Abstract
Background
The use of inflammatory biomarkers to delineate the type of lung inflammation present in asthma is increasingly common. However, the impact of obesity on these markers is unknown.
Objectives
We aimed to determine the impact of obesity on conventional markers of inflammation in asthma.
Methods
We performed secondary analysis of data from 652 patients previously enrolled in two ACRN trials. We performed linear correlations between biomarkers and logistic regression analysis to determine the predictive value of IgE, blood eosinophils and FeNO in relationship to sputum eosinophils (>2%), as well as to determine if cut points existed that would maximize the sensitivity and specificity for predicting sputum eosinophilia in the three weight groups.
Results
Overall, statistically significant but relatively weak correlations were observed among all four markers of inflammation. Within obese subjects, the only significant correlation found was between IgE and blood eosinophils (r=0.33, p<0.001); furthermore, all other correlations between inflammatory markers were approximately 0, including correlations with sputum eosinophils. In addition, the predictive value of each biomarker alone or in combination was poor in obese subjects. In fact in obese subjects, none of the inflammation biomarkers significantly predicted the presence of high sputum eosinophils. Obese asthma subjects have lower cut points for IgE, (268IU), FeNO (14.5ppb) and blood eosinophils (96 cells/ul) than all other groups.
Conclusions
In obese asthma, conventional biomarkers of inflammation are poorly predictive of eosinophilic airway inflammation. As such, biomarkers currently used to delineate eosinophilic inflammation in asthma should be approached with caution in these patients.
Keywords: Asthma, Obesity, Eosinophils, Inflammatory Markers and FeNO
Introduction
An association exists between the development of asthma and increased BMI {Beuther, 2007 #221;Beuther, 2007 #221;Beuther, 2007 #221}. Obesity in asthma is associated with increased symptoms, increased disease severity, and a decreased response to conventional medications {Boulet, 2008 #425;Peters-Golden, 2006 #426;Peters-Golden, 2006 #426;Boulet, 2008 #425.;Peters-Golden, 2006 #426}. Additionally, it has been appreciated that obese patients with asthma demonstrate phenotypic heterogeneity similar to that seen in lean individuals {Sutherland, 2012 #1538}. The most commonly accepted and well-described phenotype of asthma is one encompassed by eosinophilic inflammation resulting from type 2 cells that include CD4 and innate lymphoid cells that produce the cytokines interleukin (IL)-4, IL-5 and IL-13, among others {Woodruff, 2009 #361;Lambrecht, 2015 #430}. Eosinophilic inflammation is also associated with the presence of atopy, increased FeNO, serum IgE, sputum eosinophils, and peripheral eosinophils {Bousquet, 1990 #398;Fahy, 2009 #399}. Obesity is known to affect immune responses and alter T cell function and eosinophil migration, and may affect type II responses in a subgroup of obese patients with asthma {Agrawal, 2011 #427;Michalek, 2011 #429;Calixto, 2010 #400}.
Inflammatory biomarkers such as sputum eosinophils, blood eosinophils, periostin, IgE, and fraction of exhaled nitric oxide (FeNO) are increasingly being used to identify eosinophilic asthma phenotypes and predict response to therapy {Ortega, 2014 #372;Michils, 2008 #132;Kharitonov, 1996 #128;Dweik, 2011 #98;Deykin, 2005 #122;Corren, 2011 #243}. The gold standard of eosinophilic inflammation is the identification of eosinophils in airway tissue via bronchoscopy and analysis of endobronchial biopsies. However, this test is invasive and costly, limiting its use as a phenotyping tool. Sputum eosinophils have been extensively utilized in clinical trials as a marker of tissue eosinophilia, but induced sputum is generally unavailable for use as a clinical tool. In clinical trials, sputum eosinophils have rapidly gained favor as being predictive of important asthma outcomes, although the correlation between sputum and tissue eosinophils is not always consistent with a proportion of patients who demonstrate discordance between sputum and tissue eosinophilia {Jia, 2012 #112;Lemiere, 2006 #431}. In this study, we considered sputum eosinophils to be the gold standard of eosinophilic inflammation.
The limited availability of sputum eosinophils in clinical settings has led to increased interest in surrogate markers of inflammation, however these markers are not always accurately predictive of the presence of eosinophilic or neutrophilic inflammation{Hastie, 2013 #1561}. In addition, the effect of obesity on the ability of biomarkers to accurately depict underlying eosinophilic inflammation is unknown. For instance, studies have shown that sputum eosinophils and FeNO predict response to inhaled corticosteroids (ICS) {Deykin, 2005 #122;Brightling, 2005 #408;Brightling, 2005 #408;McGrath, 2012 #289;Dweik, 2011 #98}, and treatment strategies aimed at reducing sputum eosinophils result in a reduction in asthma exacerbation rates {Baigelman, 1983 #377;Petsky, 2007 #404}. However, two recent publications report discordance between submucosal eosinophilia and both bronchoalveolar lavage and sputum eosinophilia in obese patients with asthma {Desai, 2013 #349} {van der Wiel, 2014 #423}. FeNO, a biomarker shown to correlate with eosinophilic inflammation {Lemiere, 2006 #431}, is low in obese asthma and therefore may not be predictive {Komakula, 2007 #279;Maniscalco, 2015 #401}, but data are conflicting {Ciprandi, 2014 #402}. Blood eosinophils have become gradually accepted as a useful biomarker of asthma severity and are significantly decreased in response to anti-IgE and anti-IL-5 therapies {Massanari, 2010 #118;Pavord, 2012 #113;Ortega, 2014 #372}. The data supporting the use of blood eosinophils to predict response to ICS are mixed and widespread utilization of blood eosinophils as a biomarker has yet to occur {Meijer, 2002 #405;Pascoe, 2015 #407}. IgE has been a target of asthma therapy, but it appears that clinical characteristics including a history of exacerbation, need for high dose ICS, and the presence of low lung function are better predictors of response to omalizumab than serum IgE levels {Bousquet, 2004 #409} {Visness, 2009 #411;Fitzpatrick, 2012 #412}. However, a recent report did suggest that peripheral eosinophils >260 /µl and FeNO >19.5 ppb, as well as serum periostin >50 mg/ml (a biomarker associated with interleukin-13) were associated with response to Xolair{Hanania, 2013 #1415}.
Given the possible discordance between peripheral biomarkers and pulmonary inflammation in obese asthmatic patients, we hypothesized that the presence of obesity in asthma would decrease the ability of FeNO, serum IgE and peripheral blood eosinophil counts to predict sputum eosinophilia, which could subsequently decrease the ability to predict treatment responses.
Methods
Study Design
The Duke University IRB approved this study (Protocol number Pro00056566) and data were obtained from NHLBI BioLINCC. Secondary analyses of data from a common run-in period in two Asthma Clinical Research Network (ACRN) trials were performed (n=652), including 1) Best Adjustment Strategy for Asthma over Long Term (BASALT, ClinicalTrials.gov number: NCT00495157, n=363){Calhoun, 2012 #337} and 2) Tiotropium Bromide as an Alternative to Increased Inhaled Corticosteroid in Patients Inadequately Controlled on a Lower Dose of Inhaled Corticosteroid (TALC, ClinicalTrials.gov number: NCT00565266, n=289){Peters, 2010 #179}.
The inclusion criteria included age ≥ 18 years; a physician diagnosis of asthma confirmed by positive methacholine, or the presence of reversibility of the forced expiratory volume in one second (FEV1) by 12% and 200 ml after four puffs of inhaled albuterol; a baseline FEV1 of more than 40% predicted; and a smoking history of less than ten pack years. Exclusion criteria included the presence of other lung disease, presence of respiratory tract infection, or a significant asthma exacerbation within four weeks of study entry. During the run-in period, lung function testing, including spirometry pre- and post-bronchodilator FEV1 and methacholine testing, was performed. FeNO was measured and sputum induction was completed for differential cell counts. Additionally, all subjects had a serum IgE, peripheral eosinophil count and skin prick testing performed. Subjects completed the Asthma Control Questionnaire (ACQ), Asthma Quality of Life Questionnaire (AQLQ), Asthma Evaluation Questionnaire (AEQ) and Asthma Symptom Utility Index (ASUI) during the run-in period.
Statistical Analysis
Descriptive statistics were performed including the mean (standard deviation) or median (25th percentile, 75th percentile) to describe continuous variables dependent on variable distribution. The count (percentage) was used to describe categorical variables with non-missing values. Body mass index (BMI) was treated as both a continuous and categorical variable with three categories: lean (BMI≤24.9), overweight (BMI 25–29.9), and obese (BMI≥30). The Kolmogorov-Smirnov test was used to determine if continuous variables were normally distributed. The Kruskal-Wallis or F-test was used to make comparisons across BMI categories as appropriate followed by pairwise comparisons between weight categories, while the chi-square test was used to compare categorical variables. The prevalence of eosinophilic inflammation was defined as the presence of an elevated FeNO at two thresholds values [>50 and >25 parts per billion (ppb)], or sputum eosinophils >2%, or blood eosinophils >300 cells/µl {Bacci, 2012 #441;Pavord, 2012 #113;Dweik, 2011 #98}. Subjects were assigned to non-eosinophilic status as long as all three factors were non-missing and lower than the set threshold. Pearson’s correlation coefficients with 95% confidence intervals (based on Fisher’s Z transformation) were used to determine the relationship among FeNO, IgE, sputum eosinophils, and blood eosinophils overall and within each weight category. Logistic regression analysis was then used to determine if FeNO, IgE and blood eosinophils were predictive of high sputum eosinophils (>2%) overall and within each weight category. Regression splines were used to determine if the relationship between each biomarker and high sputum level was linear, revealing that the natural log transformation provided an approximate linear relationship. Each regression model was adjusted for study (BASALT, TALC), gender, age, BMI, and race (Caucasian, non-Caucasian).
A logistic regression model was then used to determine the value (cut point) that best predicted high sputum eosinophils for each biomarker. The values were determined by maximizing the area under the receiver operating characteristic curve (AUC), the sensitivity and the specificity. The regression models were applied across all patients and by weight group.
We did not adjust for multiple comparisons as all analyses were exploratory, thus a p-value < 0.05 was considered statistically significant. All data analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Demographics and Baseline Characteristics
Six hundred and fifty-two subjects were included in the analyses. Significantly more female subjects were found in the obese (70.1%) and lean asthma groups (72.0%) compared to the overweight (59.5%) group (p=0.014) (Table 1). The median age for patients who were obese (40 years) or overweight (36 years) was higher compared to lean subjects (30 years) (p<0.001), furthermore, a larger proportion of obese patients (55.6%) were diagnosed with asthma at >12 years of age (p=0.002) compared to lean (39.3%) or overweight (50.5%) subjects. Lean (69.7%) and overweight (67.2%) asthmatics were more likely to be Caucasian compared to obese subjects (47.7%) (p<0.001). Smoking history, presence of atopy via skin prick testing, and use of inhaled corticosteroids at baseline did not differ significantly across BMI groups.
Table 1.
Demographic Data of Participants by Weight Group
| Lean (N=211) |
Overweight (N=198) |
Obese (N=243) |
P-value | |
|---|---|---|---|---|
| Female Gender (%) | 148 (70.1) | 118 (59.6) | 175 (72.0) | 0.014* |
| Age (years) | 30 (24, 42) | 36 (27, 47) | 40 (30, 49) | <0.001* |
| Caucasian Race (%) | 147 (69.7) | 133 (67.2) | 116 (47.7) | <0.001* |
| Age at Asthma Diagnosis (years) | 9 (4, 18) | 12 (5, 24) | 13 (5, 25) | 0.012* |
| Age ≥12 years at Asthma Diagnosis (%) | 83 (39.3) | 100 (50.5) | 135 (55.6) | 0.002* |
| Asthma Duration (years) | 19 (11, 26) | 21 (14, 29) | 21 (13, 32) | 0.052 |
| No Smoking History (%) | 169 (80.1) | 149 (75.2) | 186 (76.5) | 0.474 |
| No Inhaled corticosteroid use at baseline (%) | 204 (96.7) | 194 (98.0) | 236 (97.1) | 0.718 |
| Positive Skin Prick Test (%) | N=207 191 (92.3) | N=194 180 (92.8) | N=235 211 (89.8) | 0.483 |
Denotes a statistically significant difference across BMI categories. Data are presented as n (%) or as median (25th percentile, 75th percentile).
Lean=BMI ≤24.9; Overweight=BMI 25–29.9; Obese=BMI ≥30.
Lean (20.1 ppb) and overweight (22.9 ppb) subjects had significantly higher median FeNO levels in comparison to obese (16.5 ppb) asthma subjects (p<0.001). FeNO levels did not differ significantly between lean and overweight subjects (p=0.068). Lean (145 IU/ml) and overweight (125 IU/ml) subjects also had significantly higher median IgE levels compared to obese (96 IU/ml) subjects (p=0.042), while IgE levels did not differ significantly between lean and overweight subjects (p=0.611). In addition, blood (p=0.796) and sputum eosinophil (p=0.358) counts did not differ significantly across BMI groups (Table 2).
Table 2.
Comparison of Biomarkers by Weight Group
| Lean (N=211) |
Overweight (N=198) |
Obese (N=243) |
P-value | ||
|---|---|---|---|---|---|
| Blood Eosinophils (cells/µl) | N=200 | N=191 | N=231 | 0.796 | |
| Median (Q1, Q3) | 178 (100, 251) | 171 (100, 290) | 174 (100, 260) | ||
| Sputum Eosinophils (%) | N=138 | N=126 | N=150 | 0.358 | |
| Median (Q1, Q3) | 0.6 (0.1, 2.0) | 0.6 (0.0, 1.7) | 0.4 (0.0, 1.2) | ||
| IgE (IU/mL) | N=199 | N=182 | N=225 | 0.042* | |
| Median (Q1, Q3) | 145 (61, 350) | 125 (59, 360) | 96 (38, 274) | ||
| FENO (ppb) | N=194 | N=175 | N=216 | <0.001* | |
| Median (Q1, Q3) | 20.1 (14.3, 29.2) | 22.9 (14.4, 33.1) | 16.5 (11.8, 24.2) | ||
Denotes a statistically significant difference across BMI categories. Data are presented as n (%) or as median (25th percentile, 75th percentile). Pairwise group differences are described in the text.
IgE=Immunoglobulin E; FENO=Fraction of exhaled nitric oxide; Lean=BMI ≤24.9; Overweight=BMI 25–29.9; Obese=BMI ≥30.
Baseline lung function measurements were found to be significantly lower in obese asthma subjects compared to lean and overweight asthma subjects, including pre-bronchodilator forced vital capacity (FVC), pre-bronchodilator FEV1 (absolute and %predicted), post-albuterol FEV1 (absolute and %predicted), post-ipratropium FEV1 (absolute and %predicted) (all p<0.001) (Table 3). We also determined the prevalence of reversible airway obstruction and hyper-responsiveness as a function of BMI. Across all subjects, we found a low prevalence of reversible airflow obstruction to both albuterol and ipratropium with only approximately 35% of patients demonstrating reversibility. The remaining 65% of subjects were enrolled in the studies on the basis of a positive methacholine challenge test with a PC20 <16 mg/ml, in the absence of reversibility. BMI group had no impact on the prevalence (p=0.750) or response to methacholine PC20 (p=0.967) nor on the reversibility to ipratropium (p=0.098), however, reversibility to albuterol (p=0.038) was more prevalent within the overweight (40.5%) and obese (42.8%) groups compared to the lean group (30.7%).
Table 3.
Baseline Lung Function Measurements by Weight Group
| Lean (N=211) |
Overweight (N=198) |
Obese (N=243) |
P-value | ||
|---|---|---|---|---|---|
| FVC Pre-bronchodilator (L) | N=204 | N=188 | N=230 | <0.001* | |
| Median (Q1, Q3) | 3.8 (3.3, 4.4) | 3.9 (3.3, 4.7) | 3.3 (2.7, 3.9) | ||
| FEV1 Pre-bronchodilator (L) | N=204 | N=188 | N=230 | <0.001* | |
| Median (Q1, Q3) | 2.9 (2.4, 3.4) | 2.7 (2.3, 3.2) | 2.3 (1.9, 2.9) | ||
| FEV1 Pre-bronchodilator (% predicted) | N=204 | N=188 | N=230 | <0.001* | |
| Mean (SD) | 85.2 (13.6) | 80.6 (14.7) | 76.4 (13.3) | ||
| FEV1 Post-albuterol (L) | N=192 | N=173 | N=215 | <0.001* | |
| Median (Q1, Q3) | 3.2 (2.7, 3.6) | 3.0 (2.6, 3.5) | 2.6 (2.2, 3.2) | ||
| FEV1 Post-albuterol (% predicted) | N=192 | N=173 | N=215 | <0.001* | |
| Mean (SD) | 93.8 (12.9) | 90.5 (13.7) | 85.3 (13.3) | ||
| Reversibility to Albuterol (%) | N=192 59 (30.7%) | N=173 74 (42.8%) | N=215 87 (40.5%) | 0.038* | |
| FEV1 Post-ipratropium (L) | N=202 | N=192 | N=235 | <0.001* | |
| Median (Q1, Q3) | 3.2 (2.6, 3.6) | 3.0 (2.5, 3.5) | 2.6 (2.2, 3.1) | ||
| FEV1 Post-ipratropium (% predicted) | N=202 | N=192 | N=235 | <0.001* | |
| Mean (SD) | 93.2 (12.8) | 89.1 (13.7) | 84.4 (13.0) | ||
| Reversibility to Ipratropium (%) | N=196 62 (31.6%) | N=182 77 (30.3%) | N=223 84 (37.7%) | 0.098 | |
| Methacholine PC20 (mg/ml) | N=153 | N=141 | N=179 | 0.750 | |
| Median (Q1, Q3) | 1.2 (0.5, 4.0) | 1.1 (0.5, 2.8) | 1.2 (0.6, 3.4) | ||
| Response to Methacholine (≤8 mg/ml) (%) | N=153 145 (94.8%) | N=141 133 (94.3%) | N=179 170 (95.0%) | 0.967 | |
Denotes a statistically significant difference across BMI categories. Data are presented as n (%), mean (standard deviation) or as median (25th percentile, 75th percentile).
FEV1=Forced expiratory volume in one second; FVC=Forced vital capacity; Reversibility is defined as an improvement of 12% and 200 ml in FEV1 or FVC; PC20=Provocative concentration of methacholine that causes a 20% drop in FEV1.
Obese asthma subjects had a higher symptom burden as indicated by higher asthma symptom scores including higher Asthma Evaluation Questionnaire (AEQ; p=0.010), Asthma Control Questionnaire (ACQ; p<0.001), and Asthma Symptom Utility Index (ASUI; p=0.005) scores compared to lean and overweight subjects. In addition, obese subjects had a significant concomitant decrease in the quality of life scores (AQLQ) (p<0.001). Although these symptom burden results are statistically significant, the differences were small and therefore may not be clinically meaningful (Table 4).
Table 4.
Asthma Symptom and Quality of Life Questionnaire Scores
| Lean (N=211) |
Overweight (N=198) |
Obese (N=243) |
P-value | ||
|---|---|---|---|---|---|
| Average AEQ Score | N=211 | N=198 | N=242 | 0.010* | |
| Median (Q1, Q3) | 0.33 (0.00, 0.67) | 0.33 (0.00, 1.00) | 0.67 (0.00, 1.00) | ||
| Average ACQ Score | N=207 | N=197 | N=240 | <0.001* | |
| Median (Q1, Q3) | 0.67 (0.33, 1.17) | 0.83 (0.50, 1.33) | 1.00 (0.50, 1.67) | ||
| Average ASUI Score | N=207 | N=197 | N=240 | 0.005* | |
| Median (Q1, Q3) | 0.75 (0.50, 1.11) | 0.75 (0.57, 1.00) | 0.88 (0.57, 1.25) | ||
| Average AQLQ Score | N=211 | N=198 | N=243 | <0.001* | |
| Median (Q1, Q3)) | 6.38 (5.78, 6.62) | 6.19 (5.56, 6.62) | 6.09 (5.19, 6.53) | ||
Denotes a statistically significant difference across BMI categories. Pairwise group differences are described in the text. Data are presented as median (25th percentile, 75th percentile).
ACQ=Asthma Control Questionnaire; AEQ=Asthma Evaluation Questionnaire; AQLQ=Asthma Quality of Life Questionnaire; ASUI=Asthma Symptom Utility Index.
The prevalence of eosinophilic inflammation according to FeNO threshold (>25 ppb or >50 ppb), sputum eosinophils >2% or blood eosinophils >300 cells/µl was significantly lower in obese compared to lean and overweight asthma subjects (p=0.010) if the FeNO threshold was >25 ppb. If the FeNO threshold was increased to >50 ppb, then the eosinophilic inflammation was not significantly different (p=0.311) across BMI categories (Table 5).
Table 5.
Prevalence of Eosinophilic Inflammation by FeNO Threshold
| Lean (N=211) |
Overweight (N=198) |
Obese (N=243) |
P-value | ||
|---|---|---|---|---|---|
| FeNO Threshold >25 ppb | N=191 | N=177 | N=212 | 0.010* | |
| Eosinophilic | 99 (51.8) | 103 (58.2) | 91 (42.9) | ||
| FeNO Threshold >50 ppb | N=189 | N=137 | N=165 | 0.311 | |
| Eosinophilic | 68 (35.9) | 59 (33.3) | 61 (28.9) | ||
Denotes a statistically significant difference across BMI categories. Data are presented as n (%). Eosinophilic if FENO >25 ppb or >50 ppb (two thresholds); or sputum eosinophils >2%; or blood eosinophils >300 cells/µl. The absence of all three denotes non-eosinophilic status.
Correlation between FeNO, IgE, blood eosinophils and sputum eosinophils overall and within BMI groups
Overall, statistically significant but relatively weak correlations were observed among all four markers of inflammation, ranging from r=0.17 (IgE and FeNO) to r=0.22 (sputum eosinophils and both blood eosinophils and FeNO) as demonstrated in Table 6. Pearson’s correlations were then determined within each weight category. Lean asthma subjects were noted to have the strongest correlations between inflammation markers with significant correlations found between all biomarkers except sputum eosinophils and IgE. The strongest correlation within the lean group occurred between sputum eosinophils and FeNO (r=0.43; p<0.001). As BMI increased, the number of significant associations between biomarkers diminished. As such, overweight subjects only demonstrated significant but relatively weak correlations between sputum eosinophils and IgE (r=0.41; p<0.001) and blood eosinophils and both sputum eosinophils (r=0.33; p<0.001) and FeNO (r=0.016; p=0.039). Within obese subjects, the only significant correlation found was between IgE and blood eosinophils (r=0.33, p<0.001); furthermore, all other correlations between inflammatory markers were approximately 0, including correlations with sputum eosinophils.
Table 6.
Correlation of the Eosinophilia Markers Overall and by Weight Group
| Weight Category |
Marker | Sputum Eosinophils |
FENO | IgE |
|---|---|---|---|---|
| Overall | Blood Eosinophils | 0.22* (0.13, 0.31) | 0.18* (0.09, 0.26) | 0.19* (0.11, 0.27) |
| Sputum Eosinophils | 0.22* (0.13, 0.32) | 0.12* (0.02, 0.22) | ||
| FeNO | 0.17* (0.08, 0.25) | |||
| Lean | Blood Eosinophils | 0.31* (0.15, 0.46) | 0.31* (0.18, 0.44) | 0.15* (0.01, 0.29) |
| Sputum Eosinophils | 0.43* (0.28, 0.56) | 0.09 (−0.08, 0.26) | ||
| FENO | 0.32* (0.18, 0.44) | |||
| Overweight | Blood Eosinophils | 0.33* (0.16, 0.48) | 0.16* (0.01, 0.30) | 0.11 (−0.03, 0.26) |
| Sputum Eosinophils | 0.16 (−0.03, 0.33) | 0.41* (0.24, 0.55) | ||
| FeNO | 0.11 (−0.05, 0.26) | |||
| Obese | Blood Eosinophils | 0.00 (−0.16, 0.16) | 0.01 (−0.12, 0.15) | 0.33* (0.20, 0.44) |
| Sputum Eosinophils | −0.02 (−0.19, 0.14) | −0.02 (−0.19, 0.14) | ||
| FeNO | 0.03 (−0.11, 0.17) |
Denotes a statistically significant correlation. Data presented as the Pearson correlation coefficient with 95% confidence intervals.
Predicting high sputum eosinophils (>2%) overall and within BMI groups
Logistic regression models for FeNO, quantitative IgE and blood eosinophils adjusted for study, age, gender, race and BMI were used to predict high sputum eosinophils (>2%) across all subjects and within each weight category (Table 7). Overall blood eosinophils [OR=1.44; 95% CI: (1.06–1.96)], IgE [OR=1.25; 95% CI: (1.03–1.51)] and FeNO [OR=2.06; 95% CI: (1.32–3.23)] significantly predicted high sputum eosinophils. When each weight category was modeled separately, IgE was poorly predictive of high sputum eosinophils regardless of weight, while FeNO was predictive of high sputum eosinophils but only in lean asthma subjects [OR 3.89; 95% CI: (1.56, 9.65)]. Lastly, blood eosinophils were most predictive of high sputum eosinophils in lean [OR 1.67; 95% CI: (0.98, 2.85)] and overweight [OR 3.01; 95% CI: (1.35, 6.73)] asthma subjects but not obese subjects [OR 0.96; 95% CI: (0.67, 1.38)]. In fact, none of the inflammation biomarkers significantly predicted the presence of high sputum eosinophils in asthmatic obese patients.
Table 7.
Predicting High Sputum Eosinophils (>2%) by Weight Category Utilizing the Natural Log of each Inflammation Biomarker
| Biomarker | BMI Category |
OR | Lower 95% CI |
Upper 95% CI |
P-value | C-index |
|---|---|---|---|---|---|---|
| Log(IgE) | Overall | 1.25 | 1.03 | 1.51 | 0.024* | 0.646 |
| Lean | 1.28 | 0.93 | 1.76 | 0.125 | 0.684 | |
| Overweight | 1.14 | 0.83 | 1.56 | 0.434 | 0.623 | |
| Obese | 1.32 | 0.89 | 1.96 | 0.161 | 0.659 | |
| Log(FeNO) | Overall | 2.06 | 1.32 | 3.23 | 0.002* | 0.662 |
| Lean | 3.89 | 1.56 | 9.65 | 0.004* | 0.762 | |
| Overweight | 1.61 | 0.75 | 3.44 | 0.220 | 0.639 | |
| Obese | 1.58 | 0.67 | 3.77 | 0.298 | 0.632 | |
| Log(Blood Eosinophils) | Overall | 1.44 | 1.06 | 1.96 | 0.018* | 0.667 |
| Lean | 1.67 | 0.98 | 2.85 | 0.059 | 0.744 | |
| Overweight | 3.01 | 1.35 | 6.73 | 0.007* | 0.718 | |
| Obese | 0.96 | 0.67 | 1.38 | 0.838 | 0.607 |
Denotes a statistically significant correlation. Each logistic regression model is adjusted for study, gender, race, age, and BMI.
We then utilized a multivariable logistic regression model to determine the predictive value of using all three inflammatory biomarkers in the model while still adjusting for the same baseline descriptors as above. FeNO continued to be predictive of sputum eosinophilia but only in lean subjects [OR=6.02; 95% CI: (1.86, 19.51); p=0.003]. Blood eosinophils were no longer predictive in lean subjects when all three biomarkers were considered. In overweight subjects, blood eosinophils was most predictive of sputum eosinophilia [OR=3.33; 95% CI: (1.36, 7.78); p=0.008], indicating that blood eosinophils continue to be predictive of sputum eosinophils after accounting for the other markers. The combination of all three biomarkers did not result in any improvements in the ability to predict high sputum eosinophils in obese subjects.
Cut points for predicting high sputum eosinophils (>2%) overall and by weight group
We performed analyses to determine if cut points existed for each biomarker individually (FeNO, IgE and blood eosinophils) for predicting high sputum eosinophils across all subjects and by weight group (Table 8). The cut-points for IgE, FeNO and blood eosinophils that maximize sensitivity and specificity differ by weight category. Obese asthma subjects have lower cut points for IgE, (268IU), FeNO (14.5ppb) and blood eosinophils (96 cells/ul) than all other groups.
Table 8.
Cut Points for Predicting High Sputum Eosinophils (>2%) by Weight Category
| Maximize AUC | Maximize Sensitivity | Maximize Specificity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker | BMI Category |
Cut Point |
SENS | SPEC | Cut Point |
SENS | SPEC | Cut Point |
SENS | SPEC |
| Log(IgE) | Overall | 268 | 0.46 | 0.73 | 5 | 1.00 | 0.02 | 5000 | 0.01 | 0.997 |
| Lean | 277 | 0.43 | 0.75 | 10 | 1.00 | 0.04 | 5000 | 0.04 | 0.99 | |
| Overweight | 605 | 0.35 | 0.88 | 5 | 1.00 | 0.01 | 2841 | 0.00 | 0.99 | |
| Obese | 268 | 0.53 | 0.76 | 15 | 1.00 | 0.12 | 4761 | 0.00 | 0.99 | |
| Log(FeNO) | Overall | 17.1 | 0.78 | 0.43 | 9.2 | 1.00 | 0.09 | 166.9 | 0.01 | 1.00 |
| Lean | 17.1 | 0.93 | 0.44 | 9.5 | 1.00 | 0.07 | 166.9 | 0.03 | 1.00 | |
| Overweight | 31.2 | 0.42 | 0.78 | 11.0 | 1.00 | 0.19 | 103.9 | 0.04 | 1.00 | |
| Obese | 14.5 | 0.79 | 0.42 | 9.2 | 1.00 | 0.10 | 94.7 | 0.00 | 0.99 | |
| Log(Blood Eosinophils) | Overall | 195 | 0.70 | 0.57 | 1 | 1.00 | 0.00 | 897 | 0.00 | 0.997 |
| Lean | 195 | 0.75 | 0.66 | 1 | 1.00 | 0.00 | 630 | 0.04 | 1.00 | |
| Overweight | 400 | 0.36 | 0.95 | 70 | 1.00 | 0.13 | 897 | 0.00 | 0.99 | |
| Obese | 96 | 0.25 | 0.85 | 486 | 1.00 | 0.02 | 1 | 0.10 | 0.97 | |
AUC=Area under the receiver operator characteristics (ROC) curve; SENS=Sensitivity; SPEC=Specificity.
Discussion
The principal finding of this study was that conventional markers of eosinophilic inflammation were poorly predictive of sputum eosinophilia in obese asthma subjects. Consistent with other studies, we found that obesity is associated with a more significant respiratory symptom burden and poorer quality of life scores concomitant with lower lung function, even in this population of patients with mild to moderate asthma. Further, we demonstrated that obese asthma subjects are more likely to be female, older age and have adult onset asthma.
Our data revealed no differences in baseline sputum and blood eosinophil levels on the basis of obesity. However there were significant differences in IgE and FeNO. Similar to currently published literature, obesity appeared to result in lower FeNO levels but, contrary to our expectations based on available literature, IgE levels were lower in obese than lean subjects with asthma {Maniscalco, 2015 #401;Visness, 2009 #411}. Obese asthma subjects had significantly lower prevalence of eosinophilic inflammation defined as sputum eosinophils > 2% or blood eosinophils > 300 cells/ml or FeNO >25ppb (p=0.01). However, when a higher FeNO threshold is used (>50ppb) there are no significant differences in the prevalence of eosinophilia (Table 5). There were no significant differences in the prevalence of eosinophilic inflammation on the basis of obesity and additionally, greater than 85% of subjects in the study demonstrated positive skin prick testing. Although age of onset has been reported as being a key factor in differentiating the eosinophilic and non-eosinophilic obesity phenotypes, our data did not reveal any differences in inflammatory markers on the basis of age of onset in obese subjects {Holguin, 2011 #351}.
Furthermore, our data revealed increasingly poor correlations between markers of inflammation as subjects become gradually more obese. Whereas lean asthma subjects demonstrated a high degree of correlation between FeNO, blood eosinophils, sputum eosinophils and IgE, these associations became increasingly poor as BMI increased. As a result, obese patients only had a significant correlation between IgE and blood eosinophils. One can postulate that this was secondary to alterations in surrogate markers that led to increased discordance between the actual inflammatory milieu in the compartments of the lung and the surrogate measurements that we obtained clinically. For instance, nitric oxide levels were reduced in obesity and this may have been related to the presence of underlying oxidative stress and subsequent changes in NOS signaling associated with increased asymmetric dimethyl arginine that could result in a lack of concordance between tissue eosinophilia, inflammation and FeNO levels {Komakula, 2007 #279;Holguin, 2013 #350}. We postulate that the mechanisms that mediate inflammation in a proportion of eosinophilic asthmatics that are obese is similar to lean asthmatics. However obese subjects are at higher risk of inaccurate phenotyping on the basis of surrogate markers of inflammation. These surrogate markers are indirect measures of inflammation that may be influenced by conditions in the lungs of obese subjects such as the presence of higher levels of oxidative stress{Holguin, 2010 #722} and adipokine mediated alterations of eosinophil chemotaxis and survival {Kato, 2011 #1539;Takeda, 2012 #1540;Kim, 2014 #1557}. These unique influences of obesity have the potential to influence the accuracy of surrogate markers at detecting compartmental eosinophilia.
Additionally, our study was limited by the reliance on sputum and not tissue eosinophils, as the gold standard test for eosinophilic inflammation in the lung. The variability in sputum eosinophilia on repeated measures and the potential discordance with tissue eosinophilia, particularly in obesity, has been reported and could be a confounding variable {Desai, 2013 #349;Peters, 2013 #418;van der Wiel, 2014 #423;McGrath, 2012 #289}. Therefore, we cannot be reassured that sputum eosinophilia reliably reflects lung tissue eosinophils; this remains a subject for future study. Other limitations include the retrospective data analysis of a cross sectional rather than longitudinal dataset.
We determined the ability of FeNO, blood eosinophils and IgE to predict sputum eosinophils (>2%). Sputum eosinophils have been shown to predict response to inhaled corticosteroids and elevations in this marker are associated with increased exacerbations {Bacci, 2006 #119;Brightling, 2005 #408;Petsky, 2007 #404}. Given the potential clinical importance of these markers in identifying treatment responders, adjusting therapies and predicting outcomes, we performed nominal logistic regression modeling to determine the ROC characteristics of each biomarker. None of the inflammation markers (FeNO, blood eosinophils and IgE) significantly predicted high sputum eosinophils. We noted a decrease in the ROC AUC with each of these biomarkers with increasing obesity with only blood eosinophils having an AUC > 0.70 for overweight subjects. From the standpoint of precision medicine, these results suggest that obese patients could be inaccurately assigned to non-eosinophilic phenotypes and possibly be excluded from receiving therapies for their asthma that could facilitate improved outcomes. Indeed, most targeted therapies currently in development rely on surrogate biomarkers to identify potential responders to therapy {Pavord, 2012 #113;Ortega, 2014 #372;Castro, 2014 #365}.
In conclusion, asthma is a heterogeneous disease regardless of the presence of obesity. However, obesity has a significant impact on the ability of currently available biomarkers of inflammation to accurately detect the type of underlying inflammation present in the lung. It is, therefore, imperative that the potential confounding effect of obesity be taken into consideration when interpreting the results of FeNO, IgE, blood and sputum eosinophil measurements. Moreover, these results underscore the need to identify more sensitive biomarkers in this population, perhaps serum periostin, Dipeptidyl Peptidase 4 (DPP4) or specific cytokine measurements that might permit more precise and individualized therapy {Marijsse, 2014 #422;Bobolea, 2015 #1401;Y. Zhang, 2014 #1426}.
FIG 1.
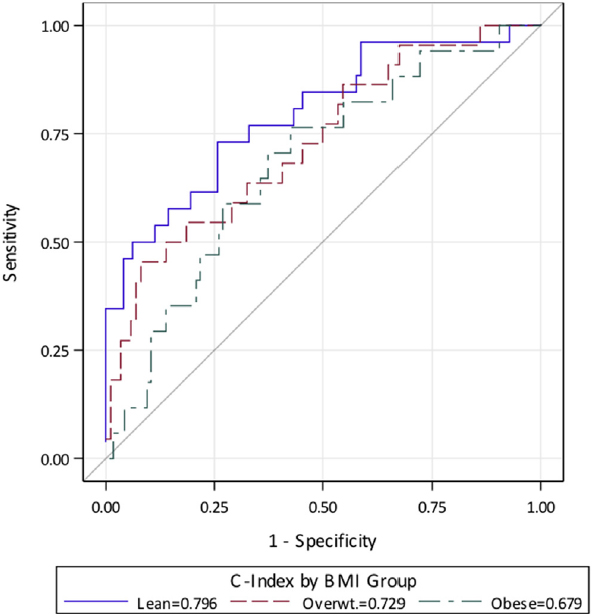
Receiver operating characteristic curves by weight group adjusted for all biomarkers of inflammation.
Clinical Implications.
The reliance on inflammatory markers to identify responders to various asthma therapies and thus to enable personalized treatment of asthma patients makes accurate characterization of inflammation essential in obese asthma.
Capsule Summary.
Obesity is associated with decreased sensitivity of blood eosinophils, IgE and FeNO in characterizing eosinophilia. Reliance on peripheral markers to make decisions regarding therapies targeting eosinophilia should therefore be approached with caution in obese asthma.
Acknowledgments
Funding: The study is supported by the National Heart Lung, and Blood Institute/National Institutes of Health Asthma Research Network Grant U10 HL074225, U10 HL074227, U10 HL074231, U10 HL074204, U10 HL074212, U10 HL074073, U10 HL074206, U10 HL074208, and U10 HL07421. NIH Training grants # 5 T32 AI 007062-36 and NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC).
Trial Registration: ClinicalTrials.gov number: NCT00495157 and NCT00565266.
Abbreviations
- ACRN
Asthma Clinical Research Network
- BMI
Body mass index
- IgE
Immunoglobulin E
- FeNO
Fraction of exhaled nitric oxide
- CD4
Cluster of differentiation 4
- IL
Interleukin
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- PC20
provocative concentration of methacholine that causes a 20% drop in FEV1.
- AEQ
Asthma Evaluation Questionnaire
- ACQ
Asthma Control Questionnaire
- ASUI
Asthma Symptom Utility Index
- ICS
Inhaled corticosteroids
- AUC
Area under the curve
- OR
Odds ratio
- DPP4
Dipeptidyl peptidase 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions:
The following authors contributed to the concept and design, analysis and interpretation of data: Njira Lugogo MD1, Noah Agada MD1, Cynthia L Green1, Kevin J. Anstrom PhD1, Run Zhou MS1, Siyun Yang MS1, Siyi Zhang MS1, and Monica Kraft MD12
The following authors contributed to the drafting of work and critical revision of the manuscript for intellectual content: Njira Lugogo MD1, Susanne Meghdadpour PhD1, Cynthia L Green1, Kevin Anstrom PhD1, Noah Agada MD1, Elliot Israel MD2, Richard Martin MD3, Robert F. Lemanske, Jr. MD4, Homer Boushey MD5, Stephen C. Lazarus MD5, Stephen I. Wasserman MD6, Mario Castro MD7, William Calhoun MD8, Stephen P. Peters MD PhD9, Emily DiMango MD10, Vernon Chinchilli PhD11, Nikolina Icitovic MAS11, Susan Kunselman MA11, Tonya S. King PhD11 and Monica Kraft MD12
The following authors had accountability for all aspects of the work related to accuracy and integrity: Njira Lugogo MD1 and Monica Kraft MD12.
References
- 1.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175(7):661–6. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulet LP. Influence of obesity on the prevalence and clinical features of asthma. Clinical and investigative medicine Medecine clinique et experimentale. 2008;31(6):E386–90. doi: 10.25011/cim.v31i6.4926. [DOI] [PubMed] [Google Scholar]
- 3.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27(3):495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland ER, Goleva E, King TS, Lehman E, Stevens AD, Jackson LP, et al. Cluster analysis of obesity and asthma phenotypes. PLoS One. 2012;7(5):e36631. doi: 10.1371/journal.pone.0036631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambrecht BN, Hammad H. The immunology of asthma. Nature immunology. 2015;16(1):45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323(15):1033–9. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 8.Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009;6(3):256–9. doi: 10.1513/pats.200808-087RM. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal A, Mabalirajan U, Ahmad T, Ghosh B. Emerging interface between metabolic syndrome and asthma. American journal of respiratory cell and molecular biology. 2011;44(3):270–5. doi: 10.1165/rcmb.2010-0141TR. [DOI] [PubMed] [Google Scholar]
- 10.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. Journal of immunology. 2011;186(6):3299–303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calixto MC, Lintomen L, Schenka A, Saad MJ, Zanesco A, Antunes E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol. 2010;159(3):617–25. doi: 10.1111/j.1476-5381.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortega H, Li H, Suruki R, Albers F, Gordon D, Yancey S. Cluster analysis and characterization of response to mepolizumab. A step closer to personalized medicine for patients with severe asthma. Annals of the American Thoracic Society. 2014;11(7):1011–7. doi: 10.1513/AnnalsATS.201312-454OC. [DOI] [PubMed] [Google Scholar]
- 13.Michils A, Baldassarre S, Van Muylem A. Exhaled nitric oxide and asthma control: a longitudinal study in unselected patients. Eur Respir J. 2008;31(3):539–46. doi: 10.1183/09031936.00020407. [DOI] [PubMed] [Google Scholar]
- 14.Kharitonov SA, Yates DH, Barnes PJ. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1996;153(1):454–7. doi: 10.1164/ajrccm.153.1.8542158. [DOI] [PubMed] [Google Scholar]
- 15.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deykin A, Lazarus SC, Fahy JV, Wechsler ME, Boushey HA, Chinchilli VM, et al. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol. 2005;115(4):720–7. doi: 10.1016/j.jaci.2004.12.1129. [DOI] [PubMed] [Google Scholar]
- 17.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365(12):1088–98. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 18.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130(3):647–54. e10. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemiere C, Ernst P, Olivenstein R, Yamauchi Y, Govindaraju K, Ludwig MS, et al. Airway inflammation assessed by invasive and noninvasive means in severe asthma: eosinophilic and noneosinophilic phenotypes. J Allergy Clin Immunol. 2006;118(5):1033–9. doi: 10.1016/j.jaci.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. 2013;132(1):72–80. doi: 10.1016/j.jaci.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brightling CE, Green RH, Pavord ID. Biomarkers predicting response to corticosteroid therapy in asthma. Treatments in respiratory medicine. 2005;4(5):309–16. doi: 10.2165/00151829-200504050-00002. [DOI] [PubMed] [Google Scholar]
- 22.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185(6):612–9. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baigelman W, Chodosh S, Pizzuto D, Cupples LA. Sputum and blood eosinophils during corticosteroid treatment of acute exacerbations of asthma. The American journal of medicine. 1983;75(6):929–36. doi: 10.1016/0002-9343(83)90871-9. [DOI] [PubMed] [Google Scholar]
- 24.Petsky HL, Kynaston JA, Turner C, Li AM, Cates CJ, Lasserson TJ, et al. Tailored interventions based on sputum eosinophils versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev. 2007;(2):CD005603. doi: 10.1002/14651858.CD005603.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Respir Crit Care Med. 2013;188(6):657–63. doi: 10.1164/rccm.201208-1470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Wiel E, Ten Hacken NH, van den Berge M, Timens W, Reddel HK, Postma DS. Eosinophilic inflammation in subjects with mild-to-moderate asthma with and without obesity: disparity between sputum and biopsies. Am J Respir Crit Care Med. 2014;189(10):1281–4. doi: 10.1164/rccm.201310-1841LE. [DOI] [PubMed] [Google Scholar]
- 27.Komakula S, Khatri S, Mermis J, Savill S, Haque S, Rojas M, et al. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res. 2007;8:32. doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maniscalco M, Zedda A, Faraone S, Cristiano S, Sofia M, Motta A. Low alveolar and bronchial nitric oxide in severe uncomplicated obesity. Obesity research & clinical practice. 2015 doi: 10.1016/j.orcp.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Ciprandi G, Schiavetti I, Bellezza Fontana R, Sorbello V, Ricciardolo FL. Overweight and obesity as risk factors for impaired lung function in patients with asthma: A real-life experience. Allergy and asthma proceedings : the official journal of regional and state allergy societies. 2014;35(4):e62–71. doi: 10.2500/aap.2014.35.3773. [DOI] [PubMed] [Google Scholar]
- 30.Massanari M, Holgate ST, Busse WW, Jimenez P, Kianifard F, Zeldin R. Effect of omalizumab on peripheral blood eosinophilia in allergic asthma. Respir Med. 2010;104(2):188–96. doi: 10.1016/j.rmed.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–9. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 32.Meijer RJ, Postma DS, Kauffman HF, Arends LR, Koeter GH, Kerstjens HA. Accuracy of eosinophils and eosinophil cationic protein to predict steroid improvement in asthma. Clin Exp Allergy. 2002;32(7):1096–103. doi: 10.1046/j.1365-2222.2002.01412.x. [DOI] [PubMed] [Google Scholar]
- 33.Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. The Lancet Respiratory medicine. 2015;3(6):435–42. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 34.Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378–86. doi: 10.1378/chest.125.4.1378. [DOI] [PubMed] [Google Scholar]
- 35.Visness CM, London SJ, Daniels JL, Kaufman JS, Yeatts KB, Siega-Riz AM, et al. Association of obesity with IgE levels and allergy symptoms in children and adolescents: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2009;123(5):1163–9. 9 e1–4. doi: 10.1016/j.jaci.2008.12.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzpatrick S, Joks R, Silverberg JI. Obesity is associated with increased asthma severity and exacerbations, and increased serum immunoglobulin E in inner-city adults. Clin Exp Allergy. 2012;42(5):747–59. doi: 10.1111/j.1365-2222.2011.03863.x. [DOI] [PubMed] [Google Scholar]
- 37.Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–11. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 38.Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. Jama. 2012;308(10):987–97. doi: 10.1001/2012.jama.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715–26. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bacci E, Latorre M, Cianchetti S, Bartoli M, Costa F, Di Franco A, et al. Transient sputum eosinophilia may occur over time in non-eosinophilic asthma and this is not prevented by salmeterol. Respirology. 2012;17(8):1199–206. doi: 10.1111/j.1440-1843.2012.02242.x. [DOI] [PubMed] [Google Scholar]
- 41.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127(6):1486–93. e2. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holguin F, Comhair SA, Hazen SL, Powers RW, Khatri SS, Bleecker ER, et al. An association between L-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. Am J Respir Crit Care Med. 2013;187(2):153–9. doi: 10.1164/rccm.201207-1270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holguin F, Fitzpatrick A. Obesity, asthma, and oxidative stress. J Appl Physiol. 2010;108(3):754–9. doi: 10.1152/japplphysiol.00702.2009. [DOI] [PubMed] [Google Scholar]
- 44.Kato H, Ueki S, Kamada R, Kihara J, Yamauchi Y, Suzuki T, et al. Leptin has a priming effect on eotaxin-induced human eosinophil chemotaxis. Int Arch Allergy Immunol. 2011;155(4):335–44. doi: 10.1159/000321195. [DOI] [PubMed] [Google Scholar]
- 45.Takeda M, Ueki S, Kato H, Konno Y, Chihara M, Itoga M, et al. Obesity and eosinophilic inflammation: does leptin play a role. Int Arch Allergy Immunol. 2012;158(Suppl 1):87–91. doi: 10.1159/000337799. [DOI] [PubMed] [Google Scholar]
- 46.Kim SH, Sutherland ER, Gelfand EW. Is there a link between obesity and asthma? Allergy Asthma Immunol Res. 2014;6(3):189–95. doi: 10.4168/aair.2014.6.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters MC, Fahy JV. Type 2 immune responses in obese individuals with asthma. Am J Respir Crit Care Med. 2013;188(6):633–4. doi: 10.1164/rccm.201307-1360ED. [DOI] [PubMed] [Google Scholar]
- 48.Bacci E, Cianchetti S, Bartoli M, Dente FL, Di Franco A, Vagaggini B, et al. Low sputum eosinophils predict the lack of response to beclomethasone in symptomatic asthmatic patients. Chest. 2006;129(3):565–72. doi: 10.1378/chest.129.3.565. [DOI] [PubMed] [Google Scholar]
- 49.Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. The Lancet Respiratory medicine. 2014;2(11):879–90. doi: 10.1016/S2213-2600(14)70201-2. [DOI] [PubMed] [Google Scholar]
- 50.Marijsse GS, Seys SF, Schelpe AS, Dilissen E, Goeminne P, Dupont LJ, et al. Obese individuals with asthma preferentially have a high IL-5/IL-17A/IL-25 sputum inflammatory pattern. Am J Respir Crit Care Med. 2014;189(10):1284–5. doi: 10.1164/rccm.201311-2011LE. [DOI] [PubMed] [Google Scholar]
- 51.Bobolea I, Barranco P, Del Pozo V, Romero D, Sanz V, Lopez-Carrasco V, et al. Sputum periostin in patients with different severe asthma phenotypes. Allergy. 2015;70(5):540–6. doi: 10.1111/all.12580. [DOI] [PubMed] [Google Scholar]
- 52.Zhang CU Y, Burchard EG, Chu H, Seibold MA. The Asthma Biomarker Dipeptidyl Peptidase 4 (dpp4) Is Il-13 Inducible In Airway Epithelial Cells And Inhibits Rhinovirus Infection. Am J Respir Crit Care Med. 2014;189:A4875. [Google Scholar]


