Abstract
In this work, we intended to inhibit the biofilm synthesis and the metabolism of Gram-positive and Gram-negative bacteria using two highly available wastes (stem and marc) obtained after the manufacturing of Torrontes wine at Cafayate, Argentina. Wine wastes contain a significant amount of bioactive compounds, mainly phenolic compounds, which makes them a potential source of compounds with beneficial properties to human health, as they could inhibit the virulence of pathogenic bacteria or protect the tissue against oxidative stress. Marc and stem extracts of Torrontes wine were evaluated for their ability to inhibit the metabolism and biofilm production of Pseudomonas aeruginosa and Staphylococcus aureus strains. The phytochemical composition and antioxidant activity of these extracts were also determined. The methanol and ethyl acetate extracts, which contained the highest amount of total polyphenolic, exhibited the highest scavenging capacity of ABTS and nitric oxide and the strongest Fe3+ reducing power and exhibited the highest level of inhibition of the biofilm formation and of the metabolic activity in bacterial biofilm. We also noticed a positive correlation between phenolic compounds content, the antioxidant activity, and the anti-biofilm capacity of the winemaking wastes. These results display the potentiality of wine wastes to prevent or reduce the formation of biofilm. Moreover, their abundance makes them an attractive and affordable source of antibiofilm and antioxidant agents.
1. Introduction
Biofilms are complex communities of bacteria embedded in a self-produced matrix that attach to inert or living surfaces [1]. The biofilm is an important microbial survival strategy that enables bacteria to colonize habitats, to survive to external stress, and to synchronize the bacterial population through quorum sensing to function as a multicellular organism. At the same time, it allows them to be less sensitive to antimicrobial agents [2].
Depending on the surface where the biofilm develops, it can produce cystic fibrosis, infect wounds, resulting in chronic infections that are resistant to antibiotics, or contaminate medical devices, which is the main cause of hospital infections. The biofilm formation is also an important source of concern at the food industries, since it can develop on different surfaces of the industrial machinery, becoming the principal source of foodborne diseases. In both cases, the principal concern is the impossibility of removing the biofilm using commercial cleaners or antimicrobials, since biofilm not only allows the bacterial colonization of surfaces but also enhances the bacterial community's resistance to antimicrobial agents, as well as to the host's immune system.
Staphylococcus aureus is a major human pathogen that is responsible for several diseases that vary from minor skin infections to life-threatening syndromes [3]. Their infective circle depends greatly on their ability to form biofilm and is the principal source of hospital infections caused by implants and the poor sanitization of medical devices [4]. Additionally, Staphylococcus aureus is the most found bacteria in contaminant biofilms on surfaces that are usually in contact with food, even on stainless steel [5].
Pseudomonas aeruginosa has been identified as the principal biofilm-forming opportunistic pathogen in chronic wounds, cystic fibrosis, as well as in several chronic infections [6, 7]. A major concern is the fact that P. aeruginosa produces biofilm, as well as several virulence factors, coordinately by quorum sensing when it is contaminating foods, but the addition of chemical preservatives to control biofilm is highly questioned because of their doubtful safety to human health [8]. Foodborne bacteria are a recalcitrant source of infections, and over 80% of persistent bacterial infections are associated with biofilms formed in the food manufacturing industries [9].
Many compounds are investigated as biofilm inhibitors [8]; however, only few of them are considered to be safe according to Codex Alimentarius to be used in the medicine and food industries. As a result, some essential oils and condiments have risen as potential natural inhibitors of biofilm [10, 11]. Nonetheless, these products have a high commercial cost that makes them nonprofitable to be used for this purpose. Thus, the alternative of using wastes from the food industry as biofilm inhibitors is an economically promising and environmentally friendly alternative. In this context, wine wastes present the potential to be used as biofilm inhibitors due to their richness in bioactive compounds, highlighting the possibility of producing manufactured products with a commercial benefit.
Wine production is a very important agricultural activity around the world. This industry generates a vast amount of wastes, such as grape stalks (stem), grape marc, exhausted yeast, wine lee, and high loaded wastewater [12]. The grape marc is the main component of the winery organic wastes, representing between 60 and 70% of the total disposed [13]. Typically, this waste has a high water content (ca. 60%), but on a dry basis it is comprised of skin (ca. 51%), seeds (ca. 47%), and stalks (ca. 2%) [14]. However, the specific composition of grape marc depends on the wine type, the soil, weather, and topography in which the grape is grown, as well as the winemaking techniques [15].
The industrial wastes cause a serious disposal problem and can generate pollution problems. Nevertheless, wine wastes contain bioactive compounds that might impart health benefits, opening the possibility of developing new affordable products to be incorporated into food or pharmaceutical products [16]. Various studies have been performed to explore the nature of the bioactive compounds present in these residues; the presence of polyphenols is standing out, which have been associated with the bioactive properties of grapes due to their antioxidant, anti-inflammatory, anticarcinogenic, and antibacterial activities [17–20].
On the other hand, the nature of the solvent used to extract the bioactive constituents plays an important role in the extraction process [17, 21]. Thus, using solvents of different polarities would allow a more extensive evaluation of the bioactivity of all the compounds present in the wastes. The grape's wastes are a cheap, abundant, and valuable raw material for the recovery of biologically interesting compounds. Moreover, products based on grape components are already being commercialized as supplements, as powder or capsules, which would support the safe use of compounds derived from wine wastes as part of medicinal or food products [22].
In this work, we aim to study the capacity of two wastes (stem and marc) obtained from the manufacturing of Torrontes wine at Cafayate (Argentina) to inhibit the biofilm synthesis and the metabolism of Gram-positive and Gram-negative bacteria. Moreover, numerous studies have demonstrated a strong and positive correlation between the phenolic compounds content and the antioxidant potential of grapes [23, 24]. Nevertheless, according to our knowledge, there is no information about the correlation between the antioxidant activity, the anti-biofilm capacity, and the phenolic compounds content of winemaking wastes. As a result, we also intend to determine the chemical composition of these extracts and their antioxidants properties.
2. Materials and Methods
2.1. Extractions
Wine wastes (marc and stem) were extracted using solvents of different polarities in order to obtain several extracts containing different classes of compounds. A successive extraction was developed using hexane, chloroform, ethyl acetate, and methanol. The solvent removal was performed by vacuum evaporation using a rotary evaporator (at 30°C). The soluble principle (SP) of each extract was suspended with dimethylsulfoxide (DMSO, Sigma Aldrich) for the chemical and biological analysis.
2.2. Phytochemical Analysis
2.2.1. Determination of Total Phenolic and Nonflavonoid Compounds
Total phenolics were determined using the Folin-Ciocalteu method [25, 26]. The reaction mixture contained, in each extract, distilled water, Folin-Ciocalteu reagent, and sodium carbonate (15.9% w/v). It was maintained at 50°C for 5 min in a water bath and the absorbance was measured at 765 nm. Gallic acid was used for the standard curve (R2 = 0.997, p ≤ 0.05), and results were expressed as μg gallic acid equivalents per mg of soluble principle (μg GAE/mg SP).
Nonflavonoid phenolics were measured according to Torres Carro et al. [26] by quantifying the total phenolic content remaining in the supernatant after the precipitation of the flavonoids with acidic formaldehyde for 24 h. Results were expressed as μg GAE/mg SP.
2.2.2. Determination of Flavonoid Compounds
Total flavonoids were estimated using the method described by Popova et al. [27]. Samples were put to react with ethanol and a 5% AlCl3 ethanolic solution. After 30 min at room temperature, the absorbance was measured at 420 nm. Quercetin was used for the standard curve (R2 = 0.999, p ≤ 0.05), and results were expressed as μg quercetin equivalents per mg of soluble principle (μg QE/mg SP).
2.2.3. Determination of Condensed Tannins
The total condensed tannins content was determined using 4-dimethylaminocinnamaldehyde (DMAC) according to Prior et al. [28]. Each extract, dissolved in DMSO, reacted with 0.1% DMAC and the total volume (600 μL) was completed with acidified ethanol 0.1%. The mixture was put to react for 25 min at 30°C, and the absorbance was measured using a spectrophotometer at 640 nm. Proanthocyanidin B2 was used as standard drug, and results were expressed in μg of proanthocyanidin B2 equivalents per mg of soluble principle (μg PB2E/mg SP) (R2 = 0.989, p ≤ 0.05).
2.3. Microbiological Analysis
2.3.1. Microorganisms
Pseudomonas aeruginosa ATCC 27853 and a strain HT5, resistant to several antibiotics, aztreonam (30 mg), ceftazidime (30 mg); cefepime (30 mg), ciprofloxacin (5 mg); gentamicin (10 mg); imipenem (10 mg), meropenem (10 mg), and piperacillin-tazobactam (110 mg), but sensitive to amikacin (30 mg), were used. The strains were grown for 24 h at 37°C in Luria-Bertani (LB) medium. In addition, Staphylococcus aureus ATTC 6538, and a strain HT1, methicillin-resistant, were also used. These strains were grown in Müller-Hinton (MH) medium for 24 h at 37°C.
2.3.2. Bacterial Growth
Overnight cultures of both P. aeruginosa strains were diluted to reach an OD of 0.125 ± 0.02 at 560 nm in LB medium. Concurrently, overnight cultures of S. aureus strains were diluted to reach an OD of 0.13 ± 0.03 at 560 nm in MH medium. The diluted cultures (190 μL) were placed in each of the 96 wells of a microtiter polystyrene plate. Solutions containing 10 and 100 μg/mL of marc and stem extracts were prepared separately in DMSO/distilled water (1:1), and 10 μL of each one was pipetted into the wells individually. After 24 h incubation at 37°C, bacterial growth was measured at 560 nm, using a microtiter plate reader (Multiskan Go, Thermo). A DMSO/water (1:1) solution added to the diluted culture was employed as a growth control, and the antibiotic ciprofloxacin was incorporated into the bioassay at 5 μg/mL as a positive control.
2.3.3. Biofilm Formation Assay
The biofilm quantification was done using a micro method based on a crystal violet stain according to a protocol previously reported [29] with several modifications [11]. Ciprofloxacin, a known biofilm inhibitor, was incorporated into the bioassay [30]. Then, the specific biofilm, which express the amount of biofilm that each bacterium forms, was calculated as the ratio between the biofilm production (measured at OD 595 nm) and the bacterial growth (measured at 560 nm) [31].
2.3.4. Biofilm Metabolic Activity Assay
The metabolic activity of the biofilm formed by the bacterial strains assayed in this work was determined using a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) reduction assay with some modifications [32]. Shortly, 200 μL of P. aeruginosa and S. aureus cultures were incubated for 24 h at 37°C. The biofilm generated after 24 h incubation was gently removed and the plates were air-dried. Afterward, 10 μL of the above-mentioned concentration of marc and stem extracts (10 and 100 μg/mL) was incorporated into each well containing 190 μL of PBS (pH 6.5) and was incubated for 24 h at 37°C. Then, the microplate was washed again and 100 μL of MTT solution (0.5 mg/mL) was pipetted into each well and incubated for 5 h at 37°C under sterile conditions. The insoluble purple formazan salt (obtained by enzymatic hydrolysis of MTT by the dehydrogenase enzyme) was dissolved with DMSO, and the absorbance was measured at 570 nm using the microplate reader (Multiskan Go, Thermo).
2.4. Antioxidant Assays
2.4.1. ABTS Scavenging Activity
The total antioxidant capacity of the samples was determined by the 2,2-azino-bis-(3-ehylbenzothiazoline-6-sulphonic acid) di-ammonium salt (ABTS) radical cation method as described by Torres Carro et al. [33]. An ABTS+• solution (absorbance of 0.700) was added to a microplate containing the extracts (5–400 μg/mL) and was mixed thoroughly. The reaction mixture was kept at room temperature for 1 min and the absorbance was immediately recorded at 734 nm. The percentage of scavenging by the samples was compared to the negative control (DMSO).
Results were expressed as percentage of ABTS+• scavenging, which was calculated using the following equation:
| (1) |
where A0 is the absorbance of the control and As is the absorbance of the samples. The percentage of scavenging was plotted as a function of concentration, obtaining the concentration of sample required to scavenge 50% of the radical (SC50). Quercetin (2–20 μg/mL) and ascorbic acid (0.3–3 μg/mL) were used as positive controls.
2.4.2. Nitric Oxide Scavenging Activity
The ability of the extracts to scavenge the nitric oxide (NO) released by sodium nitroprusside under light was determined spectrophotometrically according to Torres Carro et al. [33]. Different concentrations of the extracts (200–500 μg/mL) were mixed with sodium phosphate buffer (0.2 M; pH 7.4) and sodium nitroprusside (100 mM). The reaction mixture was incubated for 15 min at 37°C. Then, Griess reagent was added and the absorbance of the formed chromophore was measured at 550 nm. The SC50 (concentration necessary to scavenge 50% of NO) was calculated using a regression curve. Ascorbic acid was used as positive control (10–100 μg/mL).
2.4.3. Fe+++ to Fe++ Reducing Power
The ability of the extracts to reduce Fe+++ was assessed according to D'Almeida et al. [34]. The extract solutions (10–250 μg/mL) were mixed with 416 μL of 1% aqueous potassium ferricyanide and sodium phosphate buffer (0.1 M; pH 6.3) was added to reach a final volume of 1 mL. After 10 min of incubation at 50°C, 416 μL of 10% trichloroacetic acid was added, and the mixture was centrifuged at 1000 xg for 10 min. Finally, 416 μL of the upper layer was mixed with 416 μL of water and 83 μL of 0.1% aqueous FeCl3. The absorbance was recorded at 700 nm after 10 min of incubation at room temperature. The percentage of reducing power was plotted against the concentration, and a linear regression analysis was carried out. The RC50 is the concentration necessary to reduce 50% of the Fe3+ and was obtained by interpolation from linear regression analysis. Ascorbic acid (2–16 μg/mL) was used as positive control.
2.4.4. Iron Chelating Capacity
The chelation of ferrous ions by the extracts was determined according to Torres Carro et al. [35]. Briefly, 6 μL of 2 mM FeSO4 was added to different concentration of the extracts (100–700 μg/mL) or positive control Na2EDTA (5–20 μg/mL) and ultrapure water to a final volume of 143 μL. The reaction was initiated by the addition of 7 μL of 5 mM ferrozine solution which forms a colored complex with Fe2+. The mixture was shaken and maintained at room temperature for 10 min. The absorbance was measured using a microplate reader at 562 nm, and the percentage of inhibition of the complex formation was calculated. The chelating concentration 50% (CC50) is the concentration at which 50% of the iron is chelated and was obtained by interpolation from linear regression analysis.
2.5. Statistical Analysis
All of the assays were carried out in triplicate or quadruplicate and data are presented as mean values ± SD. The statistic software InfoStat (Student Version, 2011) was employed to evaluate the significance of differences between groups. The criterion of statistical significance was taken as p ≤ 0.05. The correlation studies were also analyzed using InfoStat (Student Version, 2011).
3. Results and Discussion
Bioactive substances are compounds characterized by their beneficial properties on human health. A natural substance is considered bioactive if it has a measurable biological activity and has a beneficial effect on health; in accordance with this, several secondary metabolites are recognized as bioactive compounds [36].
Among the most representative and well-known secondary metabolites derived from plants that have a beneficial effect on human health are the phenolic compounds. In the particular case of wine, its phytochemical composition has been extensively studied and reported. It is mostly constituted by phenolic acids, anthocyanins, flavonols, flavanols, tannins, stilbenes, etc. [37, 38]. Among the industrial byproduct from the winemaking process, grape seeds are the main component (38%–52% of dry matter) of the grape marc, whose polyphenolic composition depends mostly on the winemaking process [39]. Another important byproduct of the winemaking industry is the stem. Studies have determined that the composition of this waste consists mostly on flavonols, hydroxycinnamic acids, anthocyanins, and stilbenes [40]. For the phytochemical studies carried out in this work, we measured the most common and abundant groups of phenolic compounds present in plants to observe variations in the composition depending on the polarity of the solvent system used. As expected, a variation of the content of the different phenolic compounds was observed, increasing from the less polar solvent (hexane) to the most polar (methanol) (Table 1). The methanol extract of marc exhibited the largest proportion of polyphenols, with a content 4- to 100-folds higher than the rest of the samples. In Table 1 a variation on the proportion of the different types of polyphenols between the marc and stem extracts can also be noticed, which is probably related to a variable distribution of phenolic compounds throughout different parts of the plant. Moreover, the ethyl acetate and methanolic extracts of stem were particularly rich in nonflavonoid compounds (77 and 75% of the total, respectively). The stem extracts also showed the largest proportion of flavonoids, while the condensed tannins were more abundant in the marc samples.
Table 1.
Phytochemical screening.
| Sample | Total phenolics |
Non-flavonoid phenolics |
Flavonoids phenolics |
Condensed Tannins |
|---|---|---|---|---|
| (μg GAE/mg SP) | (μg GAE/mg SP) | (μg QE/mg PS) | (μg PB2E/mg SP) | |
| Grape Marc extract | ||||
| Hexane | 1.5 ± 0.2a | 0.6 ± 0.1a | 1.0 ± 0.2a | 0.1 ± 0.1a |
| Chloroform | 4.2 ± 0.2a | 1.1 ± 0.3a | 2.3 ± 0.1d | 0.2 ± 0.3a |
| Ethyl acetate | 33.3 ± 1.3b | 23.0 ± 1.1d | 1.8 ± 0.2b,c | 54.4 ± 3.7b |
| Methanol | 157.7 ± 3.1d | 13.4 ± 0.3c | 2.2 ± 0.1c,d | 131.8 ± 12.9c |
|
| ||||
| Grape Stem extract | ||||
| Hexane | 5.6 ± 0.6a | 1.1 ± 0.2a | 3.7 ± 0.1e | 0.2 ± 0.2a |
| Chloroform | 25.9 ± 0.5b | 7.0 ± 0.3b | 4.6 ± 0.1f | 0.8 ± 0.1a |
| Ethyl acetate | 42.5 ± 4.7c | 32.6 ± 0.3e | 5.9 ± 0.2g | 6.3 ± 0.1a |
| Methanol | 42.1 ± 0.7c | 31.5 ± 0.3e | 1.7 ± 0.1b,c | 9.7 ± 0.2a |
GAE: gallic acid equivalents, QE: quercetin equivalents, PB2E: proanthocyanidin B2 equivalents. SP: soluble principle. Values are reported as mean ± S.D. Different letters in the same column show significant differences among each treated group, according to Tukey's test (p ≤ 0.05).
Secondary metabolites like phenolic compounds are well known for their broad range of bioactivities. This property, along with their relatively safe nature, makes them an attractive target for the development of new bioproducts aimed to the cosmetic, health, and food industries.
On the other hand, industrial wastes represent an important source of pollution that lead to serious disposal problems and demand high processing costs. Therefore, it is necessary to seek for alternatives to exploit these wastes in a bid to generate a profit out of them and to reduce the amount of residues that are disposed. Wine wastes contain a vast amount of bioactive compounds known for their health benefits as antioxidants, anti-inflammatories, anticarcinogenic, etc. In accordance with this, in the present work, we evaluated the capacity of extracts of different polarities to prevent and inhibit the formation of biofilm and the growth of two bacteria that produce biofilm.
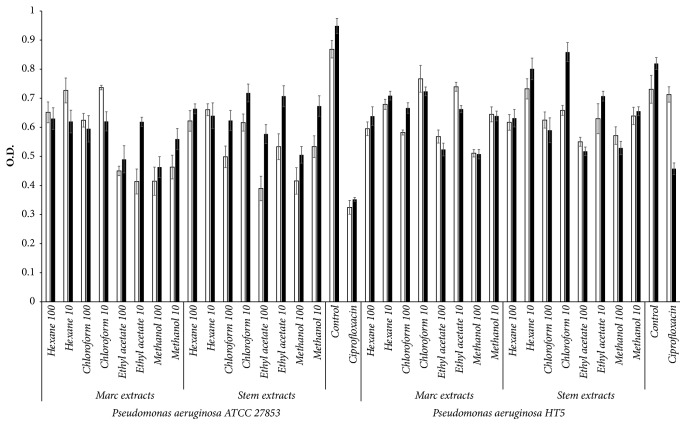
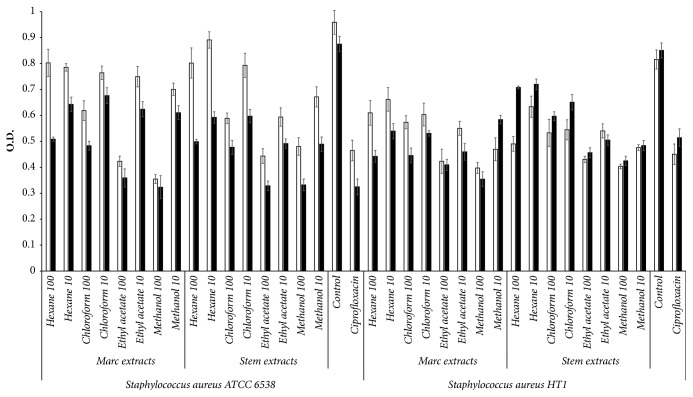
We studied the effect of the wine wastes extracts on two biofilm producing bacteria, S. aureus and P. aeruginosa, and we compared their effect on antibiotic-resistant strains and sensitive strains of both microorganisms. As shown in Figures 1 and 2, the reduction of the amount of biofilm observed appears to be due to the inhibition of biofilm production more than a depletion of viable cells, which is corroborated by the decrease noticed on the specific biofilm values. The stronger inhibition of the biofilm production was displayed by the most polar extracts of both wastes at 100 μg/mL, with inhibition rates ranging from 39% to 51% for P. aeruginosa ATTC 27853, 35–38% for P. aeruginosa HT5, 59–63% for S. aureus ATCC 6538, and 50–58% for S. aureus HT1. It is important to highlight that the effect on Gram-positive strains was similar to or higher than the one observed for the control, ciprofloxacin, which exhibited a level of inhibition of 63% for S. aureus ATCC and 40% for HT1 (p ≤ 0.05). One remarkable detail was that the inhibition of the biofilm formation by the wastes was higher than their capacity to inhibit the growth of all the strains, resulting in an ability to reduce the specific biofilm, a characteristic that was not observed on the antibiotic drugs tested. Only the concentration of 10 μg/mL of marc methanol extract inhibited the growth of S. aureus resistant strain in a similar way to the antibiotic (31%, p ≤ 0.05).
Figure 1.
Effect of the marc and stem extracts at 10 and 100 μg/mL on the viability at 570 nm (□) and biofilm production at 595 nm (■) of Pseudomonas aeruginosa ATCC 27853 and HT5 after 24 h of incubation. Control: Pseudomonas aeruginosa with the vehicle of the extracts. Data are presented as mean ± SD from three different experiments.
Figure 2.
Effect of the marc and stem extracts at 10 and 100 μg/mL on the viability at 570 nm (□) and biofilm production at 595 nm (■) of Staphylococcus aureus ATCC 6538 and HT1 after 24 h of incubation. Control: Staphylococcus aureus with the vehicle of the extracts. Data are presented as mean ± SD from three different experiments.
As for the viability of the different strains in the biofilm, the methanol and ethyl acetate extracts of marc and stem (100 μg/mL) exhibited the higher levels of inhibition of P. aeruginosa ATTC 27853 and both strains of S. aureus viability (47–63% of inhibition, p ≤ 0.05). Moreover, a higher or equal inhibitory capacity was also observed for the S. aureus strains compared to the ciprofloxacin. Even though the effect observed for P. aeruginosa HT5 was lower (22–30%, p ≤ 0.05), it was still considerable taking into account the fact that ciprofloxacin did not decrease the metabolic activity of this strain in the biofilm (viability assay).
According to what was described above, we can conclude that the polar extracts of both wastes are overall more active than the less polar ones. Their major effect could be explained by their higher content of polyphenols, whose antimicrobial and antibiofilm activities have already been demonstrated [41]. Our studies have shown a positive correlation between the content of tannins in the marc extracts and their capacity to inhibit the synthesis of biofilm by S. aureus and P. aeruginosa strains (r = 0.8–0.85). While we observed a positive correlation between the content of nonflavonoids in the stem extracts and the inhibition of biofilm formation (r = 0.78–0.99), the same correlation was observed between this phenolic compound and the depletion of viability (r = 0.82–0.86) exhibited by the stem extracts on all the strains tested.
Studies have demonstrated that quorum sensing triggers cells response to oxidative stress by inducing the synthesis of scavenging enzymes [42]. It is also one of the main sources of heterogeneity in biofilm since each cell is exposed to different levels of ROS and activates their own scavenging mechanisms in response to the variable stress. ROS may also stir up adaptive mechanisms that are more effective in the biofilm environment than in a unicellular form of life and prompt dispersal of cells from biofilm [42]. Therefore, scavenging oxidative species may help to prevent biofilm formation and might explain in part the antibiofilm activity exhibited by the samples evaluated in the present work.
The antioxidant activity of phenolic compounds is one of their most well-known and studied bioactivities. A wide range of mechanisms are involved in this process and the structure of the different molecules that are joint under the group of the polyphenols is determinant. In this work, we observed that the polar polyphenols present in the methanol and ethyl acetate extracts appear to exert their antioxidant activity by scavenging radicals or by reducing metal ions. As seen in Table 2, the most active sample in scavenging the ABTS·+ radical was marc's methanol extract with a SC50 of 10.6 ± 0.4 μg/mL. This sample was more than 6-fold more active than the rest of the polar extracts of both stem and marc and about 13-fold more active than chloroform fraction of stem, the only nonpolar fraction that was able to scavenge the ABTS·+ radical. On the other hand, the ethyl acetate extract of marc showed a similar scavenging capacity than stem's methanol and ethyl acetate extracts (Table 2). As for the iron reducing power (Table 2), the most active sample was also marc's methanol extract, with a RC50 4- to 6-fold lower than the rest of the polar fractions of both samples. None of the nonpolar fractions of both samples reached the RC50 up to the maximum concentration tested. All the samples have a limited capacity to scavenge NO radicals and did not reach the SC50 values up to a maximum concentration tested (500 μg/mL). Nonetheless, marc's methanol and ethyl acetate extracts were the only fractions that reached the SC25 up to the maximum concentration tested.
Table 2.
Antioxidant activity of the stem and marc extracts.
| Sample |
ABTS radical scavenging SC50 (μg/mL) |
Fe
3+
Reducing power RC50 (μg/mL) |
Fe
2+
Chelating capacity CC25 (μg/mL) |
NO Scavenging capacity SC 25 (μg/mL) | |
|---|---|---|---|---|---|
| Marc | Hexane | - | - | 173.3 ± 6.9c | - |
| Chloroform | - | - | 172.6 ± 9.9c | - | |
| Ehtyl acetate | 65.3 ± 0.4c | 160.0 ± 4.7c | - | 437.2± 0.5c | |
| Methanol | 10.6 ± 0.4b | 27.3 ± 0.1a | 426.1 ± 20.9d | 267.3± 32.4b | |
|
| |||||
| Stem | Hexane | - | - | 173.5 ± 4.1c | - |
| Chloroform | 138.7 ± 1.9d | - | 109.2 ± 10.3b | - | |
| Ethyl acetate | 64.2 ± 0.9c | 106.0 ± 1.4b | 124.1 ± 17.9b | - | |
| Methanol | 66.2 ± 2.5c | 140.9 ± 1.3b,c | 661.3 ± 2.3e | - | |
|
| |||||
| Quercetin | 3.6 ± 0.5a | ||||
| Ascorbic acid | 1.9 ± 0.4a | 5.4± 0.03a | 29.9 ± 0.7a | ||
| Na 2 EDTA | 5.0 ± 0.3a | ||||
ABTS·+ radical scavenging concentration (SC), Fe3+ reducing concentration (RC), Fe2+ chelating concentration (CC), NO Scavenging capacity (SC) of marc and stem extracts. Values (mean ± SDE, n = 3) in the same column followed by the same letter are not significantly different (Tuckey's test, p ≤ 0.05).
While nonpolar fractions appear to exert their antioxidant capacity mostly by chelating metal ions, as seen in Table 2, the most active fraction was the chloroform extract of stem, which was 1.6-fold more active than the rest of the nonpolar fraction, and was up to 4-6-fold more active than the methanol extracts of stem and marc. However, the only fraction that reached the SC50 up to the maximum concentration tested was the hexane fraction of marc (SC50 = 500.0 ± 3.7 μg/mL), while the ethyl acetate fraction of marc was not able to chelate the Fe2+ at all the concentration tested. It has been proven that a specific structure is needed for a molecule to be able to chelate metals, which limits the number of molecules that exhibits this property [43]. The presence of these types of molecules would allow controlling the oxidative stress in biofilms, since reactive oxygen species (ROS) are also generated through a redox reaction led by low molecular weight iron and iron ligands [44].
Correlation studies showed a positive correlation between the iron chelating activity with the content of nonflavonoids (r = 0.98, p ≤ 0.05) for marc samples. On the other hand, we observed a positive correlation between the content of tannins versus ABTS scavenging capacity for marc samples (r = 0.93, p ≤ 0.05) and a positive correlation between the content of total phenolics (r = 0.93, p ≤ 0.05), nonflavonoids (r = 0.98, p ≤ 0.05), and tannins (r = 0.91, p ≤ 0.05) versus the ABTS scavenging capacity of the stem samples. There was a positive correlation between the content of total phenolic compounds (r = 0.91, p ≤ 0.05) and tannins (r = 0.99, p ≤ 0.01) versus iron reducing power in marc samples. Furthermore, there was a positive correlation with the content of nonflavonoids (r = 0.98, p ≤ 0.05) for the stem samples.
4. Conclusions
In this work, we evaluated the potential use of different extracts of grape's stem and marc of Torrontes white wine produced in Cafayate, Argentina. These byproducts inhibited the biofilm production, as well as the metabolic activity of P. aeruginosa and S. aureus strains in the biofilm environment. The major inhibition of the biofilm formation and the metabolic activity of all the strains were exerted by the polar extracts of marc and stem extracts at 100 μg/mL. We also evaluated the antioxidant capacity of these extracts since it was proven that there is a correlation between oxidative stress and biofilm synthesis. The methanol and ethyl acetate extracts, which showed the highest content of polyphenolics, exhibited the strongest scavenging capacity of ABTS and NO, as well as the highest Fe3+ reducing power. Moreover, in accordance with these results, the correlation studies showed a positive correlation between the content of phenolic compounds, the antioxidant activity, and the antibiofilm capacity of the winemaking wastes. These results display the potentiality of wine wastes to be used to prevent or to reduce the formation of biofilm. Furthermore, their abundance makes them an attractive and affordable source of antibiofilm agents for the healthcare and food industries.
Acknowledgments
The authors acknowledge the financial support from the SCAIT-UNT (Project Codes 26D 552-1 and G533), the Agencia Nacional de Promoción Científica y Técnica, ANPCyT (Project Code PICT 3136), and the Consejo Nacional de Investigaciones Científicas y Técnicas, CONICET (Project Codes PIP 00533 and 00590), AGROVALOR Project N° 25 Ministerio de Agricultura, Ganaderia y Pesca (MAGyP), and Ministerio de Educacion (ME) of Argentine.
Abbreviations
- ABTS:
2,2-Azino-bis-(3-ehylbenzothiazoline-6-sulphonic acid) di-ammonium salt
- NO:
Nitric oxide
- ROS:
Reactive oxygen species
- DMSO:
Dimethyl sulfoxide
- SP:
Soluble principle
- GAE:
Gallic acid equivalents
- QE:
Quercetin equivalents
- PB2E:
Proanthocyanidin B2 equivalents
- MTT:
3-[4,5-Dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide
- PBS:
Phosphate buffered saline
- MH:
Marc hexane
- MC:
Marc chloroform
- MA:
Marc ethyl acetate
- MM:
Marc methanol
- SH:
Stem hexane
- SC:
Stem chloroform
- SA:
Stem ethyl acetate
- SM:
Stem methanol.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors have declared that there are no conflicts of interest.
Authors' Contributions
Carolina María Viola and Romina Torres-Carro have the same participation. María Rosa Alberto and Mario Eduardo Arena contributed equally to the manuscript
References
- 1.Costerton J. W., Stewart P. S., Greenberg E. P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Vlamakis H., Chai Y., Beauregard P., Losick R., Kolter R. Sticking together: Building a biofilm the Bacillus subtilis way. Nature Reviews Microbiology. 2013;11(3):157–168. doi: 10.1038/nrmicro2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer G. L. Staphylococcus aureus: a well-armed pathogen. Clinical Infectious Diseases. 1998;26(5):1179–1181. doi: 10.1086/520289. [DOI] [PubMed] [Google Scholar]
- 4.Parsek M. R., Singh P. K. Bacterial biofilms: an emerging link to disease pathogenesis. Annual Review of Microbiology. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues J. B., Souza N. T., Scarano J. O., et al. Efficacy of using oregano essential oil and carvacrol to remove young and mature Staphylococcus aureus biofilms on food-contact surfaces of stainless steel. LWT- Food Science and Technology. 2018;93:293–299. doi: 10.1016/j.lwt.2018.03.052. [DOI] [Google Scholar]
- 6.Ammons M. C. B., Ward L. S., Dowd S., James G. A. Combined treatment of Pseudomonas aeruginosa biofilm with lactoferrin and xylitol inhibits the ability of bacteria to respond to damage resulting from lactoferrin iron chelation. International Journal of Antimicrobial Agents. 2011;37(4):316–323. doi: 10.1016/j.ijantimicag.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arena M. E., Ramos A. N., Valdez J. C. Regulación del quórum sensing de Pseudomonas por productos naturales. Saabrücken, Germany: Academic Publishing GmbH & Co.; 2012. [Google Scholar]
- 8.Luciardi M. C., Pérez Hernández M. V., Muruaga N., Bardón A., Arena M. E., Cartagena E. Volatiles from Subtropical Convolvulaceae That Interfere with Bacterial Cell-to-Cell Communication as Potential Antipathogenic Drugs. Evidence-Based Complementary and Alternative Medicine. 2016;2016:8. doi: 10.1155/2016/7890260.7890260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssens J. C. A., Steenackers H., Robijns S., et al. Brominated furanones inhibit biofilm formation by Salmonella enterica serovar Typhimurium. Applied and Environmental Microbiology. 2008;74(21):6639–6648. doi: 10.1128/AEM.01262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilabert M., Cartagena E., Escobar G., Bardón A., Arena M. E. Volatile terpenoids from water pepper (Polygonum punctatum) against Pseudomonas aeruginosa and Staphylococcus aureus Virulence strategies. Global Journal of Agricultural Innovation, Research & Development. 2014;1:3–10. [Google Scholar]
- 11.Luciardi M. C., Blázquez M. A., Cartagena E., Bardón A., Arena M. E. Mandarin essential oils inhibit quorum sensing and virulence factors of Pseudomonas aeruginosa. LWT- Food Science and Technology. 2016;68:373–380. doi: 10.1016/j.lwt.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 12.Bustamante M. A., Moral R., Paredes C., Pérez-Espinosa A., Moreno-Caselles J., Pérez-Murcia M. D. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Management. 2008;28(2):372–380. doi: 10.1016/j.wasman.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Zhang N., Hoadley A., Patel J., Lim S., Li C. Sustainable options for the utilization of solid residues from wine production. Waste Management. 2017;60:173–183. doi: 10.1016/j.wasman.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Duba K. S. Supercritical technologies for the valorization of wine industry by-products. Trento, Italy: Department of Civil, Environmental and Mechanical Engineering, University of Trento; 2015. [Google Scholar]
- 15.Apolinar-Valiente R., Romero-Cascales I., Gómez-Plaza E., López-Roca J. M., Ros-García J. M. The composition of cell walls from grape marcs is affected by grape origin and enological technique. Food Chemistry. 2015;167:370–377. doi: 10.1016/j.foodchem.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Amorin F. L., Rocha I. S., Ferreira E. S., Machado B. A. S., Umsza-Guez M. A. Technological prospecting related to related patent deposits bioactive compounds present on grapes. Cadernos de Prospecção. 2015;8(4):801–807. [Google Scholar]
- 17.Burin V. M., Ferreira-Lima N. E., Panceri C. P., Bordignon-Luiz M. T. Bioactive compounds and antioxidant activity of Vitis vinifera and Vitis labrusca grapes: evaluation of different extraction methods. Microchemical Journal. 2014;114:155–163. doi: 10.1016/j.microc.2013.12.014. [DOI] [Google Scholar]
- 18.Sahpazidou D., Geromichalos G. D., Stagos D., et al. Anticarcinogenic activity of polyphenolic extracts from grape stems against breast, colon, renal and thyroid cancer cells. Toxicology Letters. 2014;230(2):218–224. doi: 10.1016/j.toxlet.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 19.Lingua M. S., Fabani M. P., Wunderlin D. A., Baroni M. V. In vivo antioxidant activity of grape, pomace and wine from three red varieties grown in Argentina: its relationship to phenolic profile. Journal of Functional Foods. 2016;20:332–345. doi: 10.1016/j.jff.2015.10.034. [DOI] [Google Scholar]
- 20.Martillanes S., Rocha-Pimienta J., Cabrera-Bañegil M., Martín-Vertedor D., Delgado-Adámez J. Application of phenolic compounds for food preservation: Food additive and active packaging. In: Soto-Hernandez M., Palma-Tenango M., Garcia-Mateos M. R., editors. Phenolic compounds biological activity. Mexico: InTech; 2017. pp. 39–58. [Google Scholar]
- 21.Pinelo M., Rubilar M., Jerez M., Sineiro J., Núñez M. J. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. Journal of Agricultural and Food Chemistry. 2005;53(6):2111–2117. doi: 10.1021/jf0488110. [DOI] [PubMed] [Google Scholar]
- 22.Machado B. A. S., Silva C. C., Guedes C. M. C., Umsza-Guez M. A., Cirqueira M. G., Oliveira R. S. Process for the preparation of concentrate rich in bioactive compounds and product obtained Invention Patent National Institute of Industrial Property. BR1020140302425. 2014 [Google Scholar]
- 23.Vijaya Kumar Reddy C., Sreeramulu D., Raghunath M. Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Research International. 2010;43(1):285–288. doi: 10.1016/j.foodres.2009.10.006. [DOI] [Google Scholar]
- 24.Minatel I. O., Borges C. V., Ferreira M. I., et al. Chapter 1: Phenolic compounds biological activity. In: Soto-Hernandez M., Palma-Tenango M., Garcia-Mateos M. R., editors. Phenolic compounds: Functional properties, impact of processing and bioavailability. InTech: Mexico; 2017. pp. 1–24. [Google Scholar]
- 25.Singleton V. L., Orthofer R., Lamuela-Raventós R. M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 26.Torres Carro R., Isla M. I., Ríos J. L., Giner R. M., Alberto M. R. Anti-inflammatory properties of hydroalcoholic extracts of Argentine Puna plants. Food Research International. 2015;67:230–237. doi: 10.1016/j.foodres.2014.11.012. [DOI] [Google Scholar]
- 27.Popova M., Silici S., Kaftanoglu O., Bankova V. Antibacterial activity of Turkish propolis and its qualitative and quantitative chemical composition. Phytomedicine. 2005;12(3):221–228. doi: 10.1016/j.phymed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Prior R. L., Fan E., Ji H., et al. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. Journal of the Science of Food and Agriculture. 2010;90(9):1473–1478. doi: 10.1002/jsfa.3966. [DOI] [PubMed] [Google Scholar]
- 29.O'Toole G. A., Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Molecular Microbiology. 1998;28(3):449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 30.Sandasi M., Leonard C. M., Van Vuuren S. F., Viljoen A. M. Peppermint (Mentha piperita) inhibits microbial biofilms in vitro. South African Journal of Botany. 2011;77(1):80–85. doi: 10.1016/j.sajb.2010.05.011. [DOI] [Google Scholar]
- 31.Amaya S., Pereira J. A., Borkosky S. A., Valdez J. C., Bardón A., Arena M. E. Inhibition of quorum sensing in Pseudomonas aeruginosa by sesquiterpene lactones. Phytomedicine. 2012;19(13):1173–1177. doi: 10.1016/j.phymed.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Jadhav S., Shah R., Bhave M., Palombo E. A. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. Food Control. 2013;29(1):125–130. doi: 10.1016/j.foodcont.2012.05.071. [DOI] [Google Scholar]
- 33.Torres Carro R., D'Almeida R. E., Isla M. I., Alberto M. R. Antioxidant and anti-inflammatory activities of Frankenia triandra (J. Rémy) extracts. South African Journal of Botany. 2016;104:208–214. doi: 10.1016/j.sajb.2015.09.020. [DOI] [Google Scholar]
- 34.D'Almeida R. E., Isla M. I., De L. Vildoza E., Quispe C., Schmeda-Hirschmann G., Alberto M. R. Inhibition of arachidonic acid metabolism by the Andean crude drug Parastrephia lucida (Meyen) Cabrera. Journal of Ethnopharmacology. 2013;150(3):1080–1086. doi: 10.1016/j.jep.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Torres-Carro R., Isla M. I., Thomas-Valdes S., Jiménez-Aspee F., Schmeda-Hirschmann G., Alberto M. R. Inhibition of pro-inflammatory enzymes by medicinal plants from the Argentinean highlands (Puna) Journal of Ethnopharmacology. 2017;205:57–68. doi: 10.1016/j.jep.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Muñoz Quezada S., Gómez Llorente C., Gil Hernández A. Compuestos bioactivos de los alimentos de origen vegetal y obesidad. Nutrición Clínica en Medicina. 2010;4(3):138–152. [Google Scholar]
- 37.Mihailovic-Stanojevic N., Savikin K., Zivkovic J., et al. Moderate consumption of alcohol-free red wine provide more beneficial effects on systemic haemodynamics, lipid profile and oxidative stress in spontaneously hypertensive rats than red wine. Journal of Functional Foods. 2016;26:719–730. doi: 10.1016/j.jff.2016.08.051. [DOI] [Google Scholar]
- 38.Motilva M.-J., Macià A., Romero M.-P., Rubió L., Mercader M., González-Ferrero C. Human bioavailability and metabolism of phenolic compounds from red wine enriched with free or nano-encapsulated phenolic extract. Journal of Functional Foods. 2016;25:80–93. doi: 10.1016/j.jff.2016.05.013. [DOI] [Google Scholar]
- 39.Garcia-Jares C., Vazquez A., Lamas J., Pajaro M., Alvarez-Casas M., Lores M. Antioxidant White Grape Seed Phenolics: Pressurized Liquid Extracts from Different Varieties. Antioxidants. 2015;4(4):737–749. doi: 10.3390/antiox4040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barros A., Gironés-Vilaplana A., Teixeira A., et al. Evaluation of grape (Vitis vinifera L.) stems from Portuguese varieties as a resource of (poly)phenolic compounds: A comparative study. Food Research International. 2014;65:375–384. doi: 10.1016/j.foodres.2014.07.021. [DOI] [Google Scholar]
- 41.Jagani S., Chelikani R., Kim D.-S. Effects of phenol and natural phenolic compounds on biofilm formation by Pseudomonas aeruginosa. Biofouling. 2009;25(4):321–324. doi: 10.1080/08927010802660854. [DOI] [PubMed] [Google Scholar]
- 42.Gambino M., Cappitelli F. Mini-review: Biofilm responses to oxidative stress. Biofouling. 2016;32(2):167–178. doi: 10.1080/08927014.2015.1134515. [DOI] [PubMed] [Google Scholar]
- 43.Khokhar S., Owusu Apenten R. K. Iron binding characteristics of phenolic compounds: Some tentative structure-activity relations. Food Chemistry. 2003;81(1):133–140. doi: 10.1016/S0308-8146(02)00394-1. [DOI] [Google Scholar]
- 44.Thomas C., Mackey M. M., Diaz A. A., Cox D. P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Report. 2009;14(3):102–108. doi: 10.1179/135100009X392566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.