Abstract
Pericytes heterogeneity is based on their morphology, distribution, and markers. It is well known that pericytes from different organs may have distinct embryonic sources. Yamazaki et al. (2017) using several transgenic mouse model reveal by cell-lineage tracing that pericytes are even more heterogeneous than previously appreciated. This study shows that pericytes from within the same tissue may be heterogeneous in their origin. Remarkably, a subpopulation of embryonic dermal pericytes derives from the hematopoietic lineage, an unexpected source. Reconstructing the lineage of pericytes is central to understanding development, and also for the diagnosis and treatment of diseases in which pericytes play important roles.
Keywords: pericytes, stem cells, origin, embryonic, plasticity
Approximately one hundred years ago, Karl Wilhelm Zimmermann named a population of contractile cells pericytes because they were primarily located around blood vessels (Zimmermann, 1923). The word pericyte derives from the Greek kytos, a hollow vessel, appropriately describing a cell surrounding a blood vessel. Back then, these cells were identified mainly by their anatomical location and morphology. Pericytes have long projections that encircle the vessel walls which are widely scattered in all tissues (Hirschi and D’Amore, 1996). They surround endothelial cells and communicate with them along the length of the blood vessels by physical contact and paracrine signaling (Diaz-Flores et al., 1991).
Defining a specific molecular marker for pericytes has been challenging. Until recently, light and electron microscopy were the only techniques able to visualize them, thus limiting the knowledge acquired from those studies. In the last years, with the advent of fluorescent and confocal microscopy, technologies combining anatomical location, expression of surface markers, and genetic lineage tracing enabled the discovery of pericytes’ varying, sometimes unexpected, roles in health and disease (Birbrair et al., 2015). It is already known that pericytes stabilize blood vessels and participate in vascular development, maturation, remodeling, architecture, and permeability (Enge et al., 2002; Hellstrom et al., 2001; Leveen et al., 1994; Lindahl et al., 1997; Soriano, 1994). Additionally, they regulate blood flow (Pallone and Silldorff, 2001; Pallone et al., 1998; Pallone et al., 2003), and, in the central nervous system, collaborate with astrocytes to maintain the functional integrity of the blood brain barrier (Al Ahmad et al., 2011; Armulik et al., 2010; Bell et al., 2010; Cuevas et al., 1984; Daneman et al., 2010; Dohgu et al., 2005; Kamouchi et al., 2011; Krueger and Bechmann, 2010; Nakagawa et al., 2007; Nakamura et al., 2008; Shimizu et al., 2008). Pericytes also may affect immune function by regulating lymphocyte activation and by phagocytic activity (Balabanov et al., 1999; Balabanov et al., 1996; Bouchard et al., 1997; Castejon, 2011; Fabry et al., 1993; Fisher, 2009; Hasan and Glees, 1990; Jeynes, 1985; Kim et al., 2006; Thomas, 1999; Tu et al., 2011; Verbeek et al., 1995). Interestingly, strong evidence identified pericytes as stem cells capable to form several other cell types (Birbrair et al., 2017a; Birbrair and Delbono, 2015; Birbrair et al., 2017b; Birbrair et al., 2014a; Birbrair et al., 2013a, b, c; Birbrair et al., 2013d, 2014b, 2015; Birbrair et al., 2014c; Brighton et al., 1992; Collett et al., 2003; Crisan et al., 2008; Davidoff et al., 2004; Dellavalle et al., 2011; Dellavalle et al., 2007; Diaz-Flores et al., 1992; Doherty et al., 1998; Dore-Duffy et al., 2006; Farrington-Rock et al., 2004; Feng et al., 2011; Olson and Soriano, 2011; Richardson et al., 1982; Tang et al., 2008).
Pericytes differ in their embryonic origin between tissues (Armulik et al., 2011; Sims, 1991, 2000). Very little is known about the exact identity of pericyte ancestors within developing tissues, and there is evidence for numerous distinct developmental sources (Armulik et al., 2011). Lineage tracing studies indicate that pericytes in the cephalic region and thymus are of neuroectodermal origin (Foster et al., 2008; Muller et al., 2008; Simon et al., 2012; Trost et al., 2013; Zachariah and Cyster, 2010); while in lung, heart, liver and gut, the mesothelium is the main source of perivascular cells (Armulik et al., 2011; Asahina et al., 2011; Cai et al., 2008; Khan et al., 2016; Mellgren et al., 2008; Que et al., 2008; Zhou et al., 2008). In most other organs, pericytes derive from the mesoderm; specifically, the sclerotomal compartment (Armulik et al., 2011; Asahina et al., 2011; Bergwerff et al., 1998; Etchevers et al., 2001; Korn et al., 2002; Que et al., 2008; Wilm et al., 2005; Winkler et al., 2011; Yamanishi et al., 2012).
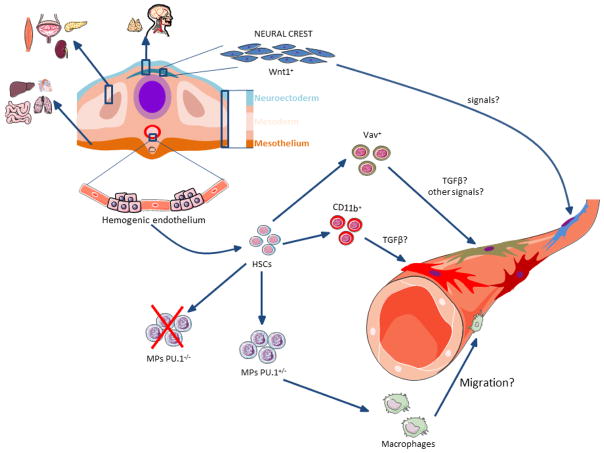
Understanding the origin and the processes that drive pericyte formation is a central question in developmental biology. Whether all pericytes from the same tissue have the same ancestry remains unknown. Nevertheless, in a recent article in Cell Reports, Yamazaki and colleagues showed that a pericyte subpopulation within the embryonic skin derives from an unexpected source (Yamazaki et al., 2017). The authors used in vivo lineage-tracing technologies to track specifically neural crest-, endothelial-, and hematopoietic-derived cells. These experiments suggested that during development the sources of tissue pericytes are heterogeneous. Strikingly, some of the pericytes in the embryonic skin and brain had hematopoietic origin (Yamazaki et al., 2017). Furthermore, the authors showed defective pericyte development in a mouse model with a known impairment of the myeloid lineage, suggesting that cells from this lineage contribute to pericyte formation in ectodermal organs (Yamazaki et al., 2017). Additionally, this study unravels an important signal (TGFβ) necessary for hematopoietic progenitors to differentiate into pericytes (Yamazaki et al., 2017). This study brings a new possible ancestor for pericytes, and reopens the discussions about pericytes’ heterogeneity. These cells are heterogeneous not only in their morphology, distribution, molecular markers and function, but also in their origin even within the same tissue.
Pericytes have been anatomically defined by their perivascular location in the blood vessel wall in close contact with endothelial cells (Feng et al., 2011; Sa-Pereira et al., 2012). However, not all perivascular cells are pericytes. Besides smooth muscle cells, other cellular types have been described as perivascular: i.e. adventitial cells (Crisan et al., 2012), fibroblasts (Soderblom et al., 2013), and macrophages (Bechmann et al., 2001; Guillemin and Brew, 2004). Classical electron microscopy studies of pericytes reveal their location under the vascular basal lamina (Allsopp and Gamble, 1979), in contrast to other perivascular cells. None of pericyte markers are specific, since they are also expressed by other cell types; and their expression in pericytes is highly dependent on the developmental stages (Armulik et al., 2011). Thus, pericitic markers used in this study could refer to other cell populations. For instance, PDGFRβ is a known marker of other cell types, such as fibroblasts (Soderblom et al., 2013; Spitzer et al., 2012); while NG2 proteoglycan could be expressed in macrophages (Yotsumoto et al., 2015). Additionally, pericytes that do not express NG2 were also recently described (Stark et al., 2013). Recent studies discovered new molecular markers for pericytes, such as Gli1 (Kramann et al., 2015; Kramann et al., 2017) and Tbx18 (Guimaraes-Camboa et al., 2017). Whether the perivascular cells derived from hematopoietic progenitors in the embryonic skin are pericytes still needs to be clarified. The combination of pericyte molecular markers with immunolabeling of the basal lamina in genetic lineage tracing models will confirm the nature of those cells.
Surprisingly, Yamazaki and colleagues found that perivascular cells were labeled in Vav-Cre/R26REYFP mice, but not in Tie2-Cre/R26REYFP mice (Yamazaki et al., 2017). It is known that Tie2 gene is expressed by endothelial cells (Maisonpierre et al., 1997; Schnurch and Risau, 1993). However, hematopoietic cells also express Tie2 (Arai et al., 2004; Takakura et al., 1998). Consistent with this, Tie2-Cre mice display Cre recombinase in both endothelial cells and hematopoietic cells, especially in hematopoietic stem cells (HSCs) (Constien et al., 2001; de Lange et al., 2008; Kisanuki et al., 2001; Tang et al., 2010). During development, both endothelium and definitive HSCs which form all hematopoietic cells, arise from a shared precursor, the hemogenic endothelium (Chen et al., 2009; Hirschi, 2012; Medvinsky and Dzierzak, 1996; Nguyen et al., 2014; Rafii et al., 2016). Due to this, it is virtually impossible to avoid some Cre recombinase activity in hematopoietic cells when using endothelial specific promoters with constitutively active Cre recombinase. Similarly, Vav-Cre strains have been shown to target both hematopoietic and endothelial cells (Croker et al., 2004; de Boer et al., 2003; Georgiades et al., 2002). It will be interesting to explore whether the embryonic hematopoietic cells that originate dermal pericytes derive from a different source than the hemogenic endothelium.
Interestingly, a recent study shows that cardiac endothelial cells give rise to ~20% of pericytes in the murine embryonic heart (Chen et al., 2016). Thus, the developmental sources of pericytes are more heterogeneous than previously appreciated. These surprising findings raise the possibility that distinct subsets of pericytes, depending on their developmental origin, could differentially contribute to different pathological conditions.
Additionally, to examine which specific hematopoietic cells form pericytes, CD11b-Cre/TdTomato mice were analyzed (Yamazaki et al., 2017). Nevertheless, pericytes may express CD11b in culture (Balabanov et al., 1996), as well as after stroke (Ozen et al., 2014). Thus, although pericytes in the skin vasculature are labeled in this genetic tracing mouse model (Yamazaki et al., 2017), whether dermal pericytes express CD11b earlier during development or if they derive from non-pericyte CD11b+ cell populations remains to be elucidated.
Although the authors show that dermal myeloid progenitors differentiate into pericytes in culture (Yamazaki et al., 2017), recent studies have shown that cells’ behavior in vitro could be completely different from their functionality in vivo (Guimaraes-Camboa et al., 2017; Snippert and Clevers, 2011; van Berlo et al., 2014). Artificial conditions in the dish which characterize cell culture systems may activate differentiation potential that could be not shared by these same endogenous cells in vivo under physiological conditions (Guimaraes-Camboa et al., 2017; Snippert and Clevers, 2011; van Berlo et al., 2014).
Thus, the plasticity observed in vitro might be simply a consequence of the artificial cell culture microenvironment. Based on this, a recent study has challenged the current view about pericytes’ capacity to differentiate into other cell types and reopened the discussion about pericytes’ plasticity (Birbrair et al., 2017a; Guimaraes-Camboa et al., 2017).
Furthermore, Yamazaki and colleagues used a transgenic mouse model (PU.1 knockout) in which severe impairment of the myeloid lineage was previously reported (McKercher et al., 1996; Scott et al., 1994). In those mice, F4/80+ macrophages were absent from the skin. Although the vascular network covered by endothelial and smooth muscle cells appeared normal, these vessels had a reduction in pericytes (Yamazaki et al., 2017). Interestingly, the reduction in the number of pericytes was approximatelly 50%, while the proportion of dermal pericytes derived from the hematopoietic lineage seems to correspond to approximately one fourth of all pericytes in the skin. It will be interesting to explore whether the absence of one pericyte subpopulation may influence the development of other pericitic subtypes in the same tissue but of different origin.
PERSPECTIVES/FUTURE DIRECTIONS
Pericytes development and survival are regulated by several signals coming from other cells, i.e. platelet-derived growth factor-β (PDGF-β) (Leveen et al., 1994), transforming growth factor-β1 (TGFβ) (Gaengel et al., 2009), heparin-binding epidermal growth factor (HB-EGF)(Stratman et al., 2010); stromal-derived factor 1-a (SDF-1α)(Song et al., 2009); Sonic hedgehog (Shh)(Nielsen and Dymecki, 2010); Jagged-1 (Jag-1)(Liu et al., 2009); Ephrin (Salvucci et al., 2009). Macrophages produce several of these molecules (Arango Duque and Descoteaux, 2014; Coulthard et al., 2012; Edwards et al., 2009; Goh et al., 2009; Heldin and Westermark, 1999; Pereira et al., 2013). Future studies will address whether the lack of macrophage-derived signals may affect pericytes survival.
Also, it remains unknown whether pericytes at early stages of skin development express PU.1 gene; and whether the absence of pericytes in PU.1 knockout mice is due to autonomous efect on pericytes. These issues may be addressed by using pericyte-specific inducible CreER driver, such as Tbx18-CreERT2 recently described (Guimaraes-Camboa et al., 2017), crossed to PU.1 floxed mouse (Iwasaki et al., 2005). In the resulting mice, Tbx18-CreERT2/PU.1fl/fl, PU.1 could be deleted specifically in pericytes at different developmental stages.
Mukouyama group found that TGFβ signaling is required for the differentiation of hematopoietic cells into pericytes in the embryonic skin, by deleting the gene for TGFβ receptor specifically in hematopoietic cells (Yamazaki et al., 2017). The primary sources of TGFβ in the skin remains unknown. Blood vessels form highly branched and ramified networks with nerves extending into almost every part of our body (Carmeliet and Tessier-Lavigne, 2005). The functional interdependence between the two systems is reflected in their close anatomic apposition (Bates et al., 2003; Lewis, 1902; Quaegebeur et al., 2011). The nervous system provides precise control of vascular diameter and blood flow. Blood vessels and nerves can crosstalk to one another and stimulate each other’s growth by neurotrophic or angiogenic guidance signals, respectively (Butler et al., 2010). Ingrowth of nerves precedes arterial formation, which follows axons branching pattern in the embryonic skin (Li et al., 2013; Mukouyama et al., 2002). The most prevalent cell type in peripheral nerves is the Schwann cell. In the bone marrow, Schwann cells maintain HSCs in the quiescent state through the production of activated TGFβ. It remains unknown whether during embryonic skin development perineural cells (Schwann cells) regulate the formation of perivascular cells (pericytes) through TGFβ production.
Within the same tissue, pericytes were characterized as heterogeneous based on their phenotype, molecular markers, distribution, and function (Armulik et al., 2011; Sims, 1991, 2000; Stark et al., 2013). For instance, in the adult skeletal muscle, two pericyte subtypes were identified based on their expression of Nestin-GFP. They differ in their differentiation potential; while type-1 pericytes (Nestin GFP−/NG2 DsRed+) can form fat and fibroblasts, type-2 pericytes (Nestin GFP+/NG2 DsRed+) have myogenic, neurogenic and angiogenic potential (Birbrair et al., 2014a; Birbrair et al., 2013a, c; Birbrair et al., 2014b, 2015; Birbrair et al., 2014c). Whether those same subtypes are present during embryogenesis remains unknown. And, more interestingly, further studies will reveal the origin of skeletal muscle pericytes subpopulations.
Two varieties of bone marrow pericytes were distinguished according to their location in the blood vessels: arteriolar and sinusoidal (Birbrair and Frenette, 2016). Arteriolar and sinusoidal pericytes can be separated in Nestin-GFP transgenic mice according to Nestin-GFP transgene expression level (Birbrair et al., 2011; Kunisaki et al., 2013). Sinusoidal pericytes express low levels of the Nestin-GFP transgene, thus are denominated Nestin-GFP dim cells. Arteriolar pericytes express high levels of the Nestin-GFP transgene, thus are denominated Nestin-GFP bright cells. Additionally, arteriolar pericytes express the pericytic marker NG2 proteoglycan, and do not express leptin receptor; while sinusoidal pericytes express leptin receptor, but lack NG2 expression (Kunisaki et al., 2013). Interestingly, although both pericyte subtypes produce the chemokine C-X-C motif ligand 12 (CXCL12), only Cxcl12 derived from arteriolar pericytes is importante for HSC maintenance (Asada et al., 2017). The embryonic origin and the developmental relationship of bone marrow pericyte subpopulations remain to be elucidated.
In the spinal cord, pericytes that express the glutamate aspartate transporter Glast differ from those that express desmin and αSMA (Goritz et al., 2011). After spinal cord injury, only Glast+ pericytes increase in number and form the core of the scar, suggesting that the role of spinal cord pericytes’ subpopulations differ in tissue repair after CNS injury (Goritz et al., 2011). Nevertheless, whether spinal cord pericytes have distinct origins is still unknown.
Pericytes’ potential to differentiate into several cell types has been established by numerous studies; and the general consensus holds that pericytes are cells with high plasticity; although a recent study challenges this concept (Birbrair et al., 2017a; Guimaraes-Camboa et al., 2017). Future studies should address whether this hematopoietic lineage-derived pericyte subpopulation vary in its differentiation capability in comparison to other pericytes from the same tissue. Pericyte-intrinsic changes may be reversible or not but, either way, represent another source of heterogeneity; a pericyte subpopulation could be more prone to differentiate or to enter apoptosis than another.
Furthermore, it will be interesting to test whether this differentiation from hematopoietic cells into pericytes during development could be reversed under certain pathological circumstances; are pericytes able to form hematopoietic cells?
In addition to genetic cell fate mapping, transcriptomic and single cell analysis represent fundamental tools that will help us understand the roles and the origins of pericyte subpopulations within the same tissue. This understanding may bring new approaches for several pathologic conditions as pericytes are present in all tissues and play important roles related to tissue turnover and regeneration. Taking their diversity into account, pericytes will be crucial in advancing our understanding of development, disease and aging.
Figure 1. Heterogeneity in the pericytes origins.
Pericytes are present around blood vessels in several tissues, such as brain, heart, lungs, skeletal muscle, pancreas, intestine, bone marrow, kidney, and others. During the embryonic development, pericytes in the head, cephalic region and thymus originate from the neuroectodermis, the ones found in gut, liver, lungs and heart are derived from the mesothelium, while the mesoderm gives rise to pericytes in other organs (such as kidneys, liver and pancreas). The study of Yamazaki and colleagues now suggests that surprisingly a subgroup of pericytes may derive from the hematopoietic lineage (Yamazaki et al., 2017). With the appearance of state of art technologies, such as new pericyte-lineage tracing mouse models, the true origin of pericytes subgroups will likely be revealed with much greater details in future studies.
Highlights.
Pericytes from within the same tissue may be heterogeneous in their origin.
A subpopulation of embryonic dermal pericytes derives from the hematopoietic lineage.
Acknowledgments
Alexander Birbrair is supported by a grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); Akiva Mintz is supported by the National Institute of Health (1R01CA179072-01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13-121-01-CDD); Osvaldo Delbono is supported by a grant from the National Institute of Health/National Institute on Aging (AG13934).
Footnotes
DISCLOSURES
The authors indicate no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:693–705. doi: 10.1038/jcbfm.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp G, Gamble HJ. An electron microscopic study of the pericytes of the developing capillaries in human fetal brain and muscle. Journal of anatomy. 1979;128:155–168. [PMC free article] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Frontiers in immunology. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma’ayan A, Frenette PS. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nature cell biology. 2017 doi: 10.1038/ncb3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K, Zhou B, Pu WT, Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53:983–995. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanov R, Beaumont T, Dore-Duffy P. Role of central nervous system microvascular pericytes in activation of antigen-primed splenic T-lymphocytes. Journal of neuroscience research. 1999;55:578–587. doi: 10.1002/(SICI)1097-4547(19990301)55:5<578::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Washington R, Wagnerova J, Dore-Duffy P. CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvascular research. 1996;52:127–142. doi: 10.1006/mvre.1996.0049. [DOI] [PubMed] [Google Scholar]
- Bates D, Taylor GI, Minichiello J, Farlie P, Cichowitz A, Watson N, Klagsbrun M, Mamluk R, Newgreen DF. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Developmental biology. 2003;255:77–98. doi: 10.1016/s0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. The European journal of neuroscience. 2001;14:1651–1658. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC. Neural crest cell contribution to the developing circulatory system: implications for vascular morphology? Circulation research. 1998;82:221–231. doi: 10.1161/01.res.82.2.221. [DOI] [PubMed] [Google Scholar]
- Birbrair A, Borges I, Sena I, Almeida G, Meirelles L, Gonçalves R, Mintz A, Delbono O. How plastic are pericytes? Stem cells and development. 2017a doi: 10.1089/scd.2017.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Delbono O. Pericytes are Essential for Skeletal Muscle Formation. Stem cell reviews. 2015;11:547–548. doi: 10.1007/s12015-015-9588-6. [DOI] [PubMed] [Google Scholar]
- Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Annals of the New York Academy of Sciences. 2016 doi: 10.1111/nyas.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Sattiraju A, Zhu D, Zulato G, Batista I, Nguyen VT, Messi ML, Solingapuram Sai KK, Marini FC, Delbono O, Mintz A. Novel Peripherally Derived Neural-Like Stem Cells as Therapeutic Carriers for Treating Glioblastomas. Stem cells translational medicine. 2017b;6:471–481. doi: 10.5966/sctm.2016-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O. Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PloS one. 2011;6:e16816. doi: 10.1371/journal.pone.0016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem cell research & therapy. 2014a;5:122. doi: 10.1186/scrt512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem cells and development. 2013a;22:2298–2314. doi: 10.1089/scd.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Experimental cell research. 2013b;319:45–63. doi: 10.1016/j.yexcr.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem cell research. 2013c;10:67–84. doi: 10.1016/j.scr.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. American journal of physiology Cell physiology. 2013d;305:C1098–1113. doi: 10.1152/ajpcell.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Frontiers in aging neuroscience. 2014b;6:245. doi: 10.3389/fnagi.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 2015;128:81–93. doi: 10.1042/CS20140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O. Type-2 pericytes participate in normal and tumoral angiogenesis. American journal of physiology Cell physiology. 2014c;307:C25–38. doi: 10.1152/ajpcell.00084.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard BA, Shatos MA, Tracy PB. Human brain pericytes differentially regulate expression of procoagulant enzyme complexes comprising the extrinsic pathway of blood coagulation. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:1–9. doi: 10.1161/01.atv.17.1.1. [DOI] [PubMed] [Google Scholar]
- Brighton CT, Lorich DG, Kupcha R, Reilly TM, Jones AR, Woodbury RA., 2nd The pericyte as a possible osteoblast progenitor cell. Clinical orthopaedics and related research. 1992:287–299. [PubMed] [Google Scholar]
- Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nature reviews Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Castejon OJ. Ultrastructural pathology of cortical capillary pericytes in human traumatic brain oedema. Folia Neuropathol. 2011;49:162–173. [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang H, Liu Y, Adams S, Eilken H, Stehling M, Corada M, Dejana E, Zhou B, Adams RH. Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nature communications. 2016;7:12422. doi: 10.1038/ncomms12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett G, Wood A, Alexander MY, Varnum BC, Boot-Handford RP, Ohanian V, Ohanian J, Fridell YW, Canfield AE. Receptor tyrosine kinase Axl modulates the osteogenic differentiation of pericytes. Circulation research. 2003;92:1123–1129. doi: 10.1161/01.RES.0000074881.56564.46. [DOI] [PubMed] [Google Scholar]
- Constien R, Forde A, Liliensiek B, Grone HJ, Nawroth P, Hammerling G, Arnold B. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis. 2001;30:36–44. doi: 10.1002/gene.1030. [DOI] [PubMed] [Google Scholar]
- Coulthard MG, Morgan M, Woodruff TM, Arumugam TV, Taylor SM, Carpenter TC, Lackmann M, Boyd AW. Eph/Ephrin signaling in injury and inflammation. The American journal of pathology. 2012;181:1493–1503. doi: 10.1016/j.ajpath.2012.06.043. [DOI] [PubMed] [Google Scholar]
- Crisan M, Corselli M, Chen WC, Peault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012 doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L, Cluse LA, Sutherland KD, Hartley L, Williams E, Zhang JG, Hilton DJ, Nicola NA, Alexander WS, Roberts AW. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- Cuevas P, Gutierrez-Diaz JA, Reimers D, Dujovny M, Diaz FG, Ausman JI. Pericyte endothelial gap junctions in human cerebral capillaries. Anat Embryol (Berl) 1984;170:155–159. doi: 10.1007/BF00319000. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Muller D. Progenitor cells of the testosterone-producing Leydig cells revealed. The Journal of cell biology. 2004;167:935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, Kioussis D. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. European journal of immunology. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- de Lange WJ, Halabi CM, Beyer AM, Sigmund CD. Germ line activation of the Tie2 and SMMHC promoters causes noncell-specific deletion of floxed alleles. Physiological genomics. 2008;35:1–4. doi: 10.1152/physiolgenomics.90284.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nature communications. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nature cell biology. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Lopez-Alonso A, Gonzalez R, Varela H. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clinical orthopaedics and related research. 1992:280–286. [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol. 1991;6:269–286. [PubMed] [Google Scholar]
- Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1998;13:828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Edwards JP, Zhang X, Mosser DM. The expression of heparin-binding epidermal growth factor-like growth factor by regulatory macrophages. Journal of immunology. 2009;182:1929–1939. doi: 10.4049/jimmunol.0802703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. The EMBO journal. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol. 1993;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6503–6508. doi: 10.1073/pnas.1015449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. Pericyte signaling in the neurovascular unit. Stroke. 2009;40:S13–15. doi: 10.1161/STROKEAHA.108.533117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, Blackburn C, Kioussis D, Coles M. Contribution of neural crest-derived cells in the embryonic and adult thymus. Journal of immunology. 2008;180:3183–3189. doi: 10.4049/jimmunol.180.5.3183. [DOI] [PubMed] [Google Scholar]
- Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- Georgiades P, Ogilvy S, Duval H, Licence DR, Charnock-Jones DS, Smith SK, Print CG. VavCre transgenic mice: a tool for mutagenesis in hematopoietic and endothelial lineages. Genesis. 2002;34:251–256. doi: 10.1002/gene.10161. [DOI] [PubMed] [Google Scholar]
- Goh F, Irvine KM, Lovelace E, Donnelly S, Jones MK, Brion K, Hume DA, Kotze AC, Dalton JP, Ingham A, Sweet MJ. Selective induction of the Notch ligand Jagged-1 in macrophages by soluble egg antigen from Schistosoma mansoni involves ERK signalling. Immunology. 2009;127:326–337. doi: 10.1111/j.1365-2567.2008.02979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. Journal of leukocyte biology. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans SM. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell stem cell. 2017 doi: 10.1016/j.stem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M, Glees P. The fine structure of human cerebral perivascular pericytes and juxtavascular phagocytes: their possible role in hydrocephalic edema resolution. J Hirnforsch. 1990;31:237–249. [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiological reviews. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. The Journal of cell biology. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK. Hemogenic endothelium during development and beyond. Blood. 2012;119:4823–4827. doi: 10.1182/blood-2011-12-353466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovascular research. 1996;32:687–698. [PubMed] [Google Scholar]
- Iwasaki H, Somoza C, Shigematsu H, Duprez EA, Iwasaki-Arai J, Mizuno S, Arinobu Y, Geary K, Zhang P, Dayaram T, Fenyus ML, Elf S, Chan S, Kastner P, Huettner CS, Murray R, Tenen DG, Akashi K. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood. 2005;106:1590–1600. doi: 10.1182/blood-2005-03-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeynes B. Reactions of granular pericytes in a rabbit cerebrovascular ischemia model. Stroke. 1985;16:121–125. doi: 10.1161/01.str.16.1.121. [DOI] [PubMed] [Google Scholar]
- Kamouchi M, Ago T, Kitazono T. Brain pericytes: emerging concepts and functional roles in brain homeostasis. Cell Mol Neurobiol. 2011;31:175–193. doi: 10.1007/s10571-010-9605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, Pinho S, Ciero P, Nakahara F, Ma’ayan A, Bergman A, Merad M, Frenette PS. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science. 2016;351:176–180. doi: 10.1126/science.aad0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Tran ND, Li Z, Yang F, Zhou W, Fisher MJ. Brain endothelial hemostasis regulation by pericytes. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:209–217. doi: 10.1038/sj.jcbfm.9600181. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Developmental biology. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Korn J, Christ B, Kurz H. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. The Journal of comparative neurology. 2002;442:78–88. doi: 10.1002/cne.1423. [DOI] [PubMed] [Google Scholar]
- Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell stem cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD. Gli1+ Pericyte Loss Induces Capillary Rarefaction and Proximal Tubular Injury. Journal of the American Society of Nephrology: JASN. 2017;28:776–784. doi: 10.1681/ASN.2016030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58:1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes & development. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Lewis WH. The development of the arm in man. Am J Anat. 1902;1:145–185. [Google Scholar]
- Li W, Kohara H, Uchida Y, James JM, Soneji K, Cronshaw DG, Zou YR, Nagasawa T, Mukouyama YS. Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin. Developmental cell. 2013;24:359–371. doi: 10.1016/j.devcel.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Liu H, Kennard S, Lilly B. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circulation research. 2009;104:466–475. doi: 10.1161/CIRCRESAHA.108.184846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, Maki RA. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. The EMBO journal. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Mellgren AM, Smith CL, Olsen GS, Eskiocak B, Zhou B, Kazi MN, Ruiz FR, Pu WT, Tallquist MD. Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circulation research. 2008;103:1393–1401. doi: 10.1161/CIRCRESAHA.108.176768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Muller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N, Iannarelli P, Richardson WD, Wegner M, Rodewald HR. Neural crest origin of perivascular mesenchyme in the adult thymus. Journal of immunology. 2008;180:5344–5351. doi: 10.4049/jimmunol.180.8.5344. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, Kataoka Y, Niwa M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27:687–694. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kamouchi M, Kitazono T, Kuroda J, Matsuo R, Hagiwara N, Ishikawa E, Ooboshi H, Ibayashi S, Iida M. Role of NHE1 in calcium signaling and cell proliferation in human CNS pericytes. American journal of physiology Heart and circulatory physiology. 2008;294:H1700–1707. doi: 10.1152/ajpheart.01203.2007. [DOI] [PubMed] [Google Scholar]
- Nguyen PD, Hollway GE, Sonntag C, Miles LB, Hall TE, Berger S, Fernandez KJ, Gurevich DB, Cole NJ, Alaei S, Ramialison M, Sutherland RL, Polo JM, Lieschke GJ, Currie PD. Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature. 2014;512:314–318. doi: 10.1038/nature13678. [DOI] [PubMed] [Google Scholar]
- Nielsen CM, Dymecki SM. Sonic hedgehog is required for vascular outgrowth in the hindbrain choroid plexus. Developmental biology. 2010;340:430–437. doi: 10.1016/j.ydbio.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LE, Soriano P. PDGFRbeta signaling regulates mural cell plasticity and inhibits fat development. Developmental cell. 2011;20:815–826. doi: 10.1016/j.devcel.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen I, Deierborg T, Miharada K, Padel T, Englund E, Genove G, Paul G. Brain pericytes acquire a microglial phenotype after stroke. Acta neuropathologica. 2014;128:381–396. doi: 10.1007/s00401-014-1295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallone TL, Silldorff EP. Pericyte regulation of renal medullary blood flow. Exp Nephrol. 2001;9:165–170. doi: 10.1159/000052608. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Silldorff EP, Turner MR. Intrarenal blood flow: microvascular anatomy and the regulation of medullary perfusion. Clin Exp Pharmacol Physiol. 1998;25:383–392. doi: 10.1111/j.1440-1681.1998.tb02220.x. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol. 2003;284:F253–266. doi: 10.1152/ajprenal.00304.2002. [DOI] [PubMed] [Google Scholar]
- Pereira TA, Xie G, Choi SS, Syn WK, Voieta I, Lu J, Chan IS, Swiderska M, Amaral KB, Antunes CM, Secor WE, Witek RP, Lambertucci JR, Pereira FL, Diehl AM. Macrophage-derived Hedgehog ligands promotes fibrogenic and angiogenic responses in human schistosomiasis mansoni. Liver international: official journal of the International Association for the Study of the Liver. 2013;33:149–161. doi: 10.1111/liv.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16626–16630. doi: 10.1073/pnas.0808649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RL, Hausman GJ, Campion DR. Response of pericytes to thermal lesion in the inguinal fat pad of 10-day-old rats. Acta Anat (Basel) 1982;114:41–57. doi: 10.1159/000145577. [DOI] [PubMed] [Google Scholar]
- Sa-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Molecular neurobiology. 2012;45:327–347. doi: 10.1007/s12035-012-8244-2. [DOI] [PubMed] [Google Scholar]
- Salvucci O, Maric D, Economopoulou M, Sakakibara S, Merlin S, Follenzi A, Tosato G. EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood. 2009;114:1707–1716. doi: 10.1182/blood-2008-12-192294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurch H, Risau W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development. 1993;119:957–968. doi: 10.1242/dev.119.3.957. [DOI] [PubMed] [Google Scholar]
- Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- Shimizu F, Sano Y, Maeda T, Abe MA, Nakayama H, Takahashi R, Ueda M, Ohtsuki S, Terasaki T, Obinata M, Kanda T. Peripheral nerve pericytes originating from the blood-nerve barrier expresses tight junctional molecules and transporters as barrier-forming cells. Journal of cellular physiology. 2008;217:388–399. doi: 10.1002/jcp.21508. [DOI] [PubMed] [Google Scholar]
- Simon C, Lickert H, Gotz M, Dimou L. Sox10-iCreERT2: a mouse line to inducibly trace the neural crest and oligodendrocyte lineage. Genesis. 2012;50:506–515. doi: 10.1002/dvg.22003. [DOI] [PubMed] [Google Scholar]
- Sims DE. Recent advances in pericyte biology--implications for health and disease. Can J Cardiol. 1991;7:431–443. [PubMed] [Google Scholar]
- Sims DE. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–846. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Clevers H. Tracking adult stem cells. EMBO reports. 2011;12:113–122. doi: 10.1038/embor.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, Lee JK. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci. 2013;33:13882–13887. doi: 10.1523/JNEUROSCI.2524-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N, Huang Y, Shi H, Yuan S, Ding Y, Song X, Fu Y, Luo Y. Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1alpha/CXCR4 axis. Cancer research. 2009;69:6057–6064. doi: 10.1158/0008-5472.CAN-08-2007. [DOI] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes & development. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Spitzer TL, Rojas A, Zelenko Z, Aghajanova L, Erikson DW, Barragan F, Meyer M, Tamaresis JS, Hamilton AE, Irwin JC, Giudice LC. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biology of reproduction. 2012;86:58. doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nature immunology. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–4730. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura N, Huang XL, Naruse T, Hamaguchi I, Dumont DJ, Yancopoulos GD, Suda T. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 1998;9:677–686. doi: 10.1016/s1074-7613(00)80665-2. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Harrington A, Yang X, Friesel RE, Liaw L. The contribution of the Tie2+ lineage to primitive and definitive hematopoietic cells. Genesis. 2010;48:563–567. doi: 10.1002/dvg.20654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Brain Res Rev. 1999;31:42–57. doi: 10.1016/s0165-0173(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Trost A, Schroedl F, Lange S, Rivera FJ, Tempfer H, Korntner S, Stolt CC, Wegner M, Bogner B, Kaser-Eichberger A, Krefft K, Runge C, Aigner L, Reitsamer HA. Neural crest origin of retinal and choroidal pericytes. Investigative ophthalmology & visual science. 2013;54:7910–7921. doi: 10.1167/iovs.13-12946. [DOI] [PubMed] [Google Scholar]
- Tu Z, Li Y, Smith DS, Sheibani N, Huang S, Kern T, Lin F. Retinal pericytes inhibit activated T cell proliferation. Investigative ophthalmology & visual science. 2011;52:9005–9010. doi: 10.1167/iovs.11-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek MM, Westphal JR, Ruiter DJ, de Waal RM. T lymphocyte adhesion to human brain pericytes is mediated via very late antigen-4/vascular cell adhesion molecule-1 interactions. Journal of immunology. 1995;154:5876–5884. [PubMed] [Google Scholar]
- Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–5328. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nature neuroscience. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi E, Takahashi M, Saga Y, Osumi N. Penetration and differentiation of cephalic neural crest-derived cells in the developing mouse telencephalon. Dev Growth Differ. 2012;54:785–800. doi: 10.1111/dgd.12007. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Nalbandian A, Uchida Y, Li W, Arnold TD, Kubota Y, Yamamoto S, Ema M, Mukouyama YS. Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-beta Signaling in Developing Skin Vasculature. Cell reports. 2017;18:2991–3004. doi: 10.1016/j.celrep.2017.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto F, You WK, Cejudo-Martin P, Kucharova K, Sakimura K, Stallcup WB. NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization. Oncoimmunology. 2015;4:e1001204. doi: 10.1080/2162402X.2014.1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328:1129–1135. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann KW. Der feinere Bau der Blutkapillaren. Z Anat Entwicklungsgesch. 1923;68:29–109. [Google Scholar]