inc.). Industrial support in the development of PAT technology is critical for accelerating its commercialization and clinical translation.
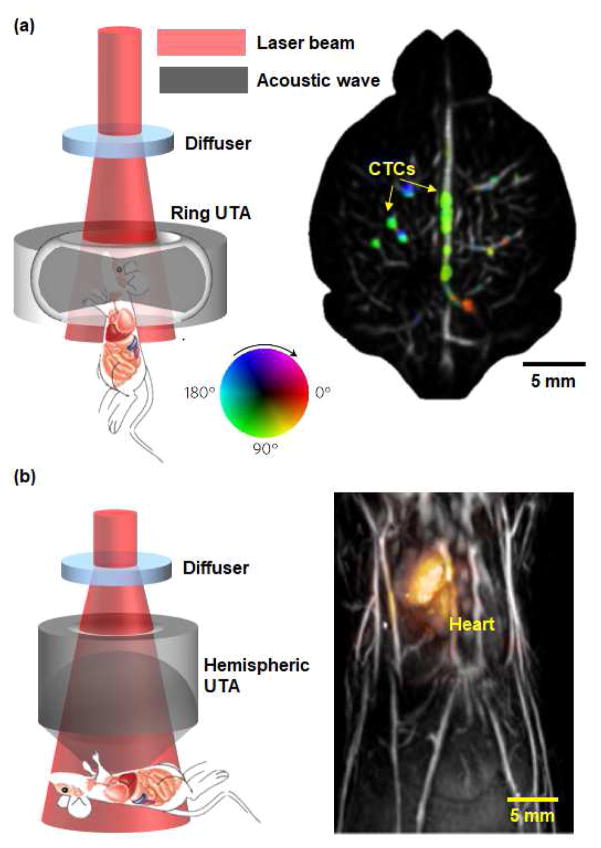
Here, we highlight several recent technological breakthroughs in PAT. (1) Real-time, whole-body small animal imaging has been achieved due to high-speed laser sources and data acquisition systems [20, 21]. We reported a panoramic PACT system with a 125 μm in-plane resolution, 50 Hz 2D frame rate, and 48 mm penetration depth, which is capable of capturing circulating tumor cells in mouse brains (Fig. 1a) [20]. Fehm et al. developed a 3D PACT system to capture the dynamics of an entire heart beat with a 100 Hz 3D frame rate within a 1.5 cm3 volume (Fig. 1b) [21]. These real-time, whole-body PAT systems are extremely powerful when tracking exogenously labeled drug molecules in pharmacokinetic studies on small animal models, thereby enabling biomedical researchers to test new drugs and monitor longitudinal therapy in the future. (2) The spatial resolutions of PAT have been pushing the existing limits through the use of high-frequency wideband ultrasound detection [22, 58]. Agurirre et al. recently reported an ultra-broadband PAM system for human skin imaging with a spatial resolution of 7 μm, enabled by an ultrasonic detection band of 10–180 MHz [22]. Guggenheim et al. developed a PAM system using an ultrasensitive plano-concave microresonator with an ultrasound detection band of 0–40 MHz and a large acceptance angle of 75 degrees [58]. By matching the ultrasonic detection band with the detectable PA signal spectrum, which is primarily limited by the depth of the target, ultra-wideband ultrasonic detection has enabled multi-scale PA molecular imaging with the highest possible resolutions at different depths.
Figure 1. Advances in real-time, whole-body small animal PAT.
(a) A ring-shaped ultrasonic transducer array (UTA) based panoramic PACT system with a 50 Hz 2D frame rate and 48 mm penetration depth, which is capable of capturing circulating tumor cells (CTCs) in mouse brains [20]. The colors represent the flow direction of CTCs. Flow speed is radially encoded in the color disk by hue saturation (a greater radius indicates a faster flow speed). (b) A hemispherical-shaped UTA based PACT system with a 100 Hz 3D frame rate in a 1.5 cm3 volume, which is capable of capturing the mouse heart beating [21].
Advances in signal unmixing methods
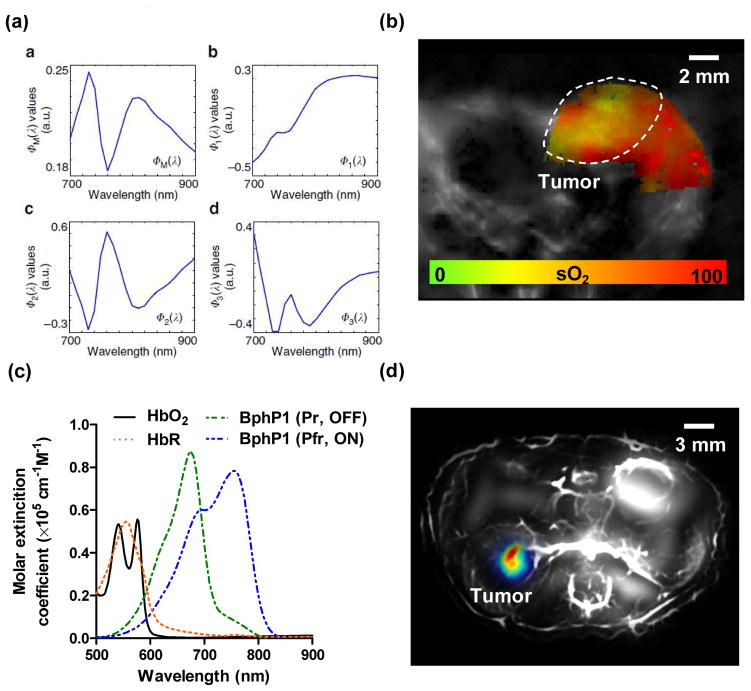
Traditionally, spectroscopic imaging is used in PAT to extract the weak signals of molecular probes from the strong background signals of blood, by taking measurements at multiple optical wavelengths [3]. However, this method performs optimally only in superficial tissue because it requires knowledge of the local optical fluence (J/m2), which is difficult to estimate in deep tissue [13, 25]. Novel methods based on two different strategies have been developed to improve the signal unmixing accuracy in PAT [3, 16, 18, 23, 25]. The first strategy focuses on optical fluence compensation with tissue property modeling [18, 23, 24, 59–72]. Instead of assuming homogenous optical properties, these advanced model-based methods typically treat the wavelength-dependent local optical fluence as another unknown parameter and then iteratively solve for the concentrations of molecular probes as an inverse problem. For example, Tzoumas et al. have recently reported an eigenspectrum-based method that has shown improved accuracy in quantifying deep-tissue blood oxygenation. This method models the local optical fluence as an affine function of only three reference base spectra (Figs. 2a–b) [73]. While this strategy has the potential to recover the concentrations of weakly-absorbing molecular probes, a large number of optical wavelengths (or reference fluence spectra) are needed, which slows down image acquisition. Moreover, the inverse problem is typically ill-posed and computationally intensive.
Figure 2. Advances in PAT signal unmixing.
(a) An eigenspectrum-based signal unmixing method assumes the local optical fluence in deep tissue can be modeled as an affine function of three reference base spectra (Φ 1, Φ 2, and Φ 3) [73]. (b) Eigenspectrum-based blood oxygenation mapping of the breast tumor in a mouse, showing the hypoxia tumor core. (c) A photoswitching-based signal unmixing method explores the two absorbing spectra of non-fluorescent protein BphP1. The constant background signals from hemoglobin can be suppressed through differential imaging [36]. (d) Photoswitching-based differential image of the BphP1-expressing tumor in a mouse kidney.
The second strategy seeks to recover the signal contribution from the molecular probes by exploring the temporal changes of the detected signals, assuming that the changes are confined only to the local molecular probes of interest [27, 74–83]. The temporal signal changes can be induced externally (e.g., light illumination) [36, 74] or internally (e.g., chemical cleavage) [75, 76, 83]. For example, several groups (including the authors’) have explored the reversible photoswitching capability of several fluorescent (Dronpa, rsTagRFP) and non-fluorescent (BphP1, AGP1) proteins [36, 74, 84]. By turning the molecular probe’s optical absorption on or off at a certain wavelength, this temporal modulation can effectively eliminate the constant background signals without needing to know the local optical fluence, thus dramatically enhance the image reconstruction robustness and detection sensitivity (Figs. 2c–d). However, the applicability of this strategy is limited to special types of molecular probes whose optical properties can be physically or chemically modulated, such as activatable nanoparticles or photoswitchable proteins [27, 36].
Advances in molecular probes made for PAT
PAT does not rely on fluorescence emission of molecules, giving it the ability to image nearly all molecules, fluorescent or not. Taking advantage of wavelength-tunable optical parametric oscillator (OPO) lasers and Ti:Sapphire lasers, PAT has been implemented to explore numerous molecular probes with primary absorption wavelengths ranging from the ultraviolet to the near-infrared (NIR) region [43]. The ideal molecular probe for PAT should have the following attributes: be specific to the biological process of interest; exhibit maximal absorption in the NIR window for deep in vivo imaging; have zero or low fluorescent quantum yield (not strict); be nontoxic to the cells; and be resistant to photobleaching. As PAT draws increasing attention from the biomedical community at large, more and more molecular probes are being developed specifically for PAT by researchers in synthetic chemistry, protein engineering, and nanotechnology. The availability of commercial PAT systems has also accelerated the adoption of these molecular probes in fundamental research areas, including cancer biology [8, 31, 33, 35, 39, 45, 85, 86], neuroscience [87–90], and regenerative medicine [91–94].
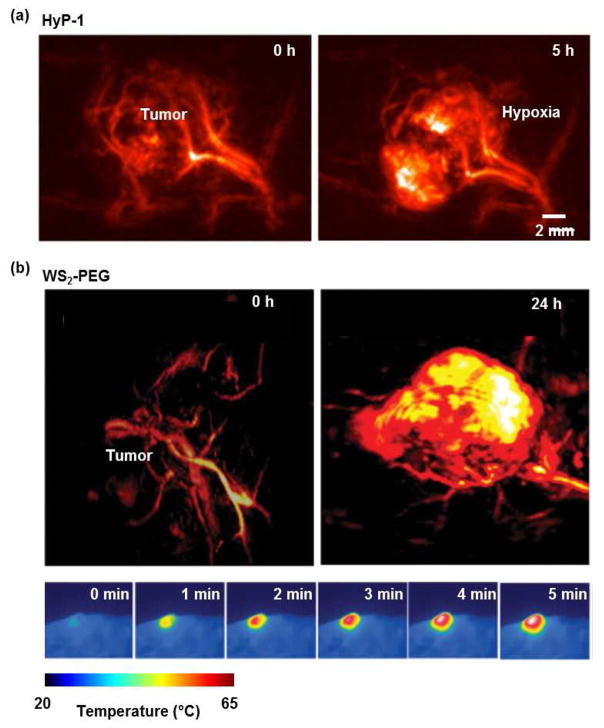
So far, three major strategies have been individually or concurrently implemented in developing PAT-specific molecular probes. (1) Aiming to maximize the penetration depth of PAT, the first strategy focuses on developing contrast agents that have strong optical absorption in the NIR wavelength range with low fluorescent quantum yield [16, 86, 95–101]. Taking advantage of melanin’s strong absorption in the NIR range, Jathoul et al. developed a tyrosinase reporter gene system that introduced the key enzyme in melanin synthesis into nonmelanogenic cells [101]. Although melanin’s relatively featureless absorption spectrum could make it hard to distinguish from intrinsic signals from hemoglobin, in vivo PAT of tyrosinase-expressing cells has shown high sensitivity [100, 101]. Zhou et al. recently developed a phosphorus phthalocyanine (P-Pc) dye that has an absorption spectrum peaking around 1000 nm [99]. P-Pc takes maximum advantage of its large molar extinction coefficient (1.1×105 cm−1 M−1 at 1064 nm) and the strong 1064 nm light from the Nd:YAG lasers, and thus has enabled deep tumor imaging in vivo. (2) Aiming to suppress the background signals from blood and improve the detection sensitivity, the second strategy focuses on developing contrast agents that can change their optical absorption in response to external or internal modulations [27, 34, 45, 75–82, 102]. Knox et al. reported an NIR agent for PA imaging of tissue hypoxia, which features an N-oxide-based trigger that can undergo facile bioreduction in the absence of oxygen and shifts the optical absorption peak from 670 nm to 760 nm (Fig. 3a) [83]. By taking ratiometric measurement, the hypoxic tissue environment (e.g., tumor and ischemia) can be imaged. (3) Aiming to improve the theranostic efficiency in personalized medicine, the third strategy focuses on developing contrast agents that have simultaneous functionalities of imaging and therapy (e.g., photothermal, photodynamic, drug delivery) [78, 82, 103–106]. Cheng et al. demonstrated PEGylated nanosheets for dual-modal CT/PAT guided photothermal therapy of tumors [103]. The strong NIR absorption of the nanosheets provides excellent signals for PAT of the tumor structure, and efficient heating for ablating the tumor cells (Fig. 3b).
Figure 3. Advances in PAT molecular probes.
(a) PACT images showing that HyP-1, an NIR hypoxia-response dye, changed its absorption peak from 670 nm to 760 nm five hours after exposure to the hypoxic environment of a breast tumor in a mouse [83]. (b) PACT images of a mouse breast tumor before and 24h after i.v. injection of WS2-PEG nanosheets, showing the accumulation of WS2-PEG in the tumor region [103]. The bottom-row images show that, when exposed to 808 nm light, the photothermal effect of WS2-PEG increased the local tumor temperature by 40 °C within 5 minutes.
Conclusion and discussions
Enabled by the advances in system implementations, signal unmixing methods, and molecular probes, PA molecular imaging has become increasingly popular in fundamental research and precision medicine. While this concise review can only cover a small portion of the exciting developments in PA molecular imaging, it has demonstrated the strong potential of this promising technology to continue growing and developing. It is also clear that the development of PAT has become a multidisciplinary effort from laser technology, ultrasound detection, high-speed electronics, mathematics, parallel computation, synthetic chemistry, protein engineering, and nanotechnology. The rapid growth of PAT technologies and their broad applications in biomedical research have, in turn, triggered new opportunities for each discipline.
With a series of long-standing engineering challenges overcome, we believe that PA molecular imaging will see even faster growth in the coming years. In particular, we anticipate four key breakthroughs. (1) PA molecular imaging at depths around the optical dissipation limit (~10 cm) will be possible by developing molecular probes that can strongly absorb light in the NIR optical window, while other intrinsic tissue components present the least optical attenuations [107–109]. For example, the effective attenuation coefficient spectrum of human breast tissue has a minimum near 730 nm. Moreover, when the optical scattering effect is compensated for by using wavefront engineering technologies [110, 111], PA molecular imaging may approach a sufficient penetration beyond 10 cm. (2) Single-molecule detection by PAT is highly promising using novel ultrasonic detectors with high piezoelectric efficiency (for piezoelectric ultrasound receivers) or high Q-factors (for optical ultrasound receivers) [58, 112, 113]. (3) Quantitative PA molecular imaging with high accuracy at depths will be enabled by new imaging methods and mathematical models that can better map the optical properties of the tissue [73]. (4) Finally, PA molecular imaging of neural activities in the deep brain will be achieved by using novel genetically encodable indicators of action potentials or surrogates (e.g., voltage- or calcium-sensitive proteins) with strong absorption in the NIR spectral region [114].
Highlights.
Photoacoustic tomography is inherently capable of molecular imaging with high spatial resolution, deep penetration depth, and high detection sensitivity.
Recent advances in PAT system development have made critical breakthroughs in real-time small animal whole-body imaging.
Novel methods based on compensating for the optical fluence or extracting the signal temporal changes have been developed to improve the signal unmixing accuracy in PAT.
Innovative strategies that aim at improving the detection sensitivity have been individually or concurrently implemented in developing PAT-specific molecular probes.
Acknowledgments
This work was supported in part by National Institutes of Health grants DP1 EB016986 (NIH Director’s Pioneer Award), R01 CA186567 (NIH Director’s Transformative Research Award), R01 EB016963, U01 NS090579 (BRAIN Initiative), and U01 NS099717 (BRAIN Initiative) as well as National Science Foundation grant 1255930 (all to L.W.), and by Duke MEDx research fund and startup fund (to J.Y.). The authors appreciate Emelina Vienneau’s kind help with editing the manuscript. L.W. has a financial interest in Microphotoacoustics, Inc., CalPACT, LLC, and Union Photoacoustic Technologies, Ltd., which, however, did not support this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as
• of special interest
•• of outstanding interest
- 1.Bell AG. Upon the production and reproduction of sound by light. American Journal of Science. 1880;20(118):305–324. [Google Scholar]
- 2.Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335(6075):1458–62. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox B, Laufer JG, Arridge SR, Beard PC. Quantitative spectroscopic photoacoustic imaging: a review. Journal of Biomedical Optics. 2012;17(6):061202. doi: 10.1117/1.JBO.17.6.061202. [DOI] [PubMed] [Google Scholar]
- 4.Greneur CL, Sagot B. Biomedical Photoacoustic Imaging Patent Landscape. 2015 [Google Scholar]
- 5.Taruttis A, Ntziachristos V. Advances in real-time multispectral optoacoustic imaging and its applications. Nature Photonics. 2015;9(4):219–227. [Google Scholar]
- 6.Zackrisson S, van de Ven SMWY, Gambhir SS. Light In and Sound Out: Emerging Translational Strategies for Photoacoustic Imaging. Cancer Research. 2014;74(4):979–1004. doi: 10.1158/0008-5472.CAN-13-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang LV. Tutorial on photoacoustic microscopy and computed tomography. Ieee Journal of Selected Topics in Quantum Electronics. 2008;14(1):171–179. [Google Scholar]
- 8.Copland JA, Eghtedari M, Popov VL, Kotov N, Mamedova N, Motamedi M, Oraevsky AA. Bioconjugated gold nanoparticles as a molecular based contrast agent: Implications for imaging of deep tumors using optoacoustic tomography. Molecular Imaging and Biology. 2004;6(5):341–349. doi: 10.1016/j.mibio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Mallidi S, Larson T, Aaron J, Sokolov K, Emelianov S. Molecular specific optoacoustic imaging with plasmonic nanoparticles. Optics Express. 2007;15(11):6583–6588. doi: 10.1364/oe.15.006583. [DOI] [PubMed] [Google Scholar]
- 10.De La Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, Levi J, Smith BR, Ma TJ, Oralkan O, Cheng Z, Chen XY, Dai HJ, Khuri-Yakub BT, Gambhir SS. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nature Nanotechnology. 2008;3(9):557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Li ML, Oh JT, Xie XY, Ku G, Wang W, Li C, Lungu G, Stoica G, Wang LV. Simultaneous molecular and hypoxia imaging of brain tumors in vivo using spectroscopic photoacoustic tomography. Proceedings of the Ieee. 2008;96(3):481–489. The first demonstration of PA molecular imaging in vivo. [Google Scholar]
- 12.Li PC, Wang CRC, Shieh DB, Wei CW, Liao CK, Poe C, Jhan S, Ding AA, Wu YN. In vivo Photoacoustic Molecular Imaging with Simultaneous Multiple Selective Targeting Using Antibody-Conjugated Gold Nanorods. Optics Express. 2008;16(23):18605–18615. doi: 10.1364/oe.16.018605. [DOI] [PubMed] [Google Scholar]
- 13.Razansky D, Baeten J, Ntziachristos V. Sensitivity of molecular target detection by multispectral optoacoustic tomography (MSOT) Medical Physics. 2009;36(3):939–945. doi: 10.1118/1.3077120. [DOI] [PubMed] [Google Scholar]
- 14.Razansky D, Distel M, Vinegoni C, Ma R, Perrimon N, Koster RW, Ntziachristos V. Multispectral opto-acoustic tomography of deep-seated fluorescent proteins in vivo. Nature Photonics. 2009;3(7):412–417. [Google Scholar]
- 15.Homan K, Kim S, Chen YS, Wang B, Mallidi S, Emelianov S. Prospects of molecular photoacoustic imaging at 1064 nm wavelength. Optics Letters. 2010;35(15):2663–2665. doi: 10.1364/OL.35.002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razansky D, Deliolanis NC, Vinegoni C, Ntziachristos V. Deep Tissue Optical and Optoacoustic Molecular Imaging Technologies for Pre-Clinical Research and Drug Discovery. Current Pharmaceutical Biotechnology. 2012;13(4):504–522. doi: 10.2174/138920112799436258. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Rajian JR, Cheng JX. Spectroscopic Imaging of Deep Tissue through Photoacoustic Detection of Molecular Vibration. Journal of Physical Chemistry Letters. 2013;4(13):2177–2185. doi: 10.1021/jz400559a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzoumas S, Nunes A, Deliolanis NC, Ntziachristos V. Effects of multispectral excitation on the sensitivity of molecular optoacoustic imaging. Journal of Biophotonics. 2015;8(8):629–637. doi: 10.1002/jbio.201400056. [DOI] [PubMed] [Google Scholar]
- 19.Hui J, Li R, Phillips EH, Goergen CJ, Sturek M, Cheng JX. Bond-selective photoacoustic imaging by converting molecular vibration into acoustic waves. Photoacoustics. 2016;4(1):11–21. doi: 10.1016/j.pacs.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Li L, Zhu L, Ma C, Lin L, Yao J, Wang L, Maslov K, Zhang R, Chen W, Shi J, Wang LV. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nature Biomedical Engineering. 2017;1:0071. doi: 10.1038/s41551-017-0071. The first panoramic PACT system that can provide real-time imaging of small-animal whole-body dynamics, with sub-millimeter resolutions. The ring-shaped 512-elements ultrasonic transducer array surrounded the animal and covered the entire cross-sectional region. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Fehm TF, Dean-Ben XL, Ford SJ, Razansky D. In vivo whole-body optoacoustic scanner with real-time volumetric imaging capacity. Optica. 2016;3(11):1153–1159. The first demonstration of 3D PACT with real-time imaging of small-animal regional dynamics. The semi-spherical 256-elements ultrasonic transducer array provided near-isotropic spatial resolutions over a 1.5 cm3 tissue volume. However, 256 elements are considered too sparse for a 2D array, tending to cause imaging artifacts outside the central zone of the 3D field of view covered by the array. [Google Scholar]
- 22•.Aguirre J, Schwarz M, Garzorz N, Omar M, Buehler A, Eyerich K, Ntziachristos V. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nature Biomedical Engineering. 2017;1:0068. The utilization of an ultra-wideband ultrasonic transducer enabled high-resolution PAM of human skins at different depths. [Google Scholar]
- 23.Tzoumas S, Deliolanis NC, Morscher S, Ntziachristos V. Unmixing Molecular Agents From Absorbing Tissue in Multispectral Optoacoustic Tomography. Ieee Transactions on Medical Imaging. 2014;33(1):48–60. doi: 10.1109/TMI.2013.2279994. [DOI] [PubMed] [Google Scholar]
- 24.Tzoumas S, Kravtsiv A, Gao Y, Buehler A, Ntziachristos V. Statistical Molecular Target Detection Framework for Multispectral Optoacoustic Tomography. Ieee Transactions on Medical Imaging. 2016;35(12):2534–2545. doi: 10.1109/TMI.2016.2583791. [DOI] [PubMed] [Google Scholar]
- 25.Beard PC, Laufer JG, Cox B, Arridge SR. Quantitative Photoacoustic Imaging: Measurement of Absolute Chromophore Concentrations for Physiological and Molecular Imaging. In: Wang LV, editor. Photoaccoustic Imaging and Spectroscopy. Vol. 144. Boca Raton: Crc Press-Taylor & Francis Group; 2009. pp. 121–143. [Google Scholar]
- 26.Weber J, Beard PC, Bohndiek SE. Contrast agents for molecular photoacoustic imaging. Nat Methods. 2016;13(8):639–50. doi: 10.1038/nmeth.3929. [DOI] [PubMed] [Google Scholar]
- 27.Levi J, Kothapalli SR, Ma TJ, Hartman K, Khuri-Yakub BT, Gambhir SS. Design, Synthesis, and Imaging of an Activatable Photoacoustic Probe. Journal of the American Chemical Society. 2010;132(32):11264–11269. doi: 10.1021/ja104000a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayer CL, Chen YS, Kim S, Mallidi S, Sokolov K, Emelianov S. Multiplex photoacoustic molecular imaging using targeted silica-coated gold nanorods. Biomedical Optics Express. 2011;2(7):1828–1835. doi: 10.1364/BOE.2.001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de la Zerda A, Kim JW, Galanzha EI, Gambhir SS, Zharov VP. Advanced contrast nanoagents for photoacoustic molecular imaging, cytometry, blood test and photothermal theranostics. Contrast Media & Molecular Imaging. 2011;6(5):346–369. doi: 10.1002/cmmi.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan D, Pramanik M, Wickline SA, Wang LHV, Lanza GM. Recent advances in colloidal gold nanobeacons for molecular photoacoustic imaging. Contrast Media & Molecular Imaging. 2011;6(5):378–388. doi: 10.1002/cmmi.449. [DOI] [PubMed] [Google Scholar]
- 31.Kircher MF, de la Zerda A, Jokerst JV, Zavaleta CL, Kempen PJ, Mittra E, Pitter K, Huang RM, Campos C, Habte F, Sinclair R, Brennan CW, Mellinghoff IK, Holland EC, Gambhir SS. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nature Medicine. 2012;18(5):829–U235. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei CW, Lombardo M, Larson-Smith K, Pelivanov I, Perez C, Xia J, Matula T, Pozzo D, O’Donnell M. Nonlinear contrast enhancement in photoacoustic molecular imaging with gold nanosphere encapsulated nanoemulsions. Applied Physics Letters. 2014;104(3):4. doi: 10.1063/1.4862461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balasundaram G, Ho CJH, Li K, Driessen W, Dinish US, Wong CL, Ntziachristos V, Liu B, Olivo M. Molecular photoacoustic imaging of breast cancer using an actively targeted conjugated polymer. International Journal of Nanomedicine. 2015;10:387–397. doi: 10.2147/IJN.S73558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao QQ, Pu KY. Emerging Designs of Activatable Photoacoustic Probes for Molecular Imaging. Bioconjugate Chemistry. 2016;27(12):2808–2823. doi: 10.1021/acs.bioconjchem.6b00641. [DOI] [PubMed] [Google Scholar]
- 35.Onoe S. Development of Molecular Probes for Spatio-temporal Analysis of in Vivo Tumor with Photoacoustic Imaging. Yakugaku Zasshi-Journal of the Pharmaceutical Society of Japan. 2016;136(3):491–498. doi: 10.1248/yakushi.15-00249. [DOI] [PubMed] [Google Scholar]
- 36••.Yao J, Kaberniuk AA, Li L, Shcherbakova DM, Zhang R, Wang L, Li G, Verkhusha VV, Wang LV. Multiscale photoacoustic tomography using reversibly switchable bacterial phytochrome as a near-infrared photochromic probe. Nat Methods. 2016;13(1):67–73. doi: 10.1038/nmeth.3656. The first demonstration of switchable non-fluorescent protein BphP1 for PA molecular imaging. The switching capability of BphP1 enabled suppression of background signals from hemoglobin and thus improved the detection sensitivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu LM, Qin H. Photoacoustic molecular imaging with functional nanoparticles. Journal of Innovative Optical Health Sciences. 2017;10(4):12. [Google Scholar]
- 38.Liu Y, Wang S, Ma Y, Lin J, Wang HY, Gu YQ, Chen XY, Huang P. Ratiometric Photoacoustic Molecular Imaging for Methylmercury Detection in Living Subjects. Advanced Materials. 2017;29(17):5. doi: 10.1002/adma.201606129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson KE, Bachawal SV, Abou-Elkacem L, Jensen K, Machtaler S, Tian L, Willmann JK. Spectroscopic Photoacoustic Molecular Imaging of Breast Cancer using a B7-H3-targeted ICG Contrast Agent. Theranostics. 2017;7(6):1463–1476. doi: 10.7150/thno.18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beard P. Biomedical photoacoustic imaging. Interface Focus. 2011;1(4):602–31. doi: 10.1098/rsfs.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang LV, Yao J. A practical guide to photoacoustic tomography in the life sciences. Nature methods. 2016;13(8):627–638. doi: 10.1038/nmeth.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim C, Favazza C, Wang LHV. In Vivo Photoacoustic Tomography of Chemicals: High-Resolution Functional and Molecular Optical Imaging at New Depths. Chemical Reviews. 2010;110(5):2756–2782. doi: 10.1021/cr900266s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber J, Beard PC, Bohndiek SE. Contrast agents for molecular photoacoustic imaging. Nature Methods. 2016;13(8):639–650. doi: 10.1038/nmeth.3929. [DOI] [PubMed] [Google Scholar]
- 44.Gujrati V, Mishra A, Ntziachristos V. Molecular imaging probes for multi-spectral optoacoustic tomography. Chemical Communications. 2017;53(34):4653–4672. doi: 10.1039/c6cc09421j. [DOI] [PubMed] [Google Scholar]
- 45.Wilson KE, Wang TY, Willmann JK. Acoustic and Photoacoustic Molecular Imaging of Cancer. Journal of Nuclear Medicine. 2013;54(11):1851–1854. doi: 10.2967/jnumed.112.115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taruttis A, Ntziachristos V. Optoacoustic Molecular Imaging: Methods and Applications. In: Wang RK, Tuchin VV, editors. Advanced Biophotonics: Tissue Optical Sectioning. Boca Raton: Crc Press-Taylor & Francis Group; 2014. pp. 449–474. [Google Scholar]
- 47.Liu YJ, Nie LM, Chen XY. Photoacoustic Molecular Imaging: From Multiscale Biomedical Applications Towards Early-Stage Theranostics. Trends in Biotechnology. 2016;34(5):420–433. doi: 10.1016/j.tibtech.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao J, Wang LV. Photoacoustic Brain Imaging: from Microscopic to Macroscopic Scales. Neurophotonics. 2014;1(1):011003. doi: 10.1117/1.NPh.1.1.011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mallidi S, Luke GP, Emelianov S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol. 2011;29(5):213–21. doi: 10.1016/j.tibtech.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai X, Zhang YS, Xia Y, Wang LV. Photoacoustic Microscopy in Tissue Engineering. Materials today. 2013;16(3):67–77. doi: 10.1016/j.mattod.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ripoll J, Koberstein-Schwarz B, Ntziachristos V. Unleashing Optics and Optoacoustics for Developmental Biology. Trends Biotechnol. 2015;33(11):679–91. doi: 10.1016/j.tibtech.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Strohm EM, Moore MJ, Kolios MC. Single cell photoacoustic microscopy: a review. Selected Topics in Quantum Electronics, IEEE Journal of. 2015;(99):1. [Google Scholar]
- 53.Wang LHV, Hu S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science. 2012;335(6075):1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang LV. Multiscale photoacoustic microscopy and computed tomography. Nature Photonics. 2009;3(9):503–509. doi: 10.1038/nphoton.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herzog E, Taruttis A, Beziere N, Lutich AA, Razansky D, Ntziachristos V. Optical Imaging of Cancer Heterogeneity with Multispectral Optoacoustic Tomography. Radiology. 2012;263(2):461–468. doi: 10.1148/radiol.11111646. [DOI] [PubMed] [Google Scholar]
- 56.Kim JW, Galanzha EI, Shashkov EV, Moon HM, Zharov VP. Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nature Nanotechnology. 2009;4(10):688–694. doi: 10.1038/nnano.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laufer J, Johnson P, Zhang E, Treeby B, Cox B, Pedley B, Beard P. In vivo preclinical photoacoustic imaging of tumor vasculature development and therapy. Journal of Biomedical Optics. 2012;17(5):056016. doi: 10.1117/1.JBO.17.5.056016. [DOI] [PubMed] [Google Scholar]
- 58•.Guggenheim JA, Li J, Allen TJ, Colchester RJ, Noimark S, Ogunlade O, Parkin IP, Papakonstantinou I, Desjardins AE, Zhang EZ, Beard PC. Ultrasensitive planoconcave optical microresonators for ultrasound sensing. Nature Photonics. 2017;11(11):714–719. The development of an ultra-sensitive all-optical ultrasound detector for PA and ultrasound imaging, especially for endoscopic applications. [Google Scholar]
- 59.Cox BT, Laufer JG, Beard PC. Quantitative Photoacoustic Image Reconstruction using Fluence Dependent Chromophores. Biomedical Optics Express. 2010;1(1):201–208. doi: 10.1364/BOE.1.000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao L, Sun Y, Jiang HB. Transport-based quantitative photoacoustic tomography: simulations and experiments. Physics in Medicine and Biology. 2010;55(7):1917–1934. doi: 10.1088/0031-9155/55/7/009. [DOI] [PubMed] [Google Scholar]
- 61.Zemp RJ. Quantitative photoacoustic tomography with multiple optical sources. Applied Optics. 2010;49(18):3566–3572. doi: 10.1364/AO.49.003566. [DOI] [PubMed] [Google Scholar]
- 62.Bauer AQ, Nothdurft RE, Erpelding TN, Wang LHV, Culver JP. Quantitative photoacoustic imaging: correcting for heterogeneous light fluence distributions using diffuse optical tomography. Journal of Biomedical Optics. 2011;16(9):096016. doi: 10.1117/1.3626212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li SF, Montcel B, Liu WY, Vray D. Analytical model of optical fluence inside multiple cylindrical inhomogeneities embedded in an otherwise homogeneous turbid medium for quantitative photoacoustic imaging. Optics Express. 2014;22(17):20500–20514. doi: 10.1364/OE.22.020500. [DOI] [PubMed] [Google Scholar]
- 64.Song NN, Deumic C, Da Silva A. Considering sources and detectors distributions for quantitative photoacoustic tomography. Biomedical Optics Express. 2014;5(11):3960–3974. doi: 10.1364/BOE.5.003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malone E, Powell S, Cox BT, Arridge S. Reconstruction-classification method for quantitative photoacoustic tomography. Journal of Biomedical Optics. 2015;20(12):126004. doi: 10.1117/1.JBO.20.12.126004. [DOI] [PubMed] [Google Scholar]
- 66.Hochuli R, Powell S, Arridge S, Cox B. Quantitative photoacoustic tomography using forward and adjoint Monte Carlo models of radiance. Journal of Biomedical Optics. 2016;21(12):126004. doi: 10.1117/1.JBO.21.12.126004. [DOI] [PubMed] [Google Scholar]
- 67.Liu YB, Jiang HB, Yuan Z. Two schemes for quantitative photoacoustic tomography based on Monte Carlo simulation. Medical Physics. 2016;43(7):3987–3997. doi: 10.1118/1.4953185. [DOI] [PubMed] [Google Scholar]
- 68.Venugopal M, van Es P, Manohar S, Roy D, Vasu RM. Quantitative photoacoustic tomography by stochastic search: direct recovery of the optical absorption field. Optics Letters. 2016;41(18):4202–4205. doi: 10.1364/OL.41.004202. [DOI] [PubMed] [Google Scholar]
- 69.An L, Saratoon T, Fonseca M, Ellwood R, Cox B. Statistical independence in nonlinear model-based inversion for quantitative photoacoustic tomography. Biomedical Optics Express. 2017;8(11):5297–5310. doi: 10.1364/BOE.8.005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brochu FM, Brunker J, Joseph J, Tomaszewski MR, Morscher S, Bohndiek SE. Towards Quantitative Evaluation of Tissue Absorption Coefficients Using Light Fluence Correction in Optoacoustic Tomography. Ieee Transactions on Medical Imaging. 2017;36(1):322–331. doi: 10.1109/TMI.2016.2607199. [DOI] [PubMed] [Google Scholar]
- 71.Nykanen O, Pulkkinen A, Tarvainen T. Quantitative photoacoustic tomography augmented with surface light measurements. Biomedical Optics Express. 2017;8(10):4380–4395. doi: 10.1364/BOE.8.004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shan TQ, Qi J, Jiang M, Jiang HB. GPU-based acceleration and mesh optimization of finite-element-method-based quantitative photoacoustic tomography: a step towards clinical applications. Applied Optics. 2017;56(15):4426–4432. doi: 10.1364/AO.56.004426. [DOI] [PubMed] [Google Scholar]
- 73••.Tzoumas S, Nunes A, Olefir I, Stangl S, Symvoulidis P, Glasl S, Bayer C, Multhoff G, Ntziachristos V. Eigenspectra optoacoustic tomography achieves quantitative blood oxygenation imaging deep in tissues. Nature Communications. 2016;7:12121. doi: 10.1038/ncomms12121. This study reported a new mathematic method for quantifying the absorption coefficients in PA Molecular imaging, in which the local optical fluence was modeled as a linear combination of three independent eigenspectra. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stiel AC, Dean-Ben XL, Jiang YY, Ntziachristos V, Razansky D, Westmeyer GG. High-contrast imaging of reversibly switchable fluorescent proteins via temporally unmixed multispectral optoacoustic tomography. Optics Letters. 2015;40(3):367–370. doi: 10.1364/OL.40.000367. [DOI] [PubMed] [Google Scholar]
- 75.Dragulescu-Andrasi A, Kothapalli SR, Tikhomirov GA, Rao JH, Gambhir SS. Activatable Oligomerizable Imaging Agents for Photoacoustic Imaging of Furin-Like Activity in Living Subjects. Journal of the American Chemical Society. 2013;135(30):11015–11022. doi: 10.1021/ja4010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirasawa T, Iwatate RJ, Kamiya M, Okawa S, Urano Y, Ishihara M. Multispectral photoacoustic imaging of tumours in mice injected with an enzyme-activatable photoacoustic probe. Journal of Optics. 2017;19(1):14. [Google Scholar]
- 77.Jeon M, Song WT, Huynh E, Kim J, Kim J, Helfield BL, Leung BYC, Goertz DE, Zheng G, Oh J, Lovell JF, Kim C. Methylene blue microbubbles as a model dual-modality contrast agent for ultrasound and activatable photoacoustic imaging. Journal of Biomedical Optics. 2014;19(1):8. doi: 10.1117/1.JBO.19.1.016005. [DOI] [PubMed] [Google Scholar]
- 78.Liang XL, Fang L, Li XD, Zhang X, Wang F. Activatable near infrared dye conjugated hyaluronic acid based nanoparticles as a targeted theranostic agent for enhanced fluorescence/CT/photoacoustic imaging guided photothermal therapy. Biomaterials. 2017;132:72–84. doi: 10.1016/j.biomaterials.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 79.Miao QQ, Lyu Y, Ding D, Pu KY. Semiconducting Oligomer Nanoparticles as an Activatable Photoacoustic Probe with Amplified Brightness for In Vivo Imaging of pH. Advanced Materials. 2016;28(19):3662–3668. doi: 10.1002/adma.201505681. [DOI] [PubMed] [Google Scholar]
- 80.Morgounova E, Shao Q, Hackel BJ, Thomas DD, Ashkenazi S. Photoacoustic lifetime contrast between methylene blue monomers and self-quenched dimers as a model for dual-labeled activatable probes. Journal of Biomedical Optics. 2013;18(5):8. doi: 10.1117/1.JBO.18.5.056004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang JJ, Zhen X, Upputuri PK, Pramanik M, Chen P, Pu KY. Activatable Photoacoustic Nanoprobes for In Vivo Ratiometric Imaging of Peroxynitrite. Advanced Materials. 2017;29(6):8. doi: 10.1002/adma.201604764. [DOI] [PubMed] [Google Scholar]
- 82.Zhang LW, Gao S, Zhang F, Yang K, Ma QJ, Zhu L. Activatable Hyaluronic Acid Nanoparticle as a Theranostic Agent for Optical/Photoacoustic Image-Guided Photothermal Therapy. Acs Nano. 2014;8(12):12250–12258. doi: 10.1021/nn506130t. [DOI] [PubMed] [Google Scholar]
- 83••.Knox HJ, Hedhli J, Kim TW, Khalili K, Dobrucki LW, Chan J. A bioreducible N-oxide-based probe for photoacoustic imaging of hypoxia. Nature Communications. 2017;8(1):1794. doi: 10.1038/s41467-017-01951-0. This study developed the first near-infrared PA probe Hyp-1 that could report the local hypoxic microenvironment. In a low-oxygen environment, Hyp-1 can be converted into red-Hyp-1 with a different optical absorption spectrum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dortay H, Märk J, Wagener A, Zhang E, Grötzinger C, Hildebrandt P, Friedrich T, Laufer J. SPIE BiOS. SPIE; 2016. Dual-wavelength photoacoustic imaging of a photoswitchable reporter protein. [Google Scholar]
- 85.Sano K. Development of Molecular Probes Based on Iron Oxide Nanoparticles for in Vivo Magnetic Resonance/Photoacoustic Dual Imaging of Target Molecules in Tumors. Yakugaku Zasshi-Journal of the Pharmaceutical Society of Japan. 2017;137(1):55–60. doi: 10.1248/yakushi.16-00228. [DOI] [PubMed] [Google Scholar]
- 86.Xiang LZ, Yuan Y, Xing D, Ou ZM, Yang SH, Zhou FF. Photoacoustic molecular imaging with antibody-functionalized single-walled carbon nanotubes for early diagnosis of tumor. Journal of Biomedical Optics. 2009;14(2):7. doi: 10.1117/1.3078809. [DOI] [PubMed] [Google Scholar]
- 87.Dean-Ben XL, Gottschalk S, Sela G, Shoham S, Razansky D. Functional optoacoustic neuro-tomography of calcium fluxes in adult zebrafish brain in vivo. Optics Letters. 2017;42(5):959–962. doi: 10.1364/OL.42.000959. [DOI] [PubMed] [Google Scholar]
- 88•.Dean-Ben XL, Sela G, Lauri A, Kneipp M, Ntziachristos V, Westmeyer GG, Shoham S, Razansky D. Functional optoacoustic neuro-tomography for scalable whole-brain monitoring of calcium indicators. Light-Science & Applications. 2016;5:7. doi: 10.1038/lsa.2016.201. The first report of PA imaging of neural activities in vivo, using a fluorescent calcium-sensitive indicator GCaMP. However, using a single wavelength of 488 nm, it is likely that the detected PA signal changes included the contributions from the concurrent hemodynamic responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rasheed N, Cressman JR, Chitnis PV. Feasibility of using RH795 dye for photoacoustic imaging of neuro-electrical activity. In: Luo Q, Ding J, editors. Neural Imaging and Sensing. Spie-Int Soc Optical Engineering; Bellingham: 2017. [Google Scholar]
- 90.Zhang RY, Rao B, Rong HY, Raman B, Wang LV. In vivo photoacoustic neuronal imaging of odor-evoked calcium signals in the drosophila brain (Conference Presentation) In: Oraevsky AA, Wang LV, editors. Photons Plus Ultrasound: Imaging and Sensing 2016. Spie-Int Soc Optical Engineering; Bellingham: 2016. [Google Scholar]
- 91.Avigo C, Flori A, Armanetti P, Di Lascio N, Kusmic C, Jose J, Losi P, Soldani G, Faita F, Menichetti L. Strategies for non-invasive imaging of polymeric biomaterial in vascular tissue engineering and regenerative medicine using ultrasound and photoacoustic techniques. Polymer International. 2016;65(7):734–740. [Google Scholar]
- 92.Ishihara M, Sato M, Sato S, Kikuchi T, Mitani G, Kaneshiro N, Ishihara M, Mochida J, Kikuchi M. Usefulness of the photoacoustic measurement method for monitoring the regenerative process of full-thickness defects in articular cartilage using tissue-engineering technology. In: Jacques SL, Roach WP, editors. Optical Interactions with Tissue and Cells Xvi. Spie-Int Soc Optical Engineering; Bellingham: 2005. pp. 288–291. [Google Scholar]
- 93.Nam SY, Chung E, Suggs LJ, Emelianov SY. Combined Ultrasound and Photoacoustic Imaging to Noninvasively Assess Burn Injury and Selectively Monitor a Regenerative Tissue-Engineered Construct. Tissue Engineering Part C-Methods. 2015;21(6):557–566. doi: 10.1089/ten.tec.2014.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharkey J, Scarfe L, Comenge J, Brillant N, Burton N, Antoine D, Wilm B, Levy R, Park K, Murray P. Utilising multispectral optoacoustic tomography (MSOT) to assess organ function and track labelled therapeutic cells for regenerative medicine therapies in vivo. Human Gene Therapy. 2017;28(8):A19–A19. [Google Scholar]
- 95.Nie LM, Chen M, Sun XL, Rong PF, Zheng NF, Chen XY. Palladium nanosheets as highly stable and effective contrast agents for in vivo photoacoustic molecular imaging. Nanoscale. 2014;6(3):1271–1276. doi: 10.1039/c3nr05468c. [DOI] [PubMed] [Google Scholar]
- 96.Pu KY, Shuhendler AJ, Jokerst JV, Mei JG, Gambhir SS, Bao ZN, Rao JH. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nature Nanotechnology. 2014;9(3):233–239. doi: 10.1038/nnano.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stahl T, Bofinger R, Lam I, Fallon KJ, Johnson P, Ogunlade O, Vassileva V, Pedley RB, Beard PC, Hailes HC, Bronstein H, Tabor AB. Tunable Semiconducting Polymer Nanoparticles with INDT-Based Conjugated Polymers for Photoacoustic Molecular Imaging. Bioconjugate Chemistry. 2017;28(6):1734–1740. doi: 10.1021/acs.bioconjchem.7b00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang HN, Liu CB, Gong XJ, Hu DH, Lin RQ, Sheng ZH, Zheng CF, Yan M, Chen JQ, Cai LT, Song L. In vivo photoacoustic molecular imaging of breast carcinoma with folate receptor-targeted indocyanine green nanoprobes. Nanoscale. 2014;6(23):14270–14279. doi: 10.1039/c4nr03949a. [DOI] [PubMed] [Google Scholar]
- 99••.Zhou Y, Wang DP, Zhang YM, Chitgupi U, Geng JM, Wang YH, Zhang YZ, Cook TR, Xia J, Lovell JF. A Phosphorus Phthalocyanine Formulation with Intense Absorbance at 1000 nm for Deep Optical Imaging. Theranostics. 2016;6(5):688–697. doi: 10.7150/thno.14555. A PA molecular probe that absorbs strongly at 1064 nm, which is the fundamental wavelength of the Nd: YAG lasers widely used in PAT systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100••.Krumholz A, VanVickle-Chavez SJ, Yao JJ, Fleming TP, Gillanders WE, Wang LHV. Photoacoustic microscopy of tyrosinase reporter gene in vivo. Journal of Biomedical Optics. 2011;16(8):080503. doi: 10.1117/1.3606568. The first demonstrations of high-sensitivity PAT of nonmelanogenic mammalian tissues that transiently (Ref. 100) and stably (Ref. 101) expressed melanin, respectively, using a tyrosinase-based gene reporter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101••.Jathoul AP, Laufer J, Ogunlade O, Treeby B, Cox B, Zhang E, Johnson P, Pizzey AR, Philip B, Marafioti T, Lythgoe MF, Pedley RB, Pule MA, Beard P. Deep in vivo photoacoustic imaging of mammalian tissues using a tyrosinase-based genetic reporter. Nature Photonics. 2015;9(4):239–246. The first demonstrations of high-sensitivity PAT of nonmelanogenic mammalian tissues that transiently (Ref. 100) and stably (Ref. 101) expressed melanin, respectively, using a tyrosinase-based gene reporter. [Google Scholar]
- 102.Levi J, Kothapalli SR, Bohndiek S, Yoon JK, Dragulescu-Andrasi A, Nielsen C, Tisma A, Bodapati S, Gowrishankar G, Yan XR, Chan C, Starcevic D, Gambhir SS. Molecular Photoacoustic Imaging of Follicular Thyroid Carcinoma. Clinical Cancer Research. 2013;19(6):1494–1502. doi: 10.1158/1078-0432.CCR-12-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng L, Liu JJ, Gu X, Gong H, Shi XZ, Liu T, Wang C, Wang XY, Liu G, Xing HY, Bu WB, Sun BQ, Liu Z. PEGylated WS2 Nanosheets as a Multifunctional Theranostic Agent for in vivo Dual-Modal CT/Photoacoustic Imaging Guided Photothermal Therapy. Advanced Materials. 2014;26(12):1886–1893. doi: 10.1002/adma.201304497. [DOI] [PubMed] [Google Scholar]
- 104.Emelianov S, Mallidi S, Larson T, Sokolov K. Photoacoustic Imaging and Therapy Utilizing Molecular Specific Plasmonic Nanoparticles. In: Wang LV, editor. Photoaccoustic Imaging and Spectroscopy. Vol. 144. Boca Raton: Crc Press-Taylor & Francis Group; 2009. pp. 399–407. [Google Scholar]
- 105.Lyu Y, Fang Y, Miao QQ, Zhen X, Ding D, Pu KY. Intraparticle Molecular Orbital Engineering of Semiconducting Polymer Nanoparticles as Amplified Theranostics for in Vivo Photoacoustic Imaging and Photothermal Therapy. Acs Nano. 2016;10(4):4472–4481. doi: 10.1021/acsnano.6b00168. [DOI] [PubMed] [Google Scholar]
- 106.Wang B, Su J, Karpiouk A, Yeager D, Emelianov S. Intravascular Photoacoustic and Ultrasound Imaging: From Tissue Characterization to Molecular Imaging to Image-Guided Therapy. In: Suri JS, Kathuria C, Molinari F, editors. Atherosclerosis Disease Management. New York: Springer; 2011. pp. 787–816. [Google Scholar]
- 107.Sordillo LA, Pu Y, Pratavieira S, Budansky Y, Alfano RR. Deep optical imaging of tissue using the second and third near-infrared spectral windows. Journal of Biomedical Optics. 2014;19(5):056004. doi: 10.1117/1.JBO.19.5.056004. [DOI] [PubMed] [Google Scholar]
- 108.Wilson RH, Nadeau KP, Jaworski FB, Tromberg BJ, Durkin AJ. Review of short-wave infrared spectroscopy and imaging methods for biological tissue characterization. J Biomed Opt. 2015;20(3):030901. doi: 10.1117/1.JBO.20.3.030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bashkatov AN, Genina EA, Kochubey VI, Tuchin VV. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. Journal of Physics D-Applied Physics. 2005;38(15):2543–2555. [Google Scholar]
- 110.Horstmeyer R, Ruan HW, Yang CH. Guidestar-assisted wavefront-shaping methods for focusing light into biological tissue. Nature Photonics. 2015;9(9):563–571. doi: 10.1038/nphoton.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lai P, Wang L, Tay JW, Wang LV. Photoacoustically guided wavefront shaping for enhanced optical focusing in scattering media. Nat Photonics. 2015;9(2):126–132. doi: 10.1038/nphoton.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dong BQ, Chen SY, Zhang Z, Sun C, Zhang HF. Photoacoustic probe using a microring resonator ultrasonic sensor for endoscopic applications. Optics Letters. 2014;39(15):4372–4375. doi: 10.1364/OL.39.004372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xie ZX, Tian C, Chen SL, Ling T, Zhang C, Guo LJ, Carson PL, Wang XD. 3D high resolution photoacoustic imaging based on pure optical photoacoustic microscopy with microring resonator. In: Oraevsky AA, Wang LV, editors. Photons Plus Ultrasound: Imaging and Sensing 2014. 2014. [Google Scholar]
- 114.Miller EW. Small molecule fluorescent voltage indicators for studying membrane potential. Current Opinion in Chemical Biology. 2016;33:74–80. doi: 10.1016/j.cbpa.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]