Abstract
A long-standing goal of molecular imaging is to visualize cellular function within the context of living animals, necessitating the development of reporter genes compatible with deeply penetrant imaging modalities such as ultrasound and magnetic resonance imaging (MRI). Until recently, no reporter genes for ultrasound were available, and most genetically encoded reporters for MRI were limited by metal availability or relatively low sensitivity. Here we review how these limitations are being addressed by recently introduced reporter genes based on air-filled and water-transporting biomolecules. We focus on gas-filled protein nanostructures adapted from buoyant microbes, which scatter sound waves, perturb magnetic fields and interact with hyperpolarized nuclei, as well as transmembrane water channels that alter the effective diffusivity of water in tissue.
Introduction
Molecular imaging seeks to visualize the location and function of cells and molecules within a variety of biological settings, including deep inside intact animals. Within this context, much of the powerful repertoire of genetically encoded reporters and sensors based on green fluorescent protein (GFP) and its analogues has limited utility due to the strong scattering and absorption of light by tissue. In his influential 2003 perspective titled “Imagining Imaging’s Future” Roger Tsien recognized this limitation and predicted that “the prevalence and success of GFP indicate that comparable revolutions might result from genetic sequences that robustly encode image contrast for other methods.” [1] Indeed, reporter genes for noninvasive deep-tissue imaging modalities such as ultrasound and magnetic resonance imaging (MRI) could have great value in both basic biomedical research and the development of cellular diagnostics and therapeutics. However, no such reporter genes have so far achieved the prevalence of GFP, and new ideas are therefore needed to spark the envisioned revolutions.
This review summarizes progress on two recently introduced classes of genetically encoded contrast agents for ultrasound and MRI that operate via new biophysical principles. One class is based on gas-filled proteins derived from buoyant microbes, which serve as the first reporter genes for ultrasound and produce contrast in susceptibility-based MRI and hyperpolarized xenon MRI. The second class is based on water channels such as aquaporin, whose overexpression in mammalian cells is brightly detectable with diffusion-based MRI. These reporter genes use fundamental properties of air and water to introduce new forms of contrast to the field of molecular imaging, providing unique capabilities for visualizing cellular function in vivo.
Proteins with air: gas vesicles as acoustic reporter genes
Until very recently, no reporter genes were available for ultrasound, a versatile modality capable of imaging centimeters-deep into soft tissue with spatial and temporal resolution on the order of 100 μm and 1 ms. In addition to being one of the most widely used modalities in medicine, ultrasound scales to smaller model organisms to enable basic and translational research. Recent advances in equipment and signal processing have provided ultrasound with the ability to image faster (down to tens of μs) and more precisely (below 10 μm with super-localization techniques) [2–4] (ref 2*). The classic contrast agents used in ultrasound are micron-sized bubbles of gas stabilized by a lipid or protein shell, which scatter sound waves due to their differential density and compressibility relative to water [5]. Could similar physical principles be embodied in a genetic sequence?
In 2014, it was discovered that a unique class of gas-filled protein nanostructures known as gas vesicles (GVs), which evolved in certain photosynthetic microbes as a means to achieve buoyancy in water, could produce ultrasound contrast *[6]. GVs are cylindrical or spindle-shaped gas-filled compartments with dimensions on the order of 200 nm, surrounded by a 2-nm thick protein shell [7] (Fig. 1A, B). This shell allows gases dissolved in the surrounding media to exchange freely in and out, while preventing water from forming a liquid inside the GV due to the strong hydrophobicity of the shell’s interior face. GVs are encoded in diverse organisms by operons of 8–14 genes, comprising a mixture of structural proteins and assembly factors [8]. Because they contain gas, it was hypothesized that GVs could scatter sound waves and produce ultrasound contrast. Indeed, this ability was demonstrated using GVs isolated from cyanobacteria and haloarchaea [6]. Building on this initial discovery, several other studies have been undertaken to understand the acoustic properties of GVs [9], to engineer them through genetic and biochemical modifications [10,11] (*ref. 10), to devise ultrasound imaging techniques tailored to distinguish their signal from background [12], and to characterize their in vivo biodistribution as purified, injectable agents [13]. These studies revealed remarkable non-linear acoustic properties and engineering versatility, enabling selective detection, multiplexed imaging and molecular targeting.
Figure 1. Acoustic Reporter Genes.
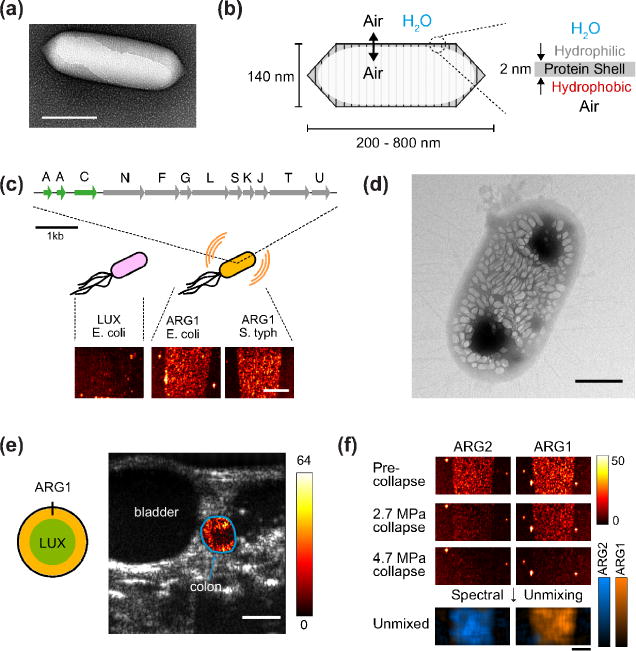
(a) Transmission electron micrograph (TEM) of an individual gas vesicle from A. flos-aquae. (b) Diagram of the structure and composition of a gas vesicle. (c) Engineered genetic construct, ARG1, comprising genes from A. flos aquae (green) and genes from B. megaterium (gray) to produce ultrasound-detectable gas vesicles in heterologous bacteria. (Bottom) Ultrasound images of E. coli and S. typhimurium expressing ARG1 or the luminescent LUX operon. (d) TEM of a an E. coli Nissle 1917 cell expressing ARG1. (e) Ultrasound image of live mouse with ARG1-expressing E. coli arranged in the colon as indicated in diagram. Color map represents collapse-subtracted contrast within the colon region of interest (outlined in blue), overlaid on grayscale anatomical image. (f) Ultrasound images of E. coli expressing ARG1 or ARG2 before and after the application of two different collapse pressures. (Bottom) Unmixed contrast maps corresponding to each type of bacterium. Scale bars represent 150 nm (a), 2 mm (c), 500 nm (d), 2.5 mm (e) and 2 mm (f). Data adapted with permission from Ref. 14.
In parallel, a major effort was undertaken to express GVs heterologously as acoustic reporter genes, initially in commensal and pathogenic bacteria being developed as microbial diagnostic and therapeutic agents **[14]. This was challenging because nearly a dozen genes need to be co-expressed at correct stoichiometry, fold into functional proteins and self-assemble into correctly shaped nanostructures. Initial attempts to transplant GV-forming genes from cyanobacteria to Escherichia coli were unsuccessful, while the expression of a more E. coli-compatible gene cluster from Bacillus megaterium [15] yielded small GVs producing little ultrasound contrast. The breakthrough was to combine structural genes from cyanobacteria with assembly factor genes from B. megaterium, resulting in correctly assembled GVs with size, shape and acoustic properties producing strong ultrasound contrast (Fig. 1, C–D). E. coli and Salmonella typhimurium expressing the resulting acoustic reporter genes could be imaged at concentrations on the order of 108 cells ml−1, representing a volume fraction of approximately 0.01%, and could be visualized in vivo in the gastrointestinal (GI) tract (Fig. 1E) and inside tumor xenografts. Furthermore, it was possible to distinguish two versions of the gene cluster from each other based on their acoustic properties, enabling multiplexed imaging (Fig. 1F).
While this initial development of acoustic reporter genes opens exciting new possibilities in the fields of ultrasound and molecular imaging, additional work must be done to maximize the acoustic contrast obtained from GV expression and apply this technology to imaging microbes in vivo in real basic biology, diagnostic and therapeutic scenarios. In addition, another breakthrough is needed to express GVs in mammalian cells, which poses additional challenges due to the differences in gene expression machinery between eukaryotes and prokaryotes.
Air in a magnet: gas vesicles as reporter genes for susceptibility-based and hyperpolarized MRI
Relative to ultrasound, much more work has been done to develop reporter genes for MRI, starting with pioneering work on enzymes that convert synthetic T1 contrast agents to forms with higher relaxivity[16], overexpression of the iron storage protein ferritin [17,18] and the detection of proteins with large numbers of exchangeable protons using chemical exchange saturation transfer (CEST) [19]. However, while these technologies continue to improve, they face intrinsic limitations due to their reliance on metal co-factors, and difficulty in being distinguished from background tissue contrast at low concentrations [20]. New contrast mechanisms are therefore needed to help overcome these limitations.
Air is well known as a source of contrast in T2-based MRI, with air-filled tissues such as lungs and nasal cavities generating unwanted image “blackouts” due to the differential magnetic susceptibility of air and water (set apart by ~ 9 ppm). Could air be turned into a source of genetically encodable MRI contrast? This concept was recently demonstrated using GVs, whose air contents produce microscale magnetic field gradients in their vicinity (Fig. 2A), efficiently dephasing aqueous protons and resulting in contrast in T2-weighted and quantitative susceptibility images (Fig. 2B) **[21]. However, what really distinguishes this contrast from that produced by metal-based T2 reporters is that it can be made to disappear: when ultrasound is applied above a threshold level, the GVs collapse, the air inside them dissolves, and their MRI contrast vanishes (Fig. 2C). This acoustically modulated MR imaging paradigm allows the contrast produced by the GVs to be distinguished from background, solving a major problem with existing contrast agents. In addition, it allows the acoustic properties of GVs to be utilized in MRI, for example enabling multiplexed imaging via serial acoustic collapse of GVs with engineered collapse thresholds (Fig. 2, D–E).
Figure 2. Gas-based MRI reporters.
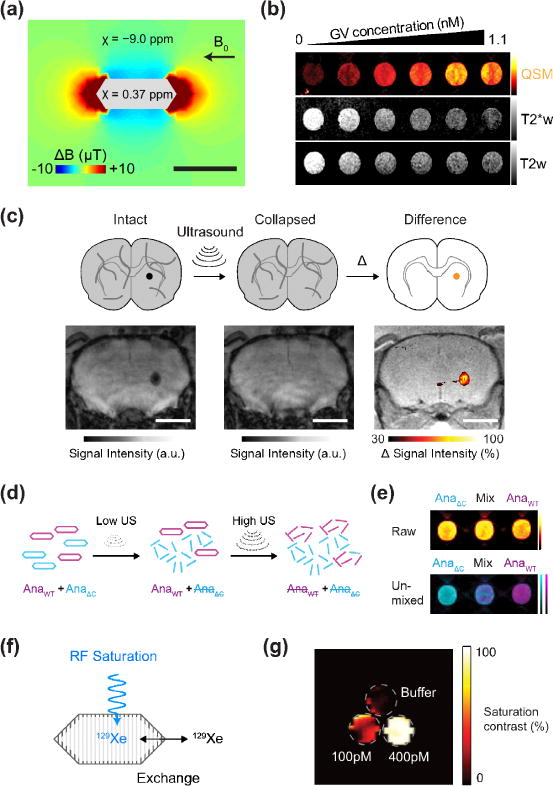
(a) Finite element model of the magnetic field gradient produced by a single air-filled GV in water exposed to a horizontal magnetic field (B0 = 7 Tesla). (b) Quantitative susceptibility map (QSM), T2*-weighted (T2*w) and T2-weighted (T2w) images of wells containing GVs at concentrations ranging from 0 to 1.1 nM. The QSM color scale ranges linearly from −2 to +50 parts per billion (ppb), and T2*w and T2w images have linear scales adjusted for optimal contrast. (c) Diagram and representative images of acoustically modulated MRI imaging of GVs injected in mouse brain. A T2*-weighted image is acquired followed by a second one after the application of ultrasound, and the difference between these images reveals contrast specific to the GVs. This difference image is overlaid on an anatomical image. (d) Schematic of the pressure-based multiplexing paradigm, wherein sequential ultrasound pulses are applied between MR images. The low-pressure ultrasound (Low US) selectively collapses AnaΔC GVs and eliminates their MRI contrast; subsequently, high-pressure ultrasound (High US) collapses AnaWT GVs. (e) Representative QSM images taken before ultrasound application in wells containing AnaWT, AnaΔC or a 1:1 mixture of the two, followed by an unmixed image indicating the quantity of each of the two populations in each well. (f) Diagram of 129Xe exchanging between the gas phase inside the GV and the dissolved phase in surrounding media. A radiofrequency (RF) pulse at the chemical shift of 129Xe in the GV saturates its signal, which is transferred by exchange to the bulk. (g) Saturation contrast image of a three-compartment phantom containing 400pM GVs, 100pM GVs and buffer. Panels (a)–(e) adapted with permission from ref. 21. Panel (g) adapted with permission from ref. 26.
Is it possible to go beyond air? A major limitation on the sensitivity of conventional MRI arises from the low equilibrium polarization of nuclear spins (10−5–10−6), meaning that only a few of the available spins in a biological sample contribute to the MRI signal. This limitation can be overcome with hyperpolarization – the preparation of certain nuclear spins in a non-equilibrium state of near-unity polarization with the applied field[22–24]. One such nucleus used in biological imaging is the noble gas 129Xe, which is biocompatible and can be delivered to tissues via inhalation. In 2006, it was discovered that synthetic contrast agents acting on 129Xe can be detected at nanomolar concentrations using hyperpolarized CEST (HyperCEST)[25], inspiring the search for reporter genes that could serve a similar purpose. As a protein compartment allowing its gas contents to be in dynamic exchange with the surrounding media, GVs made a natural candidate (Fig. 2E). Indeed, it was shown in 2014 that GVs can be detected with HyperCEST at picomolar concentrations – a level unprecedented for MRI contrast agents (Fig. 2F) *[26]. Although other xenon-binding proteins capable of HyperCEST have since been identified [27,28] (* for ref 27), GVs continue to provide unique capabilities, including elastic contrast via their ability to scale 129Xe binding capacity according to the ideal gas law to match the concentration of xenon in solution – an important property for in vivo applications with variable xenon delivery. In addition to 129Xe, other hyperpolarized nuclei such as 13C may also be substrates for reporter gene detection [29,30].
Calling nature’s plumber: water channels as reporter genes for diffusion-weighted MRI
In addition to the advances described above, one of the newest forms of MRI contrast arises from a far more ubiquitous class of proteins: aquaporins. These transmembrane channels, present in all domains of life, enable the selective diffusion of water across lipid bilayers, providing a way for cells to transport water, deform and respond to changes in osmolarity [31]. In a 2016 study, it was hypothesized that the overexpression of aquaporins in mammalian tissues would significantly increase the effective diffusivity of water in such tissues by minimizing the impact of the primary diffusion barrier – cell membranes (Fig. 3A) **[32]. This was expected to produce contrast in diffusion-weighted MRI. In fact, overexpressing aquaporin 1 (AQP1) in several mammalian cell lines produced increases of up to 200% in the apparent diffusion coefficient of water when these cells were imaged as pellets (Fig. 3B). Concentrations of the protein below 500 nM were sufficient for detection. Even when maximally expressed, AQP1 was found to have no effect on cell viability or morphology, as expected for a passive water channel under osmotically balanced conditions.
Figure 3. Diffusion-based MRI reporters.
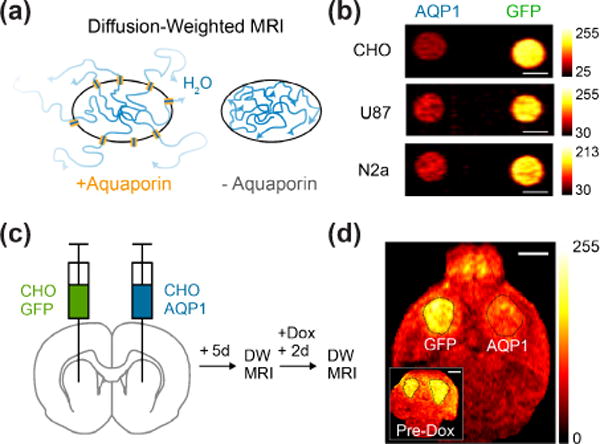
(a) Illustration of diffusion-based reporter gene mechanism. Cells overexpressing aquaporin have facilitated transport of water in and out of the cell, thereby increasing the effective diffusivity. (b) Diffusion-weighted MR image of pellets of CHO, U87 and N2a cells genetically modified to express AQP1 or GFP. Darker voxels indicate faster diffusion. (c) Illustration of in vivo injection of CHO cells to form brain tumors comprising cells with doxycycline (dox)-dependent expression of GFP or AQP1; after tumor growth, and preliminary imaging, expression is induced and the mice are imaged again. (d) Diffusion-weighted MR images of mouse before and after dox administration. The tumor expressing AQP1 appears darker after gene expression is induced. Data adapted with permission from ref. 32.
A key insight to making aquaporin work as a reporter involved setting the diffusion timescale in the MRI sequence to longer than 100 ms, allowing water to effectively sample the cell membrane as a diffusion barrier. Another important aspect of this contrast mechanism is that it involves water transport among cells and therefore depends on the fraction of cells in a given voxel expressing the reporter gene. Simulation and experiments showed that this fraction can be as small at 10%. The functionality of AQP1 as a reporter gene in vivo was demonstrated with its chemically induced expression in a brain tumor model (Fig. 3C). Together with another water channel published shortly after this work *[33], aquaporin represents a new category of MRI reporter genes that is sensitive, compact, autologous to its host, nonmetallic and non-toxic.
Outlook
As described in this article, the armory of reporter genes available for non-invasive molecular imaging has expanded to include proteins with air and water as their primary sources of contrast. By leveraging the unique properties of these materials, reporter genes are for the first time available for ultrasound imaging, acoustically modulated susceptibility-based MRI, hyperpolarized xenon MRI, and diffusion-weighted imaging. Whether any of these constructs achieves the impact of GFP remains to be seen. Since the initial demonstration of GFP as a fluorescent reporter, the engineering of its genetic sequence and mining of additional proteins with similar properties produced the toolkit that today is indispensable in biological imaging. Analogously, advances in the molecular engineering of GVs are needed to maximize the impact of these genetic constructs in deep-tissue imaging. The native biodiversity of GVs sets the stage for such engineering: for example, the particle volume of GVs varies by an order of magnitude between those encoded in haloarchaea and B. megaterium [11], and could potentially be reduced or expanded further through mutagenesis [34]. Exploiting this phenotypic diversity could yield GVs with material properties optimized for each imaging modality: the ability of the protein shell to harmonically scatter sound waves for ultrasound imaging, the optimal particle size and shape for susceptibility-based MRI, and the optimal diffusivity of gas across the shell for hyperpolarized xenon MRI. Additionally, a mechanistic understanding of the GV assembly process, especially in the context of heterologous expression in target cell types, will be important for their utility as imaging agents in challenging in vivo settings. Likewise, tapping into the evolutionary heritage and diversity of aquaporin genes [35] could yield proteins with enhanced or conditional water transport for improved sensitivity or functional sensing. Revolutions in biomolecular ultrasound and several forms of MRI await the results of this further research.
Acknowledgments
We thank members of the Shapiro Laboratory for helpful discussions. Related work in the Shapiro laboratory is also supported by the Heritage Medical Research Institute, the National Institutes of Health, the Defense Advanced Research Projects Agency, the Jacobs Institute for Molecular Engineering in Medicine, the Caltech Center for Environmental Microbial Interactions, the Human Frontiers Science Program, the Burroughs Wellcome Fund, the Pew Scholarship in the Biomedical Sciences, the Sontag Foundation, the Packard Fellowship for Science and Engineering. A.F. is supported by the Natural Sciences and Engineering Research Council of Canada PGSD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript.
COMPETING INTERESTS
The authors declare no competing financial interests.
References
- 1.Tsien RY. Imagining imaging’s future. Nat Rev Mol Cell Biol. 2003;9:S16–S21. [PubMed] [Google Scholar]
- 2*.Errico C, Pierre J, Pezet S, Desailly Y, Lenkei Z, Couture O, Tanter M. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature. 2015;527:499–502. doi: 10.1038/nature16066. This paper describes the development of super-localized ultrasound with sub-wavelength spatial resolution. [DOI] [PubMed] [Google Scholar]
- 3.Foster FS, Pavlin CJ, Harasiewicz KA, Christopher DA, Turnbull DH. Advances in ultrasound biomicroscopy. Ultrasound in Medicine & Biology. 2000;26:1–27. doi: 10.1016/s0301-5629(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 4.Tanter M, Fink M. Ultrafast imaging in biomedical ultrasound. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2014;61:102–119. doi: 10.1109/TUFFC.2014.6689779. [DOI] [PubMed] [Google Scholar]
- 5.Frinking PJA, Bouakaz A, Kirkhorn J, Ten Cate FJ, de Jong N. Ultrasound contrast imaging: current and new potential methods. Ultrasound in Medicine & Biology. 2000;26:965–975. doi: 10.1016/s0301-5629(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 6*.Shapiro MG, Goodwill PW, Neogy A, Yin M, Foster FS, Schaffer DV, Conolly SM. Biogenic gas nanostructures as ultrasonic molecular reporters. Nature Nanotechnology. 2014;9:311–316. doi: 10.1038/nnano.2014.32. This paper provides the first report of gas vesicles as contrast agents for ultrasound. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsby A. Gas vesicles. Microbiological reviews. 1994;58:94–144. doi: 10.1128/mr.58.1.94-144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfeifer F. Distribution, formation and regulation of gas vesicles. Nat Rev Microbiol. 2012;10:705–715. doi: 10.1038/nrmicro2834. [DOI] [PubMed] [Google Scholar]
- 9.Cherin M, Melis JS, Bourdeau RW, Yin M, Kochmann DM, Foster FS, Shapiro MG. Acoustic behavior of Halobacterium salinarum gas vesicles in the high frequency range: experiments and modeling. Ultrasound in Medicine and Biology. 2017;43:1016–1030. doi: 10.1016/j.ultrasmedbio.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Lakshmanan A, Farhadi A, Nety SP, Lee-Gosselin A, Bourdeau RW, Maresca D, Shapiro MG. Molecular Engineering of Acoustic Protein Nanostructures. ACS Nano. 2016;10:7314–7322. doi: 10.1021/acsnano.6b03364. This paper introduces molecular engineering approaches to tuning the properties and functionality of gas vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakshmanan A, Lu GJ, Farhadi A, Nety SP, Kunth M, Lee-Gosselin A, Maresca D, Bourdeau RW, Yin M, Yan J. Preparation of biogenic gas vesicle nanostructures for use as contrast agents for ultrasound and MRI. Nature protocols. 2017;12:2050. doi: 10.1038/nprot.2017.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maresca D, Lakshmanan A, Lee-Gosselin A, Melis JM, Ni Y, Bourdeau RW, Kochmann DM, Shapiro MG. Nonlinear Ultrasound Imaging of Nanoscale Acoustic Biomolecules. Applied Physics Letters. 2017;110 doi: 10.1063/1.4976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floc’h JL, Zlitni A, Bilton HA, Yin M, Farhadi A, Janzen NR, Shapiro MG, Valliant JF, Foster FS. In vivo Biodistribution of Radiolabeled Acoustic Protein Nanostructures. Molecular Imaging and Biology. 2017:1–10. doi: 10.1007/s11307-017-1122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Bourdeau RW, Lee-Gosselin A, Lakshmanan A, Farhadi A, Nety S, Kumar SR, Shapiro MG. Acoustic reporter genes for non-invasive imaging of microorganisms in mammalian hosts. Nature. 2018;553:86–90. doi: 10.1038/nature25021. This paper describes the development of gas vesicles as acoustic reporter genes in engineered bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Cannon MC. Gas vesicle genes identified in Bacillus megaterium and functional expression in Escherichia coli. J Bacteriol. 1998;180:2450–2458. doi: 10.1128/jb.180.9.2450-2458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louie AY, Hüber MM, Ahrens ET, Rothbächer U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In vivo visualization of gene expression using magnetic resonance imaging. Nature biotechnology. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 17.Cohen B, Dafni H, Meir G, Harmelin A, Neeman M. Ferritin as an Endogenous MRI Reporter for Noninvasive Imaging of Gene Expression in C6 Glioma Tumors. Neoplasia. 2005;7:109–117. doi: 10.1593/neo.04436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance imaging. Nature medicine. 2005;11:450–454. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- 19.Gilad AA, McMahon MT, Walczak P, Winnard PT, Raman V, van Laarhoven HW, Skoglund CM, Bulte JW, van Zijl PC. Artificial reporter gene providing MRI contrast based on proton exchange. Nature biotechnology. 2007;25:217–219. doi: 10.1038/nbt1277. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee A, Davis HC, Ramesh P, Lu GJ, Shapiro MG. Biomolecular MRI Reporters: evolution of new mechanisms. Progress in Nuclear Magnetic Resonance Spectroscopy. 2017;102–103:32–42. doi: 10.1016/j.pnmrs.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Lu GJ, Farhadi A, Szablowski JO, Lee-Gosselin A, Barnes SR, Lakshmanan A, Bourdeau RW, Shapiro MG. Acoustically modulated magnetic resonance imaging of gas-filled protein nanostructures. Nature Materials. 2018 doi: 10.1038/s41563-018-0023-7. in press. This paper describes biomolecular reporters for acoustically modulated susceptibility-based MRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barskiy DA, Coffey AM, Nikolaou P, Mikhaylov DM, Goodson BM, Branca RT, Lu GJ, Shapiro MG, Telkki V-V, Zhivonitko VV, et al. NMR Hyperpolarization Techniques of Gases. Chemistry. 2017;23:725–751. doi: 10.1002/chem.201603884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ardenkjær-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of> 10,000 times in liquid-state NMR. Proceedings of the National Academy of Sciences. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowers CR, Weitekamp DP. Transformation of symmetrization order to nuclear-spin magnetization by chemical reaction and nuclear magnetic resonance. Physical Review Letters. 1986;57:2645–2648. doi: 10.1103/PhysRevLett.57.2645. [DOI] [PubMed] [Google Scholar]
- 25.Schröder L, Lowery TJ, Hilty C, Wemmer DE, Pines A. Molecular imaging using a targeted magnetic resonance hyperpolarized biosensor. Science. 2006;314:446–449. doi: 10.1126/science.1131847. [DOI] [PubMed] [Google Scholar]
- 26*.Shapiro MG, Ramirez RM, Sperling LJ, Sun G, Sun J, Pines A, Schaffer DV, Bajaj VS. Genetically encoded reporters for hyperpolarized xenon magnetic resonance imaging. Nature chemistry. 2014;6:629–634. doi: 10.1038/nchem.1934. This paper describes the first reporter genes for hyperpolarized 129Xe MRI based on gas vesicles. [DOI] [PubMed] [Google Scholar]
- 27*.Wang Y, Roose BW, Palovcak EJ, Carnevale V, Dmochowski IJ. A Genetically Encoded β‐ Lactamase Reporter for Ultrasensitive 129Xe NMR in Mammalian Cells. Angewandte Chemie International Edition. 2016;55:8984–8987. doi: 10.1002/anie.201604055. This paper describes a reporter gene for hyperpolarized 129Xe MRI based on a small protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roose B, Zemerov S, Dmochowski I. Nanomolar small-molecule detection using a genetically encoded 129 Xe NMR contrast agent. Chemical Science. 2017;8:7631–7636. doi: 10.1039/c7sc03601a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrick PS, Kettunen MI, Tee SS, Rodrigues TB, Serrao E, Timm KN, McGuire S, Brindle KM. Detection of transgene expression using hyperpolarized 13C urea and diffusion‐weighted magnetic resonance spectroscopy. Magnetic resonance in medicine. 2015;73:1401–1406. doi: 10.1002/mrm.25254. [DOI] [PubMed] [Google Scholar]
- 30.Dzien P, Tee SS, Kettunen MI, Lyons SK, Larkin TJ, Timm KN, Hu DE, Wright A, Rodrigues TB, Serrao EM. 13C magnetic resonance spectroscopy measurements with hyperpolarized [1–13C] pyruvate can be used to detect the expression of transgenic pyruvate decarboxylase activity in vivo. Magnetic resonance in medicine. 2015;76:391–401. doi: 10.1002/mrm.25879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agre P, Bonhivers M, Borgnia MJ. The aquaporins, blueprints for cellular plumbing systems. Journal of Biological Chemistry. 1998;273:14659–14662. doi: 10.1074/jbc.273.24.14659. [DOI] [PubMed] [Google Scholar]
- 32**.Mukherjee A, Wu D, Davis HC, Shapiro MG. Non-invasive imaging using reporter genes altering cellular water permeability. Nature Communications. 2016;7:13891. doi: 10.1038/ncomms13891. This paper describes aquaporin as a reporter gene for diffusion-based MRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Schilling F, Ros S, Hu D-E, D’Santos P, McGuire S, Mair R, Wright AJ, Mannion E, Franklin RJ, Neves AA. MRI measurements of reporter-mediated increases in transmembrane water exchange enable detection of a gene reporter. Nature Biotechnology. 2017;35:75–80. doi: 10.1038/nbt.3714. This paper describes the urea transporter as a reporter gene for diffusion-based MRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strunk T, Hamacher K, Hoffgaard F, Engelhardt H, Zillig MD, Faist K, Wenzel W, Pfeifer F. Structural model of the gas vesicle protein GvpA and analysis of GvpA mutants in vivo. Molecular Microbiology. 2011;81:56–68. doi: 10.1111/j.1365-2958.2011.07669.x. [DOI] [PubMed] [Google Scholar]
- 35.Finn RN, Cerda J. Evolution and functional diversity of aquaporins. The Biological Bulletin. 2015;229:6–23. doi: 10.1086/BBLv229n1p6. [DOI] [PubMed] [Google Scholar]


