Abstract
Age-related hearing decline typically includes threshold shifts as well as reduced wave I auditory brainstem response (ABR) amplitudes due to cochlear synaptopathy/neuropathy, which may compromise precise coding of suprathreshold speech envelopes. This is supported by findings with older listeners, who have difficulties in envelope and speech processing, especially in noise. However, separating the effects of threshold elevation, synaptopathy, and degradation by noise on physiological representations may be difficult. In the present study, the effects of notched, low- and high-pass noise on envelope following responses (EERs) in aging were compared when sound levels (aged: 85 dB SPL; young: 60–80 dB SPL) were matched between groups peripherally, by matching wave I ABR amplitudes, or centrally by matching EFR amplitudes. Low-level notched noise reduced EFRs to sinusoidally amplitude modulated (SAM) tones in young animals for notch widths up to 2 octaves. High-pass noise above the carrier frequency reduced EFRs. Young animals showed EFR reductions at lower noise levels. Low-pass noise did not reduce EERs in either young or aged animals. High-pass noise may affect EER amplitudes in young animals more than aged by reducing the contributions of high-frequency sensitive inputs. EERs to SAM tones in modulated noise (NAM) suggest that neurons of young animals can synchronize to NAM at lower sound levels and maintain dual AM representations better than older animals. The overall results show that EER amplitudes arc strongly influenced by aging and the presence of a competing sound that likely reduces or shifts the pool of responsive neurons.
Keywords: Age-related hearing loss, Synaptopathy, Neuropathy, Amplitude modulation, Masking, Envelope-following responses
Introduction
Temporal characteristics of sound play an important role in segregating complex sounds [Itatani and Klump, 2009]. Temporal cues, such as amplitude or frequency modulation (AM or FM) rate and modulation depth, are used by the auditory system for speech recognition and sound perception in challenging settings, including in the presence of competing sounds, background noise and reverberation [Shannon et al., 1995, Zeng et al., 2005, Snell and Frisina, 2000, Srinivasan and Zahorik, 2014]. These challenges are exacerbated for older listeners in noisy settings [Duqucsnoy, 1983] and in reverberant environments [Duquesnoy and Plomp, 1980, Náblek and Robinson, 1982, Shinn-Cunningham et al., 2013], even in individuals without elevated audiometric thresholds [Dubno et al., 2002, HcTfer and Freyman, 2008]. Additional psychoacoustic studies have shown that older normal-hearing subjects have poor performance for tasks relying on temporal cues of supra-threshold sounds and speech-in-noise tests [Ruggles et al., 2012, Grose and Manio, 2010, Strelcyk and Dau, 2009, Frisina and Frisina, 1997, Strousc et al., 1998, Dubno, 1984]. These behavioral studies are consistent with electrophysiological evidence in humans and animals showing that auditory temporal processing deficits may be a major factor contributing to difficulties understanding speech in noise experienced by older adults [Parthasarathy and Bartlett, 2011, Anderson et al., 2012, Soros et al., 2009, Presacco et al., 2016b, Bidelman, 2016].
To study noise effects on temporal processing, envelope-following responses (EFRs) can be used. EFRs are shown to arise primarily from phase-locking activities of afferents to the midbrain and [Herdman et al., 2002, Kuwada et al., 2002, Picton et al., 2003, Chandrasekaran and Kraus, 2010, Parthasarathy and Bartlett, 2012, Cunningham et al., 2001, Aiken and Picton, 2008, Parthasarathy et al., 2010, Parthasarathy and Bartlett, 2011, Parthasarathy and Bartlett, 2012, Dolphin and Mountain, 1993]. Exploring evoked potentials in response to simple and complex sounds in noise might help to reveal age effects on speech-in-noise perception and potential mechanisms involved [Henry and Lucas, 2010a, Henry and Lucas, 2010b, Parthasarathy et al., 2010, Mehraei et al., 2016]. Recently, an auditory midbrain study revealed that neural thresholds of AΜ envelope synchrony were consistent with behavioral thresholds of vowel-like sound discrimination both in noise and in quiet [Henry et al., 2017).Most studies of AΜ representations with aging are in quiet [Walton et al., 2002, Shaddock Palombi et al., 2001], but how aging alters envelope synchrony in background noise is unclear. Moreover, in this study and other human psychoacoustic studies [Festen, 1990, Christiansen et al.,2013], behavioral perception (either detection or discrimination) of stimuli in fluctuating or amplitude modulated noise has shown to be better than in steady-state noise. However, there is less exploration on neural representations of modulated noise and the extent to which the representations of two temporally modulated sounds are coded separately or shift between responsive pools of neurons.
In the past, most electrophysiological studies in aging were performed using stimuli presented at equal sound levels [Boettcher et al., 2001, Purcell et al., 2004, Clinard ct al., 2010] or similar sensation levels [Parthasarathy et al., 2010, Parthasarathy and Bartlett, 2011]. At equal sound levels, younger and older listeners might perceive a stimulus differently due to hearing threshold differences and therefore different sensation levels. At similar sensation levels, each subject may have different numbers of neurons activated by and synchronized to the stimulus due to age-related cochlear synaptopathy and/or neuropathy [Sergevenko et al., 2013]. To tease apart peripheral and central contributions to temporal processing deficits as well as to provide a “fairer” comparison for younger and older groups, we compared EFRs of young and aged animals using sound levels that matched wave I amplitudes of auditory brainstem responses (ABR) to 8 kHz tones, which was the carrier in these experiments (matched peripheral neural activation), or EFR, amplitudes to sinusoidally amplitude modulated (SAM) tones at 100 % depth (matched central neural activation) [Lai et al., 2017]. In our previous study [Lai et al., 2017], the average stimulus intensities in young animals to match the responses evoked by 85 dB stimuli in aged animals were 66.3+/−1.33(mean /- SEM) dB SPL (matched wave I amplitude) and 69.9+/−1.34 dB SPL (matched EFR, amplitude). For AM depth processing in quiet (EFR, amplitudes as a function of modulation depth), we observed minimal age-related differences in AM depth processing at matched peripheral or central activation for AM frequency of 256 Hz. For AM modulation frequency processing in quiet (EFR, amplitude as a function of AM frequency), we observed enhanced EFR,s with aging at 100 % but not 25 % depth at matched wave I amplitude for AM frequencies of 16–90 Hz.
These results suggested that matched activation could preserve or boost EFR, amplitudes in older animals in quiet, but far less is known about how that will affect neural responses in noise. Here, we further explore the effect of noise on temporal processing in aging at matched peripheral or central neural activation by presenting a 256 Hz SAM 8 kHz tone in the presence of notched, low- or high-pass noise. In addition, to further understand simultaneous neural representations of target AΜ sound and noise, sinusoidally amplitude modulated noise (NAM) was also used inplace of the steady-state noise.We hypothesized that larger age-related differences in temporal processing will be observed when noise is added in the background.
Methods and Materials
Subjects
Young (3–6 months, 13 male and 5 female) and aged (21–24 months, 14 male and 9 female) Fischer-344 rats obtained from Taconie/NIA colony were used. The animal numbers used for each type of recording session are shown in each respective figure legend. All animals were housed in the animal care facility during the period of this study in a relatively quiet and standard condition. All protocols were approved by the Purdue Animal Care and Use Committee (PACUC-1111000167).
Experimental procedures
The ABR, and EFR, recording protocols and procedures were similar to our previous studies and more details can be found in Lai et al. (2017), and Parthasarathy and Bartlett (2012). All auditory evoked potential recordings were performed in a 9’x9’ double-walled anechoic chamber (Industrial Acoustic Corporation). Anesthesia was induced by 4 % isoflurane. Animals were transferred to the stage after they had lost the righting reflex.A water circulating warming blanket (Kent Scientific), set at 37°C, was placed under animals to maintain body temperature. Subdermal needle electrodes (Ambu) were placed in a two-channel configuration while maintaining under 1.5–2 % isollurane. For channel 1, a positive electrode was placed along the midline of the forehead in the Cz to Fz position. For channel 2, another positive electrode was placed horizontally C3 to C4 along the interaural line, which straddles the IC bilaterally. A negative, reference electrode was placed under the ipsilateral ear along the mastoid while a ground electrode was placed in the nape of the neck. Electrode impedance was checked using a low-impedance amplifier (RA4LI, Tucker Davis Technologies or TDT) to confirm recording impedances less than 3 kΩ. Subsequently, animals were injected (intramuscular) with dexmedetomidine (Dexdomitor, 0.2 mg/kg), an α-adrenergic agonist acting as a sedative and an analgesic, before removing from anesthesia. A 15-minute waiting period was performed to allow the effect of the anesthesia to wear off. Animals were then recorded with various stimulus conditions. The sedative effect of dexmedetomidine typically lasted for 2.5–3 hours. During this stage, animals were usually immobile but they could still respond to foot pinch.
Stimuli were presented free-field to the right ear of animal at a distance of 115 cm from a Bower and Wilkins DM601 speaker. All stimuli were generated using SigCenRP (TDT) or MATLAB (MathWorks) with a sampling rate of 100 kHz. The speaker was calibrated using a Bruel Kjaer microphone placed at the position of the rat’s head during recording and using SigCal software (TDT). Stimulus presentation and response acquisition were conducted using BioSig software (TDT). Stimulus waveforms were delivered via a multichannel processor (RX6, TDT) to an amplifier (SA1, TDT) and then to the speaker. Digitized response waveforms were recorded with a multichannel recording and stimulation system (Rz5, TDT). Evoked responses were filtered and analyzed using BioSig or extracted as text files before analyzing with custom written program in MATLAB.
Auditory stimuli
Matching of peripheral activation
To obtain 8 kHz hearing thresholds and to measure wave I amplitudes for peripheral matching, ABR,s were recorded using 8 kHz pure tones of 2 ms duration (0.5 ms cos2 rise/fall time), alternating polarity. The pure tones were presented at at a rate of 26.6/see. A 30-ms acquisition window was used and 1500 repetitions were collected to obtain an average response for each ABR, at each sound level. Stimulus intensity was presented from 95 dB SPL to 15 dB SPL in 5-dB steps. The average 8 kHz thresholds of young and aged animals used in this study were 23+/− 1.3 (mean +/ SEM) dB SPL and 39.5 +/− 1.5 dB SPL, respectively. Meanwhile, the average ABR,thresholds for clicks were 50.1+/− 2.1 dB SPL in the aged and 28.7+/− 1.6 dB SPL in the young. ABR, wave I amplitudes reflect a combined measure of the number of activated ANFs and their synchrony [Rowe, 1981, Chen and Chen, 1991]. Previous studies have shown that ABR, wave I amplitudes were correlated with the number of surviving ANFs and IHC-ANF synapses [Kujawa and Liberman, 2009, Lin et al., 2011]. Therefore,ABR, wave I amplitude at each sound level was measured and used as an indicator for the amount of activated auditory nerve fiber (ANFs). The median of tone 8 kHz /ABR, wave I amplitudes at 85 dB SPL from aged animals was calculated and used for stimulus intensity matching of peripheral activation in young animals in subsequent EFR, recordings. Wave I amplitudes are larger and more reliable in small mammals than in humans, and we have shown that wave I amplitudes are easily measurable at suprathreshold sound levels [Parthasarathy and Bartlett, 2012, Lai et al., 2017].
Matching of central activation
SAM stimuli with a carrier frequency of 8 kHz and 200 ms duration (5 ms cos2 risc/fall time) were used to elicit EFR,s. An 8 kHz carrier frequency was used because 8 kHz falls within the most sensitive regions of the rat’s audiogram and age-related changes in threshold are relatively small [Parthasarathy et al., 2014]. Stimuli were presented at a rate of 3.1/see, and 200 repetitions were acquired using a 300-ms acquisition window to obtain an average EFR, response. SAM stimuli modulated at 100 % depth and 256 Hz were presented at 85 dB SPL to all aged animals to obtain a median EFR, magnitude. In young animals, the same SAM stimuli were presented from 60 to 85 dB SPL in 5-dB steps. This sound level range allowed us to identify the sound intensity for matching in each young animals that we tested. Young animals’ EFR, magnitudes were measured to determine the stimulus intensity for each individual animal that matched to the median of EFR, amplitudes of aged animals. The selected stimulus intensities were then used for that young individual in subsequent EFR, recordings.
Stimuli
Varying AM depth in quiet:
SAM stimuli of 256 Hz AM frequency (AMF) were presented in quiet. The modulation depths of the stimuli were 0 dB (100 %), −2.5 dB (75 %), −6 dB (50 %), −12 dB (25 %), −18 dB (12,5 %), −24 dB (6.25 %) and −30 dB (3.125 %).
Varying AM depth in noise:
The 256 Hz SAM stimuli were presented in notched noise (broadband noise of 0.1–40 kHz) at 40 dB signal-to-noise (SNR,) ratio. This SNR, value was selected based on a preliminary test showing that the EFR, magnitude at 40 dB SNR, was about 10 % below maximal EFR,s in young animals, whereas lower SNR,s dramatically reduced EFR, amplitudes in younger animals (as shown in panels A and B of Figs. 3 and 5). Four types of notch widths centering at 8 kHz were used: 0, 0.5, 1, and 2 octaves. The AM depths of the SAM stimuli were varied from 6.25 to 75 %. The same 256 Hz SAM stimuli, at either 100 % or 50 % AM depth, were also presented in the presence of low-pass (100 Hz up to a cut-off at 0.5-octave below 8 kHz (5658 Hz) with a 40 dB/ octave roll-off slope) or high-pass (cut-off at 0.5-octave above 8 kHz (11314 Hz up to 40 kHz) broadband noise at SNR, values of 60 to 0 dB in 10-dB steps. In addition to steady-state broadband noise, we also used a noise carrier modulated at 71 Hz, 100 % depth. The noise carrier remained unchanged but the AMF (71 Hz) of noise was selected to be different and not harmonically related to the AMF (256 Hz) of the SAM tone stimulus. In all eases where an SAM stimulus was presented in noise, the noise waveforms had the same onset and offset as the SAM signal (i.e. simultaneously gated). The noise was generated independently (not frozen) for eaeh trial to ensure that noise did not contribute significantly to the responses evoked by the SAM signal. To generate a SAM signal in notched, low- or high-pass noise, we fixed the sound level of the SAM signal and changed the overall level of the noise to achieve the desired SNR, value. For notched noise, spectrum level of the noise was held constant but the overall level of noise as notch width increased did not reduce more than 2 dB SPL.
Figure 3: High-pass noise produced differential effects on EFRs of young and aged animals while low-pass noise had little or no effect.
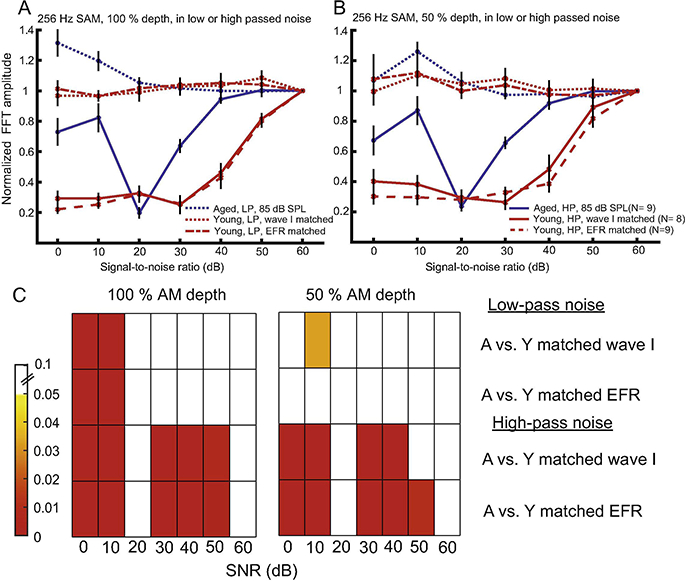
High-pass noisc decreased EFRs ofyoung animals at < 50 dB SNR. In aged animals, EFR3s decreased for 30 and 20 dB SNRs but increased for 10 and 0 dB SNRs. Similar trends were observed for 256 Hz SAM tone with either (A) 100 or (B) 50 % ΛΜ depth presented in noise. Note that all EFR, amplitudes in noise were normalized to EFR, amplitudes at 60 dB SNR, in eaeh animal. The statistical results of multiple comparisons between young and aged animals using rmAN()VA are reported in panel (C). The numbers on the left of the color maps indicate p-values and their corresponding colors. In the text on the right of the color maps, Λ indicates the aged and Y indicates the young. The data points and error bars are LS means +/− standard error of mean.
Figure 5: Time and frequency responses showed EFRs of a young animal was largely reduced by high-pass noise at 0 dB SNR.
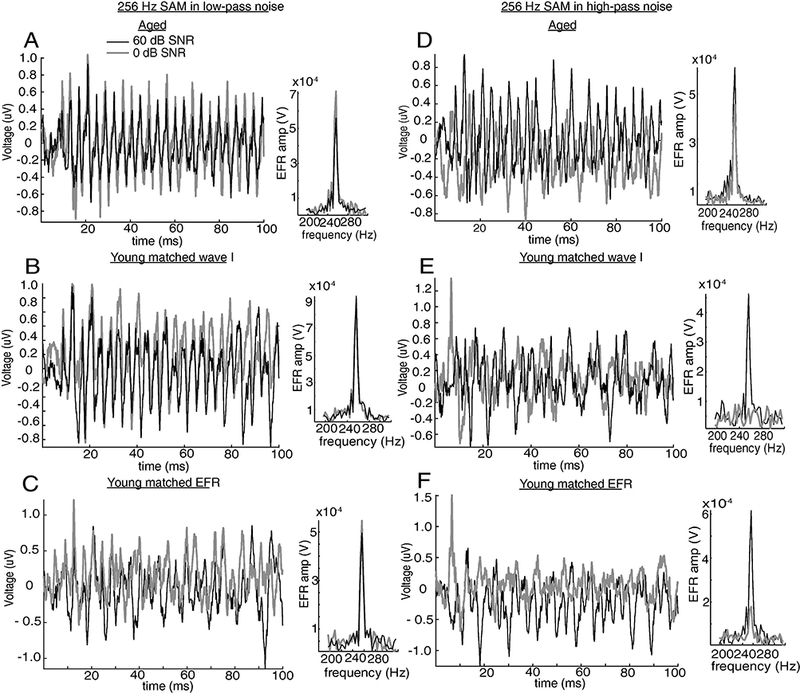
EFR,s from an individual representative aged (A and D) and young (B and E for matched peripheral activation; C and F for matched central activation) animal recorded with 256 Hz SAM tones in low- (A-C) or high-pass (D-F) steady-state noise at 0 (gray) or 60 (black) dB SNR,. The frequency response of 256 Hz for each condition was shown on the right side of the corresponding time waveform.
Data analysis
EFR magnitude measurements
All collected EFR,s were low-pass filtered at 3000 Hz. As demonstrated in a previous study (Parthasarathy and Bartlett 2012), channel 2 produces larger EFR responses for lower AM frequencies (≤ 100 Hz) and is sensitive to isoflurane anesthesia, consistent with rostral auditory generators, including the IC. In contrast, channel 1 produces larger EFR, responses for higher AM frequencies (≥ 100 Hz) and is relatively insensitive to isoflurane, consistent with primarily brainstem auditory generators. Therefore, for EFR,s evoked by the 256 Hz SAM stimuli, the responses of channel 1 were analyzed and high-pass filtered at 80 Hz. For EFR,s elicited by the 71 Hz NAM stimuli, the responses of channel 2 were analyzed and high-pass filtered at 30 Hz. Filtered data were then exported as text files and analyzed using custom written MATLAB programs. Fast Fourier transforms (FFT) were performed on the time-domain waveform from 10 to 190 ms relative to stimulus onset to exelude transient ABR,s at the beginning and end. The maximum magnitude of the evoked response at one of the three frequency bins (3 Hz/bin) around 256 Hz was measured as the peak FFT amplitude. The noise floor was calculated as the average magnitude of five frequency bins above and below the central three bins. Λ peak response was taken to be significantly above noise level when it is at least 10 dB above the noise floor.
Statistical analysis
Repeated measures ANOVAs (rmANOVAs) were performed to compare EFR, FFT amplitudes of young and aged groups as well as across different stimulus conditions using custom written scripts in SAS (Proc MIXED, SAS Institute, Cary, NC, USA). Main effects and interactions effects of each factor were analyzed based on comparisons of least squares (LS) means. Data distributions were checked for normality using normal probability plots of the residuals (proc UNIVARIATE). The differences in LS means with a confidence level of 95 % was used when reporting significant differences. LS means +/− standard error of mean are shown in the figures.
Results
Temporal processing in different types of background noise conditions
In the presence of notched noise
SAM tones at 256 Hz and modulation depths from −24 (6.25 %) to −2.5 (75 %,) dB were presented in notched noise with widths of 0.5, 1 or 2 octaves at 40 dB SNR. The same SAM stimuli were presented in noise without notch (0 width) as well in order to observe the additional effects of energetic masking around characteristic frequency. At this SNR,, it is expected that primarily lower-threshold frequency regions around 8–16 kHz will be stimulated [Parthasarathy et al., 2014). The sound levels for the SAM tone carrier in young animals matched peripheral or central neural activation to the aged. The average sound levels for the corresponding matching conditions in young animals were 66.3+/−1.33 (mean +/− SEM) dB SPL (matched wave I amplitude) and 69.9+/− 1.34 dB SPL (matched EFR, amplitude) [Lai et al., 2017]. EFR, amplitudes evoked by the SAM stimuli in the absence or presence of noise were plotted as a function of stimulus AM depth and are shown in Figure 1. At both equivalent peripheral and central activation, EFR,s to SAM of young and aged animals were similar in quiet and they reduced as SAM depth decreased. In young animals, EFR, amplitudes of SAM stimuli at −2.5 (75 %) and −6 dB (50 %) were decreased significantly by noise at matched peripheral activation (Fig. 1Λ). For matched central activation, EFR, amplitudes of SAM stimuli at −2.5 (75 %) to −12 dB (25 %) were reduced significantly by noise (Fig. 1B). In aged animals, however, EFRs elicited by the SAM stimuli were insensitive to noise and were comparable to EFR,s elicited by the SAM stimuli in quiet (Fig. 1C). This may be due in part because the noise was subthreshold in some aged animals (6 out of 10 animals). Moreover, in contrast to expectation, notch width had no effect on EFR, amplitudes in young animals despite suprathreshold noise levels for either. Similar results were observed for noise with different notch widths as well as for no notch.
Figure 1: Noise (at a SNR value of 40 dB), regardless of notch width, reduced EFRs of young animals at larger modulation depths (−2.5 and −6 dB).
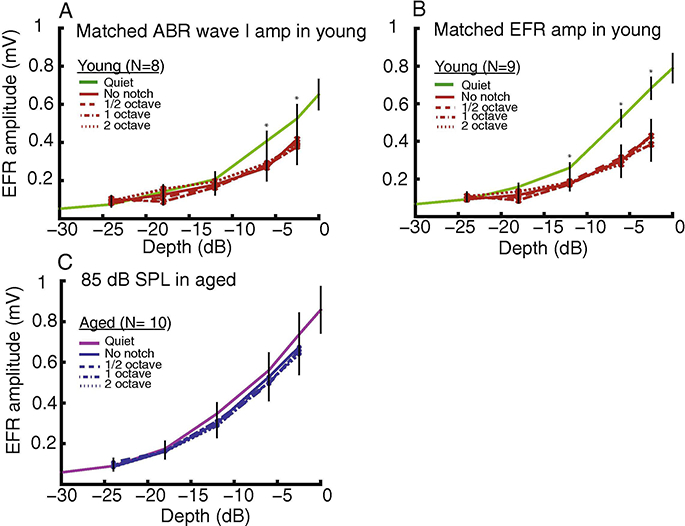
Reduction of EFR,s by noise was observed in both equivalent (A) peripheral and (B) central activation in young animals. In contrast, EFR,s of (C) aged animals were not affected by noise. The asterisks indicate p < 0.05 for EFRs in quict vs. in noise using least squares means comparison from rmANOVA. The data points and error bars are LS means /- standard error of mean.
An FFT peak response at the 256 Hz AM frequency was considered significant if it was at least 10 dB above the noise floor. Linear interpolation was performed between the lowest AM depth that had an EFR, response with SNR, > 10 dB relative to the noise floor and the next lowest AM depth. The AM depth at which the interpolated line intersected 10 dB SNR, was considered the threshold depth. The estimated average AM depth thresholds are shown in Fig. 2. In all notch types, aged animals had similar mean AM depth thresholds and they were significantly lower than young animals at both matched peripheral (F =18.56, df= 1/21, p =0.0002) and central (F = 13.74, df =1/22, p = 0.002) activation. When we compared the noise floors of young and aged animals, they were observed to be comparable at most conditions (data not shown). Lower FFT peak amplitudes in young animals contributed to their higher AM depth thresholds than the aged. In young animals, mean ΛΜ depth thresholds showed a trend of being higher for noise without notch. However, according to rmAN0VA, multiple comparisons of mean AΜ depth thresholds for the type of matching as well as for all notch widths were all not significantly different within young animals.
Figure 2: Mean modulation depth neurometric thresholds (in dB) based on EFRs in the young were lower for 256 Hz AM depth processing in quiet than in noise.
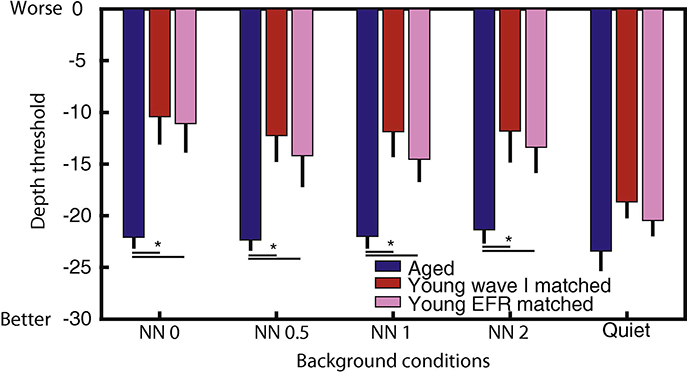
For young animals, there was a trend of mean modulation depth thresholds being slightly better in notched noise (NN0.5, NN1, NN2) than noise without notch (NN()) and the thresholds measured in quiet were significantly higher than in noise. The mean modulation depth thresholds in aged animals were similar for 256 Hz AM depth processing in noise with various notch widths and in quiet. Thresholds were determined at the cut-off of 10 dB above noise floor for the 256 Hz FFT peak. Error bars are standard error of means.
The rightmost side of Fig. 2 shows the mean neurometric thresholds (in dB) of the young and aged EFRs for ΛΜ depth processing at 256 Hz in quiet. When rmAN()VAs were performed on all SNR, thresholds of EFR,s recorded in quiet and in noisy conditions (Fig. 2), the young SNR, thresholds in quiet for both matching types were significantly lower (better) than in noise. At equivalent peripheral activation, there were significant main effects of Age (F = 21.14, df = 1/21, p =0.0002) and Background condition (F = 6.54, df = 4/59, p < 0.05) as well as an interaction effect of Age*Background condition (F = 3.19, df = 4/59, p =0.02). At equivalent central activation, there were only significant main effects of Age (F = 12.8, df = 1/22, p = 0.002) and Background condition (F = 4.8, df = 4/62, p = 0.002). However, there was no significant main or interaction effect in aged animals in quiet and noisy conditions.
In the presence of low- or high-pass steady-state noise
We were also interested to investigate how changes in noise spectrum would affect EFR,s. Two types of noise spectra were used: low-pass (0.15.657 kHz) and high-pass (11.314–40 kHz) noise. The edge of the noise was selected to be 0.5 octaves away from the 8 kHz carrier. Fig. 3 reports the effects of low- and high-pass noise, from 0 to 60 dB SNR,, on EFR, amplitudes in all animals for both peripheral and central matching. EFR, amplitudes to 256 Hz SAM obtained at each SNR, were normalized to EFR, amplitudes recorded at 60 dB SNR,, where the noise should be below threshold for physiological stimulation. Low-pass noise had little effect on EFR, amplitudes, regardless of SNR, or age, except for an increase in EFR, amplitude at the highest noise levels in aged animals (Fig. 3A). This does not appear to be due to stimulus artifacts (see Figs. 5 and 8). In contrast, high-pass noise decreased EFR, amplitudes of young animals at SNR,s less than 50 dB SNR, while EFR, amplitudes of aged animals were decreased only for 30 and 20 dB SNR,s. A “recovery” of EFR, amplitudes was observed at 10 and 0 dB SNR, high-pass noise in aged animals. These EFR, amplitudes were larger than EFR, amplitudes at 20 dB SNR, but were still smaller than EFR, amplitudes at 40–60 dB SNR,. The EFR,s in time and frequency domains of individual representative young and aged animals can be found in Fig. 5.
Figure 8: Time and frequency responses showed differential effects of high-pass NAM on EFRs at 0, 30 and 60 dB SNR.
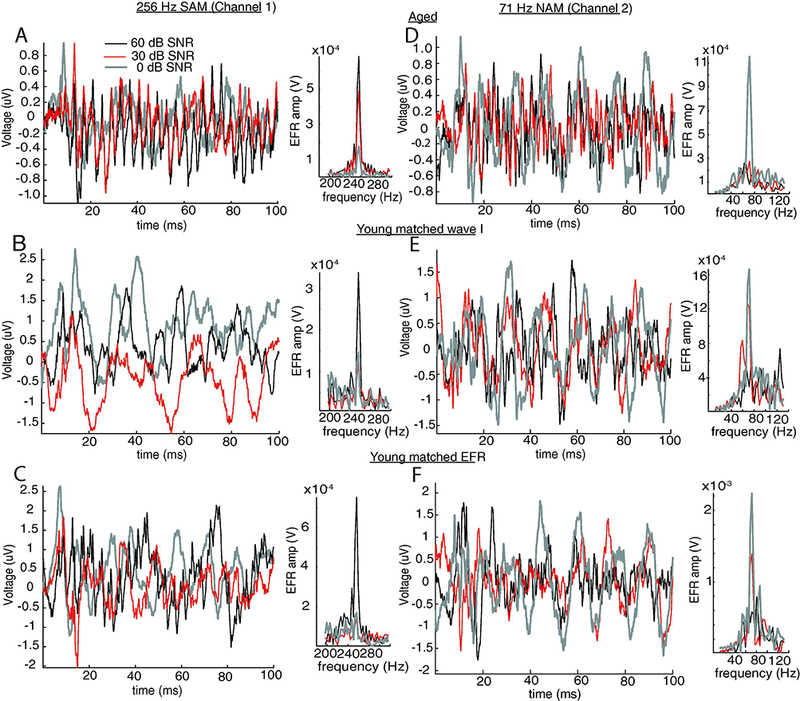
EFRs from an individual representative aged (A and D) and young (B and E for matched peripheral activation; C and F for matched central activation) animal recorded with 256 Hz SAM tones in 71 Hz NAM at 0 (gray), 30 (red) and 60 (black) dB SNR. Panels on the left (A-C) are EFRs recorded using channel 1 (better in picking up 256 Hz responses) while panels of the right (D-F) are EFR,s recorded using channel 2 (better in picking up 71 Hz responses). The frequency response of 256 Hz or 71 Hz for each condition was shown on the right side of the corresponding time waveform.
The statistical significances for multiple comparisons between young and aged animals estimated using rmAN0VA are shown in Fig. 3C. For high-pass noise, the rmAN0VA results showed that differences between the aged versus the young were statistically significant (p = 0.0002) for most SNR values (≤ 50 dB). Table 1 summarizes the F and p values of all significant main and interaction effects obtained from the results of rmAN0VA. In addition, Fig. 4 shows the mean SNR, thresholds in aged and young animals at matched wave I or EFR, amplitudes for 100 or 50 % ΛΜ depth processing in high-pass noise. Three aged animals showed EFR, amplitudes with SNR,s which were uniformly high (> 20 dB) regardless of stimulus SNR. Although we verified that these were not due to stimulus artifacts based on time domain waveforms of the EFR, responses (see Fig. 5 and 8), the thresholds of these three aged animals could not be estimated. The SNR, thresholds for 100 % AM depth were lower than 50 % depth in young animals. This is because a much larger response was elicited by a 256 Hz SAM tone at 100 % AM depth in young animals. However, SNR, thresholds were not different between 50 and 100 % depth in aged animals.
Table 1:
Significant main and interaction effects of multiple comparisons for EFRs in low- or high-pass noise using rmANOVA are summarized in the table. F and p values for each effect are reported in parentheses.
| Sig. main effect (F value. p value) | Sig. interaction effects (F value. p value) | |
|---|---|---|
| 256 Hz SAM 100 % depth processing in HP noise | ||
| A vs. Y matched wave I | Age (4411, < 0.0001) | Age*SNR (19.23, <0.0001) |
| SNR (79.85, < 0.0001) | ||
| A vs. Y matched EFR | Age (67.26, <0.0001) | Age*SNR (21.81, <0.05) |
| SNR (81.22, <0.0001) | ||
| 256 Hz SAM 50 % depth processing in HP noise | ||
| A vs. Y matched wave I | Age(24.04.< 0.05) | Age*SNR (9.31, <0.0001) |
| SNR (52.94, <0.0001) | ||
| A vs. Y matched EFR | Age (56.19 <0.0001) | Age*SNR (15.11 <0.0001) |
| SNR (67.56, <0.0001) | ||
| 256 Hz SAM 100 % depth processing in LP noise | ||
| A vs. Y matched wave I | SNR (3.56.< 0.05) | Age*SNR (9.17, <0.0001) |
| Avs. Y matched EFR | SNR (5.59, <0.0001) | Age*SNR (9.06, < 0.0001) |
| 256 Hz SAM 50 % depth proeessing.in LP noise | ||
| A vs. Y matched wave I | SNR (5..39.< 0.0001) | Age*SNR (2.26.< 0.05) |
| A vs. Y matched EFR | SNR (4.85.< 0.05) | Age*SNR (2.96, <0.05) |
Figure 4: In young animals, SNR thresholds were higher (poorer) than aged animals for SAM tones in the presence of high-pass steady-state noise.

The bar graph shows mean SNR, thresholds in aged and young animals for 256 Hz SAM tone (100 or 50 % AΜ depth) processing in high-pass noise at matched wave I or EFR, amplitudes. SNR, thresholds of young animals varied with modulation depth whereas SNR, thresholds of older animals did not. Thresholds were determined at the cut-off of 10 dB above noise floor for the 256 Hz FFT peak. Error bars are standard error of means.
In the presence of amplitude modulated high-pass noise
In order to track the ability to simultaneously represent two AMFs in the evoked potentials [Parthasarathy et al., 2016], we amplitude modulated high-pass noise at 71 Hz. All EFR,s to 256 Hz SAM were normalized to the EFR,s obtained at 60 dB SNR,. Fig. 6A and B show normalized EFR, amplitudes evoked by the 256 Hz SAM tone presented in amplitude modulated high-pass noise (NAM) at 71 Hz for 0 to 60 dB SNR,. Meanwhile, EFR,s to 71 Hz NAM were normalized to the EFR,s obtained at 0 dB SNR,. Fig. 6C and D show normalized EFR, amplitudes elicited by the 71 Hz NAM as a function of SNR, value. With increasing SNR, at values above 20 dB, EFR, amplitude to 256 Hz SAM increased while EFR, amplitudes to NAM showed a concomitant decrease in amplitude. For SNR, of 0–20 dB, EFR, amplitudes to NAM decreased but EFR, amplitudes to SAM plateaued and did not increase. In young animals, the trends of EFR, amplitudes to 256 Hz SAM in high-pass NAM were similar to the trends of EFR, amplitudes to 256 Hz SAM in high-pass steady-state noise. In aged animals, however, the “recovery” of EFR, amplitudes at 0 and 10 dB SNRs ΝAΜ was not observed as compared to steady-state noise (cf. Fig. 3 and Fig. 6). The EFR,s in time and frequency domains of individual representative young and aged animals can be found in Fig. 8.
Figure 6: EFRs to 71 Hz NAM increased concurrently with a decrease of EFRs to 256 Hz SAM, indicating dual representations of the two AM sources.

Similar trends were observed for 256 Hz SAM tone with either (A) 100 or (B) 50 % ΛΜ depth presented in high-pass NAM (71 Hz AMF, 100 % depth). The trends of EFR,s to 71 Hz (C and D) as a function of SNR, were similar in aged and young animals for both matching types. Note EFR, amplitudes to 256 Hz SAM were normalized to their respective EFR, amplitudes at 60 dB SNR, while EFR,s to 71 Hz NAM were normalized to their respective EFR,s at 0 dB SNR,. The asterisks indicate p < 0.05 for the young peripheral matching vs. the aged while the pound sign indicates p < 0.05 for the young central matching vs. the aged. Statistically significant differences were obtained using least squares means comparison from rmANOVA. Arrowheads marked the mean SNR, thresholds of EFR,s for each group. The data points and error bars are LS means +/− standard error of mean.
When EFR,s were measured for 256 Hz SAM processing in NAM, aged animals had higher EFR,s across most SNRs. In contrast, aged animals had lower 71 Hz responses at 20–60 dB SNRs. Table 2 summarizes the F and p values of main and interaction effects that were significant as shown in the residts of rmANOVA. Main effects of Age and SNR, as well as interaction effects of Age*SNR, were significant in all the comparisons. Moreover, Fig. 7 shows the mean SNR, thresholds in young (at matched wave I or EFR, amplitudes) and aged animals for EFR,s in response to 256 Hz SAM signal (100 or 50 % AM depth) in high-pass NAM. A lower SNR, threshold indicates a better sensitivity to the SAM. In contrast, a higher SNR, threshold (i.e. lower noise level) indicates a better sensitivity to the NAM. Aged animals had lower mean SNR, thresholds for EFRs to SAM signals but young animals had higher (better) mean SNR, thresholds for EFR,s to NAM. The AM depth of the 71 Hz NAM was set at 100 % for all conditions.
Table 2:
Significant main and interaction effects of multiple comparisons for EFRs in ΝΛΜ using rmANOVA are summarized in the table. F and p values for each effect are reported in parentheses.
| Sig. main effect (F value. p value) | Sig. interaction effects (F value. p value) | |
|---|---|---|
| 256 Hz SAM 100 % depth processing in HP NAM | ||
| A vs. Y matched vave I | Age (8.28,< 0.05) | Age*SNR (6.72, <0.05) |
| SNR (136.85,< 0.0001) | ||
| A vs. Y matched EFR | Age (25.03,< 0.05) | Age*SNR (15.20, <0.0001) |
| SNR (157.44,< 0.0001) | ||
| 256 Hz SAM 50 % depth processing in HP NAM | ||
| A vs. Y matched wave I | Age (6.08, < 0.05) | Age*SNR (8.27, <0.0001) |
| SNR (138.01, < 0.0001) | ||
| A vs. Y matched EFR | Age (24.17, < 0.05) | Age*SNR (17.60, <0.0001) |
| SNR (184.95, < 0.0001) | ||
| 71 Hz NAM processing in 256 Hz SAM 100 % depth | ||
| A vs. Y matched wave I | Age (26.94,< 0.05) | Age*SNR (5.65,< 0.0001) |
| SNR (38.69. 0.0001) | ||
| A vs. Y matched EFR | Age (25.88,< 0.05) | Age*SNR (3.39,< 0.05) |
| SNR (49.25, <0.0001) | ||
| 71 Hz NAM processing in 256 Hz SAM 50 % depth | ||
| Age. Y matched wave I | Age (24.34. 0.05) | Age*SNR (3.92, <0.05) |
| SNR (35.7, <0.0001) | ||
| Age. Y matched EFR | Age (30.13. 0.0001) | Age*SNR (2.96, <0.05) |
| SNR (40.55, <0.0001) | ||
Figure 7: Aged animals had lower mean SNR thresholds for EFRs to 256 Hz SAM signal (i.e. more sensitive to lower SAM sound levels) but young animals had higher mean SNR thresholds for EFRs to 71 Hz NAM (i.e. more sensitive to lower NAM sound levels).

The bar graph shows mean SNR, thresholds for EFR,s to SAM signal (left) or NAM (right) in all the tested groups presented with 256 Hz SAM tone (100 or 50 % AΜ depth) in high-pass ΝAΜ. Thresholds were determined at the cut-off of 10 dB above noise floor for the 71 and 256 Hz FFT peak. Error bars are standard error of means.
Discussion
Age-related changes in temporal processing
Speech recognition in humans degrades progressively with age [Jerger, 1973]. Furthermore, speech intelligibility performance of elderly males declines significantly with age even after adjusting for average hearing thresholds [Dubno et al., 1997]. In fact, simply controlling differences in hearing threshold may not control differences in supra-threshold temporal processing [Gifford et al., 2007]. In our previous study, we investigated temporal processing of young and aged animals in a quiet condition at equivalent peripheral or central neural activation [Lai et al., 2017]. We observed larger EFR, amplitudes in older animals for matched activation at 100 % AΜ modulation depth. Recently, a study revealed more degradations in frequency-following responses to a speech syllable in noise and larger reductions in cortical responses to speech envelope in competing sounds in clinically normal-hearing older adults [Presacco et al., 2016b]. Temporal processing deficits have also been associated with reduced identification of words and sentences presented in steady-state and modulated noise [Cordonsalant and Fitzgibbons, 1993, helfer and Vargo, 2009]. In these studies, the masking noise was either wideband or overlapping spectrally with the target stimulus. To further our investigations on temporal processing in aging, we compared EFR,s to SAM tones in a wider range of conditions, i.e. addition of various types of background noise, at equivalent peripheral or central neural activation in this study.
The idea of matching peripheral activation is different from an equal sensation level (SL) paradigm. It aims to achieve an equivalent amount of neural synchronization and activation in the auditory nerve (AΝ) response, which will ideally illustrate changes in central activation, such as ABR, and EFR, responses, to equivalent AΝ activation. Matching central activation of the EFR, or FFR,, on the other hand, suggests that the collective synchronization of the subcortical auditory system is similar across conditions. Then, it becomes possible to determine if there are differential changes when the stimulus changes, such as with the addition of noise. Both peripheral and central auditory neural responses (up to the auditory midbrain) are taken into consideration when matching central activation. In an equal SL paradigm, SL above hearing threshold would be matched in each subject, but, depending on the growth of amplitude with SL, evoked amplitudes could be very different even at same SL, as seen in some aging studies [Shaheen et al., 2015]. Since the hearing threshold was measured as the lowest sound level in which a sound can be detected in ABR, measurements, it involves neural responses of both the peripheral and central auditory systems. If we tried to match SL among young and aged animals in this study, the sound levels used for young animals would be approximately 68.5 dB SPL (85 dB SPL was used in aged animals and this corresponded to a SL of 45.5 dB). In our study, the sound level difference for matching peripheral versus central neural activation was approximately 3–4 dB. Despite the modest difference in this case, we feel that it is an important distinction for continued work in aging, noise-induced hearing loss and pathology (e.g. blast, tinnitus) and other instances where synaptopathy may be present.
Temporal processing in steady-state noise
Exploring evoked potentials in response to simple and complex sounds in noise might unveil age effects on speech-in-noise perception and potential mechanisms involved [Henry and Lucas, 2010a, Henry and Lucas, 2010b, Parthasarathy et al., 2010, Mehraei et al., 2016]. We recorded EFRs elicited by SAM stimuli presented in a background of off-frequency notched noise (Fig. 1). Notched noises with notches centered around the 8 kHz tone were applied at 40 dB SNR, to generate a more challenging listening condition [Bharadwaj et al., 2015, Mehraei et al., 2016]. Regardless of notch width within the range tested, we observed young animals having lower EFRs in notched noise than their EFRs in quiet, whereas aged EFRs were largely unaffected by what in some eases was subtrheshold noise for the aged animals (Fig. 1). Surprisingly, we did not observe any effect of notch width on the EFR,s that were recorded in notched noise from young animals. In our study, we consistently observed lower EFR,s in young animals in a background of notched noise (Fig. 1) or high-pass steady-state noise (Fig. 3) at a SNR, value of 30–40 dB. Presaoeo et al. (2016) also reported significant reductions in midbrain responses at noisy conditions in their younger subjects. This is in contrast to cortical studies that show increased responses in older listeners, which may be due to non-selectivc or poorly controlled excitation [Herrmann et al., 2016, Presacco et al., 2016b, Presacco et al., 2016a]. Our past study using SAM tones (256 Hz) in wideband noise showed significantly lower normalized EFR,s in young than aged animals at SNR,s of 10–20 dB [Parthasarathy et al., 2010, Parthasarathy et al., 2016).An important difference between that study and the present study is that the presence of a second narrowband carrier did not abolish the EFR, response to the other tone carrier, whereas the broadband noise carrier, even if it did not overlap spectrally, abolished the SAM EFR, responses at low SNR,s (Fig. 6) [Parthasarathy et al., 2016].
Although outer hair cell dysfunction may be a contributing factor to observations in the current study, it is unlikely to be the primary factor in determining EFR, amplitudes at most of the sound levels used. Studies of noise-induced sensorineural hearing loss have shown that outer hair cell dysfunction can lead to enhanced neural coding of AM in auditory evoked potentials [Zhong et al., 2014] and increased ANF phase-locking to AM [Kale and Heinz, 2010]. In addition, noise exposure shifted basilar membrane input-output (I/O) functions from compressive to linear growth for mid-level tones [Zhong et al., 2014]. In our study, the SAM signal was set at 85 dB SPL in the aged and 65–70 dB SPL in the young. According to our previous otoacoustic emission data in aging (Fig. 2A in Lai and Bartlett(2015)), these sound levels are within the compressive regions of the I/O functions of young and aged animals. Furthermore, since we performed ABR, wave I amplitude matching to produce comparable ANF activation, our observations of higher EFR,s in the aged in noisy conditions may reflect a stronger central contribution than the phenomena described in noise-induced sensorineural hearing loss. At the highest noise levels in aged animals, it is possible that we are additionally exciting the high-threshold portions of auditory nerve fibers that will contribute to the larger EFR, amplitudes at low SNR-
Past studies of auditory evoked potentials and psychoacoustics reported reduced periodicity coding in aging and better sound perception in noise in younger individuals [He et al., 2007, He et al., 2008, Grose and Mamo, 2012, Parthasarathy and Bartlett, 2012, Ben-David et al., 2012]. However, the protocols of these studies did not completely account for age-related changes in peripheral ΛΝ activities or central gain. Physiological thresholds to steady-state noise or ΝAΜ were more sensitive in younger animals by 25–30 dB, probably due to their lower hearing thresholds. One possible implication from the current study and our prior study [Parthasarathy et al., 2016] is that EFR, amplitude reduction in the young does not necessarily imply that they are worse at detecting temporal modulations in noise. Rather, it suggests that a smaller population of neurons are specifically representing the AΜ sound, whereas a substantial number of neurons are responding in a non-phase-locked manner to the unmodulated noise (Fig. 3). Responses to SAM tones in modulated noise (Fig. 6) further support that different AΜ sources were phase-locked by different groups of neurons, which in turn would aid in extraction of the SAM probe from noise or segregation of multiple AM sources, even when sound levels are disparate. In support of this idea, younger animals were also able to encode periodicity of modulated noise at lower noise intensities (or higher SNR,s, Figs. 6 and 7). These results suggest that reductions in EFR, amplitudes or spectral components should be interpreted carefully, especially for sounds presented in noise or with complex spectra. Furthermore, Fig. 3 and 5 support the idea that the lower frequency tails of neurons tuned to higher frequencies drive the EFR, in quiet conditions but become driven by the noise, leading to a desynchronization of those neurons to the SAM tone and a reduction in the EFR,.
Temporal processing in steady-state versus amplitude modulated noise
Speech recognition in the presence of a steady-state masker is very similar for young and older listeners with similar audibility [Dubno et al., 2002, Taka-hashi and Bacon, 1992, Bacon et al., 1998]. However, when comparing speech recognition in modulated versus steady-state noise maskers, young normal-hearing listeners generally have better performance in modulated noise maskers [Dubno et al., 2002, Dubno et al., 2003, Takahashi and Bacon, 1992, Shen et al., 2015, Gifford et al., 2007, Vermeire et al., 2016, Festen, 1990, Gustafsson and Arlinger, 1994]. Although older listeners can also benefit from temporal interruptions in modulated noise to improve speech recognition, the magnitude of the fluctuating masker benefit (FMB) is less than that observed in younger subjects [Takahashi and Bacon, 1992, Dubno et al., 1997]. One hypothesis is that normal-hearing listeners with good temporal acuity can effectively perform “dip-listening” during the momentary higher signal-to-noisc ratio at the valleys of the fluctuating envelope of modulated maskers [Gifford et al., 2007].
To assess the electrophysiological equivalent of listening in the dips, or the FMB, we measured SNR, thresholds for EFRs evoked by SAM signal in high-pass NAM versus steady-state noise. We found that SNR, thresholds obtained in a background of NAM were found to be lower than those in steady-state noise in the aged and the young at matched ABR, wave I amplitude (compare Figs. 2 and 4). The magnitudes of modulation masking release in terms of SNR, threshold were about 6.5–10 dB in aged animals and about 13 dB in young animals (matched ABR, wave I amplitude) for both 100 and 50 % depth processing. For matched EFR, amplitude in young animals, the FMBs of SNR, thresholds were only a few decibels. This could be because higher sound levels were used for EFR, amplitude matching, and modulation masking release is more apparent when temporal cues are less salient (i.e. at lower sound levels).
Our results for EFR,s recorded in modulated or unmodulated noise are consistent with the previous findings that changes in EFR,s are associated with changes in stimulus SNR, [Billings et al., 2009, Hiraumi et al., 2008). Λ significant main effect of SNR, was obtained from rmANOVAs in EFR,s recorded in steady-state noise or ΝAΜ (Figs. 1 and 2). The interaction effect of Age*SNR, was also significant in almost all noisy conditions. Older animals generally had lower SAM sound level thresholds (more resistant to addition of NAM, Figs. 6 and 7) than the young animals. In contrast, younger animals were able to represent the NAM periodicity at significantly higher SNR, (lower relative noise levels, Fig. 7). Although EFR, amplitudes to SAM decreased in young animals even at 30–50 dB SNR, (Fig. 3), they were still above the noise floor and detection thresholds. EFR, amplitudes reflect temporal fidelity of a population of neurons, so EFR, amplitude reduction indicates dispersion in population neural envelope coding when there are multiple AM sources [Parthasarathy et al., 2016). The young had a mean click ABR, threshold of ~ 28 dB SPL. With peripheral or central matching, NAM sound levels at their effective SNR thresholds were about 25 dB SPL (~ 40–45 dB SNR), which was very close to their hearing thresholds. The young SNR, thresholds for NAM were in the range of 40–45 dB, this indicates that they were able to phase-lock to 71 Hz ΝAΜ once noise levels reached their hearing thresholds. Comparison of hearing thresholds to ΝAΜ phase-locking thresholds using a ranksum test showed no statistically significant difference in the young, but the ΝΛΜ phase-locking thresholds were significantly higher in the aged for 100 % SAM processing in NAM. All these also suggest that young animals could segregate multiple AM sources starting at 40–45 dB SNR, down to approximately 20 dB SNR,. It was surprising that the dual representation was lost for SNR, 20 dB. This may be due to the combined factors of saturating high spontaneous rate, high CF AN fibers and the large contribution of the tails of high CF AN fibers to EFR, responses [Parthasarathy et al., 2016]. For aged animals, since their mean click ABR threshold was ~ 50 dB SPL, they should response to noise carriers or transients starting from sound levels near 50 dB SPL or SNR, values near 35 dB. However, based on Table 6, they were not phase-locking to 71 Hz NAM until noise levels increased to over 60 dB SPL. These findings imply that the aged neural populations could detect the presence of NAM at 30 dB SNR, but were unable to synchronize simultaneously to the two AM sources.
Consideration of potential neural mechanisms
Recording studies have suggested that dip listening, stream segregation and neural suppression (or inhibition) are three of the multiple possible neurophysiological mechanisms that correlate with behavioral detection of SAM signals in steady-state or amplitude modulated noise [Bohlen et al., 2014, Coense and Feng, 2()12]. Precise neurophysiological representations of pitch and timing are important for speech-in-noise listening [Anderson et al., 2011]. Inhibition has been suggested to contribute to signal detection for a modulated masker in the dorsal cochlear nuclei (DCN) [Neuert, 2004], and decreased GABAergic inhibition in the IC and DCN of rats has been reported to contribute to age-related deficits in subcortical encoding of pitch and timing [Caspary et al., 1995, Caspary et al., 2005, R,abang et al., 2012]. In this study, we observed a decrease in the young EFRs by noise at lower noise intensities. The presence of a noise (steady-state noise or NAM) masker reduces neurons’ spike rate and disrupts temporal discharge pattern to the target SAM signal via suppression or inhibition [Cocnsc and Feng, 2012, Neuert, 2004]. Although suppression or inhibition decreases neural responses to SAM sounds in the presence of a noise masker, the cxcitatory/inhibitory balance has been proposed to serve in identifying signal periodicity [Biirek and van Hemmen, 2009], sharpen neural spike timing, enhance temporal precision and decrease random background neural activities [Ncucrt, 2004, Wehr and Zador, 2003]. As a whole, we believe that age-related changes of the cxcitatory/inhibitory balance in the central auditory system, whether driven by synaptic changes, changes in membrane properties or other factors, contributes to nonspecific and higher EFRs at high noise levels in the aged animals.
Nelken et al. (1999) showed that a time varying masker causes synchronized responses of a population of neurons while simultaneous presentation of a SAM signal causes the neurons tuned to the signal carrier to switch synchronization to the modulation frequency of the signal. This phenomenon is indeed similar to what we observed for temporal processing in a background of NAM. As high-pass NAM level increased (Fig. 6), we speculate that high-frequency neurons synchronized to ΝAΜ and only a specific group of neurons that were closer to 8 kHz faithfully synchronized to the target SAM. Fig. 9 shows a schematic of proposed mechanisms in the peripheral and central auditory systems, respectively, in response to SAM signals presented in steady-state noise or NAM. In the peripheral auditory system, young animals have lower thresholds as well as sharper and narrower tuning curves which cause them to have higher sensitivity to noise at low intensities. Therefore, young animals were sensitive to the presence of noise even at 40–50 dB SNR. In contrast, broadening of auditory neurons’ tuning curves and an upward shift of thresholds render aged animals to be unaffected by low-level noise. This could be one of the reasons that noise reduced EFR,s only at moderate levels (20–30 dB SNR,) in aged animals. These effects were also represented in the central auditory system, where there were responsive neurons tuned to the 8 kHz carrier as well as neurons tuned to other CFs but phase-locked to the AMF (256 Hz) of the SAM signal in quiet or at low-level noise. As noise intensity increased, more neurons, especially high-frequency neurons, were engaged by high-pass noise and desynchronized from 256 Hz, regardless of age group, but it is not clear in which regions this will occur most. Potentially owing to decreased inhibition in aged animals, high-frequency neurons may have nonspecifically phase-locked to SAM at higher levels of steady-state noise (Fig. 3A and B, 0–10 dB SNR,). This similar phenomenon was also observed in aged animals in high-level low-pass noise. In contrast, we did not observe these nonspecific responses in young animals potentially because they have an intact cxcitatory/inhibitory balance. When the SAM signal was presented with a high-pass NAM masker, high-frequency neurons synchronized to the AMF (71 Hz) of NAM. The EFRs to ΝAΜ were higher in the young than the aged because young animals have stronger neural synchronous activities, and matching was performed for the SAM signal but not for the NAM, such that the strong threshold shifts at higher frequencies were not accounted for in the aged animals. Moreover, coupling of reduced EFR,s to SAM signal with increased EFR,s to NAM in young animals at higher SNR,s (Fig. 6) indicates that they were able to segregate different AM sources at ~ 40 dB SNR, where noisc levels were at the young hearing thresholds. In contrast, aged animals were only able to segregate NAM from SAM at noise levels that were higher than their hearing threshold (based on click ABR, thresholds). The trend of SAM-evoked EFR,s as a function of SNR, was similar in both NAM and steady-state noise for young and aged animals except that nonspecific SAM-evoked EFR,s at 0–10 dB SNR,s were not observed in NAM in aged animals (cf. Figs. 3 and 6). This could be due to high-frequency neurons phase-locked to NAM and did not incur nonspecific rate increases upon which the SAM signal could produce small synchronized modulations.
Figure 9: Young animals are more sensitive to noise but this permits them to have dual representations of AM sources even at lower noise levels.

Proposed mechanisms for (A) peripheral and (B) central auditory systems for EFRs elicited in a background of steady-state noise or NAM in young and aged animals. In the peripheral auditory system, young animals have lower thresholds as well as sharper and narrower tuning curves which cause them to have higher sensitivity to noise at low intensities. In the central auditory system, where there were responsive neurons tuned to the 8 kHz carrier as well as neurons tuned to other frequencies but phase-locked to 256 Hz AMF of the SAM signal in quiet. As noise intensity increased, more neurons, especially high-frequency neurons, were engaged to high-pass noise or ΝAΜ and desynchronized from 256 Hz.
Conclusions
Introduction of noise background substantially affects temporal representations and increases age-related differences, even after compensating between the age groups in quiet. For SAM depth processing in notched noise, young animals generally had lower EFRs at larger ΛΜ depths at both equivalent peripheral and eentral activation. High-pass noise decreased EFRs of young animals at higher SNR,s (lower noise levels) compared to aged animals while low-pass noise generally did not decrease EFR,s of either age group. Nonspecific responses were observed in aged animals at high steady-state noise intensities possibly as a result of age-related reduction in neural inhibitory function. EFRs to ΝAΜ showed that young animals had lower noise level thresholds than aged animals at both the matching conditions. Taken together, temporal processing in high-pass steady-state noise or ΝAΜ reveals that young animals were sensitive to noise at lower noise intensities and were able to synchronize to low-level ΝAΜ in the presence of the SAM signal. There are three different regimes in this study. In the tone-dominated, high SNR, regime, the noise is subthreshold in the young and subthreshold or at threshold in the aged. The effects of noise on the tone carrier EFR, are minimal. In the dual-representation, medium SNR, regime, corresponding to about 20–40 dB SNR, in the young and 10–25 dB SNR, in the aged, neurons are responsive and synchronized to both the tone and noise carrier AM. Finally, in the noise-dominated, low SNR, regime, responses to the noise carrier largely abolish the tone carrier EFR,, even though this occurs at positive SNR,s where the tone is louder than the noise (Fig. 6). In the current study, there was only a 3–4 dB difference between sound levels used for peripheral versus eentral activation, so the interpretation will focus primarily on matched peripheral activation. There may, however, be cases where this divergence is more pronounced. For matched peripheral activation, measured as similar wave I ABR, amplitudes, the idea is that the transiently driven population rates and envelope synchronies of all contributing AΝ neurons are similar. The overall similarity in sensitivity to masking for matched central activation suggests that for aging, masking effects for sustained midbrain responses are also similar. For the tone-dominated regime, all participating neurons measured by the EFR, are responding only to the tone AΜ. High spontaneous rate AΝ libers near the tone carrier frequency will have saturated rates and low vector strengths. Low spontaneous rate neurons near the tone carrier frequency will have high rates and higher vector strengths. However, it is likely that these units form only a small component of the EFR, response [Parthasarathy et al., 2016]. In dual-representation and noise-dominated regimes, the responses of neurons with CFs above the tone carrier shift towards the noise response, reducing the tone ΛΜ EFR, and increasing the noise AΜ EFR,. It is likely that the extended dual-representation range in younger animals represents a higher proportion of low-spontaneous rate fibers [Bharadwaj et al., 2014] and lower thresholds of the low-frequency tails of high CF neurons. The noise-dominated regime at both ages results from the dominance of high CF neurons in generating the EFR, response, such that switching to these neurons to responding to the noise carrier removes them from pool of neurons responding to the tone carrier and diminishes the tone AΜ EFR,.
Highlights.
Noise reduces EFRs to SAM tones of the young more than the aged at matched neural activation.
High-pass noise reduces EFRs of young animals at lower noise intensities than in the aged.
The results are consistent with EFRs generated by activities of high-frequency neurons.
Young subjects can maintain dual AM representations even at lower stimulus levels.
EFR amplitude alone may not imply better neural representations for complex sounds.
Acknowledgements
This study was supported by a grant from the National Institutes of Health [NIDCD R01DC011580]. The authors would like to thank Alexandra L.
Footnotes
Disclosure Statement
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [Aiken and Picton, 2008] Aiken SJ and Picton TW (2008). Envelope and spectral frequency-following responses to vowel sounds. Hearing Research, 245(1–2):35–47. [DOI] [PubMed] [Google Scholar]
- [Anderson et al. , 2012] Anderson S, Parbery-Clark A, White-Sehwoeh T, and Kraus N (2012). Aging affects neural precision of speech encoding. J Neurosci, 32:14156–14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Anderson et al. , 2011] Anderson S, Parbery-Clark A, Yi HC, and Kraus N (2011). A neural basis of spccch-in-noisc perception in older adults. Ear and Hearing, 32(6):750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Bacon et al. , 1998] Bacon S, Opie J, and Montoya D (1998). The effects of hearing loss and noise masking on the masking release for speech in temporally complex backgrounds. Journal ofSpeech Language and Hearing Research, 41(3):549–63. [DOI] [PubMed] [Google Scholar]
- [Ben-David et al. , 2012] Ben-David BM, Tse VYY, and Schneider BA (2012). Does it take older adults longer than younger adults to perceptually segregate a speech target from a background masker? Hearing Research, 290(1–2):55–63. [DOI] [PubMed] [Google Scholar]
- [Bharadwaj et al. , 2015] Bharadwaj HM, Masud S, Meliraei G, Verhulst S, and Shinn-Cunningham BG (2015). Individual differences reveal correlates of hidden hearing deficits. Journal of’ Neuroscience, 35(5):2161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Bharadwaj et al. , 2014] Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, and Shinn-Cunningham BC (2014). Cochlear neuropathy and the coding of supra-thrcshold sound. Frontiers in systems neuroscience, 8:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Bidelman, 2016] Bidelman CM (2016). Relative contribution of envelope and line structure to the subcortical encoding of noise-degraded speech. The Journal of the Acoustical Society of America, 140(4):EL358–EL363. [DOI] [PubMed] [Google Scholar]
- [Billings et al. , 2009] Billings CJ, Tremblay KL, Stecker GC, and Tolin WM (2009). Human evoked cortical activity to signal-to-noise ratio and absolute signal level. Hearing Research, 254(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Boettcher et al. , 2001] Boettcher FA, Poth EA, Mills JH, and Dubno JR (2001). The amplitude-modulation following response in young and aged human subjects. Hearing Research, 153(1–2):32–42. [DOI] [PubMed] [Google Scholar]
- [Bohlen et al. , 2014] Bohlen P, Dylla M, Timms C, and Ramachandran R,. (2014). Detection of Modulated Tones in Modulated Noise by Nonhuman Primates. Journal of’ the Association for Research in Otolaryngology, 15(5):801–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Burek and van Hemmen, 2009] Biirck M and van Hemmen JL (2009). Neuronal identification of signal periodicity by balanced inhibition. Biological Cybernetics, 100(4):261–70. [DOI] [PubMed] [Google Scholar]
- [Caspary et al. , 1995] Caspary DM, Milbrandt JC, and Helfert RH (1995). Central auditory aging - CABA changes in the inferior colliculus. Experimental Gerontology, 30(3–4):349–60. [DOI] [PubMed] [Google Scholar]
- [Caspary et al. , 2005] Caspary DM, Sehatteman TA, and Hughes LF(2005). Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: Role of inhibitory inputs. Journal of Neuroscience, 25(47):10952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Chandrasekaran and Kraus, 2010] Chandrasekaran B and Kraus N (2010). The scalp-recorded brainstem response to speech: neural origins and plasticity. Psychophysiology, 47(2):236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Chen and Chen, 1991] Chen T and Chen S (1991). Generator study of brainstem auditory evoked potentials by a radiofrequency lesion method in rats. Experimental brain research, 85(3):537–42. [DOI] [PubMed] [Google Scholar]
- [Christiansen et al. , 2013] Christiansen C, MacDonald EN, and Dau T (2013). Contribution of envelope periodicity to release from speech-on-speech masking. The Journal of the Acoustical Society of America, 134(3):2197–204. [DOI] [PubMed] [Google Scholar]
- [Clinard et al. , 2010] Clinard CC, Tremblay KL, and Krishnan AR,. (2010). Aging alters the perception and physiological representation of frequency: Evidence from human frequency-following response recordings. Hearing Research, 264(1–2):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Cunningham et al. , 2001] Cunningham J, Nicol T, Zecker SC, Brad-low A, and Kraus N (2001). Neurobiologic responses to speech in noise in children with learning problems: deficits and strategies for improvement. Clin Neurophysiol, 112(5):758–67. [DOI] [PubMed] [Google Scholar]
- [Dolphin and Mountain, 1993] Dolphin WF and Mountain DC (1993). The envelope following response (EFR) in the Mongolian gerbil to sinusoidally amplitude-modulated signals in the presence of simultaneously gated pure-tones. The Journal of the Acoustical Society of America, 94(6):3215–30. [DOI] [PubMed] [Google Scholar]
- [Dubno et al. , 1997] Dubno J, Lee F, Matthews L, and Mills J (1997). Age-related and gender-related changes in monaural speech recognition. Hearing Research, 40(2):444 52. [DOI] [PubMed] [Google Scholar]
- [Dubno, 1984] Dubno JR,. (1984). Effects of age and mild hearing loss on speech recognition in noise. The Journal oJ’ the Acoustical Society oj America, 76(1):87 96. [DOI] [PubMed] [Google Scholar]
- [Dubno et al. , 2002] Dubno JR, Horwitz AR, and Ahlstrom JB(2002) Benefit of modulated maskers for speech recognition by younger and older adults with normal hearing. The Journal of the Acoustical Society of jAmerica, 111(6):2897– 907. [DOI] [PubMed] [Google Scholar]
- [Dubno et al. , 2003] Dubno JR, Horwitz ΛR, and Ahlstrom JB(2003) Recovery from prior stimulation: Masking of speech by interrupted noise for younger and older adults with normal hearing. The Journal of the Acoustical Society ofAmerica, 113(4):2084 94. [DOI] [PubMed] [Google Scholar]
- [Duquesnoy, 1983] Duquesnoy ΛJ (1983). The intelligibility ofscntcnccs in quiet and in noise in aged listeners. The Journal oJ’ the Acoustical Society ofAmerica, 74(4):1136 44. [DOI] [PubMed] [Google Scholar]
- [Duquesnoy and Plomp, 1980] Duquesnoy AJ and Plomp R (1980). Effect of reverberation and noise on the intelligibility of sentences in eases of presbyacusis. The Journal of the Acoustical Society ofAmerica, 68(2):537 44. [DOI] [PubMed] [Google Scholar]
- [Festen, 1990] Festen JM (1990). Effects of fluctuating noise and interfering speech on the speech-reception threshold for impaired and normal hearing. The Journal of the Acoustical Society of America, 88(4):1725 36. [DOI] [PubMed] [Google Scholar]
- [Frisina and Frisina, 1997] Frisina DR and Frisina RD (1997). Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hearing Research, 106(1–2):95 104. [DOI] [PubMed] [Google Scholar]
- [Gifford et al. , 2007] Gifford R,H, Bacon SP, and Williams EJ (2007). An Examination of Speech Recognition in a Modulated Background and of Forward Masking in Younger and Older Listeners. Journal of Speech Language and Hearing Research, 50(4):857 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Goense and Feng, 2012] Goense JBM and Feng AS (2012). Effects of Noise Bandwidth and Amplitude Modulation on Masking in Frog Auditory Midbrain Neurons. PLoS ONE, 7(2):e31589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Cordonsalant and Fitzgibbons, 1993] Cordonsalant S and Fitzgibbons PJ (1993). Temporal factors and speech recognition performance in young and elderly listeners. Journal of Speech and Hearing Research, 36(6):1276 85. [DOI] [PubMed] [Google Scholar]
- [Grose and Manio, 2010] Grose JH and Manio SK (2010). Processing of Temporal Fine Structure as a Function of Age. Ear and Hearing, 31(6):755 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Grose and Mamo, 2012] Grose JH and Mamo SK (2012). Frequency modulation detection as a measure of temporal processing: Age-related monaural and binaural effects. Hearing Research, 294(l-2):49 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Gustafsson and Arlinger, 1994] Gustafsson HA and Arlinger SD (1994). Masking of speech by amplitude-modulated noise. The Journal of the Acoustical Society of America, 95(1):518 29. [DOI] [PubMed] [Google Scholar]
- [He et al. , 2008] He NJ, Mills JH, Ahlstrom JB, and Dubno JR (2008). Age-related differences in the temporal modulation transfer function with pure-tone carriers. The Journal of the Acoustical Society of America, 124(6):3841 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [He et al. , 2007] He N.-j., Mills JH, and Dubno JR (2007). Frequency modulation detection: Effects of age, psychophysical method, and modulation waveform. The Journal of the Acoustical Society of America, 122(1):467 77. [DOI] [PubMed] [Google Scholar]
- [Helfer and Freyman, 2008] Helfer K and Freyman R (2008). Aging and speech-on-speech masking. Ear and Hearing, 29(1):87 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Hclfe and Vargo, 2009] Helfer KS and Vargo Μ (2009). Speech Recognition and Temporal Processing in Middle-Aged Women. Journal of the American Academy of Audiology, 20(4):264 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Henry et al. , 2017] Henry KS, Abrams KS, Forst J, Mender MJ, Neilans EG, Idrobo F, and Carney LH (2017). Midbrain Synchrony to Envelope Structure Supports Behavioral Sensitivity to Single-Formant Vowel-Like Sounds in Noise. Journal of the Association for Research in Otolaryngology, 18(1):165 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Henry and Lucas, 2010a] Henry KS and Lucas JR,. (2010a). Auditory sensitivity and the frequency selectivity of auditory filters in the Carolina chickadee, Poecile carolinensis. Animal Behaviour, 80(3):497 507. [Google Scholar]
- [Henry and Lucas, 2010b] Henry KS and Lucas, JR,. (2010b). Habitat-related differences in the frequency selectivity of auditory filters in songbirds. Functional Ecology, 24(3):614 24. [Google Scholar]
- [Herdman et al. , 2002] Herdman AT, Lins 0, Van Roon P, Stapells DR, Seherg M, and Picton TW (2002). Intracerebral sources of human auditory steady-state responses. Brain topography, 15(2):69 86. [DOI] [PubMed] [Google Scholar]
- [Herrmann et al. , 2016] Herrmann B, Henry MJ, Johnsrude IS, and Obleser J (2016). Altered temporal dynamics of neural adaptation in the aging human auditory cortex. Neurobiology of Aging, 45:10 22. [DOI] [PubMed] [Google Scholar]
- [Hiraumi et al. , 2008] Hiraumi H, Nagamine T, Morita T, Naito Y, Fukuyama H, and Ito J (2008). Effect of amplitude modulation of background noise on auditory-cvokcd magnetic fields. Brain Research, 1239:191 7. [DOI] [PubMed] [Google Scholar]
- [Itatani and Klump, 2009] Itatani N and Klump GM (2009). Auditory Streaming of Amplitude-Modulated Sounds in the Songbird Forebrain. Journal of Neurophysiology, 101(6):3212 3225. [DOI] [PubMed] [Google Scholar]
- [Jerger, 1973] Jerger J (1973). Audiological findings in aging. Advances in oto-rhino-laryngology, 20:115 24. [PubMed] [Google Scholar]
- [Kale and Heinz, 2010] Kale S and Heinz MG (2010). Envelope coding in auditory nerve fibers following noise-induced hearing loss. Journal of the Association for Research in Otolaryngology, 11 (4):657 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [kujawa and Liberman, 2009] Kujawa SG and Liberman MC (2009). Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. Journal of Neuroscience, 29(45):14077 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Kuwada et al. , 2002] Kuwada S, Anderson JS, Batra R, Fitzpatrick DC, Teissier N, and D’Angelo WR (2002). Sources of the scalp-recorded amplitude-modulation following response. Journal of the American Academy of Audiology, 13(4):188 204. [PubMed] [Google Scholar]
- [Lai et al. , 2017] Lai J, Sommer AL, and Bartlett EL (2017). Age-related changes in envelope-following responses at equalized peripheral or central activation. Neurobiology of aging, page [Eprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Lin et al. , 2011] Lin HW, Furman AC, Kujawa SC, and Liberman MC (2011). Primary Neural Degeneration in the Guinea Pig Cochlea After Reversible Noise-Induced Threshold Shift. Journal ofthe Association for Research in Otolaryngology, 12(5):605 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Mehraei et al. , 2016] Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, and Shinn-Cunningham BG (2016). Auditory Brainstem Response Latency in Noise as a Marker of CochlcarSynaptopathy. Journal ofNeuroscience, 36(13):3755 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Nablek and Robinson, 1982] Nablek AK and Robinson PK (1982). Monaural and binaural speech perception in reverberation for listeners of various ages. The Journal ofthe Acoustical Society of America, 71(5):1242 8. [DOI] [PubMed] [Google Scholar]
- [Nablet, 2004] Neuert V (2004). Responses of Dorsal Cochlear Nucleus Neurons to Signals in the Presence of Modulated Maskers. Journal of Neuroscience, 24(25):5789 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Parthasarathy and Bartlett, 2012] Parthasarathy A and Bartlett E (2012). Two-channel recording of auditory-evoked potentials to detect age-related deficits in temporal processing. Hearing Research, 289(l-2):52 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Parthasarathy and Bartlett, 2011] Parthasarathy A and Bartlett EL (2011). Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience, 192:619– 30. [DOI] [PubMed] [Google Scholar]
- [Parthasarathy et al. , 2010] Parthasarathy A, Cunningham PA, and Bartlett EL (2010). Age-related differences in auditory processing as assessed by amplitude-modulation following responses in quiet and in noise. Frontiers in Aging Neuroscience, 2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Parthasarathy et al. , 2014] Parthasarathy Λ, Datta J, Torres JΛ, Hopkins C, and Bartlett EL (2014). Age-related changes in the relationship between auditory brainstem responses and envelope-following responses. Journal of the Association for Research in Otolaryngology, 15(4):649 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Parthasarathy et al. , 2016] Parthasarathy A, Lai J, and Bartlett EL (2016). Age-Related Changes in Processing Simultaneous Amplitude Modulated Sounds Assessed Using Envelope Following R,esponses. Journal of the Association for Research in Otolaryngology, 17(2):119 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Picton et al. , 2003] Picton TW, John MS, Dimitrijevie A, and Purcell D (2003). Human auditory steady-state responses. International journal of audiology, 42(4):177 −219. [DOI] [PubMed] [Google Scholar]
- [Presacco et al. , 2016a] Presacco A, Simon JZ, and Anderson S (2016a). Effect of informational content of noise on speech representation in the aging midbrain and cortex. Journal of Neurophysiology, 116(5):2356 −2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Presacco et al. , 2016b] Presacco A, Simon JZ, and Anderson S (2016b). Evidenee of degraded representation of speech in noise, in the aging midbrain and eortex. Journal of Neurophysiology, 116(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Purcell et al. , 2004] Purcell DW, John SM, Sehneider BA, and Picton TW (2004). Human temporal auditory acuity as assessed by envelope following responses. The Journal of the Acoustical Society of America, 116(6):3581 93. [DOI] [PubMed] [Google Scholar]
- [Rabang et al. , 2012] Rabang, Parthasarathy A, Venkataraman Y, Fisher ZL, Gardner SM, and Bartlett EL (2012). A Computational Model of Inferior Colliculus Responses to Amplitude Modulated Sounds in Young and Aged R,ats. Frontiers in Neural Circuits, 6(77):1– 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Rowe, 1981] Rowe MJ (1981). The brainstem auditory evoked response in neurological disease: a review. Ear and Hearing, 2(1):41– 51. [DOI] [PubMed] [Google Scholar]
- [Ruggles et al. , 2012] Ruggles D, Bharadwaj H, and Shinn-Cunningliam BG (2012). Why Middle-Aged Listeners Have Trouble Hearing in Everyday Settings. Current Biology, 22(15):1417 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Sergeyenko et al. , 2013] Sergeyenko Y, Lall K, Liberman MC, and Kujawa SC (2013). Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. Journal of Neuroscience, 33(34):13686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Palombi Shaddock et al. , 2001] Shaddock Palombi P, Backoff P, and Caspary D (2001). Responses of young and aged rat inferior colliculus neurons to sinusoidally amplitude modulated stimuli. Hearing Research2, 153(1– 2):174–80. [DOI] [PubMed] [Google Scholar]
- [Shaheen et al. , 2015] Shaheen LA, Valero MD, and Liberman MC (2015). Towards a Diagnosis of Cochlear Neuropathy with Envelope Following Responses. Journal of the Association for Research in Otolaryngology, 16(6):727–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Shannon et al. , 1995] Shannon R,V, Zeng FC, Kamath V, Wygonski J, and Ekelid M (1995). Speech recognition with primarily temporal cues. Science, 270(5234):303–4. [DOI] [PubMed] [Google Scholar]
- [Shen et al. , 2015] Shen Y, Manzano NK, and Richards VM (2015). Psychometric functions for sentence recognition in sinusoidally amplitude-modulated noises. The Journal of the Acoustical Society of America, 138(6):3613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Shinn-Cunningham et al. , 2013] Shinn-Cunningham B, Ruggles DR, and Bharadwaj H (2013). How Early Aging and Environment Interact in Everyday Listening: From Brainstem to Behavior Through Modeling. In Advances in experimental medicine and biology, volume 787, pages 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Snell and Frisina, 2000] Snell KB and Frisina DR,. (2000). Relationships among age-related differences in gap detection and word recognition. The Journal of the Acoustical Society of America, 107(3):1615–26. [DOI] [PubMed] [Google Scholar]
- [Soros et al. , 2009] Soros P, Teismann IK, Manemann E, and Lutken-honer B (2009). Auditory temporal processing in healthy aging: a magnetoencephalographic study. BMC Neuroscience, 10(34):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Srinivasan and Zahorik, 2014] Srinivasan NK and Zahorik P (2014). Enhancement of speech intelligibility in reverberant rooms: Role of amplitude envelope and temporal fine structure. The Journal of the Acoustical Society of America, 135(6):EL239–EL245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Strelcyk and Dau, 2009] Strelcyk O and Dau T (2009). Relations between frequency selectivity, temporal fine-structure processing, and speech reception in impaired hearing. The Journal of the Acoustical Society oj America, 125(5):3328 45. [DOI] [PubMed] [Google Scholar]
- [Strousc et al. , 1998] Strousc Λ, Ashmead DH, Olide RN, and Grantham DW (1998). Temporal processing in the aging auditory system. The Journal oJ’lhe Acoustical Society of America, 104(4):2385 −99. [DOI] [PubMed] [Google Scholar]
- [Takahashi and Bacon, 1992] Takahashi GA and Bacon SP (1992). Modulation detection, modulation masking, and speech understanding in noise in the elderly. Journal ofSpeech and Hearing Research, 35(6):1410–21. [DOI] [PubMed] [Google Scholar]
- [Vermeire et al. , 2016] Vermeire K, Knoop Λ, Boel C, Auwers S, Schenus L, Talavcron-R,odrigucz M, De Boom C, and De Sloovere M (2016). Speech R,ecognition in Noise by Younger and Older Adults: Effects of Age, Hearing Loss, and Temporal R,esolution. Annals of Otology, Rhinology & Laryngology, 125(4):297–302. [DOI] [PubMed] [Google Scholar]
- [Walton et al. , 2002] Walton JP, Simon H, and Frisina R,D (2002). Age-related alterations in the neural coding of envelope periodicities. Journal of neurophysiology, 88(2):565–78. [DOI] [PubMed] [Google Scholar]
- [Wehr and Zador, 2003] Wehr M and Zador AM (2003). Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature, 426(6965):442–6. [DOI] [PubMed] [Google Scholar]
- [Zeng et al. , 2005] Zeng FC, Nie K, Stickney CS, Kong YY, Vong-phoe M, Bhargave A, Wei C, and Cao K (2005). Speech recognition with amplitude and frequency modulations. Proc Natl Acad Sci U S A, 102(7):2293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Zhong et al. , 2014] Zhong Z, Henry KS, and Heinz MG (2014). Sensorineural hearing loss amplifies neural coding of envelope information in the central auditory system of chinchillas. Hearing Research, 309:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


