Abstract
Physiological stress systems and the brain rapidly develop through infancy. While the roles of caregiving and environmental factors have been studied, implications of maternal physiological stress are unclear. We assessed maternal and infant diurnal cortisol when infants were 6 and 12 months. We measured 12-month infant electroencephalography (EEG) 6–9 Hz power during a social interaction. Steeper 6-month maternal slope predicted steeper 12-month infant slope controlling for 6-month infant slope and breastfeeding. Steeper 6-month maternal slope predicted lower 6–9 Hz power. Six-month maternal area under the cuve (AUCg) was unrelated to 12-month infant AUCg and 6–9 Hz power. Psychosocial, caregiving, and breastfeeding variables did not explain results. At 6 months, maternal and infant slopes correlated, as did maternal and infant AUCg. Twelve-month maternal and infant cortisol were unrelated. Results indicate maternal slope is an informative predictor of infant physiology and suggest the importance of maternal physiological stress in this developmental period.
Keywords: Infant, Physiological Attunement, Diurnal Cortisol, Slope, EEG power
In infancy, physiological stress systems and the brain rapidly develop. Early experiences have enduring effects on both of those aspects of development (Arnsten, 2015; Bick & Nelson, 2015; McEwen, 2006). Therefore, it is important to understand factors that promote the development of healthy infant physiology. While the roles of maternal behavior and environmental experiences have been widely studied, much less is known about implications of maternal physiological stress. As development is cumulative and “builds on itself” (Sroufe, 2013), it is important to explore how early maternal physiological stress shapes the infant’s developing physiological systems.
Physiological Attunement of Maternal and Infant Physiological Stress
The role of the caregiver in regulating infant physiological stress, especially the hypothalamic-pituitary-adrenal (HPA) axis that produces the hormone cortisol, is well established (Gunnar & Donzella, 2002). As an infant’s HPA axis is developing and maturing, caregivers serve as a social buffer against the damaging physiological effects of stress and threat (Gunnar & Quevedo, 2007). Infants therefore depend on a sensitive, responsive caregiver to help them regulate physiological stress (Gunnar & Donzella, 2002).
Researchers have posited the idea of physiological attunement (or coregulation) to understand the caregiver’s role in shaping infant physiological stress. Physiological attunement reflects the idea that maternal and infant physiological systems, including the HPA axis, are in sync (Bright, Granger, & Frick, 2012; Hibel, Granger, Blair & Finegood, 2015; van Bakel & Riksen-Walraven, 2008). Indeed, it is well established that maternal and infant basal and diurnal cortisol levels are related (Benjamin Neelon, Stroo, Mayhew, Maselko, & Hoyo, 2015; Bright et al., 2012; Clearfield, Carter-Rodriguez, Merali, & Shober, 2014; Fuchs, Möhler, Resch, & Kaess, 2016; Middlemiss, Granger, Goldberg, & Nathans, 2012; Spangler, 1991; Stenius et al., 2008). Physiological attunement has been suggested to have evolutionary roots, as it is adaptive for mothers and infants to pick up on each other’s cues to external risk, resulting in attuned physiological stress. However, physiological attunement might not always be beneficial (Ruttle, Serbin, Stack, Schwartzman, & Shirtcliff, 2011). If a mother is continually physiologically stressed, then this might affect infant physiological regulation. Thus a better understanding of the mechanisms underlying links between mother and infant cortisol is needed.
Maternal and infant cortisol levels could be related due to caregiving. Mothers who are well regulated may be better able to buffer the infant from excessive cortisol output through sensitive caregiving. Thus a mother with healthy diurnal cortisol regulation may be able to be sensitive, responsive and help regulate infant stress. This buffering is not only important in situations involving threat or challenge (Atkinson et al., 2013; Laurent, Ablow, & Measelle, 2012; Ruttle et al., 2011), but also for sensitivity in daily interactions (Ben-Dat Fisher et al., 2007; Letourneau, Watson, Duffett-Leger, Hegadoren, & Tryphonopoulos, 2011; Philbrook et al., 2014; Ruttle et al., 2011).
While it is unclear whether maternal cortisol regulation relates to caregiving, there is evidence for links between caregiving and infant diurnal cortisol (Ben-Dat Fisher et al., 2007; Letourneau et al., 2011; Philbrook et al., 2014). For example, infants at both 1 and 3 months old had lower cortisol output from afternoon through the next morning at waking when their mothers were more sensitive at bedtime (Philbrook et al., 2014). This suggests that sensitive responding in a common daily situation may be particularly important for infant cortisol regulation.
Another mechanism at play could be that mothers and infants experience a similar psychosocial environment and are both physiologically responding to the environment similarly. Lower socioeconomic status (SES) has been related to higher evening cortisol in both mothers and infants (Clearfield et al., 2014) which could be due to the common daily experience of SES. Yet because a caregiving measure was not included in that study, it is possible that these results could be partially mediated by caregiving.
In addition to effects of caregiving and the psychosocial environment, physiological attunement could also reflect shared genes. Approximately 30% of the variance in waking cortisol is attributed to genetic influences (Bartels, Geus, Kirschbaum, Sluyter, & Boomsma, 2003; Ouellet-Morin et al., 2009, 2016; Van Hulle, Shirtcliff, Lemery-Chalfant, & Goldsmith, 2012) while little to zero percent of afternoon or evening cortisol is attributable to genetics (Bartels et al., 2003; Schreiber et al., 2006; Van Hulle et al., 2012). Further about 30% of cortisol slope is explained by genetics (Ouellet-Morin et al., 2016; Van Hulle et al., 2012), potentially due to the genetic influence on waking levels. However most studies have been with older children (Bartels et al., 2003; Ouellet-Morin et al., 2016; Schreiber et al., 2006; Van Hulle et al., 2012), with only one infant study that involved one waking sample on a single day (Ouellet-Morin et al., 2009).
However similarities between maternal and infant cortisol cannot fully be explained by genetics and other potential pathways include shared environment, prenatal effects, and breastfeeding. Maternal and infant cortisol levels are more strongly related than father and infant cortisol (Stenius et al., 2008). This could be because mothers spend more time with their infants, supporting the role of a shared environment. Physiological attunement could also be due to effects of the intrauterine environment on the developing fetus. Maternal prenatal cortisol relates to infant cortisol responses to a stressor (see for review Zijlmans, Riksen-Walraven, & de Weerth, 2015), but it is unclear if prenatal maternal cortisol predicts infant diurnal cortisol. Infants may be also exposed to maternal cortisol via breastfeeding (Benjamin Neelon et al., 2015) as cortisol is present in breast milk (Grey, Davis, Sandman, & Glynn, 2013; Hamosh, 2001). Further, mothers may be more attuned and sensitive from the experience of skin-to-skin contact inherent in breastfeeding (Kim et al., 2011). Cortisol attunement on one day at bedtime in a small sample was found in breastfeeding but not formula-fed infants (Benjamin Neelon et al., 2015), though more research is needed. In sum, while genetic, shared environment, prenatal, and breastfeeding influences are likely, daily experiences may also matter for mother-infant cortisol associations.
If day-to-day caregiving is a primary pathway underlying physiological attunement, then salivary cortisol variables indexing daily physiological stress are important to measure, including area under the curve with respect to ground (AUCg) and diurnal slope. By around 3 months, infants have circadian cortisol rhythms (Larson, White, Cochran, Donzella, & Gunnar, 1998; de Weerth, Zijl, & Buitelaar, 2003), supporting these measures. AUCg from waking to bed indexes cumulative cortisol exposure, with higher AUCg reflecting greater daily cortisol output. Diurnal slope indexes change in daily cortisol and is a marker of physiological regulation (Adam & Gunnar, 2001). A steep slope characterized as higher morning cortisol levels and a drop across the day, indexes healthy cortisol regulation, while a flatter slope suggests HPA axis dysfunction (Fries, Dettenborn, & Kirschbaum, 2009; Ross, Murphy, Adam, Chen, & Miller, 2014). Cortisol slopes have been related in mothers and 6-month-old infants, showing maternal and infant cortisol regulation across the day are attuned (Stenius et al., 2008).
Early Experiences and Infant Neural Activation
While there is a strong literature on effects of early environments on neural activation, assessed by electroencephalography (EEG; Lupien, McEwen, Gunnar, & Heim, 2009; Marshall, Reeb, Fox, Nelson, & Zeanah, 2008; Otero, Pliego-Rivero, Fernández, & Ricardo, 2003; Tarullo, Garvin, & Gunnar, 2011; Tomalski et al., 2013), much less is known about the role of maternal cortisol for infant EEG. EEG power is a widely used measure which assesses voltage at an electrode site and reflects global neural activation over a period of time (Molfese, Molfese, & Kelly, 2001). EEG power is quantified in frequency bands. Different bands have different functional correlates, with higher frequency power generally related to more alertness and attention (Engel, Fries, & Singer, 2001; Reid, Csibra, Belsky, & Johnson, 2007). Further, EEG is a functional measure indexing neural activation in the immediate context at the time of recording. Infant EEG power is sensitive to social context (Jones, Venema, Lowy, Earl, & Webb, 2015) and much of infant learning and engagement takes place in social situations. This suggests the relevance of recording EEG during a socially engaging context rather than a neutral baseline.
With development the spectral distribution of EEG power shifts, with increasing power concentrated in higher frequencies and less power in lower frequencies, which may reflect neurodevelopmental processes such as neuronal growth and myelination (John et al., 1980; Marshall, Bar-Haim, & Fox, 2002; Matoušek & Petersén, 1973). This developmental shift appears to be sensitive to the quality of the environment. Children who experience adversity show an excess of slow wave power and a deficit in high frequency power, suggesting delayed maturation (Marshall et al., 2008; Otero et al., 2003; Tarullo et al., 2011; Tomalski et al., 2013), while improvements in the caregiving environment are associated with increases in high frequency power (Vanderwert, Zeanah, Fox, & Nelson, 2016). Further, patterns of EEG activity in infancy and early childhood are associated with cognitive functioning (Bell, 2001; Benasich, Gou, Choudhury, & Harris, 2008; Brito, Fifer, Myers, Elliott, & Noble, 2016; Cuevas, Raj, & Bell, 2012; Gou, Choudhury, & Benasich, 2011; Tarullo et al., 2017), with high frequency activity linked to better cognitive, linguistic, and executive functioning outcomes (Benasich et al., 2008; Brito et al., 2016; Gou et al., 2011; Tarullo et al., 2017). Given the significance of early EEG patterns for later development, it is important to understand how maternal physiological stress may contribute to EEG power in infancy.
As with possible mechanisms underlying cortisol attunement, maternal cortisol could relate to infant neural activation through multiple pathways. Prenatal maternal cortisol predicts infant cognitive outcomes assessed with behavioral tasks (Davis & Sandman, 2010; Huizink, Robles de Medina, Mulder, Visser, & Buitelaar, 2003). This suggests a role for maternal cortisol in predicting underlying neural processes as well, however research is needed to understand if and how maternal postnatal cortisol relates to infant EEG. Early caregiving and maternal sensitivity relate to infant EEG power, asymmetry, and event-related potentials (ERPs) (Hane & Fox, 2006; Marshall et al., 2008; Taylor-Colls & Pasco Fearon, 2015) and lower SES relates to EEG power (Otero et al., 2003; Tomalski et al., 2013). It is possible that maternal physiological stress is playing a role in these associations such as mediating links between SES and infant EEG or influencing caregiving behavior.
Current Study
The goals of this study were (1) to understand if maternal physiological stress relates to more than one infant physiological system and (2) to disentangle potential mechanisms underlying those associations. We addressed these goals in three ways. First, we used a longitudinal design. Many studies assess maternal and infant diurnal cortisol levels at a single time point in infancy (Benjamin Neelon et al., 2015; Clearfield et al., 2014; Spangler, 1991; Stenius et al., 2008). Assessing how maternal and infant cortisol levels are related within and across time points could help clarify the pathways underlying physiological attunement. Second, we included infant cortisol and EEG power to bridge levels of analysis and broaden our understanding of the role of maternal cortisol for infant physiology. Third, we included psychosocial, breastfeeding, and mother-infant interaction variables to elucidate the mechanisms underlying any observed links between maternal cortisol and infant cortisol and EEG power.
We measured maternal and infant diurnal salivary cortisol when infants were 6 and 12 months. Slope indexed cortisol regulation and AUCg indexed cumulative cortisol exposure. At 12 months, we assessed infant EEG 6–9 Hz power during a social interaction. We anticipated that 6-month maternal slope would predict 12-month infant slope and 6-month maternal AUCg would predict 12-month infant AUCg. We expected that steeper 6-month maternal slope and smaller maternal AUCg would predict lower 12-month infant 6–9 Hz power. We explored the roles of mother-infant interaction variables, maternal stressful life events, depression, parenting stress, SES, and breastfeeding.
Methods
Participants
The sample for these analyses consisted of 65 mother-infant dyads who participated in a home visit when infants were 6 months old and a laboratory visit when infants were 12 months old. All were singletons who had no known hearing, visual, neurological, or developmental disorders (see Table 1 for demographics of the final sample).
Table 1.
Demographic Information
| Maternal age (years) | |
| M(SD) | 33.72 (4.05) |
| Infant age (months) | |
| Home Visit M (SD) | 6.68 (.45) |
| Lab Visit M (SD) | 12.12 (.71) |
| Maternal ethnicity | |
| Caucasian | 79.7 % |
| Asian | 9.4 % |
| Black | 6.3 % |
| Hispanic | 1.6 % |
| Native American | 1.6 % |
| Multiracial/Other | 1.6 % |
| Infant ethnicity | |
| Caucasian | 69.2 % |
| Asian | 3.1 % |
| Black | 4.5 % |
| Multiracial/Other | 23.1 % |
| Income-to-needs ratio; M (SD) | 5.76 (3.60) |
| Maternal education (% with at least a 4 year college degree) |
87.7 % |
| Paternal education (% with at least a 4 year college degree) |
82.6 % |
| Maternal occupational prestige (1-5 scale); M (SD) |
3.96 (1.02) |
| Paternal occupational prestige (1-5 scale); M (SD) |
3.76 (1.07) |
| Maternal Parenting Stress (36 – 180 scale); M (SD) |
73.58 (11.36) |
| Number of Stressful Life Events M (SD) |
2.34 (1.91) |
| Maternal Depression (0 - 60 scale); M (SD) |
10.23 (8.21) |
| Infants Breastfeeding at 6 months (%) | 82.8 % |
| Infants Breastfeeding at 12 months (%) | 64.6 % |
The primary goal was to assess how 6-month maternal salivary cortisol related to infant salivary cortisol and EEG power at 12 months. Therefore a dyad had to have useable maternal cortisol at 6 months and at least one of the infant outcome measures, cortisol or EEG, at 12 months. Of 130 mother-infant dyads who participated in the 6-month home visit, 97 (74.6%) returned for the laboratory visit. Of those 97, we excluded 16 because they lacked usable maternal cortisol data at 6 months and 16 because they lacked usable infant cortisol or infant EEG data at 12 months. This resulted in a final sample of 65 dyads (38 female infants).
The infants who were excluded did not significantly differ from the final sample in terms of SES, maternal age, total number of stressful life events or maternal depression. Mothers included in the final sample reported higher parenting stress (M = 73.74, SD = 11.33) compared to mothers not in the final sample (M = 69.00, SD = 11.73), t (119) = - 2.26, p = .03. Female infants were more likely to be a part of the final sample compared to male infants, X2 (1, N = 130) = 6.12, p = .01.
General Procedure
Participants were recruited from a department-maintained database of families interested in participating in research; from publically available state birth records; from online advertising; and through face-to-face recruitment events. This study was approved by the Boston University institutional review board. Visits were scheduled when infants would be well rested and informed consent was obtained.
Home visit (6 months).
Dyads completed a one-hour home visit that included questionnaires, infant behavioral measures, and a mother-infant free play interaction. Mothers were trained on home salivary collection procedures. After saliva collection was complete, research staff collected the samples.
Laboratory visit (12 months).
Families were invited to participate in a 90-minute laboratory visit. Continuous EEG was recorded for 20 minutes. The visit also included maternal questionnaires and infant behavioral measures. The same salivary collection procedure from the 6-month visit was followed.
Measures
Salivary Cortisol (6 and 12 months).
Mothers were instructed to collect salivary samples from themselves and their infants immediately upon the infant’s waking and at the infant’s bedtime (just before the last feeding) when they spent most of the day together and when neither one was sick. This sampling was repeated for 3 days. Mothers were instructed to avoid dairy and caffeine in the hour prior to sampling and to sample when it had been at least an hour since the infant was last fed and had last napped. Compliance was assessed via home diaries.
An infant-safe synthetic swab (Salimetrics, State College, PA) was placed in the infant’s mouth and mothers placed a synthetic swab in their own mouths for 60 seconds. Mothers were instructed to store samples in the freezer. Research staff collected the samples, which were frozen at −20° C until they were sent to Trier Laboratories in Germany for assay.
Two full days of sampling was the minimum for inclusion in analyses. Sixty-five mothers and 64 infants met this criterion at 6 months, 55 mothers and 55 infants met this criterion at 12 months. For 6-month cortisol, 54 mothers and 51 infants had three full days, and 11 mothers and 13 infants had two full days. For 12-month cortisol, 43 mothers and 38 infants had three full days, and 12 mothers and 17 infants had two full days.
Statistically extreme values for these dyads were not explained by illness or medications. Therefore, these values were winsorized to three standard deviations from the mean. When necessary to meet the assumptions of normality, variables were log transformed. Two variables were calculated to assess diurnal cortisol, standardized and averaged across the days of sampling: slope across the day and AUCg. Slopes were computed using the rise-over-run formula: change across the day in cortisol divided by the time elapsed from morning to bedtime sample. AUCg was calculated as an estimate of cumulative cortisol exposure using the trapezoid formula (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). We included both slope and AUCg as they provide different pieces of information about the diurnal rhythm. For example, a flat slope simply means cortisol levels did not change much from morning to evening. AUCg then provides information about cortisol exposure. Thus someone with a flat slope could have cortisol values that start high in the morning and end high in the evening resulting in a high AUCg or start low in the morning and end low in the evening, resulting in a low AUCg.
Average time since waking when the morning sample was taken was calculated for mothers (6 months: M = 24.39 mins., SD = 20.90 mins.; 12 months: M = 21.38 mins., SD = 19.39 mins.) and infants (6 months: M = 17.11 mins., SD = 16.85 mins.; 12 months: M = 16.50 mins., SD = 17.38 mins.) To control for variability in the time of sampling relative to the cortisol awakening response that peaks about 30 minutes after waking, the square of the time since wake was regressed out of slope and AUCg values, and the residual values were used in analyses.
Parenting stress (6 months).
We used the Parenting Stress Index, 4th Edition short form (Abidin, 1995). Mothers rated 36 items on a 5-point scale, which were summed to yield a Total Stress Scale (possible range 36–180). Seven questions were part of the Defensive Responding scale to assess if mothers were answering to look favorable by minimizing problems or negativity. Mothers with a score of less than 10 on this scale are considered defensive responders. Five mothers met this criterion and their scores were not included in analyses. The PSI is validated for use with parents of children aged 1 month to 12 years (Abidin, 1995).
Stressful life events (6 months).
We used the Perceived Stress Index Life Stress Subscale, associated with the Parenting Stress Index (Abidin, 1995) in which parents report whether 17 stressful life events have recently occurred in their immediate family (e.g. death of a family member). There is also space to write in stressful life events not listed.
Maternal depression (6 months).
The Center for Epidemiologic Studies-Depression Scale (CES-D; Radloff, 1977), a 20-item questionnaire was used. The CES-D has internal, concurrent, and predictive validity and is used widely with mothers of infants (Bureau, Easterbrooks, & Lyons-Ruth, 2009). The range is 0 to 60, with a clinical cutoff of 16. One participant did not complete the CES-D.
SES (6 months).
Mothers reported maternal and paternal occupation; highest maternal and paternal educational level; household composition; and household income. Occupational prestige was coded with the Job Zone coding scheme from the Occupational Information Network (O*NET, http://www.onetonline.org/help/online/zones), which ranks U. S. Census-based occupational categories on a 1–5 scale based on the education, experience, and training required. An income-to-needs ratio based on the federal poverty level was computed with income and household composition. Maternal and paternal occupational prestige were averaged as were maternal and paternal education. Finally, parent occupational prestige, parent education, and income-to-needs ratio were standardized and averaged to yield a composite SES variable.
Breastfeeding (6 and 12 months).
Infants were classified as currently breastfeeding or not receiving any breast milk, based on maternal report.
Free-play interaction (6 months).
A mother-infant interaction was assessed in the home with a 6-minute free play (Feldman, Gordon, & Zagoory‐Sharon, 2011). Mothers were provided with six standardized toys and told to play with their infants as they normally would. A video camera faced the dyad so the toys, mother’s and infant’s faces and bodies were in view. Interactions were micro-coded, frame by frame (Feldman et al., 2011), with Noldus 11.0 (The Vaggenigen, Netherlands). Interrater reliability calculated for one minute of each interaction yielded a kappa range of 84-.97. Maternal behaviors were coded every 30th of a second and behavior durations were calculated as a proportion of the 6 minutes. Positive Affect indexed when the mother demonstrated positive affect (e.g., smiling, laughter). Motherese indexed when the mother used infant-directed speech. Intrusiveness indexed the mother taking a toy away from the infant. Negative Affect indexed maternal negative facial affect. Four dyads did not have a useable free play as the mother and infant turned away from the camera.
Electrophysiological Recording and Analysis
During EEG recording, the infant was seated on the mother’s lap 24 inches from two adjacent computer monitors (measuring 14.5 inches × 12 inches) that were spaced 18 inches apart in an electrically shielded booth to prevent interference with the EEG signal. Mothers were instructed to remain silent and not to make face-to-face contact with the infant or interact socially. The experimenter was in the booth behind the computer screens, facing the infant.
Throughout EEG recording, the experimenter was face-to-face with the infant and talked warmly to the infant and responded contingently. The experimenter engaged with the infant by playing peek-a-boo, singing songs, and directing the infant’s attention to photographs of objects appearing on the computer screens while commenting on the pictures. Photographs of objects (e.g. flower, apple) were displayed using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA). Objects appeared at variable interstimulus intervals (.5 – 2 seconds) and remained on screen for 13–14.5 seconds. Because EEG is a functional measure that reflects recording context (Jones et al., 2015), we chose a context that indexes active engagement and social learning rather than a passive baseline state.
EEG was recorded to a vertex reference using NetStation software and a Net Amp 300 amplifier (Electrical Geodesics, Inc.: Eugene, OR) connected to a Geodesic Sensor Net with 128 electrodes spaced ∼ 1 cm apart over the scalp. Prior to use, the 128 lead high-density net was soaked for 10 minutes in an electrolyte solution (6cc potassium chloride/liter water) to facilitate electrical contact between the scalp and electrodes. Prior to recording, impedances were lowered by administering small amounts of the electrolyte solution to electrodes with poor contact. Electrode impedances were below the manufacturer’s recommended value of 50 kilo-ohms.
Data were sampled at 500 Hz. Offline, data were notch filtered at 60 Hz, then a high pass filter of 0.1 Hz was applied. Data were analyzed in 30-second epochs. An automatic artifact rejection paradigm excluded electrodes with excessive artifact in an epoch, generally from infant movement, if the root mean square of the EEG voltage data exceeded 175 μ V or if the amplifier was saturated any time within the epoch. Epochs with > 20 excluded electrodes were rejected.
Data were re-referenced to the average reference of the remaining electrodes prior to power analyses. EEG 6–9 Hz power was computed for each electrode using Fast Fourier Transformations for each 30-second epoch. Regional EEG power averages were computed for each scalp region (left and right frontal, central, temporal, parietal, and occipital regions) to reduce multiple comparisons. Regional demarcation was adapted based on past research (Cannon et al., 2015; Welch et al., 2014; see figure 1 for a map of electrodes by region). Good epochs were combined to yield average power values for each region. Power values were log transformed using the natural log. We assessed EEG power in 6–9 Hz which is the dominant frequency band in infant research (e.g., Marshall et al., 2002). Fifty infants had useable EEG data (M = 4.17 minutes of useable EEG data; SD = 2.41 min.)
Figure 1.
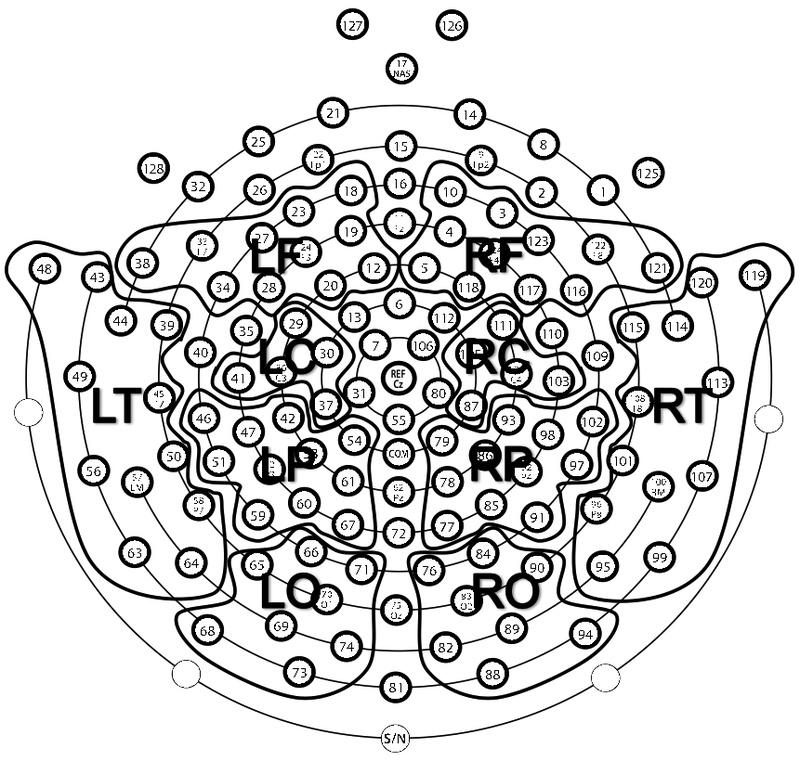
Regional demarcation used to compute EEG power values for the left and right frontal (LF, RF) central (LC, RC), temporal (LT, RT), parietal (LP, RP), and occipital (LO, RO) regions. This view is looking down on the head with the nose positioned near electrode 17.
Data Analysis Plan
Primary Analyses.
First we examined whether 6-month maternal cortisol predicted 12-month infant cortisol using separate Pearson correlations for slope and AUCg. Following significant correlations, hierarchical regressions determined unique contributions of the 6-month maternal cortisol variable to the 12-month infant cortisol variable. The corresponding 6-month infant cortisol variable was entered in the first step to assess if 6-month maternal cortisol contributed to developmental change in infant cortisol. Covariates were included if they related to the 12-month infant outcome measure (slope or AUCg). SES, maternal depression, parenting stress, stressful life events, 6 and 12-month breastfeeding statuses, and infant gender were tested as covariates as well as the corresponding 12-month maternal cortisol variable. We used Pearson correlations for continuous covariates and t-tests for gender and breastfeeding status.
Second, we assessed whether the 6-month maternal cortisol variables predicted 12-month infant 6–9 Hz power. Using repeated-measures analyses of variance (ANOVA) with region (frontal, central, temporal, parietal, occipital) and hemisphere (left, right) as within-subjects factors, 6-month maternal slope and AUCg were included in separate models as predictors. To test whether potential covariates related to infant 6–9 Hz power, we included continuous variables as covariates and dichotomous variables as between-subjects factors. Statistically, all continuous variables were entered as covariates. We use the term “predictor” for the maternal cortisol variables to distinguish from variables we treated as potential covariates (e.g., SES). In ANOVAs where the assumption of sphericity was violated, we used Greenhouse-Geisser corrections. Third, we assessed whether caregiving related to any of the physiological measures to see if they played a role in explaining relations between maternal and infant physiology.
Secondary Analyses.
We assessed stability and change in maternal and infant cortisol from 6 to 12 months. Correlations examined stability of cortisol and paired-samples t-tests examined change in cortisol over time. We assessed concurrent relations between maternal and infant cortisol at 6 and 12 months to explore physiological attunement at different time points.
Results
Primary Analyses
Maternal 6-month Cortisol Predictors of 12-month Infant Cortisol.
Six-month maternal AUCg did not relate to 12-month infant AUCg (see Table 2 for correlations of cortisol values). See Table 3 for descriptive statistics of raw mother and infant cortisol measures.
Table 2.
Correlations among cortisol measures and infant EEG
| Maternal slope-6 mo. |
Maternal AUC-6 mo. |
Maternal slope-12 mo. |
Maternal AUC-12 mo. | Infant slope- 6 mo. |
Infant AUC- 6 mo. |
Infant slope- 12 mo. |
Infant AUC- 12 mo. |
Avg. 6-9 Hz (ln) power- 12 mo. |
|
|---|---|---|---|---|---|---|---|---|---|
| Maternal slope-6 mo. |
- | ||||||||
| Maternal AUC-6 mo. |
.62*** | - | |||||||
| Maternal slope-12 mo. |
.41** | .34* | - | ||||||
| Maternal AUC-12 mo. |
.10 | .34* | .71*** | - | |||||
| Infant slope-6 mo. |
.36** | .27* | −.03 | −.08 | - | ||||
| Infant AUC-6 mo. |
.16 | .35** | −.07 | .03 | .74*** | - | |||
| Infant slope-12 mo. |
.33* | .−7 | .09 | −.06 | .10 | −.08 | - | ||
| Infant AUC-12 mo. |
−.11 | .10 | −.18 | .21 | .06 | .23† | .41** | - | |
| Average 6-9 Hz (ln) power-12 mo. |
−.32* | −.19 | −.08 | .06 | −.01 | −.05 | .03 | .33* | - |
p < .10
p < .05
p < .01
p <.001
Table 3.
Descriptive Statistics for Raw Cortisol Values
| M (SD) | Min | Max | |
|---|---|---|---|
|
Infant | |||
| 6-mo. diurnal slope (µg/dl/hour) N=64 |
.02 (.02) | −.05 | .07 |
| 6-mo. AUCg (µg/dl/hour) N=64 |
2.82 (1.38) | 1.04 | 6.68 |
| 12-mo. diurnal slope (µg/dl/hour) N=55 |
.02 (.02) | −.07 | .07 |
| 12-mo. AUCg (µg/dl/hour) N=55 |
3.62 (3.43) | 1.16 | 22.79 |
|
Mother | |||
| 6-mo. diurnal slope (µg/dl/hour) N=65 |
.02 (.01) | −.01 | .05 |
| 6-mo. AUCg (µg/dl/hour) N=65 |
1.89 (.79) | .62 | 5.95 |
| 12-mo. diurnal slope (µg/dl/hour) N=55 |
.02 (.01) | −.01 | .07 |
| 12-mo. AUCg (µg/dl/hour) N=55 |
2.02 (.98) | .14 | 7.13 |
A steeper 6-month maternal slope related to a steeper 12-month infant slope, r (53) = .33, p = .01. Next a hierarchical regression determined the unique contribution of 6-month maternal slope. Of the covariates (SES, maternal depression, parenting stress, stressful life events, 6 and 12-month breastfeeding statuses, infant gender, and 12-month maternal slope), only 6-month breastfeeding status related to 12-month infant slope, t (52) = 2.19, p = .047, such that infants breastfeeding at 6 months had a steeper 12-month slope compared to infants who did not receive any breast milk. See Table 4 for correlations between covariates and cortisol and EEG values.
Table 4.
Correlations of Psychosocial, Caregiving, and Breastfeeding Variables with Cortisol and EEG
| Maternal slope-6 mo. |
Maternal AUC-6 mo. |
Maternal slope-12 mo. |
Materna l AUC- 12 mo. |
Infant slope- 6 mo. |
Infant AUC- 6 mo. |
Infant slope- 12 mo. |
Infant AUC- 12 mo. |
Average 6-9 H (ln) power- 12 mo. |
|
|---|---|---|---|---|---|---|---|---|---|
| SES | .28* | .25* | .38** | .17 | .17 | .05 | .25† | −.05 | −.18 |
| Parenting stress |
−.03 | −.03 | .18 | .25† | .14 | .12 | −.22 | −.14 | .09 |
| Stressful life events |
−.02 | −.13 | .13 | .16 | −.29* | −.12 | .08 | .15 | −.15 |
| Maternal depression |
−.06 | −.09 | −.01 | −.12 | −.07 | .01 | −.26† | −.16 | −.01 |
| Motherese | .02 | .10 | −.06 | −.09 | −.05 | −.16 | .00 | −.25† | .14 |
| Maternal Positive affect |
.00 | −.17 | .04 | .02 | −.01 | −.11 | .20 | .12 | −.02 |
| Breastfeed ing status- 6 mo. |
−.15 | .00 | −.18 | .08 | .02 | .07 | .27* | .01 | .11 |
| Breastfeed ing status- 12 mo. |
−.18 | .03 | −.12 | .18 | .18 | .15 | −.19 | .18 | .09 |
p < .10
p < .05
p < .01
Six-month infant slope and 6-month breastfeeding status were entered in step 1 of the model. Six-month maternal slope was entered in step 2 (see Table 5). The overall model was significant, F (3, 50) = 3.70, R2 = .18, p = .02. Further, 6-month maternal slope significantly accounted for an additional 8.9 % of the variance in 12-month infant slope, F (1, 50) = 5.46, p = .02, after accounting for 6-month infant slope and 6-month breastfeeding status. This demonstrates that 6-month maternal slope predicted developmental change in infant slope from 6 to 12 months, β = .31, p = .02, and this relation was not explained by breastfeeding status.
Table 5.
Regression Predicting 12-month Infant Cortisol Slope
| Variable | B | SE | β | F-change | Δ R2 |
|---|---|---|---|---|---|
| Step 1 | 2.60† | .09 | |||
| 6-month infant slope | .13 | .13 | .14 | ||
| 6-month breastfeeding status |
7.6* | .35 | .27 | ||
| Step 2 | 5.46* | .09 | |||
| 6-month infant slope | .05 | .13 | .05 | ||
| 6-month breastfeeding status |
.67† | .34 | .25 | ||
| 6-month maternal slope | .37* | .16 | .31 |
Note. This model included 54 dyads. For the second step, F (3, 50) = 3.70, R2 = .18, p = .02.
p < .10
p < .05
Maternal 6-month Cortisol Predictors of 12-month Infant 6–9 Hz EEG Power.
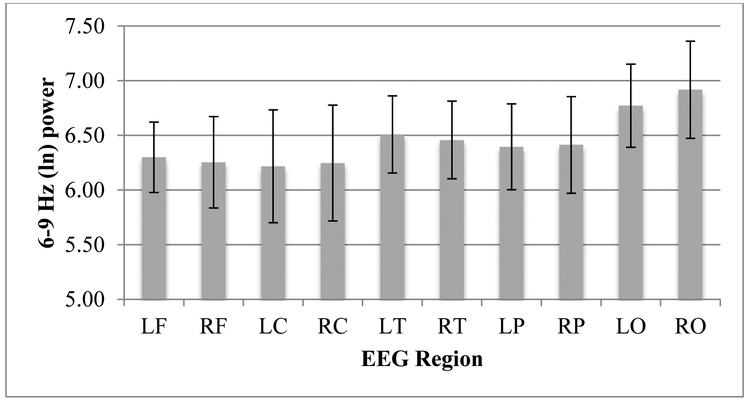
See Figure 2 for descriptives of EEG 6–9 Hz power values. Six-month maternal slope and AUCg were included as predictors in separate ANOVAs of infant 6–9 Hz power with region and hemisphere as within-subjects factors. There was a main effect of 6-month maternal slope, F (1, 48) = 5.49, p = .02, ηp = .10 (for this model, N = 50). The parameter estimates for 6-month maternal slope with each of the infant 6–9 Hz power values for each region and hemisphere were negative indicating that a steeper 6-month maternal slope (i.e., a greater magnitude of change across the day) predicted lower 12-month infant 6–9 Hz power. There were no interactions.
Figure 2.
6–9 Hz (ln) EEG power for left and right frontal (LF, RF), central (LC, RC), temporal (LT, RT), parietal, (LP, RP), and occipital (LO, RO) regions.
None of the potential covariates related to infant 6–9 Hz power so none were included in the model. There were no effects of 6-month maternal AUCg on infant 6–9 Hz power.
Relations between Maternal and Infant Physiology and Caregiving.
Maternal intrusiveness and negative affect hardly occurred; therefore they were not included in analyses. The proportions of time mothers engaged in positive affect (M = .14, SD = .15) and motherese (M = .23, SD = .19) were not related to the cortisol or EEG measures (see Table 4).
Summary.
Steeper 6-month maternal slope predicted steeper 12-month infant slope controlling for 6-month infant slope and 6-month breastfeeding status. A steeper 6-month maternal slope also predicted lower 12-month infant 6–9 Hz power. The results were not explained by psychosocial variables or maternal caregiving.
Secondary Analyses
Stability and Change in Maternal and Infant Cortisol.
Infant slope and AUCg were related concurrently at 6 and 12 months, while there were no relations between the 6 and 12-month variables. Conversely, nearly all maternal cortisol values were correlated both concurrently and over time (see Table 2). Paired samples t-tests determined that there were no changes in AUCg or slope for either mothers or infants from 6 to 12 months.
Concurrent Relations between Maternal and Infant Cortisol at 6 and 12 months.
At 6 months, maternal and infant slopes were positively related, r (62) = .36, p = .004, as were maternal and infant AUCg, r (62) = .35, p = .004. At 12 months, neither maternal and infant slopes nor maternal and infant AUCg were related.
Discussion
Using a psychobiological approach, we examined how maternal cortisol predicts two aspects of infant physiology and explored potential mechanisms underlying these associations. When infants were 6 and 12 months, we assessed maternal and infant salivary cortisol diurnal slope, indexing cortisol regulation, and AUCg, indexing cumulative cortisol exposure. At 12 months, we recorded infant EEG 6–9 Hz power during a social interaction. Consistent with our expectations, a steeper 6-month maternal slope predicted both 12-month steeper infant slope, indexing better diurnal cortisol regulation, and lower 6–9 Hz power, indexing greater neural activation. Results highlight the importance of day-to-day maternal cortisol regulation for infant physiological development. This is the first study to show that postnatal maternal cortisol predicts two distinct aspects of infant physiology and suggests a potential role of maternal slope as a biomarker of infant physiological regulation broadly construed.
The longitudinal relation between 6-month maternal slope and 12-month infant slope builds on past research showing maternal and infant cortisol are concurrently related (Benjamin Neelon et al., 2015; Bright et al., 2012; Clearfield et al., 2014; Laurent, Ablow, & Measelle, 2012; Middlemiss et al., 2012; Stenius et al., 2008). This finding suggests the importance of maternal physiological regulation in helping to buffer infants from physiological stress over time when the HPA axis is developing and maturing. This longitudinal association is noteworthy as we controlled for 6-month infant slope and 6-month breastfeeding status. Thus, 6-month maternal slope contributed to the development of infant cortisol regulation from 6 to 12 months.
Breastfeeding is a potential pathway for attunement (Benjamin Neelon et al., 2015) and infants who were breastfeeding at 6 months had steeper cortisol slopes at 12-months compared to infants not receiving breast milk. Infants may be exposed to maternal cortisol from breastfeeding (Benjamin Neelon et al., 2015) as maternal cortisol is present in breast milk (Grey et al., 2013; Hamosh, 2001). Additionally, the skin-to-skin contact involved in breastfeeding could help mothers be more attuned and sensitive (Kim et al., 2011), resulting in a steeper infant slope. Both pathways are probable, but the contributions and interplay of these pathways are unclear. For example, if a mother has dysregulated cortisol and breastfeeds, the infant may be exposed to cortisol but there may be larger benefits from the skin-to-skin experience of breastfeeding. Further, 12-month breastfeeding did not relate to 12-month infant slope, suggesting earlier breastfeeding may be important for developmental change in infant cortisol. The majority of infants were breastfeeding at 6 months. Future research including more infants not breastfeeding, assessing the amount and frequency of breast milk, and exploring the mechanisms involved is needed to explore the role of breastfeeding for cortisol attunement. While the relation between 6-month breastfeeding and 12-month infant slope became a nonsignificant trend when 6-month maternal slope was added to the model, it does suggest breastfeeding is important to consider.
The caregiving variables did not explain the relation between 6-month maternal slope and developmental change in 12-month infant slope. Specifically, maternal positive affect and motherese assessed during a non-stressful free play in the home at 6 months did not relate to maternal or infant cortisol measures, and maternal intrusiveness and negativity were almost never observed. As slope indexes daily cortisol regulation, maternal cortisol regulation may be important for cumulative caregiving and sensitivity during daily experiences such as getting ready in the morning, mealtimes, and bedtime. Days with infants are full of these normal challenges, so a key part of maternal sensitivity is to scaffold infants’ experience and help them manage arousal. Thus, maternal sensitivity during distress and challenge may be most important for shaping infant diurnal cortisol. For instance, maternal sensitivity during a bedtime routine but not in a free play was related to infant cortisol from evening to morning (Philbrook et al., 2014) and maternal and infant cortisol are more attuned during challenging compared to non-challenging interactions (Ruttle et al., 2011). Further, at-risk children in an intervention, of which a primary aim was to increase parental nurturance to child distress, had a more normal diurnal cortisol rhythm compared to children in a control group (Bernard, Hostinar, & Dozier, 2015). This evidence suggests that an important avenue of future research is to explore how caregiving during natural situations and daily challenges relates to maternal and infant diurnal cortisol.
None of the psychosocial variables (SES, maternal depression, parenting stress, stressful life events) related to 12-month infant cortisol and SES was the only psychosocial variable that related to maternal cortisol. These findings that subjective measures largely do not relate to cortisol is consistent with research finding a lack of association between biological and subjective measures of stress (Chiang et al., 2016; Desantis, Kuzawa, & Adam, 2015; Kivlighan, DiPietro, Costigan, & Laudenslager, 2008; Stalder et al., 2013). These psychosocial variables might be more relevant in a more economically diverse and strained sample. Children with a history of being in Child Protective Services (Bernard, Zwerling, & Dozier, 2015) and women with clinical levels of depression have blunted cortisol slopes (Jarcho, Slavich, Tylova-Stein, Wolkowitz, & Burke, 2013). Thus more hardship may be related to maternal and infant physiology. Likewise 6-month maternal AUCg did not relate to 12-month infant AUCg or EEG power. AUCg indexes cumulative cortisol exposure and may be more relevant in a sample with greater cumulative risk. Indeed, in an economically diverse sample, lower SES and higher parenting stress related to greater AUCg in 12–20 month infants (Saridjan et al., 2010). However, it is striking that even within our advantaged sample with few environmental risks, infant physiological development is sensitive to maternal cortisol regulation.
As these results suggest day-to-day maternal cortisol matters for infant physiology, it is important to consider practical implications and factors that may contribute to maternal dysregulation. It is likely that other measures not included in this study played a role in shaping maternal HPA axes. Adults with poorer sleep quality have flatter slopes (Kumari et al., 2009) and mothers with lower levels of social support and lower relationship functioning have more atypical diurnal cortisol patterns (Adam & Gunnar, 2001; Ben-Dat Fisher et al., 2007). Thus mothers with lower levels of these indicators might have poorer daily physiological regulation, in turn shaping infant physiology. Further, adolescent and older adults’ diurnal cortisol patterns vary with momentary emotional states assessed with daily diary responses (Adam, 2006; Adam, Hawkley, Kudielka, & Cacioppo, 2006), suggesting that maternal moods and emotions could be important for day-to-day maternal cortisol. Future research is needed to assess whether interventions to address factors such as poor maternal sleep, social support, and moods and emotions across the day could improve maternal cortisol regulation. If so, this could have practical applications such that health professionals could think about ameliorating these factors to improve maternal physiological regulation with implications for infant physiology.
Mothers collected saliva across three typical days that they spent with their infants to capture average cortisol levels and to minimize the effects of day-to-day variability in activity and stimulation. However, there are individual differences between dyads in levels of activity or novelty experienced on a typical day. Future research that collects information on daily routines is needed to assess how these factors may contribute to variability in dyadic cortisol.
A steeper maternal cortisol slope at 6 months, indexing healthy diurnal regulation, also predicted lower infant EEG 6–9 Hz power recorded during a social interaction. We assessed EEG power in 6–9 Hz as it is the dominant frequency band in infant research (Marshall et al., 2002). This band reflects slow wave activity, so lower power in the 6–9 Hz frequency band is interpreted to index greater neural activation (see for review Allen, Coan, & Nazarian, 2004). While this is the first study to show maternal cortisol relates to infant EEG power, our result generally fits in with the wider literature demonstrating that infant and child EEG power is sensitive to early experiences (Marshall et al., 2008; Otero et al., 2003; Tarullo et al., 2011; Tomalski et al., 2013; Vanderwert et al., 2016). Yet the finding is novel as our sample did not experience hardship or deprivation, which has been a focus of past research (Marshall et al., 2008; Otero et al., 2003; Tarullo et al., 2011). Our results suggest that even in an advantaged low risk sample, infant neural activation as indexed by 6–9 Hz power, is sensitive to subtle variation in experience, specifically to maternal physiological regulation. While the pathway through which maternal slope relates to infant 6–9 Hz power is unclear, it is possible that maternal slope is indexing variation in the infant’s day-to-day environment such as caregiving. Many positive early experiences help build neural architecture in early childhood (Black, 1998) and this could be a pathway through which maternal slope contributes to infant brain development. Maternal support during a challenging interaction with preschoolers has been related to structural brain development (Luby, Belden, Harms, Tillman, & Barch, 2016), highlighting the importance of caregiving experiences. Future research assessing infant EEG power, maternal cortisol, and caregiving across multiple situations throughout infancy is needed to investigate this possibility.
An additional pathway that could explain links between maternal cortisol and infant physiology is shared genes. Approximately 30% of waking cortisol levels is explained by genetic influences, while little to zero percent of evening cortisol is heritable (e.g., Bartels et al., 2003). This suggests promise of interventions to improve cortisol regulation, specifically the drop in cortisol across the day. Moreover, 6-month maternal slope had a specific association with 12-month infant slope independent of 12-month maternal slope and 6-month infant slope. This specific longitudinal relation suggests a developmental story in which maternal slope earlier in infancy shapes the infant’s developing HPA axis. This further bolsters the potential of interventions such that improving maternal slope could help with the development of the infant HPA axis over time.
We did not measure maternal prenatal cortisol so we cannot rule out a contribution of fetal programming for the developmental of infant physiology. There is some ambiguity regarding the role of the intrauterine environment for infant diurnal cortisol. A systematic review of links between prenatal maternal cortisol and infant cortisol showed not all studies find an association; moreover, all studies reviewed assessed infant cortisol reactivity to a stressor rather than diurnal cortisol (Zijlmans et al., 2015). The prenatal environment is also relevant as maternal cortisol and mental state in pregnancy have been related to infant and child brain development (Buss et al., 2012; Charil, Laplante, Vaillancourt, & King, 2010; Entringer, Buss, & Wadhwa, 2015; Li et al., 2012). Research using longitudinal designs is critical for teasing apart how prenatal and postnatal maternal cortisol contribute to both diurnal infant cortisol and EEG.
Given that the infant HPA axis rapidly develops and maternal HPA function may also be variable in the postpartum period, we assessed stability in cortisol over time. Infant slope and AUCg were concurrently related at 6 and 12 months; however, there were no relations between these infant cortisol measures across time. Conversely, nearly all maternal cortisol variables were correlated within and across time. The lack of association between 6- and 12-month infant cortisol may reflect that the HPA axis is developing and maturing throughout infancy and stable circadian cortisol rhythms are still being established (de Weerth et al., 2003). Thus maternal physiological stress may be especially important for the infant’s HPA axis in this critical developmental period. As 6-month maternal slope predicted 12-month infant slope, it is striking that 6- and 12-month month infant slopes were unrelated. This supports the potential utility of maternal slope early as a biomarker of later infant physiological regulation, as it was maternal and not infant slope at 6 months that predicted 12-month infant slope.
We assessed concurrent relations between maternal and infant cortisol at 6 and 12 months to explore diurnal physiological attunement at different time points. At 6 months, maternal and infant slopes were positively related as were maternal and infant AUCg. In contrast, neither maternal and infant slopes nor maternal and infant AUCg were related at 12 months. The 6-month findings are generally consistent with past research demonstrating links between maternal and infant slopes at 6 months (Stenius et al., 2008), cortisol levels averaged across the day in 0–7 month olds (Spangler, 1991), and midday cortisol in 5-month olds (Fuchs et al., 2016).
There is little precedent in the literature for our result that 12-month maternal and infant cortisol were unrelated. A cross-sectional study of infants aged 8–17 months (Bright et al., 2012) found AUCg for mothers and infants were related only for infants younger than one year. This is consistent with our finding that 12-month mother and infant AUCg were unrelated. To our knowledge, no other studies have assessed maternal and infant diurnal cortisol at 12 months. Maternal and child diurnal cortisol have been related in samples of 7–12 year old girls (Williams et al., 2013) and of adolescents (Papp, Pendry, & Adam, 2009), but comparing to our sample is difficult as the infant’s HPA axis is still developing stable circadian rhythms (de Weerth et al., 2003). Maternal cortisol may contribute to the infant HPA axis over time. So perhaps 12-month maternal cortisol would predict infant cortisol at a later time point (e.g., at 18 months), though future research is needed to investigate this possibility. To our knowledge, this is the first study to assess relations between maternal and infant diurnal cortisol longitudinally. Future research assessing diurnal cortisol from infancy through childhood is needed to further explore the development of dyadic cortisol relations and explore how maternal cortisol contributes to the developmental change in child cortisol rhythms.
A strength of this study was the inclusion of longitudinal physiological measures of mothers and infants and psychosocial, caregiving, and breastfeeding measures. This allowed us to assess how maternal cortisol could influence infant cortisol and neural activation over time. By bridging levels of analysis and including more than one indicator of infant physiology, we provided a broader picture of the potential implications of maternal cortisol for the infant’s developing physiological systems. While the psychosocial, breastfeeding, and caregiving variables did not explain links between maternal cortisol and infant physiology, their inclusion helps rule out potential mechanisms in this particular sample.
Future research should include a more economically diverse sample and challenging mother-infant interaction. As maternal sensitivity to infant distress may be more closely coupled with maternal physiological regulation, assessing responsiveness in a more stressful situation may elucidate how maternal cortisol shapes infant physiology. Further, as physiological attunement is thought to involve the mother and infant discerning each other’s cues, future research could investigate whether maternal and infant cue detection relates to diurnal physiological attunement. Middlemiss and colleagues (2012) found that prior to a sleep intervention, maternal and infant evening cortisol levels were related. Yet, when mothers could not hear their infant’s cries at bedtime, attunement of mother and infant cortisol was extinguished. Further, in a study of cardiovascular reactivity, infants became attuned to their mothers’ physiological response based on the mother’s affect (Waters, West, & Mendes, 2014). Perhaps mothers and infants’ abilities to detect one another’s cues could moderate attunement, though future research is needed. This could also be applied to EEG by assessing how infant neural activation varies depending on the concurrent physiological stress of the mother.
This study is the first to provide evidence that maternal cortisol predicts both infant cortisol and neural activation, indexed as lower 6–9 Hz power. Specifically, steeper 6-month maternal slope predicted steeper infant slope and lower 6–9 Hz power at 12 months. This suggests maternal physiological stress plays a role in shaping more than one aspect of infant physiology. Psychosocial, breastfeeding, and caregiving variables did not explain results. Future research is needed to elucidate the pathways through which maternal physiological stress relates to infant physiological development. Regardless of underlying mechanisms, results highlight the potential relevance of 6-month maternal slope as a biomarker of later infant physiology.
Acknowledgments
The authors thank Ryan Johnson and Leah Miller for their assistance in data collection. We are also grateful for all of the families who participated.
References
- Abidin RR (1995). Parenting stress index. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Adam EK (2006). Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology, 31(5), 664–679. 10.1016/j.psyneuen.2006.01.010 [DOI] [PubMed] [Google Scholar]
- Adam EK, & Gunnar MR (2001). Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology, 26(2), 189–208. 10.1016/S0306-4530(00)00045-7 [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, & Cacioppo JT (2006). Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences, 103(45), 17058–17063. 10.1073/pnas.0605053103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJB, Coan JA, & Nazarian M (2004). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology, 67(1–2), 183–218. 10.1016/j.biopsycho.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Arnsten AFT (2015). Stress weakens prefrontal networks: molecular insults to higher cognition. Nature Neuroscience, 18(10), 1376–1385. 10.1038/nn.4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson L, Gonzalez A, Kashy D,A, Santo Basile V, Masellis M, Pereira J, … Levitan R (2013). Maternal sensitivity and infant and mother adrenocortical function across challenges. Psychoneuroendocrinology, 38, 2943–2951. [DOI] [PubMed] [Google Scholar]
- Bartels M, Geus E. J. C. de, Kirschbaum C, Sluyter F, & Boomsma DI (2003). Heritability of Daytime Cortisol Levels in Children. Behavior Genetics, 33(4), 421–433. 10.1023/A:1025321609994 [DOI] [PubMed] [Google Scholar]
- Bell MA (2001). Brain electrical activity associated with cognitive processing during a looking version of the A-Not-B task. Infancy, 2(3), 311–330. 10.1207/S15327078IN0203_2 [DOI] [PubMed] [Google Scholar]
- Benasich AA, Gou Z, Choudhury N, & Harris KD (2008). Early Cognitive and Language Skills are Linked to Resting Frontal Gamma Power Across the First Three Years. Behavioural Brain Research, 195(2), 215–222. 10.1016/j.bbr.2008.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Dat Fisher D, Serbin LA, Stack DM, Ruttle PL, Ledingham JE, & Schwartzman AE (2007). Intergenerational predictors of diurnal cortisol secretion in early childhood. Infant and Child Development, 16(2), 151–170. 10.1002/icd.474 [DOI] [Google Scholar]
- Benjamin Neelon SE, Schou Andersen C, Schmidt Morgen C, Kamper-Jørgensen M, Oken E, Gillman MW, & Sørensen TIA (2015). Early child care and obesity at 12 months of age in the Danish National Birth Cohort. International Journal of Obesity, 39(1), 33–38. 10.1038/ijo.2014.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin Neelon SE, Stroo M, Mayhew M, Maselko J, & Hoyo C (2015). Correlation between maternal and infant cortisol varies by breastfeeding status. Infant Behavior and Development, 40, 252–258. 10.1016/j.infbeh.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Hostinar C, & Dozier M (2015). Intervention Effects on Diurnal Cortisol Rhythms of CPS-Referred Infants Persist into Early Childhood: Preschool Follow-up Results of a Randomized Clinical Trial. JAMA Pediatrics, 169(2), 112–119. 10.1001/jamapediatrics.2014.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Zwerling J, & Dozier M (2015). Effects of early adversity on young children’s diurnal cortisol rhythms and externalizing behavior. Developmental Psychobiology, n/a–n/a. 10.1002/dev.21324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, & Nelson CA (2015). Early Adverse Experiences and the Developing Brain. Neuropsychopharmacology. 10.1038/npp.2015.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE (1998). How a Child Builds Its Brain: Some Lessons from Animal Studies of Neural Plasticity. Preventive Medicine, 27(2), 168–171. 10.1006/pmed.1998.0271 [DOI] [PubMed] [Google Scholar]
- Bright MA, Granger DA, & Frick JE (2012). Do infants show a cortisol awakening response? Developmental Psychobiology, 54(7), 736–743. 10.1002/dev.20617 [DOI] [PubMed] [Google Scholar]
- Brito NH, Fifer WP, Myers MM, Elliott AJ, & Noble KG (2016). Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Developmental Cognitive Neuroscience, 19, 144–151. 10.1016/j.dcn.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau J-F, Easterbrooks MA, & Lyons-Ruth K (2009). Maternal depressive symptoms in infancy: Unique contribution to children’s depressive symptoms in childhood and adolescence? Development and Psychopathology, 21(2), 519–537. 10.1017/S0954579409000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, & Sandman CA (2012). Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences, 109(20), E1312–E1319. 10.1073/pnas.1201295109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon EN, Simpson EA, Fox NA, Vanderwert RE, Woodward AL, & Ferrari PF (2015). Relations between infants’ emerging reach-grasp competence and event-related desynchronization in EEG. Developmental Science, n/a–n/a. 10.1111/desc.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, & King S (2010). Prenatal stress and brain development. Brain Research Reviews, 65(1), 56–79. 10.1016/j.brainresrev.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Chiang JJ, Tsai KM, Park H, Bower JE, Almeida DM, Dahl RE, … Fuligni AJ (2016). Daily family stress and HPA axis functioning during adolescence: The moderating role of sleep. Psychoneuroendocrinology, 71, 43–53. 10.1016/j.psyneuen.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clearfield MW, Carter-Rodriguez A, Merali A-R, & Shober R (2014). The effects of SES on infant and maternal diurnal salivary cortisol output. Infant Behavior & Development, 37(3), 298–304. 10.1016/j.infbeh.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Cuevas K, Raj V, & Bell MA (2012). Functional connectivity and infant spatial working memory: A frequency band analysis. Psychophysiology, 49(2), 271–280. 10.1111/j.1469-8986.2011.01304.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, & Sandman CA (2010). The Timing of Prenatal Exposure to Maternal Cortisol and Psychosocial Stress is Associated with Human Infant Cognitive Development. Child Development, 81(1), 131–148. 10.1111/j.1467-8624.2009.01385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desantis AS, Kuzawa CW, & Adam EK (2015). Developmental origins of flatter cortisol rhythms: socioeconomic status and adult cortisol activity. American Journal of Human Biology, 27(4), 458–467. 10.1002/ajhb.22668 [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, & Singer W (2001). Dynamic predictions: Oscillations and synchrony in top–down processing. Nature Reviews Neuroscience, 2(10), 704–716. 10.1038/35094565 [DOI] [PubMed] [Google Scholar]
- Entringer S, Buss C, & Wadhwa PD (2015). Prenatal stress, development, health and disease risk: A psychobiological perspective—2015 Curt Richter Award Paper. Psychoneuroendocrinology, 62, 366–375. 10.1016/j.psyneuen.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Gordon I, & Zagoory‐Sharon O (2011). Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: Considering stress and affiliation components of human bonding. Developmental Science, 14(4), 752–761. 10.1111/j.1467-7687.2010.01021.x [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, & Kirschbaum C (2009). The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology, 72(1), 67–73. 10.1016/j.ijpsycho.2008.03.014 [DOI] [PubMed] [Google Scholar]
- Fuchs A, Möhler E, Resch F, & Kaess M (2016). Sex-specific differences in adrenocortical attunement in mothers with a history of childhood abuse and their 5-month-old boys and girls. Journal of Neural Transmission, 1–10. 10.1007/s00702-016-1525-6 [DOI] [PubMed] [Google Scholar]
- Gou Z, Choudhury N, & Benasich AA (2011). Resting Frontal Gamma Power at 16, 24 and 36 months Predicts Individual Differences in Language and Cognition at 4 and 5 years. Behavioural Brain Research, 220(2), 263–270. 10.1016/j.bbr.2011.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey KR, Davis EP, Sandman CA, & Glynn LM (2013). Human Milk Cortisol is Associated With Infant Temperament. Psychoneuroendocrinology, 38(7), 1178–1185. 10.1016/j.psyneuen.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, & Quevedo K (2007). The Neurobiology of Stress and Development. Annual Review of Psychology, 58(1), 145–173. 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Donzella B (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27(1–2), 199–220. [DOI] [PubMed] [Google Scholar]
- Hamosh M (2001). Bioactive Factors in Human Milk. Pediatric Clinics of North America, 48(1), 69–86. 10.1016/S0031-3955(05)70286-8 [DOI] [PubMed] [Google Scholar]
- Hane AA, & Fox NA (2006). Ordinary Variations in Maternal Caregiving Influence Human Infants’ Stress Reactivity. Psychological Science, 17(6), 550–556. 10.1111/j.1467-9280.2006.01742.x [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Blair C, Finegood ED, & The Family Life Project Key Investigators. (2015). Maternal-child adrenocortical attunement in early childhood: Continuity and change. Developmental Psychobiology, 57(1), 83–95. 10.1002/dev.21266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJH, Visser GHA, & Buitelaar JK (2003). Stress during pregnancy is associated with developmental outcome in infancy. Journal of Child Psychology and Psychiatry, 44(6), 810–818. 10.1111/1469-7610.00166 [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Slavich GM, Tylova-Stein H, Wolkowitz OM, & Burke HM (2013). Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biological Psychology, 93(1), 150–158. 10.1016/j.biopsycho.2013.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John ER, Ahn H, Prichep L, Trepetin M, Brown D, & Kaye H (1980). Developmental equations for the electroencephalogram. Science, 210(4475), 1255–1258. 10.1126/science.7434026 [DOI] [PubMed] [Google Scholar]
- Jones EJH, Venema K, Lowy R, Earl RK, & Webb SJ (2015). Developmental changes in infant brain activity during naturalistic social experiences. Developmental Psychobiology, 57(7), 842–853. 10.1002/dev.21336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, & Swain JE (2011). Breastfeeding, Brain Activation to Own Infant Cry, and Maternal Sensitivity. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 52(8), 907–915. 10.1111/j.1469-7610.2011.02406.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivlighan KT, DiPietro JA, Costigan KA, & Laudenslager ML (2008). Diurnal rhythm of cortisol during late pregnancy: Associations with maternal psychological well-being and fetal growth. Psychoneuroendocrinology, 33(9), 1225–1235. 10.1016/j.psyneuen.2008.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, & Chandola T (2009). Self-Reported Sleep Duration and Sleep Disturbance Are Independently Associated with Cortisol Secretion in the Whitehall II Study. The Journal of Clinical Endocrinology & Metabolism, 94(12), 4801–4809. 10.1210/jc.2009-0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MC, White BP, Cochran A, Donzella B, & Gunnar M (1998). Dampening of the cortisol response to handling at 3 months in human infants and its relation to sleep, circadian cortisol activity, and behavioral distress. Developmental Psychobiology, 33(4), 327–337. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, & Measelle J (2012). Taking stress response out of the box: stability, discontinuity, and temperament effects on HPA and SNS across social stressors in mother-infant dyads. Developmental Psychology, 48(1), 35–45. 10.1037/a0025518 [DOI] [PubMed] [Google Scholar]
- Letourneau N, Watson B, Duffett-Leger L, Hegadoren K, & Tryphonopoulos P (2011). Cortisol patterns of depressed mothers and their infants are related to maternal–infant interactive behaviours. Journal of Reproductive and Infant Psychology, 29(5), 439–459. 10.1080/02646838.2011.649474 [DOI] [Google Scholar]
- Li J, Wang Z-N, Chen Y-P, Dong Y-P, Shuai H-L, Xiao X-M, … Hocher B (2012). Late gestational maternal serum cortisol is inversely associated with fetal brain growth. Neuroscience & Biobehavioral Reviews, 36(3), 1085–1092. 10.1016/j.neubiorev.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Luby JL, Belden A, Harms MP, Tillman R, & Barch DM (2016). Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proceedings of the National Academy of Sciences, 201601443 10.1073/pnas.1601443113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, & Fox NA (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113(8), 1199–1208. 10.1016/S1388-2457(02)00163-3 [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Reeb BC, Fox NA, Nelson CAI, & Zeanah CH (2008). Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Development and Psychopathology, 20(03), 861–880. 10.1017/S0954579408000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoušek M, & Petersén I (1973). Automatic evaluation of EEG background activity by means of age-dependent EEG quotients. Electroencephalography and Clinical Neurophysiology, 35(6), 603–612. 10.1016/0013-4694(73)90213-7 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2006). Protective and damaging effects of stress mediators: central role of the brain. Dialogues in Clinical Neuroscience, 8(4), 367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemiss W, Granger DA, Goldberg WA, & Nathans L (2012). Asynchrony of mother-infant hypothalamic-pituitary-adrenal axis activity following extinction of infant crying responses induced during the transition to sleep. Early Human Development, 88(4), 227–232. 10.1016/j.earlhumdev.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Molfese DL, Molfese VJ, & Kelly S (2001). The use of brain electrophysiology techniques to study language: A basic guide for the beginning consumer of electrophysiology information. Learning Disability Quarterly, 24(3), 177–188. 10.2307/1511242 [DOI] [Google Scholar]
- Otero GA, Pliego-Rivero FB, Fernández T, & Ricardo J (2003). EEG development in children with sociocultural disadvantages: a follow-up study. Clinical Neurophysiology, 114(10), 1918–1925. 10.1016/S1388-2457(03)00173-1 [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Brendgen M, Girard A, Lupien SJ, Dionne G, Vitaro F, & Boivin M (2016). Evidence of a unique and common genetic etiology between the CAR and the remaining part of the diurnal cycle: A study of 14 year-old twins. Psychoneuroendocrinology, 66, 91–100. 10.1016/j.psyneuen.2015.12.022 [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Dionne G, Pérusse D, Lupien SJ, Arseneault L, Barr RG, … Boivin M (2009). Daytime Cortisol Secretion in 6-Month-Old Twins: Genetic and Environmental Contributions as a Function of Early Familial Adversity. Biological Psychiatry, 65(5), 409–416. 10.1016/j.biopsych.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Papp LM, Pendry P, & Adam EK (2009). Mother-Adolescent Physiological Synchrony in Naturalistic Settings: Within-Family Cortisol Associations and Moderators. Journal of Family Psychology : JFP : Journal of the Division of Family Psychology of the American Psychological Association (Division 43), 23(6), 882 10.1037/a0017147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbrook LE, Hozella AC, Kim B-R, Jian N, Shimizu M, & Teti DM (2014). Maternal emotional availability at bedtime and infant cortisol at 1 and 3 months. Early Human Development, 90(10), 595–605. 10.1016/j.earlhumdev.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, & Hellhammer DH (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. [DOI] [PubMed] [Google Scholar]
- Reid VM, Csibra G, Belsky J, & Johnson MH (2007). Neural correlates of the perception of goal-directed action in infants. Acta Psychologica, 124(1), 129–138. 10.1016/j.actpsy.2006.09.010 [DOI] [PubMed] [Google Scholar]
- Ross KM, Murphy MLM, Adam EK, Chen E, & Miller GE (2014). How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology, 39, 184–193. 10.1016/j.psyneuen.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Serbin LA, Stack DM, Schwartzman AE, & Shirtcliff EA (2011). Adrenocortical attunement in mother–child dyads: Importance of situational and behavioral characteristics. Biological Psychology, 88(1), 104–111. 10.1016/j.biopsycho.2011.06.014 [DOI] [PubMed] [Google Scholar]
- Saridjan NS, Huizink AC, Koetsier JA, Jaddoe VW, Mackenbach JP, Hofman A, … Tiemeier H (2010). Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? The Generation R Study. Hormones and Behavior, 57(2), 247–254. 10.1016/j.yhbeh.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Schreiber JE, Shirtcliff E, Hulle CV, Lemery-Chalfant K, Klein MH, Kalin NH, … Goldsmith HH (2006). Environmental influences on family similarity in afternoon cortisol levels: Twin and parent–offspring designs. Psychoneuroendocrinology, 31(9), 1131–1137. 10.1016/j.psyneuen.2006.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler G (1991). The emergence of adrenocortical circadian function in newborns and infants and its relationship to sleep, feeding and maternal adrenocortical activity. Early Human Development, 25(3), 197–208. 10.1016/0378-3782(91)90116-K [DOI] [PubMed] [Google Scholar]
- Sroufe LA (2013). The promise of developmental psychopathology: Past and present. Development and Psychopathology, 25(25th Anniversary Special Issue 4pt2), 1215–1224. 10.1017/S0954579413000576 [DOI] [PubMed] [Google Scholar]
- Stalder T, Bäumler D, Miller R, Alexander N, Kliegel M, & Kirschbaum C (2013). The cortisol awakening response in infants: Ontogeny and associations with development-related variables. Psychoneuroendocrinology, 38(4), 552–559. 10.1016/j.psyneuen.2012.07.015 [DOI] [PubMed] [Google Scholar]
- Stenius F, Theorell T, Lilja G, Scheynius A, Alm J, & Lindblad F (2008). Comparisons between salivary cortisol levels in six-months-olds and their parents. Psychoneuroendocrinology, 33(3), 352–359. 10.1016/j.psyneuen.2007.12.001 [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Garvin MC, & Gunnar MR (2011). Atypical EEG power correlates with indiscriminately friendly behavior in internationally adopted children. Developmental Psychology, 47(2), 417–431. 10.1037/a0021363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, Obradović J, Keehn B, Rasheed MA, Siyal S, Nelson CA, & Yousafzai AK (2017). Gamma power in rural Pakistani children: Links to executive function and verbal ability. Developmental Cognitive Neuroscience, 26, 1–8. 10.1016/j.dcn.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Colls S, & Pasco Fearon RM (2015). The Effects of Parental Behavior on Infants’ Neural Processing of Emotion Expressions. Child Development, 86(3), 877–888. 10.1111/cdev.12348 [DOI] [PubMed] [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, Karmiloff‐Smith A, … Kushnerenko E (2013). Socioeconomic status and functional brain development—Associations in early infancy. Developmental Science, 16(5), 676–687. [DOI] [PubMed] [Google Scholar]
- van Bakel HJA, & Riksen-Walraven JM (2008). Adrenocortical and behavioral attunement in parents with 1-year-old infants. Developmental Psychobiology, 50(2), 196–201. 10.1002/dev.20281 [DOI] [PubMed] [Google Scholar]
- Vanderwert RE, Zeanah CH, Fox NA, & Nelson CA (2016). Normalization of EEG activity among previously institutionalized children placed into foster care: A 12-year follow-up of the Bucharest Early Intervention Project. Developmental Cognitive Neuroscience, 17, 68–75. 10.1016/j.dcn.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulle CA, Shirtcliff EA, Lemery-Chalfant K, & Goldsmith HH (2012). Genetic and environmental influences on individual differences in cortisol level and circadian rhythm in middle childhood. Hormones and Behavior, 62(1), 36–42. 10.1016/j.yhbeh.2012.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters SF, West TV, & Mendes WB (2014). Stress Contagion Physiological Covariation Between Mothers and Infants. Psychological Science, 25(4), 934–942. 10.1177/0956797613518352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerth C. de, Zijl RH, & Buitelaar JK (2003). Development of cortisol circadian rhythm in infancy. Early Human Development, 73(1), 39–52. 10.1016/S0378-3782(03)00074-4 [DOI] [PubMed] [Google Scholar]
- Welch MG, Myers MM, Grieve PG, Isler JR, Fifer WP, Sahni R, … Stark RI (2014). Electroencephalographic activity of preterm infants is increased by Family Nurture Intervention: A randomized controlled trial in the NICU. Clinical Neurophysiology, 125(4), 675–684. 10.1016/j.clinph.2013.08.021 [DOI] [PubMed] [Google Scholar]
- Williams SR, Cash E, Daup M, Geronimi EMC, Sephton SE, & Woodruff-Borden J (2013). Exploring patterns in cortisol synchrony among anxious and nonanxious mother and child dyads: A preliminary study. Biological Psychology, 93(2), 287–295. 10.1016/j.biopsycho.2013.02.015 [DOI] [PubMed] [Google Scholar]
- Zijlmans MAC, Riksen-Walraven JM, & de Weerth C (2015). Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neuroscience & Biobehavioral Reviews, 53, 1–24. 10.1016/j.neubiorev.2015.02.015 [DOI] [PubMed] [Google Scholar]