Abstract
The gadolinium-based contrast agents widely used in diagnostic MRI exams for 30 years are all small molecule agents enter all extracellular space in tissues without providing any specific biological information. Although many “responsive agent” designs have been presented over the past 20 years or so, none have found use in clinical diagnostic medicine at this point. This review summarizes some recent approaches taken to enhance the sensitivity of such gadolinium-based agents, to target them to specific tissue components, and to create new systems for monitoring specific biological processes.
Graphical Abstract
Gadolinium-based MRI contrast agents have been widely used in clinical medicine for ~30 years. Next generation designs for targeting specific biological structures or monitoring biological processes have been demonstrated in vivo. The sensitivity of such agents has been improved by optimizing their water exchange rates and molecular motions and signal amplification via nanoparticles is beginning to show promise. Trends in the latest designs are summarized in this article.

Introduction
Gadolinium-based MRI contrast agents (CAs) were first approved for clinical use in 1988. Several first generation agents were derived from previous pharmaceutically approved ligands such as DTPA or bis-amides of DTPA not on the basis of any particular chemical design feature other than they were multidentate ligands that formed thermodynamically stable complexes with the trivalent lanthanide ions. These first generation ligands excluded all but one inner-sphere water molecule (q = 1) from interacting with the paramagnetic Gd3+ ion. It was thought that q = 2 or 3 systems were unusable due to the abundance of endogenous anions like phosphate or bicarbonate which could bind and exclude those inner-sphere water molecules. Little attention was given to other physical-chemical parameters like water exchange rates until the pioneering experiments of Merbach and co-workers [1] showed that water exchange in these q = 1 complexes occurs largely via a dissociative mechanism and much slower than originally thought. The observation that water exchange was slower than anticipated had little impact in the clinical use of those first generation agents but, as we shall see, has had a substantial impact on the design of newer types of biologically responsive agents.
The first demonstration of a “smart” or “responsive” contrast agent was a galactose conjugate (Egad) that could be cleaved by β-galactosidase to make available a blocked inner-sphere coordination site on the Gd3+ for an exchanging water molecule [2]. That first report catalyzed a flurry of activity by chemists worldwide to create other types of smart agents based on the q = 0 to q = 1 design. Again, the rate at which water molecules exchange between an inner-sphere water coordination position and bulk solvent was not considered and likely not terribly important in any of those early designs.
Another parameter one can take advantage of in the design of a responsive CA is molecular motion, typically governed by the rotational correlation time, τM. In those designs, the rate of water exchange is extremely important because the impact of slowing molecular rotation on r1 upon binding of a Gd3+ complex to a biological target can be quite limited if water exchange is either too fast or too slow. The best example of this effect is MS-325 (Ablavar®, gadofosveset). This derivative of GdDTPA has a large organic group attached to the backbone of the agent that upon binding with albumin results in an increase in r1 relaxivity from ~4 to ~50 mM−1s−1 [3]. This substantial increase in r1 is of course frequency dependent but also heavily dependent upon the rate of water exchange. This example teaches us that when considering the design of this type of “responsive” agent, it is best for the Gd3+ complex to have an optimal water exchange rate, especially when bound to its biological target.
1. Agents that respond to specific biological processes
Two recent examples come from our own work on the design of an agent that shows an increase r1 relaxivity when the agent is exposed to excess Zn2+ ions. Why Zn2+ and how might its detection be useful clinically? Zn2+ ions are important in many biological processes including enzyme catalysis, a structural element in zinc-finger proteins, and as cellular ion messengers. While much of our knowledge of the role of Zn2+ in these biological systems was derived from studies of isolated enzymes and proteins, less is known about the dynamic movement of Zn2+ ions between cellular compartments, across cell membranes, and between cells in intact tissues. The classic and best-known example of Zn2+ ion efflux from cells comes from studies of insulin secretion from pancreatic β-cells. Zn2+ is packaged with insulin in β-cell granules [4] and upon stimulation by an increase in plasma glucose, insulin is secreted and excess Zn2+ ions are also released in the extracellular spaces around β-cells such that the concentration of free, unbound Zn2+ ions in that space rises from about 40–50 μM prior to insulin secretion to ~400–500 μM during insulin secretion [5]. Hence, any agent that could detect this 10-fold increase in Zn2+ might prove useful as a biomarker of insulin secretion. Our first agent designed for this purpose was a bis-amide derivative of DOTA called GdDOTA-diBPEN [6]. The agent itself has a low affinity for HSA in the absence of Zn2+ but after binding two equivalents of Zn2+ ions with high affinity (KD = 30 nM), the resulting ternary complex then binds to HSA and this results in a substantial increase in r1. When first tested in mice, T1-weighted images showed image enhancement in abdominal regions consistent with the location of the pancreas only after a bolus of glucose was given to stimulate insulin secretion [7]. Further studies in mice pre-treated with streptozotocin (STZ) to destroy pancreatic β-cells did not show abdominal image enhancement (Fig. 1) while mice fed a high-fat diet for 12 weeks showed larger areas of enhancement, consistent with expansion of β-cell mass as the animals gained weight and stored abdominal fat. More recent studies of mice with an implanted abdomen window that holds the pancreas immediately beneath the window show distinct, punctate “hot spots” in images throughout the pancreas tail after injection of a 2nd generation, lower affinity Zn2+ agent plus glucose (Martins, et al., unpublished). Early data suggest these hot spots may reflect “first responder” islets that release insulin and Zn2+ during the first phase of insulin secretion although additional experiments will be required to prove this point.
Fig. 1.
Images of Zn2+ release during glucose-stimulated insulin secretion (GSIS) in a control versus a STZ-treated mouse. The color overlay represents those regions where image contrast was enhanced only after a bolus injection of GdDOTA-diBPEN plus glucose. The arrows refer to F = fundus stomach, S = spleen, K = kidneys. The images were from the same mouse before and 4 days after a single high-dose treatment of STZ.
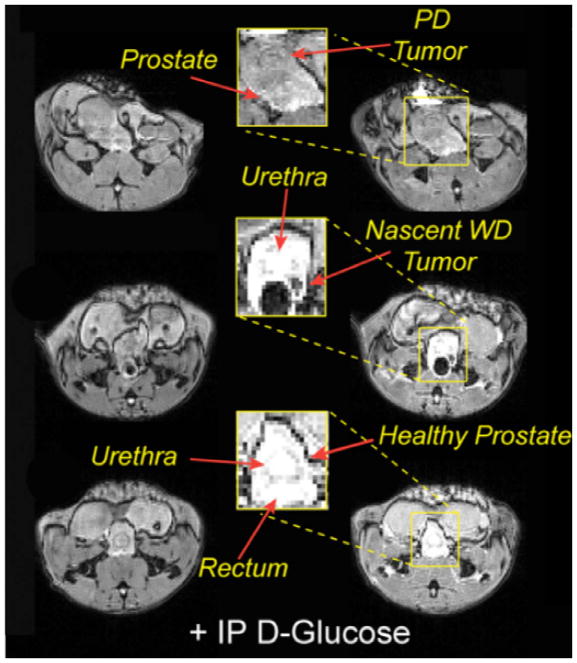
The healthy prostate contains even more Zn2+ than the pancreas and is also known to secrete Zn2+ ions into prosthetic fluids. During imaging experiments of the mouse pancreas, we were surprised to see intense image enhancement of the prostate only after glucose was given to a 4 h-fasted male mouse, much like the response seen in the pancreas. This was shown not to reflect stimulation of the prostate by insulin but rather traced to release of Zn2+ ions from secretory granules stored in prostate epithelial cells stimulated by glucose [8]. Interestingly, glucose stimulated zinc release (GSZS) from prostate cells was only detected by MRI in fasted animals and not in fed animals. Further studies in PNT1A cells, an immortalized prostate epithelial cell line, showed that sudden increase in glucose results in a redistribution of Zn2+ ions among various cell compartments and net release of Zn2+ ions from an intracellular compartment into extracellular space (S-T Lo, unpublished). Given that total tissue zinc is known to be lower in prostate cancer cells, further experiments with TRAMP mice showed that small prostate cancer lesions could be detected as hypointense spots as small as 1 mm3 in the TRAMP prostate during their progression to full malignancy (Fig. 2). It remains to be determined whether these regions reflect cells containing less stored Zn2+ ions or whether it reflects an inability of these cells to release Zn2+ in response to glucose. Nevertheless, this observation is exciting because it offers the possibility of developing a diagnostic clinical tool for early detection of prostate cancer without a tissue biopsy and for monitoring prostate cancer during therapeutic treatments.
Fig. 2.
MR images of TRAMP mouse after injection of 0.07 mmol/kg Zn2+ sensor only (left) and again after in IP injection of glucose (right). The bottom images are of a TRAMP mouse prior to development of cancer, the middle row shows images of the same mouse 8 weeks later showing a hypo-intense spot reflecting a nascent tumor proven by histology, and the upper images are of an older TRAMP mouse with a large tumor that has grown into the adjacent tissues.
2. Small molecule targeted agents
Although the concentrations of most biological receptors are too low for MRI detection by single Gd-based agent, some biological targets are certainly abundant enough to consider targeting with a MRI contrast agent. One such example is collagen, an abundant protein present in connective tissues that provides structural and mechanical support for cells. Collagen is highly regulated under most circumstances but dysregulated in many disease states including fibrosis and cancer. Excess production of collagen results in tissue stiffening or fibrosis, a common liver condition associated with hepatic hepatitis, alcohol or drug abuse. Tissue stiffness is also important in cardiac diseases associated with hypertrophy and aging. The presence of excess collagen in tissues increases mechanical stress on tissues, promotes tumor growth and the metastatic potential of cancers. Given the abundance of type 1 collagen and its importance in several diseases, this was a logical target for development of a MR agent for detection of excess collagen in tissues. One approach to target collagen was to identify collagen-binding peptides that could be further modified by addition of one or more Gd-chelates without disrupting its binding affinity with collagen. One resulting targeted agent was EP-3533, a 16 amino acid peptide containing three covalently appended Gd-chelates that binds with high affinity (KD = 1.8 μM) to collagen [9]. This agent has been used in various animal models to detect liver fibrosis [10], lung fibrosis [11], cardiac fibrosis [9,12], and most recently, pancreatic cancer related fibrosis [13]. An example of the use of this agent to identify excess collagen in myocardial infarcted regions of a mouse heart is given in Figure 3. Comparisons of this image with either an extracellular agent or a control Gd3-peptide containing a scrambled, non-targeting peptide showed quite clearly that the bright rim shown in the upper left image of Figure 3 does indeed reflect excess collagen in the tissue. Given that collagen consists of a right-handed bundle of three parallel, left-handed polyproline II-type helices, part of the success of this amplification system is that the targeted peptide can bind at multiple locations along the collagen fiber (N ~ 8) [9]. The three Gd-chelates attached to each peptide results in another ~3-fold amplification plus the fact that the r1 relaxivity of EP-3533 can be further amplified by another factor of 2–5 due to a decrease in molecular rotation upon binding of EP-3533 to the large collagen fibers. The extent to which the later amplification factor adds to the sensitivity of this agent is highly dependent upon the rate of water exchange between the inner-sphere of the paramagnetic Gd chelate and bulk water [14].
Fig. 3.
Two examples of chemical targeting of Gd-chelates for detection of fibrotic tissues by MRI. (top images) A Gd3-peptide that binds non-covalently with collagen that collects in infarcted regions of a mouse heart. (bottom images) GdOA, oxyamine derivative of GdDOTA, reacts with aldehyde-lysine groups in fibrotic lung tissue. In this example, the mouse on the left was untreated while the mouse on the right was treated with Bleomycin to generate excess reactive oxygen species to catalyze lung fibrogenesis (red areas).
A second example of a chemistry-based targeting agent also comes from the lab of Peter Caravan. In this example, a derivative of GdDOTA having a terminal oxyamine group on one acetate side-arm (GdOA) was shown to react with allysine [15], an oxidized aldehyde derivative of lysine groups in collagen formed during development of fibrosis. Ultimately, such oxidized allysine groups form cross-linked bridges between collagen fibers adding further stiffness to the tissue. To evaluate the potential of this probe to detect allysine prior to crosslinking, mice were treated with a single dose of Bleomycin to stimulate fibrogenesis in lung tissue. Chemical assays of the resulting lung tissue showed that the amount of allysine increased approximately 2-fold to an estimated concentration of ~150 μM, an amount that should be easily detected by MRI if targeted by a single Gd-chelate of modest r1 relaxivity at each allysine residue. As shown by the images in Figure 3, a single injection of GdOA into mice treated with Bleomycin 14 days prior to imaging showed sufficient collection of the agent in lung tissue to detect by MRI. Although it is too early to know whether GdOA will be generally useful for imaging the extent of active fibrogenesis in all tissue types, the concept of using simple chemical agents like GdOA for covalent targeting of specific biological products associated with disease is an important scientific advance.
3. Nanoparticle designs for amplifying MRI contrast
Covalent attachment of a Gd-chelate to larger macromolecular structures typically decreases molecular tumbling thereby increasing r1 relaxivity. Although this approach is commonly taken without any consideration of water exchange rate, this parameter is equally important in determining r1 [14]. Attachment of multiple Gd-chelates to an existing nanoparticle platform allows further amplification of r1 and has the added benefit of extending the blood clearance lifetime and hence the tissue biodistribution of such agents. The enhanced permeation and retention (EPR) effect of nanoparticle constructs plus facile targeting of a nanoparticle payload through surface modification add to the benefits of using nanoparticles to deliver MRI contrast agent to specific tissues. Gadolinium chelates have been attached via chemical or physical binding (electrostatic or encapsulation) to a variety of nanoparticles, including dendrimers [16–19], micelles [20,21], liposomes [22,23], polymeric [24–26] or inorganic nanoparticles [27,28], and nanogels [29–32]. Among those examples, the use of nanogels is particularly well suited for MRI as they are stable in aqueous media and allow facile access of water molecules throughout the particle. Their drug loading capabilities and the possibility to use degradable polymers to construct their backbone are also advantageous for biomedical applications [33]. As one example, Lux et al. [30] prepared polyacrylamide-based nanogels that incorporate acyclic and cyclic chelates as crosslinkers. The advantage of this strategy is two-fold, as the contrast agent acts as a template to formulate the nanoparticle while its incorporation in the polymeric scaffold provide thermodynamic and kinetic stabilization and improved relaxivity. A series a crosslinkers based on DTPA and DOTA scaffolds and bearing acryloyl groups were synthesized and reacted with acrylamide under reverse emulsion conditions to produce 50–100 nm nanogels (Fig. 4A). Proton Nuclear Magnetic Relaxation Dispersion (NMRD) profiles were recorded at 37°C and confirmed that Gd complexes have a decreased tumbling in the nanogel’s scaffold leading to an increase in relaxivity (Fig. 4B), thereby improving their detection in MRI phantoms at 7T (Fig. 4C). While the commercial contrast agents and small molecule crosslinkers showed similar contrast, the nanogels displayed a much brighter signal.
Fig. 4.
A) Preparation of nanogels incorporating Gd-chelates as cross-linkers through reverse emulsion. B) Nuclear magnetic relaxation dispersion (NMRD) profiles of the Gd-chelated cross-linker and Gd-chelated nanogels. C) Representative phantom MRI images at 7 T of Magnevist, Gd-chelated crosslinkers and Gd-chelated nanogels.
Interestingly, covalent incorporation of Gd-chelates into a nanogel scaffold not only increases the relaxivities of those contrast agents (up to 6.1-fold increase in r1 at 1.4 T) but also stabilizes the metal complexes against transmetallation. Specifically, the relaxivity of DTPA-based nanogels decreased by 23% after 66 h of incubation in phosphate buffer at 37°C with a large excess of Zn2+ while the relaxivity of Magnevist® decreased by 40%.
Nanoparticles do not only provide enhanced relaxivity and targeting capabilities but can also be designed as activatable MRI agents by formulating them with bioresponsive materials. Specifically, Viger et al. used hydrophobic nanoparticles to encapsulate ultrasmall (< 5 nm) gadolinium oxide (Gd2O3) nanoparticles and shield them from the aqueous environment, preventing them from accelerating the relaxation of water protons [34]. Upon exposure to a certain stimulus, the polymeric nanoparticle degrades, releases Gd2O3 NPs and allows water access, which dramatically increases the relaxivity of the contrast agent (Fig. 5A) by more than one order of magnitude. This relaxivity enhancement is greater than previous activatable small molecule Gd-chelates [35–38] because it effectively silences the contrast agent not only by prohibiting the coordination of molecules to Gd3+ in the inner sphere but also by suppressing any outer sphere relaxation rate contribution. Particles encapsulating Gd2O3 NPs (Fig. 5B) were prepared using an electrospray method and the validity of this concept was demonstrated with three different polymers: the slow degrading poly(lactic-glycolic acid) (PLGA), a pH-responsive polymer [39] and a H2O2- responsive polymer [40]. A signal activation was observed in each case after the degradation of each polymer with 9.6, 4.2 and 11-fold increase in relaxivity for PLGA (Fig. 5C), pH- (Fig. 5D) and H2O2-degradable (Fig. 5E) polymers, respectively. This work showed that an approach that consists in quenching both inner and outer sphere relaxation effects is a generalizable concept and can be applied to a variety of bioresponsive materials.
Fig. 5.
A) Schematic representation of the collective activation of encapsulated MRI agents after degradation of bioresponsive particles and interactions with water molecules. B) Representative TEM image of oxidation-sensitive nanoparticles encapsulating ultrasmall gadolinium oxide nanoparticles (scale bar = 500 nm). C) Activation of MRI contrast agents in PLGA particles after dissolution of the polymeric matrix and activation of MRI contrast agents at low pH (D) or in the presence of elevated levels of hydrogen peroxide (E) after encapsulating Gd2O3 nanoparticles in pH- and ROS-degradable polymeric particles respectively.
Another approach is to create nanoparticles from small molecule Gd-chelates in situ to increase their relaxivity in the presence of specific biomarkers. This strategy was used by Rao and coworkers [41] to detect the presence of caspase 3/7, a family of cysteine proteases that are critical components in cell apoptosis. This work employed GdDOTA conjugated to a rigid chain bearing a caspase-cleavable DEVD (Asp-Glu-Val-Asp) peptide on one side and a disulfide bond on the other end. In the presence of caspase 3/7, the intracellular reduction of the disulfide bond and the cleavage of the peptide triggered an intramolecular cyclization that yielded a more rigid and hydrophobic cyclic molecule (Fig 6A). The latter molecules subsequently self-assemble into nanoaggregates through hydrophobic interactions and pi-pi stacking to yield nanoparticles ranging from hundred to a few hundreds of nanometers (Fig. 6B). The relaxivity enhancement and enzyme specificity were demonstrated by incubating the activatable contrast agent with nine relevant cellular proteases and significantly better contrast was obtained in the case of caspase 3 and 7 (Fig. 6C). This probe was used to observe chemotherapy-induced tumor apoptosis in mice and its design can be generalized to the molecular imaging of various enzymes of interest.
Fig. 6.
A) Chemical structure of the activatable MRI contrast agent and cyclization mechanism that yields hydrophobic molecules that self-assemble into nanoparticles. B) Size distribution and TEM image of caspase-activated self-assembled particles. C) Representative phantom MRI images at 1T of activatable MRI contrast agent upon exposure to caspase-3/7 and other enzymes.
A fourth chemistry-based targeting approach used gold nanoparticles coated with a layer of 3′ end-on 24-mer-dT chains to form spherical nucleic acids (SNAs) further modified to carry other cargo [42,43]. Using this multiplexing approach, click chemistry was used to attach five GdDOTA chelates to each nucleic acid chain to give a SNA nanoparticle covered with ~100 dT chains each with a terminal chloro group plus ~500 GdDOTA chelates per particle. These SNA nanoparticles were then exposed to cells expressing the cell-surface HaloTag protein as a gene reporter [44]. The exposed chloro groups on the surface of the SNAs reacted specifically with HaloTag protein to label cells at a sufficient level to be detected by MRI. This approach may prove generally useful as a gene reporter system for cells but it is unclear at this point how such a construct might be translated in vivo.
The four nanoparticle designs summarized above demonstrate the advantages of particle constructs for amplifying the effective sensitivity of Gd-based MRI contrast agents. This brute force approach simply relies upon adding a sufficient number of agents to each nanoparticle to significantly impact T1 with little or no attention devoted to optimizing molecular rotation or water exchange rates of each particle-bound Gd-chelate. Thus, the sensitivity of these constructs could likely be improved considerably by optimizing these physical characteristics. One example of such improvement was reported by Rotz, et al. who demonstrated that the r1 relaxivity of star-shaped Au nanoparticles coated with a Gd-chelate known to have a much faster water exchange rate was considerably higher than an equivalent particle coated with a water exchange rate limited complex [44]. Interestingly, that study also showed that the shape of the Au nanoparticle also influenced the observed r1 relaxivity, with star-shaped Au particles showing about a 2–3 fold improvement over spherical particle with the same gadolinium loading.
Most early Gd-based nanoparticle designs that have been shown to work in vivo have been largely based on liposome [45] or phosphoprotein particle [46] designs where a Gd-chelate is attached to the outer lipid surface. The advantages and limitations of such designs have been reviewed elsewhere [47]. The intent of this short review was not to be comprehensive but rather to summarize a few recent nanoparticle constructs that show potential for in vivo reporters of biological activity. A good example of this was a recent report from Roger Tsien’s lab of a MRI nanoparticle construct that collects in cells only after cleavage of a peptide by an enzyme known to be over-expressed in the extracellular space of cancer cells. Given that this issue is dedicated to the research contributions of Roger Tsien, we feel it is appropriate to conclude this short article with a summary of his responsive nanoparticle design.
4. Enzyme-activated cellular uptake of nanoparticle-based MRI contrast agents
Roger Tsien was well-known for his brilliant contributions to development of optical probes but he also had a long-standing desire to translate his optical designs into MRI probes. This of course required consideration of the vast difference in sensitivity of the two imaging modalities. One recent example of his success in this area was the use of activatable cell-penetrating peptides (ACPP) that consist of a positively charged cell-penetrating petide masked by a negatively charged inhibitory peptide connected by a linker that is cleaved by cancer related enzymes [48]. Prior to enzymatic cleavage, the ACPP is not recognized by cells but after cleavage by a cancer-related enzyme such as a matrix metalloprotease, the unmasked cell-penetrating peptide then accumulates in the tumor parenchyma with some entering cells via endocytosis. Given that cell-penetrating peptides can drag along other sizeable cargo, this provided an opportunity to tag large cargo structures with optical probes [49], ultrasound [50] or MRI contrast agents [48,51], a therapeutic agent, or perhaps a combination of those. In the example shown in Figure 7, a G5 dendrimer coated with a single Cy5 for optical detection, thirty six GdDOTA molecules, and six copies of ACPP masked by a linker and peptide that could be cleaved by MMPs [51]. 24 hours after IV delivery of a single bolus of ACPP-Gd (0.036 mmol/kg based on Gd), the MR intensity in tumors was about 2-fold higher in comparison to images collected using a simple extracellular low MW Gd-chelate. Furthermore, the tumors containing the targeted ACPP-Gd were uniformly enhanced and remained enhanced for several days while the non-targeted agent showed typical tumor rim enhancement which cleared in a few hours. The ACPP-Gd construct provided clear detection of tumors as small as 0.4 mm in diameter using a 3T clinical scanner. The greater sensitivity and specificity of ACPP-Gd for detection of small tumors compared to untargeted, extracellular Gd-chelates suggests this approach could prove useful as a screening tool in some cancer patient populations.
Fig. 7.
Schematic of ACPP-Gd structure and function. MR Images were ~2-fold more intense in comparison to Gadobutrol and showed uniform contrast in tumors ranging in size from 0.4 – 5.9 mm using a 3T clinical scanner (modified from Figs 1 and 2 in reference 51).
Conclusions
This short review highlights recent strategies that aim to amplify the relaxation effects of gadolinium in order to detect biological targets (e.g., ions, enzymes) or biomarkers of disease. Bioresponsive MRI contrast agents in the form of small molecules or nanoparticles have a great potential not only to develop new diagnostic clinical tools for early detection of disease but also for monitoring important biological processes. It is now possible to detect abundant biological targets using responsive contrast agents and contrast amplification strategies.
Highlights.
Responsive MRI agents provide new insights into Zn2+ ion release from secretory tissues.
Small molecule targeted agents are sensitive enough to detect abundant biological targets.
Novel nanoparticle platforms can be used to amplify the relaxation effects of gadolinium.
Early example of a nanoparticle-based MRI agent that accumulates in cancer cells in vivo.
Acknowledgments
The authors acknowledge support from the NIH (R01 DK-095416 and P41 EB015908), the Cancer Prevention and Research Institute of Texas (CPRIT RR150010) and the Robert A. Welch Foundation (AT-584) during the writing of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Micskei K, Helm L, Brucher E, Merbach AE. Oxygen-17 NMR study of water exchange on gadolinium polyaminopolyacetates [Gd(DTPA)(H2O)]2− and [Gd(DOTA)(H2O)]− related to NMR imaging. Inorg Chem. 1993;32:3844–3850. [Google Scholar]
- 2••.Moats RA, Fraser SE, Meade TJ. A “Smart” Magnetic Resonance Imaging Agent That Reports on Specific Enzymatic Activity. Angewandte Chemie International Edition in English. 1997;36:726–728. This paper is the first example of a “smart” MRI contrast agent where the relaxivity is increased by the activity of a certain enzyme (galactose conjugate) (Egad) that could be cleaved by β-galactosidase to make available a blocked inner-sphere coordination site on the Gd3+ for an exchanging water molecule. [Google Scholar]
- 3.Lauffer RB, Parmelee DJ, Ouellet HS, Dolan RP, Sajiki H, Scott DM, Bernard PJ, Buchanan EM, Ong KY, Tyeklár Z, et al. MS-325: A small-molecule vascular imaging agent for magnetic resonance imaging. Academic Radiology. 1996;3:S356–S358. doi: 10.1016/s1076-6332(96)80583-6. [DOI] [PubMed] [Google Scholar]
- 4.Emden S, Dodson G, Cutfield J, Cutfield S. Role of zinc in insulin biosynthesis. Some possible zinc-insulin interactions in the pancreatic beta-cell. Diabetologia. 1980;19:174–182. doi: 10.1007/BF00275265. [DOI] [PubMed] [Google Scholar]
- 5.Kim BJ, Kim YH, Kim S, Kim JW, Koh JY, Oh SH, Lee MK, Kim KW, Lee MS. Zinc as a paracrine effector in pancreatic islet cell death. Diabetes. 2000;49:367–372. doi: 10.2337/diabetes.49.3.367. [DOI] [PubMed] [Google Scholar]
- 6.Esqueda AC, Lopez JA, Andreu-de-Riquer G, Alvarado-Monzon JC, Ratnakar J, Lubag AJM, Sherry AD, De Leon-Rodriguez LM. A New Gadolinium-Based MRI Zinc Sensor. J Am Chem Soc. 2009;131:11387–11391. doi: 10.1021/ja901875v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lubag AJM, De Leon-Rodriquez LM, Burgess SC, Sherry AD. Non-invasive MRI of beta-cell function using a Zn2+-responsive contrast agent. Proc Natl Acad Sci, USA. 2011;108:18400–18405. doi: 10.1073/pnas.1109649108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clavijo Jordan MV, Lo S-T, Chen S, Preihs C, Chirayil S, Zhang S, Kapur P, Li W-H, De Leon-Rodriguez LM, Lubag AJM, et al. Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate. Proceedings of the National Academy of Sciences. 2016;113:E5464–E5471. doi: 10.1073/pnas.1609450113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caravan P, Das B, Dumas S, Epstein FH, Helm PA, Jacques V, Koerner S, Kolodziej A, Shen L, Sun W-C, et al. Collagen-Targeted MRI Contrast Agent for Molecular Imaging of Fibrosis. Angewandte Chemie International Edition. 2007;46:8171–8173. doi: 10.1002/anie.200700700. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs BC, Wang H, Yang Y, Wei L, Polasek M, Schühle DT, Lauwers GY, Parkar A, Sinskey AJ, Tanabe KK, et al. Molecular MRI of collagen to diagnose and stage liver fibrosis. Journal of Hepatology. 2013;59:992–998. doi: 10.1016/j.jhep.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caravan P, Yang Y, Zachariah R, Schmitt A, Mino-Kenudson M, Chen HH, Sosnovik DE, Dai G, Fuchs BC, Lanuti M. Molecular Magnetic Resonance Imaging of Pulmonary Fibrosis in Mice. American Journal of Respiratory Cell and Molecular Biology. 2013;49:1120–1126. doi: 10.1165/rcmb.2013-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helm PA, Caravan P, French BA, Jacques V, Shen L, Xu Y, Beyers RJ, Roy RJ, Kramer CM, Epstein FH. Postinfarction Myocardial Scarring in Mice: Molecular MR Imaging with Use of a Collagen-targeting Contrast Agent. Radiology. 2008;247:788–796. doi: 10.1148/radiol.2473070975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polasek M, Yang Y, Schühle DT, Yaseen MA, Kim YR, Sung YS, Guimaraes AR, Caravan P. Molecular MR imaging of fibrosis in a mouse model of pancreatic cancer. Scientific Reports. 2017;7:8114. doi: 10.1038/s41598-017-08838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Sherry AD, Wu Y. The importance of water exchange rates in the design of responsive agents for MRI. Current Opinion in Chemical Biology. 2013;17:167–174. doi: 10.1016/j.cbpa.2012.12.012. This review highlights the impact of the water exchange rate and the importance of this parameter in the design responsive MRI contrast agents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waghorn PA, Jones CM, Rotile NJ, Koerner SK, Ferreira DS, Chen HH, Probst CK, Tager AM, Caravan P. Molecular Magnetic Resonance Imaging of Lung Fibrogenesis with an Oxyamine-Based Probe. Angewandte Chemie. 2017;129:9957–9960. doi: 10.1002/anie.201704773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaszberenyi Z, Moriggi L, Schmidt P, Weidensteiner C, Kneuer R, Merbach AE, Helm L, Toth E. Physicochemical and MRI characterization of Gd3+-loaded polyamidoamine and hyperbranched dendrimers. J Biol Inorg Chem. 2007;12:406–420. doi: 10.1007/s00775-006-0197-3. [DOI] [PubMed] [Google Scholar]
- 17••.Nicolle GM, Toth E, Schmitt-Willich H, Raduchel B, Merbach AE. The impact of rigidity and water exchange on the relaxivity of a dendritic MRI contrast agent. Chemistry. 2002;8:1040–1048. doi: 10.1002/1521-3765(20020301)8:5<1040::aid-chem1040>3.0.co;2-d. This paper describes the impact of chelate rigidity in determining the molecular tumbling rate and the relaxivity of MRI contrast agents. [DOI] [PubMed] [Google Scholar]
- 18.Rudovsky J, Hermann P, Botta M, Aime S, Lukes I. Dendrimeric Gd(III) complex of a monophosphinated DOTA analogue: optimizing relaxivity by reducing internal motion. Chem Commun (Camb) 2005:2390–2392. doi: 10.1039/b418712a. [DOI] [PubMed] [Google Scholar]
- 19.Floyd WC, 3rd, Klemm PJ, Smiles DE, Kohlgruber AC, Pierre VC, Mynar JL, Frechet JM, Raymond KN. Conjugation effects of various linkers on Gd(III) MRI contrast agents with dendrimers: optimizing the hydroxypyridinonate (HOPO) ligands with nontoxic, degradable esteramide (EA) dendrimers for high relaxivity. J Am Chem Soc. 2011;133:2390–2393. doi: 10.1021/ja110582e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kielar F, Tei L, Terreno E, Botta M. Large relaxivity enhancement of paramagnetic lipid nanoparticles by restricting the local motions of the Gd(III) chelates. J Am Chem Soc. 2010;132:7836–7837. doi: 10.1021/ja101518v. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Zhang R, Wen X, Li L, Li C. Micelles based on biodegradable poly(L-glutamic acid)-b-polylactide with paramagnetic Gd ions chelated to the shell layer as a potential nanoscale MRI-visible delivery system. Biomacromolecules. 2008;9:36–42. doi: 10.1021/bm700713p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Z, Al Zaki A, Jones IW, Hall HK, Jr, Aspinwall CA, Tsourkas A. Stabilized porous liposomes with encapsulated Gd-labeled dextran as a highly efficient MRI contrast agent. Chem Commun (Camb) 2014;50:2502–2504. doi: 10.1039/c3cc48939f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabalka GW, Davis MA, Moss TH, Buonocore E, Hubner K, Holmberg E, Maruyama K, Huang L. Gadolinium-labeled liposomes containing various amphiphilic Gd-DTPA derivatives: targeted MRI contrast enhancement agents for the liver. Magn Reson Med. 1991;19:406–415. doi: 10.1002/mrm.1910190231. [DOI] [PubMed] [Google Scholar]
- 24.Schopf E, Sankaranarayanan J, Chan M, Mattrey R, Almutairi A. An extracellular MRI polymeric contrast agent that degrades at physiological pH. Mol Pharm. 2012;9:1911–1918. doi: 10.1021/mp2005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randolph LM, LeGuyader CLM, Hahn ME, Andolina CM, Patterson JP, Mattrey RF, Millstone JE, Botta M, Scadeng M, Gianneschi NC. Polymeric Gd-DOTA amphiphiles form spherical and fibril-shaped nanoparticle MRI contrast agents. Chemical Science. 2016;7:4230–4236. doi: 10.1039/c6sc00342g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashim Z, Green M, Chung PH, Suhling K, Protti A, Phinikaridou A, Botnar R, Khanbeigi RA, Thanou M, Dailey LA, et al. Gd-containing conjugated polymer nanoparticles: bimodal nanoparticles for fluorescence and MRI imaging. Nanoscale. 2014;6:8376–8386. doi: 10.1039/c4nr01491j. [DOI] [PubMed] [Google Scholar]
- 27.Lux F, Mignot A, Mowat P, Louis C, Dufort S, Bernhard C, Denat F, Boschetti F, Brunet C, Antoine R, et al. Ultrasmall rigid particles as multimodal probes for medical applications. Angew Chem Int Ed Engl. 2011;50:12299–12303. doi: 10.1002/anie.201104104. [DOI] [PubMed] [Google Scholar]
- 28•.Alric C, Taleb J, Le Duc G, Mandon C, Billotey C, Le Meur-Herland A, Brochard T, Vocanson F, Janier M, Perriat P, et al. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. J Am Chem Soc. 2008;130:5908–5915. doi: 10.1021/ja078176p. This article reports the development of bimodal gold nanoparticles for CT and MRI. These nanoparticles consist of an inorganic gold core coated with an organic shell composed of multiple Gd chelates bound to gold via disulfide bonds. [DOI] [PubMed] [Google Scholar]
- 29.Courant T, Roullin VG, Cadiou C, Callewaert M, Andry MC, Portefaix C, Hoeffel C, de Goltstein MC, Port M, Laurent S, et al. Hydrogels incorporating GdDOTA: towards highly efficient dual T1/T2 MRI contrast agents. Angew Chem Int Ed Engl. 2012;51:9119–9122. doi: 10.1002/anie.201203190. [DOI] [PubMed] [Google Scholar]
- 30.Lux J, Chan M, Elst LV, Schopf E, Mahmoud E, Laurent S, Almutairi A. Metal Chelating Crosslinkers Form Nanogels with High Chelation Stability. J Mater Chem B Mater Biol Med. 2013;1:6359–6364. doi: 10.1039/C3TB21104E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Soleimani A, Martinez F, Economopoulos V, Foster PJ, Scholl TJ, Gillies ER. Polymer cross-linking: a nanogel approach to enhancing the relaxivity of MRI contrast agents. Journal of Materials Chemistry B. 2013;1:1027–1034. doi: 10.1039/c2tb00352j. This article demonstrates that the molecular rigidification imparted by crosslinking of a linear polymer containing pendant Gd-chelates leads to an enhanced relaxivity. [DOI] [PubMed] [Google Scholar]
- 32.Chan M, Lux J, Nishimura T, Akiyoshi K, Almutairi A. Long-Lasting and Efficient Tumor Imaging Using a High Relaxivity Polysaccharide Nanogel Magnetic Resonance Imaging Contrast Agent. Biomacromolecules. 2015;16:2964–2971. doi: 10.1021/acs.biomac.5b00867. [DOI] [PubMed] [Google Scholar]
- 33.Kabanov AV, Vinogradov SV. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed Engl. 2009;48:5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viger ML, Sankaranarayanan J, de Gracia Lux C, Chan M, Almutairi A. Collective activation of MRI agents via encapsulation and disease-triggered release. J Am Chem Soc. 2013;135:7847–7850. doi: 10.1021/ja403167p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 36.Li W-h, Fraser SE, Meade TJ. A Calcium-Sensitive Magnetic Resonance Imaging Contrast Agent. Journal of the American Chemical Society. 1999;121:1413–1414. [Google Scholar]
- 37.Tu C, Nagao R, Louie AY. Multimodal magnetic-resonance/optical-imaging contrast agent sensitive to NADH. Angew Chem Int Ed Engl. 2009;48:6547–6551. doi: 10.1002/anie.200900984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu M, Beyers RJ, Gorden JD, Cross JN, Goldsmith CR. A magnetic resonance imaging contrast agent capable of detecting hydrogen peroxide. Inorg Chem. 2012;51:9153–9155. doi: 10.1021/ic3012603. [DOI] [PubMed] [Google Scholar]
- 39.Sankaranarayanan J, Mahmoud EA, Kim G, Morachis JM, Almutairi A. Multiresponse strategies to modulate burst degradation and release from nanoparticles. ACS Nano. 2010;4:5930–5936. doi: 10.1021/nn100968e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Gracia Lux C, Joshi-Barr S, Nguyen T, Mahmoud E, Schopf E, Fomina N, Almutairi A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J Am Chem Soc. 2012;134:15758–15764. doi: 10.1021/ja303372u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye D, Shuhendler AJ, Pandit P, Brewer KD, Tee SS, Cui L, Tikhomirov G, Rutt B, Rao J. Caspase-responsive smart gadolinium-based contrast agent for magnetic resonance imaging of drug-induced apoptosis. Chem Sci. 2014;4:3845–3852. doi: 10.1039/C4SC01392A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation. Science. 2006;312:1027. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 43.Song Y, Xu X, MacRenaris K, Zhang X-Q, Mirkin C, Meade T. Multimodal Gadolinium-Enriched DNA Gold Nanoparticle Conjugates for Cellular Imaging. Angewandte Chemie (International ed in English) 2009;48:9143–9147. doi: 10.1002/anie.200904666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rotz MW, Culver KSB, Parigi G, MacRenaris KW, Luchinat C, Odom TW, Meade TJ. High Relaxivity Gd(III)–DNA Gold Nanostars: Investigation of Shape Effects on Proton Relaxation. ACS Nano. 2015;9:3385–3396. doi: 10.1021/nn5070953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanza G, Lorenz C, Fisher S, Scott M, Cacheris W, Kaufman R, Gaffney P, Wickline S. Enhanced detection of thrombi with a novel fibrin-targeted magnetic resoance imaging agent. Acad Radiol. 1998;5:S173–S176. doi: 10.1016/s1076-6332(98)80097-4. [DOI] [PubMed] [Google Scholar]
- 46.Frias JC, Williams KJ, Fisher EA, Fayad ZA. Recombinant HDL-Like Nanoparticles: A Specific Contrast Agent for MRI of Atherosclerotic Plaques. Journal of the American Chemical Society. 2004;126:16316–16317. doi: 10.1021/ja044911a. [DOI] [PubMed] [Google Scholar]
- 47••.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine. 2008;3:703–717. doi: 10.2217/17435889.3.5.703. This paper reviews the physiologic aspects of nanoparticle biodistribution and clearance mechanism. It provides an overview of different types of particles including dendrimers, liposomes and inorganic nanoparticles. The review demonstrates that particles’ size, charge and surface chemistry can be optimized to increase renal clearance and reduce biological half-life. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olson ES, Jiang T, Aguilera TA, Nguyen QT, Ellies LG, Scadeng M, Tsien RY. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proceedings of the National Academy of Sciences. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitney M, Savariar EN, Friedman B, Levin RA, Crisp JL, Glasgow HL, Lefkowitz R, Adams SR, Steinbach P, Nashi N, et al. Ratiometric activatable cell-penetrating peptides provide rapid in vivo readout of thrombin activation. Angew Chem Int Ed Engl. 2013;52:325–330. doi: 10.1002/anie.201205721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lux J, Vezeridis AM, Hoyt K, Adams SR, Armstrong AM, Sirsi SR, Mattrey RF. Thrombin-Activatable Microbubbles as Potential Ultrasound Contrast Agents for the Detection of Acute Thrombosis. ACS Appl Mater Interfaces. 2017;9:37587–37596. doi: 10.1021/acsami.7b10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.CDM, Olson E, RFM, Jiang T, Tsien R, Nguyen Q. Tumor Detection at 3 Tesla with an Activatable Cell Penetrating Peptide Dendrimer (ACPPD-Gd), a T1 Magnetic Resonance (MR) Molecular Imaging Agent. PLoS ONE. 2015;10:e0137104. doi: 10.1371/journal.pone.0137104. [DOI] [PMC free article] [PubMed] [Google Scholar]