Abstract
Periventricular leukomalacia (PVL) is a severe type of white matter damage in premature infants and the most common cause of cerebral palsy. It is generally known to be caused by hypoxia and inflammation. Currently there is no effective treatment available, in part due to that the pathogenesis of the disease has not been well understood. The p38α mitogen-activated protein kinase (MAPK) is the serine/threonine kinase and several in vitro studies demonstrated that p38 MAPK is essential for oligodendroglial differentiation and myelination. Indeed, our nerve/glial antigen 2 (NG2)-specific oligodendroglial p38α MAPK conditional knockout (CKO) mice revealed its complex roles in myelination and remyelination. To identify the specific in vivo roles of oligodendroglial p38α MAPK in PVL, we generated a mouse PVL model by combination of LPS-mediated inflammation and hypoxia-ischemia in NG2-p38α MAPK CKO mice. Our results demonstrate that a selective deletion of p38α MAPK in oligodendrocyte did not attenuate myelination defects in the mouse model of PVL. Myelination phenotype revealed by MBP immunostaining was not significantly affected in the p38α MAPK CKO mice compared to the wildtype after PVL induction. The electron microscopic images demonstrated that the microstructure of myelin structures was not significantly different between the wild type and p38α MAPK CKO mice. In addition, oligodendrocyte degeneration in the corpus callosum white matter area was unaffected in the p38α MAPK CKO during and after the PVL induction. These data indicate that p38α MAPK in oligodendrocyte has minimal effect on myelination and oligodendrocyte survival in the mouse PVL model.
Keywords: p38alpha MAPK, periventricular leukomalacia, oligodendrocyte, myelination
Graphical Abstract
INTRODUCTION
Periventricular Leukomalacia (PVL) is the most common form of white matter injury underlying cerebral palsy in premature infants from maternal-fetal infection and oxygen-deprivation (Volpe, 2001, 2003; Deng et al., 2008; Deng, 2010). The disease phenotype is characterized by necrosis, mostly due to the death of pre-myelinating oligodendrocytes and oligodendrocyte precursor cells (OPC) of white matter region near the lateral ventricle. The OPCs, which differentiate into myelin-forming oligodendrocytes, are known to be especially vulnerable to PVL injury (Haynes et al., 2003). The proper structure of oligodendrocyte and myelination is crucial for maintaining the efficient transmission of electrical nerve potential.
Several useful animal models of PVL have been reported including rabbit, dog and sheep by hypoxia and inflammation induction (Hagberg et al., 2002), though they do not mimic all facets of human PVL pathology. A hypoxic-ischemic rat PVL model was previously described using postnatal day (P) 7 rat pups (Follet et al., 2000, 2004). This rodent model mimics majority of the PVL pathology observed in human infants. The major hallmark of this rat PVL model is selective white matter injury, compared to other major stroke models that are characterized by considerable amount of gray matter infarction. Given the cost effectiveness and easy access/handling, we have developed an efficient mouse PVL model and carefully analyzed its phenotype and relevance to human PVL phenotype (Deng et al., 2008; Shen et al., 2010, 2012; Liu et al., 2011). The CNS developmental age in mice that matches with the human developmental stage of major phenotype for PVL lesions is P6–7. Indeed, our careful ischemia/LPS injection time point analysis confirmed that co-induction of LPS injection with hypoxia-ischemia at P6–7 in the mice induce a periventricular white matter lesion very similar to that seen in pediatric PVL (Deng et al., 2008).
The p38 mitogen-activated protein kinases (MAPKs) are essential mediators of stress responses and their physiological roles during oligodendrocyte development and myelination have been reported (Fragoso et al., 2003, 2007; Bhat et al., 2007; Hamanoue et al., 2007; Hossain et al., 2012). Using a variety of general p38 inhibitors, previous studies have demonstrated that p38α MAPK is important for inducing myelination in in vitro Schwann cells (Fragoso et al., 2003) and OPCs (Fragoso et al., 2007). Hossain et al. 2012 showed that p38α MAPK regulates Krox-20 to regulate Schwann cell differentiation and peripheral nerve myelination.
In addition, for the first time, we have reported the in vivo role of the p38α MAPK in normal peri/postnatal myelination process, as well as during remyelination after demyelination injury, by generating oligodendrocyte specific p38α MAPK conditional knockout (CKO) mice (Chung et al., 2015). Our study revealed a complex dual role of p38α MAPK: 1) as a positive regulator in normal oligodendrocyte development and differentiation, and 2) as a negative regulator in a white matter injury demyelination model. The p38α MAPK negatively controlled remyelination in cuprizone-induced demyelination mouse model of p38α MAPK CKO by enhancing the remyelination ability (Chung et al., 2015). We now further investigate the possibility of white matter injury protection by p38α MAPK inhibition using p38α MAPK CKO mice model of PVL.
MATERIALS AND METHODS
Generation of NG2cre p38α MAPK−/− mice
Generation of NG2cre p38α MAPK −/− (p38α MAPK CKO) mice with p38α MAPK [B6.129-Mapk14<tm1.2Otsu>] has been described previously (For details, refer to Chung et al., 2015). Briefly, Cre/loxP recombination system is used by breeding NG2/Plp-Cre mice and p38α-floxed (p38α MAPK fl/fl) mice. All mice that were used in this study were carefully maintained in accordance to the NIH guidelines for the Care and Use of Laboratory Animals. Experimental protocols used for this study were approved by the Institutional Animal Care and Use Committee at the University of California, Davis and University of Illinois at Chicago.
PVL Mouse Model
Newborn mice pups were administered with LPS (lipopolysaccharide, Sigma), a potent inflammatory agent, by intraperitoneal injections at a dose of 0.12 mg/kg body weight, twice a day, from P4 to P7. Then, at P6 or P7 the pups underwent unilateral carotid ligation (UCL) surgery to induce ischemia followed with hypoxia (H/I). This procedure caused selective white matter injury near the periventricular regions. Mice were anesthetized under ice and then underwent UCL followed by a 1 hour recovery interval during which the pups were housed with the dam and kept on a thermal blanket to maintain body temperature at 33–34 °C. For a detailed description of the protocol, please refer to (Shen et al., 2010, 2012; Liu et al., 2011).
Electron Microscopy
Mice were transcardially perfused with Karnovsky’s solution (4% PFA in PBS with 5% glutaraldehyde) and stayed overnight with post-fixation agent containing 2% osmium tetroxide in 0.1 M cacodylate buffer. The brains were dehydrated and embedded and stained with toluidine blue to locate white matter regions. The samples were cut in 70 nm and placed on Formva-coated copper grids. The sections then stained with uranyl acetate and lead citrate, and observed in a Philips CM120 Electron Microscope at 80 kV. Images were acquired to demonstrate myelion sheath and g-ratio via a high resolution CCD camera (Gatan, Pleasanton, CA).
Immunohistochemistry
Mice were aesthetized with intraperitoneal sodium pentobarbital injection (100 mg/kg, i.p.) and transcardially perfused with 0.9% NaCl in 0.1 M PBS, pH 7.4 followed by 4% PFA in 0.1 M PBS (pH 7.4). The brains were then removed from the skull and post-fixed in 4% PFA at 4°C for 48 hours. Brain tissues were cryoprotected with a series of sucrose solutions and sectioned with 40 μm thickness. The rabbit polyclonal, and mouse monoclonal anti-mouse green fluorescent protein (GFP) antibodies (1:1000, Abcam) to identify EYFP, anti-mouse MBP antibody (1:500; Sternberger and Sternberger), anti-rabbit p38 (1:1000, Abcam) and anti-human Olig2 antibody (1:500; Abcam) were used in our study.
For the Diaminobenzidine (DAB) peroxidase immunohistochemistry, sectioned sections were blocked with 10% normal goat serum and then incubated in 0.1 M PBS containing 0.1% Triton-X and the primary antibody for 16–18 hours at 4°C. Brain section were then treated with HRP-conjugated goat anti-rabbit or HRP-conjugated goat anti-mouse secondary antibodies (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 hours at room temperature. DAB (0.5 mg/ml) was used to stain with brown colors. Finally, sections were dehydrated and, cover-slipped with Entellan mounting medium (BDH Chemicals, Toronto, ON, Canada).
Brain sections for fluorescent immunohistochemistry were conducted. The sections were blocked with 10% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA), and then incubated in PBS containing 0.1% Triton-X and the primary antibody overnight. Sections were then treated with Alexa 546-conjugated goat anti-rabbit IgG, Alexa 488-conjugated goat anti-mouse IgG, and Alexa 643-conjugated goat anti-guinea pig IgG (Molecular Probes Inc., Eugene, OR), at 1:2,000 dilution. Finally, the slides were cover slipped in the mounting medium (Fluorsave Reagent, Calbiochem, La Jolla, CA).
Data Analysis
For quantification of MBP staining intensity, ImageJ was used. Fixed areas of 1mm X 1mm or 2mm X 2mm were selected to analyse the number of Olg2-positive OPCs. The total number of mice used in this study is 68 (p38 CKO: 39 and wildtype mice: 29).
EM images were presented for myelination by calculation of the G (g) ratio (the ratio of axon circumference to myelin circumference). Briefly, high magnification images of myelinated axons were obtained and imported to the ImageJ and manually measured. The g-ratio was demonstrated as the diameter of the axon (a) divided by diameter of the myelinated axon caliber (A): G-ratio = a/A. All statistical data were shown as mean ± SEM. Student’s t-test when two independent groups were compared. P values of <0.05 were considered significant.
RESULTS
Oligodendroglial p38α MAPK deletion does not attenuate periventricular myelination injury in the mouse model of PVL
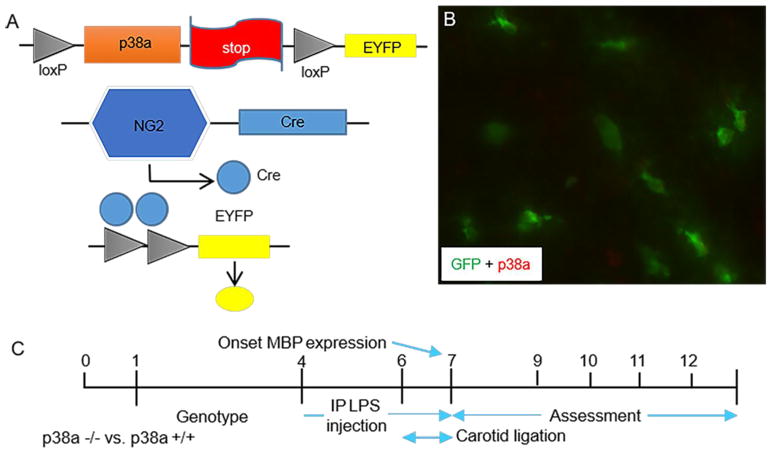
To identify in vivo role of oligodendroglial p38α MAPK in PVL-like injury, we utilized our previously generated NG2-Cre p38α CKO mice. The generation of the OPC-specific p38α gene knockout NG2cre/p38α−/− mice was described previously (Chung et al., 2015). Briefly, the p38α MAPK deletion was conducted by Cre-loxP recombination under the OPC-specific NG2 promoter (Fig. 1A). We also applied a reporter strain (EYFP) to simultaneously validate Cre recombination in these cells (Fig. 1B). The p38α MAPK CKO mice were viable with no obvious phenotypic abnormalities compared to the wildtype. A strong inflammatory agent LPS was administrated intraperitoneally and hypoxic/ischemia surgery was performed subsequently to mimic the human PVL white matter injury (Fig. 1C). We already validated and published a number of studies using our PVL model (Deng et al., 2008; Shen et al., 2010, 2012; Liu et al., 2011).
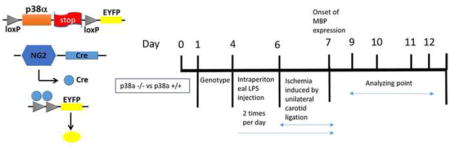
Figure 1. Schematic representation of NG2Crep38α MAPK conditional knockout mice and mouse periventricular leukomalacia (PVL) induction protocol timeline.
A: Generation of oligodendrocyte specific p38α MAPK knockout mice by breeding NG2-Cre mice and mice with p38α MAPK-floxed alleles (p38α fl/fl). Schematic knockout strategy diagram showing the targeted genetic locus of p38α MAPK gene flanked by the loxP sites. p38α MAPK gene knockout is mediated by Cre-loxP recombination that is under the control of OPC-specific NG2 promoter activity in targeted cells. Successful knockout cells express EYFP. B: Double immunohistochemistry staining confirms that p38α knockout cells (green) do not express p38α protein. C: Schematic illustrations of mouse model of PVL. Pups at postnatal day (P) 0 were utilized for genotyping. LPS (1.2 mg/kg) was injected intraperitoneally twice per day from P4 to P7 to induce inflammatory injury. Unilateral carotid ligation surgery was performed either P6 or P7 which resulted in selective white matter oligodendrocyte and myelination damage. The PVL phenotype was being examined from P8 to P15 (mostly at P12).
We first examined myelin expression in the periventricular white matter under PVL with anti-MBP (myelin basic protein) peroxidase immunohistochemistry using p38α MAPK CKO mice. PVL induction drastically decreased MBP expression both in wild type and p38α MAPK CKO mice (wild type: Fig. 2A vs. 2C; p38α CKO: Fig. 2B vs. 2D). Surprisingly, gross examination under the low-magnification microscope showed that MBP staining pattern and intensity was similar throughout the corpus callosum areas of the p38α MAPK CKO brain (Fig. 2D, F) compared to wild type sibling (Fig. 2C, E) after PVL induction. On careful examination, the staining intensity of MBP in some areas of the periventricular white matter appeared to be lightly stained in the CKO compared to p38α MAPK wildtype, the differences in general were not statistically significant. Under physiological conditions, the quantitative MBP staining intensity was 2467 ± 243 (N=5) in the p38α MAPK CKO vs 2678 ± 173 (N=7) in wildtype (Fig. 2G, t(10)=1.767, P=0.11). Following induction of PVL, The quantitative MBP staining intensity changed to 1634 ± 93 in p38α MAPK CKO (N=5) vs 1432 ± 93 (N=4) in wildtype pups (Fig. 2G, t(7)=3.237, P<0.05). These data indicate that a selective deletion of p38 MAPK alpha subtype in oligodendrocyte does not attenuate myelination defects in the mouse model of PVL.
Figure 2. No significant myelination phenotype defects were observed in the P12 p38α CKO mice compared to the wildtype control after PVL induction.
A–F: Transverse brain sections were peroxidase immunostained for anti-MBP in the p38α wildtype (A, C, E) and p38α CKO (B, D, F) at P12. PVL is induced at P6 (C, D) and compared to normal group (A, B). (E) and (F) are higher magnification views of the regions indicated in (C) and (D). MBP expression in the PVL induced mice is significantly weaker both in the wildtype (C vs A) and p38α CKO mice (D vs B). In the PVL-induced groups, no significant difference of MBP staining intensity and pattern was observed between the wildtype (C, E) and p38α CKO mice (D, F). G: Statistical representation of the MBP staining intensity in the periventricular area of the p38α wildtype and p38α CKO both in normal and PVL induced groups. MBP immunostaining intensity was quantified using image J. Under physiological conditions, the quantitative MBP staining intensity was 2467 ± 243 (N=5) in the p38α MAPK CKO vs 2678 ± 173 (N=7) in wildtype (t(10)=1.767, P=0.11). Following induction of PVL, The quantitative MBP staining intensity changed to 1634 ± 93 in p38α MAPK CKO (N=5) vs 1432 ± 93 (N=4) in wildtype pups (t(7)=3.237, P<0.05). Scale bars: B, D = 500 μm; F = 250 μm.
The microstructure of myelin was not affected in the p38α MAPK CKO mice compared to the wildtype control under PVL
Given that our previous analysis showed severe ultrastructural myelin defects in our p38α MAPK CKO mice (Chung et al., 2015), we next performed EM study. A detailed morphological observation using EM did not show any noticeable difference in myelin structure or the diameter of individual axons between the wildtype and p38α MAPK CKO mice under PVL (Fig. 3).
Figure 3. p38α CKO mice reveal no significant difference in ultrastructure of myelin compared to the wildtype control under PVL induction.
A, B: Quantification representation of axon diameters and g-ratio (the calculated ratio of individual axon diameter to fiber diameter). The axon diameters show of wildtype (3.71 ± 0.12, N=4) and p38α MAPK CKO (2.82 ± 0.23, N=5) in normal status versus wildtype (2.41 ± 0.22, N=3) and p38α MAPK CKO (2.28 ± 0.14, N=4). The average g-ratio of the nerve fibers from p38α MAPK CKO mice after PVL induction was 0.833 ± 0.067 (54 OF, N=4) compared to 0.731 ± 0.047 (64 OF, N=3) in wildtype (t(5)=2.233, P=0.07). C–J: Electron microscopy analysis of myelin structures in periventricular sections from P12 p38α CKO (D, H, F, J) and wildtype mice (C, G, E, I) under normal (C, D, G, H) and PVL induction (E, F, I, J). (G, H) and (I, J) are higher magnification views of the regions indicated in (C, D) and (E, F). The thickness of individual myelin diameter was similar in the p38α CKO (F, J) compared with wildtype (E, I) under PVL induction. Scale bars: B, F = 2 μm; D, H = 1 μm.
In p38α MAPK CKO, the morphology of myelin sheath thickness was not significantly different compared to the wildtype controls. Individual myelin bundle thickness was measured as the g-ratio (the ratio of axon diameter to fiber diameter) from different non-overlapping observational fields (OF). The PVL induction decreased axon diameters both in the wildtype and p38α MAPK CKO mice. The axon diameters show of wildtype (3.71 ± 0.12, N=4) and p38α MAPK CKO (2.82 ± 0.23, N=5) in normal status versus wildtype (2.41 ± 0.22, N=3) and p38α MAPK CKO (2.28 ± 0.14, N=4). The average g-ratio of the nerve fibers from p38α MAPK CKO mice after PVL induction was 0.833 ± 0.067 (54 OF, N=4) compared to 0.731 ± 0.047 (64 OF, N=3) in wildtype (Fig. 3J, t(5)=2.233, P=0.07), thus suggesting that the microstructure of myelin was not significantly affected in p38α MAPK CKO mice compared to the wildtype control under PVL.
The oligodendrocyte death/survival was unchanged in the p38α MAPK CKO compared to the wildtype under PVL
Immunostained sections of p38α MAPK CKO periventricular white matter using anti-EYFP (green) and anti-MBP antibodies (red) demonstrated that a considerable number of p38α knockout oligodendrocytes are present after the PVL induction (Fig. 4B). We then assessed whether oligodendroglial p38α MAPK gene deletion affects the death/survival of OPCs which in turn would contribute to myelination defects in the p38α MAPK CKO mice after PVL induction.
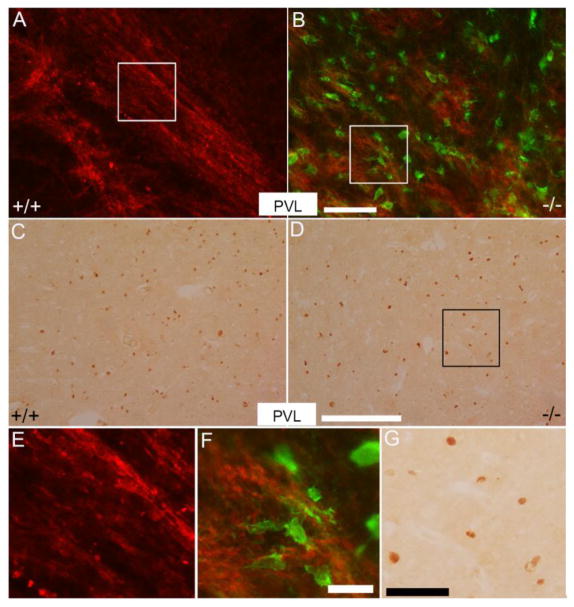
Figure 4. p38α gene deletion does not affect oligodendrocyte death/survival under PVL induction.
A, B: Double immunofluorescence stained brain samples with anti-EYFP (green) and MBP (red) in periventricular region in the p38α MAPK CKO (B) and wildtype mice (A) under PVL. Green cells represent p38α MAPK knockout cells. Note that a majority of p38α MAPK knockout cells did not express MBP. (E), (F) and (G) are higher magnification views of the areas indicated in (A), (B) and (D). C, D: Transverse sections were peroxidase stained for anti-Olig2 at P12 in the periventricular white matter of the wildtype (C) and p38α MAPK CKO mice (D) under PVL induction. Scale bars: B = 100 μm; D = 250 μm; F = 25 μm; G = 50 μm.
The numbers of Olig2-positive cells in the periventricular white matter of p38α wildtype and p38α MAPK CKO mice were examined at P12. In normal condition, Olig2-positive OPC numbers were unchanged in the p38α MAPK CKO (Fig. 4C, D). This is consistent with our previous observation (Chung et al., 2015). Quantitatively (Fig. 5), the numbers of Olig2-positive cells were 282 ± 24 per mm2 in wildtype (32 areas were examined, N=4) vs 264 ± 27 per mm2 in p38α MAPK CKO (57 areas were examined, N=6). In normal condition, the Olig2-positive oligodendrocyte cells were reduced under PVL (* t(7)=10.471, P<0.0001). But after PVL induction, the number of oligodendrocytes remained similar between p38α MAPK CKO and wildtypes (Fig. 5, t(10)=1.377, P=0.19). The numbers of Olig2-positive cells were 136 ± 18 per mm2 in wildtype (48 areas were examined, N=5) vs 114 ± 32 per mm2 in p38α MAPK CKO (82 fields within identical anatomical areas were examined, N=7). These results indicate that p38α MAPK did not appear to play a key role in oligodendrocyte death/survival during PVL induction.
Figure 5. The number of oligodendrocytes was not significantly changed between the p38α MAPK CKO and wildtypes both in normal and PVL induction.
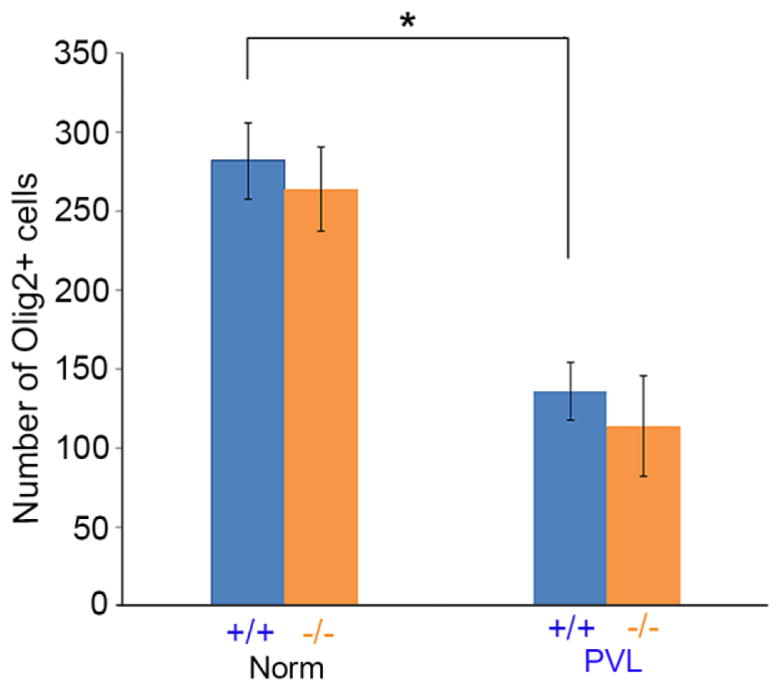
Quantification of Olig2-immunopositive cells in the periventricular white matter of the P12 wildtype and p38α MAPK CKO mice under PVL. The numbers of Olig2-positive cells were 282 ± 24 per mm2 in wildtype (N=4) vs 264 ± 27 per mm2 in p38α MAPK CKO (N=6). In normal condition, the Olig2-positive oligodendrocyte cells were reduced under PVL (* t(7)=10.471, P<0.0001). But after PVL induction, the number of oligodendrocytes remained similar between p38α MAPK CKO and wildtypes (t(10)=1.377, P=0.19). The numbers of Olig2-positive cells were 136 ± 18 per mm2 in wildtype (N=5) vs 114 ± 32 per mm2 in p38α MAPK CKO (N=7).
DISCUSSION
In this study, we identified the role of oligodendroglial p38α MAPK in oligodendrocyte cell death/survival and myelination in the mouse model of PVL using a newly generated p38α MAPK CKO mice. Despite a number of in vitro studies of p38 MAPK showing its neuroprotective properties against several forms of white matter injuries, our in vivo analysis confirms that myelination phenotype and pre-oligodendrocyte death/survival protection is not affected at a gross and ultrastructure level by the oligodendroglial p38α MAPK deletion. Our MBP immunohistochemistry showed that myelin expression was not affected in the PVL of p38α MAPK CKO mice. The EM analysis demonstrated that micro myelin structures were not significantly different between wildtype and the CKO mice. The number of surviving oligodendrocytes was also not affected either. Our data suggests that p38α MAPK in oligodendrocytes may not be a significant player in the pathology of mouse model of PVL suggesting that other cellular players might have prominent role(s) in this process.
Our previous study (Chung et al., 2015) using p38α MAPK CKO mice demonstrated that, although a myelination phenotype was not changed significantly in the knockout mice under light microscopic level, there were a number of myelination defects at the EM level. First, several myelin bundles in the corpus callosum developed abnormally, and there was a slightly delayed myelination in the white matter. These phenotypes could be, in part due to a disruption in oligodendrocyte differentiation during early peri/postnatal development as the number of oligodendrocyte precursor cells remained unchanged in these knockout mice. This observation was supported by our gene PCR analysis of several crucial transcription factors of oligodendrocyte differentiation such as Olig1, Zfp488. Additionally, consistent with previous reports, an inherent myelination defect was obvious in the primary oligodendrocyte progenitor cells isolated from p38α MAPK CKO mouse brains. These cells were not able to synthesize MBP when differentiated in vitro. Our second major finding was that p38α MAPK appears to have dual roles in myelination and remyelination and constitutes a negative regulator in remyelination of cuprizone-induced demyelination model in adult p38α MAPK CKO mice. The p38α MAPK CKO mice showed increased remyelination capability compared to control during the recovery (Chung et al., 2015).
Recent several studies reported p38αMAPK’s critical roles in oligodendrocyte and peripheral nervous system (PNS) myelinating Schwann cells differentiation and myelination. Inhibition of p38 MAPK by pharmacological agents, SB203580 prevented myelin formation in CNS oligodendrocyte and PNS Schwann cells (Fragoso et al., 2003, 2007). In the mouse model of experimental autoimmune encephalomyelitis (EAE), p38 MAPK inhibition using both p38α MAPK CKO heterozygote mice and p38α-specific inhibitor (UR-5269) have proven beneficial to suppress the progression of EAE (Namiki et al., 2012). However, previous studies mostly used less specific p38 MAPK inhibitors. For example, SB203580, the most commonly used p38 MAPK inhibitor, is not p38α specific since it blocks both p38α and p38β types, and has been known to interact with additional molecules at relatively higher concentrations (Godl et al., 2003; Fabian et al., 2005). There is a study showing that alpha and gamma subtypes play opposite roles in muscle cell differentiation (Lluis et al., 2006). Thus, we think that most p38 inhibitors cannot really reveal the alpha specific role of p38 MAPK, and an endogenous gene knockout approach is essential.
Our study did not reveal a specific role of p38α MAPK in the progression of PVL suggesting that oligodendroglial p38α MAPK is not a major player in the pathogenesis of mouse PVL model. This might be due to that the PVL induction occurs very early postnatal development and other p38 MAPK isotypes such as beta type can rescue the phenotype defects. There is also possibility that other key cellular players such as microglia and astrocytes participate and interact with p38α MAPK. A comprehensive analysis of astroglial p38α MAPK in the mouse PVL model is ongoing our newly generated astrocyte-specific p38α MAPK CKO mice. Taken together, our study will provide and narrow the basis for potential therapeutic interventions of oligodendrocyte, p38α MAPK and PVL white matter injury.
HIGHTLIGHTS.
We used a recently established mouse periventricular leukomalacia model with our newly generated p38 MAPK CKO mice.
Unexpectedly, in vivo OPC-specific p38α inhibition has minimal protection effect in the mouse model of PVL.
OPC-specific p38α inhibition did not show myelination phenotype and oligodendrocyte differences.
Our study will provide and narrow the basis for potential therapeutic interventions.
Acknowledgments
This study was supported by start-up funds from University of Illinois at Chicago College of Dentistry to S. C. and by grants from the National Institutes of Health (R01HD087566, R01HD091325) to W.D. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Abbreviations
- CKO
conditional knockout
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- GFP
green fluorescent protein
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MBP
myelin basic protein
- NG2
neuron-glial antigen 2
- OPC
oligodendrocyte precursor cell
- P
postnatal day
- PVL
periventricular leukomalacia
- UCL
underwent unilateral carotid ligation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bhat NR, Zhang P, Mohanty SB. p38 MAP kinase regulation of oligodendrocyte differentiation with CREB as a potential target. Neurochem Res. 2007;32:293–302. doi: 10.1007/s11064-006-9274-9. [DOI] [PubMed] [Google Scholar]
- Chung SH, Biswas S, Selvaraj V, Liu XB, Sohn J, Jiang P, Chen C, Chmilewsky F, Marzban H, Horiuchi M, Pleasure D, Deng W. The p38alpha mitogen-activated protein kinase is a key regulator of myelination and remyelination in the CNS. Cell Death and Dis. 2015;6:e1748. doi: 10.1038/cddis.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. Neurobiology of injury to the developing brain. Nat Rev Neurol. 2010;6:328–336. doi: 10.1038/nrneurol.2010.53. [DOI] [PubMed] [Google Scholar]
- Deng W, Pleasure J, Pleasure D. Progress in periventricular leukomalacia. Arch Neurol. 2008;65:1291–1295. doi: 10.1001/archneur.65.10.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lélias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. A small molecule kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- Follett PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA, Volpe JJ, Jensen FE. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci. 2004;24:4412–4420. doi: 10.1523/JNEUROSCI.0477-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX Attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso G, Haines JD, Roberston J, Pedraza L, Mushynski WE, Almazan G. p38 mitogen- activated protein kinase is required for central nervous system myelination. Glia. 2007;55:1531–1541. doi: 10.1002/glia.20567. [DOI] [PubMed] [Google Scholar]
- Fragoso G, Robertson J, Athlan E, Tam E, Almazan G, Mushynski WE. Inhibition of p38 mitogen-activated protein kinase interferes with cell shape changes and gene expression associated with Schwann cell myelination. Exp Neurol. 2003;183:34–46. doi: 10.1016/s0014-4886(03)00101-8. [DOI] [PubMed] [Google Scholar]
- Godl K, Wissing J, Kurtenbach A, Habenberger P, Blencke S, Gutbrod H, Salassidis K, Stein-Gerlach M, Missio A, Cotten M, Daub H. An efficient proteomics method to identify the cellular targets of protein kinase inhibitors. Proc Natl Acad Sci USA. 2003;100:15434–15439. doi: 10.1073/pnas.2535024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- Hamanoue M, Sato K, Takamatsu K. Inhibition of p38 mitogen activated protein kinase-induced apoptosis in cultured mature oligodendrocytes using SB202190 and SB203580. Neurochem Int. 2007;51:16–24. doi: 10.1016/j.neuint.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Haynes RL, Folkerth RD, Keefe RJ, Sung I, Swzeda LI, Rosenberg PA, Volpe JJ, Kinney HC. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–450. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- Hossain S, de la Cruz-Morcillo MA, Sanchez-Prieto R, Almazan G. Mitogen-activated protein kinase p38 regulates Krox-20 to direct Schwann cell differentiation and peripheral myelination. Glia. 2012;60:1130–1144. doi: 10.1002/glia.22340. [DOI] [PubMed] [Google Scholar]
- Liu W, Shen Y, Plane JM, Pleasure DE, Deng W. Neuroprotective potential of erythropoietin and its derivative carbamylated erythropoietin in periventricular leukomalacia. Exp Neurol. 2011;230:227–239. doi: 10.1016/j.expneurol.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluis F, Perdiguero E, Nebreda AR, Muñoz-Cánoves P. Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol. 2006;16:36–44. doi: 10.1016/j.tcb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Namiki K, Matsunaga H, Yoshioka K, Tanaka K, Murata K, Ishida J, Sakairi A, Kim J, Tokuhara N, Shibakawa N, Shimizu M, Wada Y, Tokunaga Y, Shigetomi M, Hagihara M, Kimura S, Sudo T, Fukamizu A, Kasuya Y. Mechanism for p38α-mediated experimental autoimmune encephalomyelitis. J Biol Chem. 2012;287:24228–24238. doi: 10.1074/jbc.M111.338541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Liu X, Pleasure DE, Deng W. Axon-glia synapses are highly vulnerable to white matter injury in the developing brain. J Neurosci Res. 2012;90:105–121. doi: 10.1002/jnr.22722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Plane JM, Deng W. Mouse models of periventricular leukomalacia. J Vis Exp. 2010;18:1951. doi: 10.3791/1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the Newborn. Saunders; Philadelphia: 2001. pp. 217–276. [Google Scholar]
- Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]