Abstract
Background
One third of patients with focal epilepsy are drug refractory and surgery may provide a cure. Seizure free outcome following surgery is dependent on the correct identification and resection of the epileptogenic zone. In patients with no visible MRI abnormality, or when pre-surgical evaluation yields discordant data, invasive Stereoelectroencephalography (SEEG) recordings may be necessary. SEEG is a procedure in which multiple electrodes are stereotactically placed in key targets within the brain to record interictal and ictal electrophysiological activity. Correlating this activity with the seizure semiology allows identification of the seizure onset zone and key structures within the ictal network. The main risk of SEEG electrode placement is haemorrhage, which occurs in 1% of patients. Planning safe SEEG electrodes requires meticulous adherence to the following constraints: 1) maximise distance from cerebral vasculature, 2) avoid crossing sulcal pial boundaries (sulci), 3) maximize grey matter sampling, 4) minimise electrode length, 5) drilling angle orthogonal to skull and 6) avoid critical neurological structures. We provide a validation of EpiNav™ Strategy and Planning™, a multimodal platform that allows automated computer-assisted planning (CAP) of SEEG electrodes by user defined regions of interest.
Methods
Thirteen consecutive patients who underwent SEEG implantation of 116 electrodes over a 15 month period were retrospectively studied. Models of the cortex, grey matter and sulci were generated from a patient specific whole brain parcellation. Vascular segmentation was performed from pre-operative MR venography. The multi-disciplinary implantation strategy and precise trajectory planning was reconstructed using CAP and compared to the implemented manual plans. Paired results for safety metric comparisons were available for 104 electrodes. External validity of the suitability and safety of electrode entry points, trajectories and target point feasibility was sought through 5 independent blinded experts from outside institutions.
Results
CAP generated electrode trajectories resulted in a statistically significant improvement in electrode length, drilling angle, grey matter sampling ratio and minimum distance from segmented vasculature and risk (p<0.05). Blinded external raters had varying opinions of trajectory feasibility which were not statistically significant and considered a mean of 69.4% of manual and 62.2% of CAP generated trajectories feasible. In 19.4% CAP generated electrodes were deemed feasible when manual electrodes were not, whereas 26.5% of manual electrode were rated feasible when CAP electrodes were unfeasible (no significant difference).
Conclusion
CAP generates clinically feasible electrode plans with statistically improved safety metrics. CAP is a useful tool for automating electrode placement for SEEG and requires operating surgeon review prior to implantation as only 62% of electrodes were rated feasible compared to 69% of manual plans, mainly due to proximity to unsegmented vasculature. Improved vascular segmentation and sulcal modelling may lead to further improvements in the feasibility of CAP generated trajectories.
Keywords: Stereoencephalography, computer assisted planning, Epilepsy evaluation, External validation, EpiNav
1. Introduction
Epilepsy is defined as “a disorder of the brain characterised by an enduring predisposition to generate epileptic seizures”11. Epilepsy can have wide ranging effects on patient’s quality of life resulting in physical injury, psycho-social dysfunction, cognitive decline and risk of death15. One third of patients with epilepsy continue to have seizures despite the use of two or more appropriately chosen antiepileptic drug schedules. These patients are defined as having drug resistant epilepsy (DRE)26. Surgical intervention can potentially cure DRE if the region from which the seizures arise, known as the epileptogenic zone (EZ), can be identified and safely removed. Chances of achieving sustained seizure freedom after epilepsy surgery are highest where there is concordance between the seizure semiology, electrophysiological investigations, imaging findings and neuropsychological assessment. In such cases patients do not require any further investigation, unless there is proximity to eloquent cortex and resective surgery can be performed. In a proportion of patients the non-invasive pre-surgical evaluation is not clear or discordant and invasive intracranial EEG recordings are required in the form of either grid / strip implantation or stereoelectroencephalography (SEEG). SEEG involves the stereotactic placement of multiple (8-16) electrodes at predefined regions of the brain to help delineate the EZ as well as the spatial and temporal network of seizure spread within the brain. A recent meta-analysis regarding the safety of SEEG implantation has shown the overall risks of complication to be 1.3% per patient. The greatest risk of SEEG is intracranial haemorrhage which had a pooled prevalence of 1% per patient16. The factors that determine the risk of haemorrhage are the initial planned trajectory and the accuracy of the implantation method. The methods currently used for SEEG implantation include stereotactic frame-based, frameless and robotic systems. There is a paucity of evidence in the literature comparing which of these methods is most accurate, but entry and target point accuracies range from 0.78 - 3.5 mm and 1.70 – 3.66 mm respectively29.
Currently electrode trajectories are planned manually to sample the regions of interest (ROI) whilst maximising grey matter contact and distance from blood vessels. This is a time consuming task that requires significant multi-disciplinary input. We have previously described the benefits of 3D multimodal imaging for manual electrode planning and an early version of computer assisted planning18,20. In the initial study, manually planned electrode implantation schemes from 18 patients (166 electrodes) were retrospectively recreated using the EpiNav™ software. The earlier version of the software required the target points for the electrodes to be manually placed on the MR image and the software would calculate the safest electrode trajectory based on the cumulative distance from segmented blood vessels along the whole trajectory19. The computer generated and manual trajectories were then rated by three independent blinded neurosurgeons as to whether they were feasible for implantation. Overall the computer generated electrodes resulted in significantly shorter intracranial length, increased distance from blood vessels, greater grey matter sampling and improved drilling angles (p<0.05 for all parameters). Of the computer generated electrodes 78.9% were deemed feasible for implantation by at least two of the three independent neurosurgeons.
Further development of the EpiNav™ software implements the ability to define entry and target zones constrained by anatomical structures25. Users can now define the region of interest by typing or clicking on an anatomical location (e.g. right amygdala) and the computer algorithm will define the safest entry and target points within the anatomical structure as a whole. Furthermore, multiple trajectories can be placed within the same anatomical structure and electrodes will be spread evenly within safe zones to maximise region sampling. This is of particular benefit in large anatomical targets, such as the cingulate cortex, or when high density sampling of a structure such as the insula or hippocampus is required. We confirmed external validity of the generated electrodes from five independent blinded epilepsy neurosurgeons, from outside institutions, who have expertise in SEEG implantation, none of whom were involved in the generation of the initial manual plans. We assessed why surgeons rated trajectories as infeasible, to gauge surgeon variability and preferences. The implantation methods used by the external raters include frame-based (JM), frameless (DN), iSYS1 (SW/CD) and Neuromate (MT) robotic implantation methods.
2. Methods
a. Subjects
We included thirteen consecutive patients who underwent manual planning of electrodes and surgical implantation between July 2015 and October 2016. Informed consent was taken from each patient prior to inclusion in the study. National Research Ethics Service Committee London approval reference: 12/LO/0377.
Patient demographics are summarized in table 1.
-
Determination of target points
All patients had been discussed in a multidisciplinary team (MDT) setting consisting of epileptologists, neurosurgeons, neuropsychologists, neuropsychiatrists and neuroradiologists. From the non-invasive presurgical evaluation the hypothesized epileptogenic zone was agreed and requirement for invasive EEG recording was determined. Patients requiring subdural grid implantation were excluded from the study. Regions for SEEG sampling were agreed between the multidisciplinary team and a list of brain regions requiring sampling were generated. The manual plans were then performed by a Consultant Neurosurgeon with subspecialty expertise in epilepsy surgery prior to final approval by the MDT.
Table 1.
Patient demographics
| Patient | Age at implantation | Sex | Hemisphere implanted | Hemispheric language dominance (fMRI) | EZ hypothesis | No. of electrodes |
|---|---|---|---|---|---|---|
| 1 | 37 | M | Left | Left | Frontal (non-lesional) | 11 |
| 2 | 27 | M | Right | Left | Frontal (non-lesional) | 8 |
| 3 | 45 | F | Right | Bilateral | Frontal (non-lesional) | 13 |
| 4 | 35 | F | Left | Left | Temporal (lesional) | 3 |
| 5 | 31 | M | Right | Left | Temporal (lesional) | 8 |
| 6 | 42 | M | Right | Left | Frontal (non-lesional) | 10 |
| 7 | 49 | F | Right | Left | Left temporal (non-lesional) | 10 |
| 8 | 61 | M | Right | Left | Frontal (non-lesional) | 11 |
| 9 | 24 | M | Right | Left | Frontal (non-lesional) | 8 |
| 10 | 42 | M | Left | Left | Frontal (non-lesional) | 6 |
| 11 | 31 | M | Bilateral | Left | Right Temporal (non-lesional) | 12 |
| 12 | 48 | F | Right | Left | Right Temporal (non-lesional) | 8 |
| 13 | 27 | M | Right | Left | Right Occipital (lesional) | 8 |
b. Multimodal imaging
MR imaging was performed on a GE 3T MR750 scanner with a 32-channel head coil. A coronal 3D T1-weighted MPRAGE scan was performed with a field-of-view (FOV) of 224×256×256 mm (AP´LR´IS) with an acquisition matrix of 224×256×256 for a voxel size of 1 mm isotropic (TE/TR/TI = 3.1/7.4/400 ms; flip angle 11°; parallel imaging acceleration factor 2). 3D-FLAIR scans were acquired with a 3D fast spin echo sequence with variable flip-angle readout (CUBE) with the same FOV and acquisition matrix for a 1 mm isotropic resolution (TR/TI/TE = 6200/1882/137 ms; echo train length of 150; parallel imaging acceleration 2 along both the in-plane and through-plane phase-encoding axes). Vascular imaging comprises a post-gadolinium T1, and phase-contrast MR angiography (MRA) and venography (MRV) scans. The axial post-gadolinium T1-weighted scan was acquired with an FSPGR sequence with a FOV of 256×256×224 mm and acquisition and reconstruction matrix of 256×256×224 (TE/TR = 3.1/7.4 ms; flip angle 11°). MRA and MRV were performed using a 3D phase-contrast sequence with a FOV of 220×220×148.8 mm with an acquisition matrix of 384×256×124 for a reconstructed voxel size of 0.43×0.43×0.60 mm (flip angle 8°; parallel imaging acceleration factor 2). To highlight the arteries the MRA was scanned with a velocity-encoding of 80 cm/s (TE/TR = 4.0/9.3 ms). For sensitivity to the venous circulation the MRV was scanned with a velocity-encoding of 15 cm/s (TE/TR = 4.8/26.4 ms), fat suppression, and a saturation band inferior to the FOV.
c. Manual planning
Manual plans were generated using volumetric T1 gadolinium enhanced images as the reference image upon which MRV images were co-registered and vessels were extracted using a previously described tensor voting framework algorithm31. Entry and target points were manually placed using axial, coronal and sagittal reconstructions and trajectories were checked using the ‘probe’s eye’ function. A 3D model of the cortical surface was used to ensure entry points were on the crown of gyri.
d. EpiNav™
Data processing and model generation: EpiNav™ is a software platform that allows multimodal image co-registration, vessel segmentation, 3D model generation, manual and automated electrode planning. T1 MPRAGE sequences were submitted for whole brain parcellation (GIF) from which cortical, grey matter and sulcal models were generated6,10. Pre-operative CT scans were used to generate skull models, which were then modified to prevent entry through the contralateral hemisphere, face, ear, posterior fossa and skull base.
The technical aspects of the CAP algorithm used in this study have been previously described24. In brief, the user defines target points as a region of interest (ROI) for electrode sampling. This can be through typing the name of the structure (e.g. right amygdala) or clicking on the ROI of the brain parcellation image. The entry ROI can be specified if a superficial target is also required (e.g. entry through the motor cortex to target the supplementary motor area), but is not obligatory. In this study the same target points, and if specified the entry points, were selected based on the requirements of the SEEG MDT planning meeting. The user defines a maximum electrode length (90 mm was applied for all electrodes), as well as a maximum drilling angle (25 degrees from orthogonal to skull). The CAP algorithm will then remove any potential electrode trajectories that do not adhere to length and angle constraints before ensuring the trajectories pass through the skull model to the target ROI. If an entry ROI is defined trajectories not passing through this ROI will also be removed. The remaining trajectories are then checked to ensure they do not collide with a critical structure such as blood vessel or sulcus. A minimum distance from vessels can be set as a safety margin by the user (3 mm was used for all electrodes in this study). The electrode trajectories that satisfy the requirements are then stratified based on risk, which is calculated as a function of the cumulative distance from vessels along the whole trajectory, optimised for grey matter contact and adjusted to avoid conflicts with other electrode trajectories. The electrode trajectories are then presented for review by the using the ‘probe’s eye’ function linked to the orthogonal planes. The resulting electrode trajectories were then iterated through using either the ‘Next Entry’ and ‘Next Target’ buttons until a feasible electrode trajectory is chosen by the user. (See Figure 1).
- Risk metric calculation: EpiNav™ provides a graphic of the minimum distance from vasculature along the length of the electrode and a quantitative representation of the following safety metrics for both manual and CAP planned electrodes which were used for electrode comparison:
- Electrode length
- Drilling angle
- Risk
- Grey-white matter sampling ratio
- Minimum distance from vessel
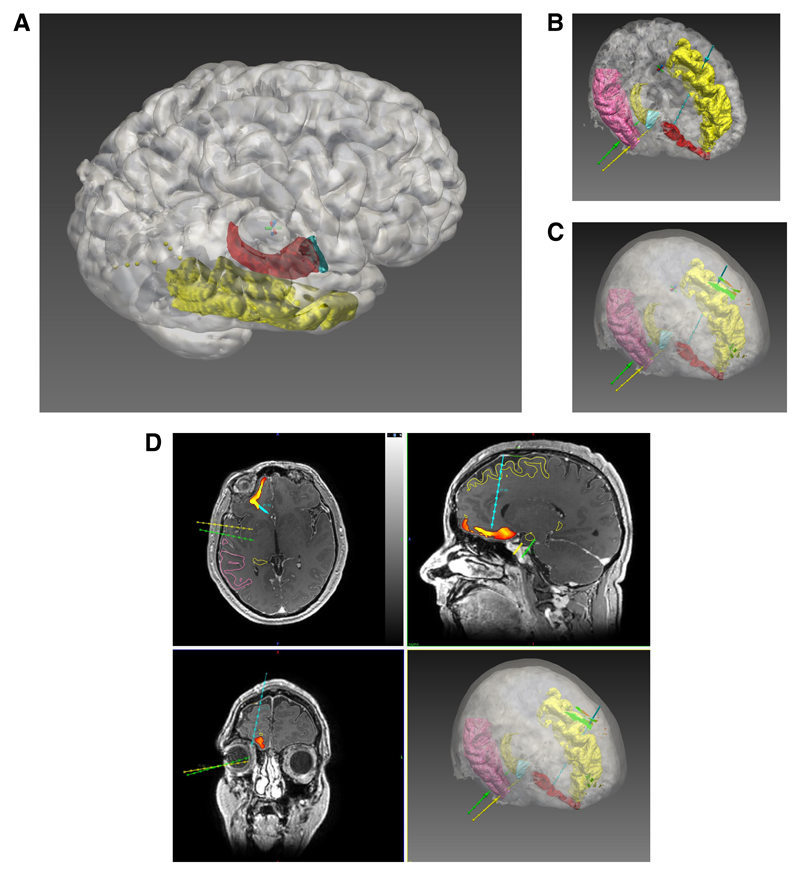
Figure 1. Computer-assisted planning.
Computer-assisted electrode generation workflow. A) Using the EpiNav Strategy™ module ROIs are automatically segmented from the parcellation image. In this example the cortex (white) is semi-transparent to allow visualisation of the underlying middle temporal gyrus (yellow), amygdala (blue) and hippocampus (red). B) Entry points and target points for the electrodes within the strategy are generated automatically based on the safety metrics defined by the user. Please note, in figures 1 B-D only three electrode trajectories are shown for clarity. Electrode colours are shown as right amygdala (yellow), right anterior hippocampus (green) and right posterior mesial orbitofrontal (blue). C) A surface risk ‘heat map’ on the scalp has been generated for the mesial orbitofrontal electrode, as an example, showing safety of potential trajectory entry points. D) Orthogonal and 3D views showing the target risk ‘heat map’ has been generated for the mesial orbitofrontal electrode, as an example, showing safe trajectory target points in orthogonal planes. Please note, in figures 1 B-D only three electrodes (right amygdala (yellow), right anterior hippocampus (green) and right posterior mesial orbitofrontal (blue)) are shown for clarity. A probe’s eye view (not shown) can then be linked to the orthogonal planes to assess the electrode trajectory further.
e. External validation
Five independent external raters who were neurosurgeons with expertise in performing SEEG implantations performed the external validation. The external raters have a range of experience with different implantation techniques including frame-based (JM), frameless (DN), iSYS1 (SW/CD) and Neuromate (MT) robotic implantation methods. A prospective power calculation based on a pilot study in which 14 electrodes from two patients were rated by a single surgeon (MT) revealed 24 electrodes were required to detect an absolute difference in risk of 0.2 assuming a standard deviation of 0.3 and a power of 0.90 to achieve a significance level p = 0.05, two-tailed. To account for a potential clustering effect a total of 13 patients were recruited. All raters appraised the same two pairs of plans (n = 32 electrodes) to assess inter-rater variability and a further 3-4 sets of paired plans (n = 34-41 electrodes) independently. All raters were blinded to the electrode trajectory generation method and were asked to provide ratings of the entry, trajectory and target feasibility for paired manual and CAP electrodes. Raters were asked to rate feasibility of each trajectory based on their current implantation practice. Given that the sampling region suitability had previously been approved by the multi-disciplinary team based on the non-invasive presurgical evaluation, the raters were only asked to comment on the surgical feasibility of electrode implantation.
f. Statistical evaluation
Risk metrics for manual and CAP electrodes were confirmed to have a normal distribution through the Shapiro-Wilks test (p>0.05). A paired Students t-test was performed for manual and CAP electrode comparisons. Clustering of electrodes within patients was assessed using a patient-specific random effects model (model 1) and the possible difference between surgeons using a fixed effect model (model 2). A generalised likelihood ratio test comparing models 1 and 2 was performed, with a resulting p-value of 0.151, indicating that there is insufficient evidence to suggest a significant difference between surgeons with regard to feasibility ratings. Feasibility ratings of electrodes generated from manual and CAP methods were compared using McNemar’s test and an odds ratio calculated.
3. Results
Thirteen consecutive patients who underwent SEEG implantation of 116 electrodes were included in the study. Manual plans were not provided for 12 electrodes due to safety concerns of reaching specified targets, however, CAP was able to generate trajectories for these electrodes. As such, paired results for safety metric comparison were available for 104 electrodes (Figure 2 and Table 2).
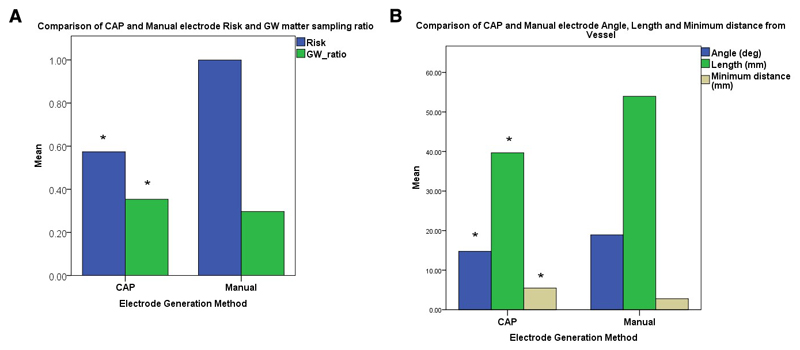
Figure 2. Comparison of CAP and manual electrode risk metrics.
A) Comparison of Risk and GM sampling ratio between CAP and manually generated electrodes showing a statistically significant reduction in Risk and improvement in GM sampling ratios. B) Comparison of trajectory angle, length and minimum distance from segmented vessels showing a statistically significant reduction in electrode trajectory length, drilling angle and increase in minimum distance from vasculature using CAP compared to manually generated electrodes. (* p<0.01)
Table 2.
Risk metric comparison between CAP and Manual plans
| Metric | CAP plan (mean +/- SD) | Manual plan (mean +/- SD) | Student’s t-test |
|---|---|---|---|
| Mean length (mm) | 39.8 +/- 14.9 | 54.0 +/- 14.7 | p = 0.001 |
| Drilling angle (deg) | 14.8 +/- 5.8 | 18.9 +/- 9.0 | p = 0.001 |
| Grey matter sampling ratio | 0.35 +/- 0.2 | 0.30 +/- 0.16 | p = 0.007 |
| Minimum distance from vessel (mm) | 5.4 +/- 3.0 mm | 2.8 +/- 1.9 mm | p < 0.001 |
| Risk | 0.57 +/- 0.39 | 1.00 +/- 0.60 | p = 0.001 |
Inter-rater variability: Surgeons rated each electrode for feasibility of the entry point, trajectory and target point. If all three ratings were deemed feasible the electrode was deemed feasible as a whole. All surgeons initially rated the same two pairs of plans (5 x 18 CAP and 5 x 14 manual electrode ratings) to assess inter-rater variability. A generalised likelihood ratio produced a test statistic of 6.72. When compared to the quantiles of Chi-squared distribution with 4 degrees of freedom a p-value of 0.11 was obtained, implying that there is insufficient evidence to suggest a difference in surgeon ratings. The remaining 98 electrode ratings pairs were then pooled.
a. Feasibility of electrode trajectories
Based on external independent ratings both manual and CAP electrodes were rated as feasible in 42.8% of cases. CAP was able to provide feasible electrodes in 19.4% whereas manual planning was able to generate a feasible electrode in 26.5% when the alternative generation method was not feasible. In 11.2% of cases both the CAP and manual electrode plans were both rated as unfeasible.
Time to generate plans
Both CAP and manual electrodes were generated using EpiNav™ which requires multimodal images to be co-registered and segmentation of vascular, sulcal and grey matter models prior to electrode planning. This time was common to both methods and depending on the number of images can take up to 60 minutes. Both CAP and manual planning requires generated electrodes to be checked using the probe’s eye and orthogonal views to ensure the electrodes are suitable and takes approximately 2 minutes per trajectory. Time for generation of the plans using the manual method varied from 2-4 hours whilst computational time for CAP varied from 34-120 seconds.
4. Discussion
a. Previous studies evaluating computer assisted planning
CAP for surgical interventions provides the potential to automate time-consuming tasks and optimise clinically significant parameters to improve the safety and efficacy of surgical interventions. Unlike human users, CAP systems provide reliable and reproducible results regardless of the institution or team providing the intervention. CAP algorithms however are only as good as the information provided to them. As a result, rigorous quality assurance is required for the imaging acquisition, post-processing and model segmentation used to generate CAP electrodes. The success of epilepsy surgery is dependent on the detection and safe resection of the epileptogenic zone. This is the minimum region of brain that is required to be resected or ablated to result in sustained seizure freedom. In cases in whom the presumed seizure onset zone cannot be accurately defined due to discrepancy or lack of clarity in the non-invasive pre-surgical evaluation (imaging studies, scalp EEG and neuropsychological investigations), invasive EEG in the form of SEEG or subdural grid / strip implantation is indicated. SEEG investigations involve the stereotactic placement of electrodes within predefined brain structures to allow for the spatial and temporal evolution of interictal and ictal activity to be recorded. This is subsequently used to guide surgical resection margins as well as functional cortical mapping. Here we describe a multimodal imaging platform for automated SEEG electrode implantation that allows for multiple electrode trajectories to be planned into anatomically defined structures, whilst avoiding conflicts with other electrodes, maintaining a user defined safety margin from cerebral vasculature, increasing cumulative distance from vessels, prevents crossing of sulcal pial boundaries and maximising grey matter sampling whilst reducing intracerebral electrode length and drilling angles.
-
Initial studies of CAP in neurosurgery were described in the 1980s for stereotactic intracranial biopsies7,13. The system described by Davies et al allowed the co-registration of pre-operative MRI scans with digital subtraction angiography and a CT scan performed once patients were placed in stereotactic frames7. The target points for the biopsies were manually placed by the surgeon and the computer system automatically calculated the stereotactic coordinates. Potential trajectories could then be simulated on anterior-posterior and lateral projections. Davies et al provided results from 447 biopsies performed in 439 patients for both supratentorial and infratentorial targets over a five year period in which a histological diagnosis was achieved in 99% and a clinically significant haemorrhage occurred in <1% (3/439).The next significant advance in CAP was through the introduction of 3D reconstructions of the cortex to allow the surgeon to choose the most appropriate surgical trajectory for the resection of supratentorial mass lesions12. Giorgi et al utilised this to plan a transfrontal approach as an alternative to a transcallosal approach for intraventricular lesions thereby preventing the neuropsychological complications related to partial corpus callosotomy13. A further iteration of this system was also used to allow manual segmentation of lesions and improve distinction between normal brain structures. Zamorano et al described the 'Wayne State University hardware and software configuration' which in addition to pre-planning surgical approaches could also be used intra-operatively with a neuronavigation system to track instruments in real time relative to the patients head30. The NeuroPlanner software also integrated multiple brain atlases within a computer assisted planning system for functional neurosurgical procedures such as thalamotomy, pallidotomy and deep brain stimulation (DBS) procedures21. This system resulted in a reduction in surgical operative time, improved targeting accuracy, reduced surgical complications and lower overall procedure cost. The prior use of clinical information to build upon and guide further surgery was described by Guo et al who developed probabilistic functional maps to guide targeting of the subthalamic nucleus for DBS14. Here the CAP automated targets and trajectories in 10 patients were compared to those developed by an experienced stereotactic neurosurgeon. The average distance between the CAP and manually planned target points was on average <2 mm. The incorporation of trajectory risk was used by Vaillant et al based on whether a particular trajectory intersected a critical brain structure and the relative weighting given to the importance of that structure28. Given that the major complications of stereotactic electrode placement includes haemorrhage and inaccuracy of targeting a structure the inaccuracy of the implantation method also requires consideration16,29. It is not sufficient therefore to calculate whether an electrode conflicts with a critical structure (such as an intracerebral vessel) but also how close the electrode passes to it along its trajectory. Cardinale et al introduced the concept of a minimum safety margin when planning SEEG electrodes based on the accuracy of the implantation method being used calculated by the following equation4:
Based on this a minimum distance of 3 mm was recommended, so that 99% of electrodes will fall within this safety margin. Once a minimum planning distance is set, risk for candidate trajectories can be calculated and represented as a heat map on the cortical surface 1,17,22. The calculation of risk however is based on the accuracy and completeness of segmentation of critical structures. In the case of cerebral vasculature a number of different vessel segmentation methods have been utilised including gadolinium enhanced MR, MR venography, MR angiography, time of flight (TOF) and DSA5. The gold-standard method is DSA but this entails an invasive procedure and radiation exposure. Non-invasive techniques visualize fewer segmented vessels but it is unclear whether this is clinically significant and whether there is a minimum vessel size that needs to be avoided. A simple weighting based on vessel size may not be appropriate as multiple factors such as stylet design, vessel tethering and vessel wall (arteries versus veins) also impact upon likelihood of haemorrhage3. Whilst reviewing complications associated with the placement of DBS electrodes Elias et al described a haemorrhagic complication rate of 10% in cases when electrodes crossed a sulcus and an intraventricular haemorrhage rate of 5% with ventricular penetration9. Beriault et al described a CAP algorithm that avoided segmented vasculature, critical neurological structures, ventricles, sulci and did not allow crossing of the midline providing qualitative safety metrics for each trajectory2. Trope et al added additional tractography and fMRI data and found that presentation of multi-modal information to the surgeon resulted in a change in trajectory for intracranial biopsies in 85% of cases27. Shenai et al described the use of CAP for the stereotactic placement of depth electrodes within the amygdalohippocampal complex in patients with epilepsy23. The system resulted one additional electrode contact being inserted within the target structure. De Momi et al described an automated system for the placement of multiple SEEG electrodes in which entry and target points are “roughly” selected and drilling angle to the skull as well as distance from other electrodes are additionally considered when calculating optimal trajectories8. Clinical validation of 26 electrodes in three patients were assessed by 4 blinded neurosurgeons and feasible electrodes were planned in 86% of cases and in 30% of cases these were preferred to manually planned electrodes. Of note CAP resulted in a significantly greater distance from vessels along the first 25 mm of the trajectory compared to the manual plans.
b. Improvements from previous work
We have previously described the EpiNav™ software platform for the automated placement of SEEG electrodes based on a user defined target and the aforementioned constraints20. We have subsequently improved upon this work by allowing entire anatomical structures to be selected as the target point based on a whole brain parcellation. This therefore allows the safest target within the anatomical structure of interest to be selected as manually placed targets may not represent the safest option. Furthermore, to improve the feasibility of electrodes and to account for different surgical preference we have allowed the user to iterate through risk stratified CAP generated electrodes. The development of a 'Next Target' or 'Next Entry' function allows the user to iterate through computed trajectories until they are satisfied with the trajectory. In line with our previous work we have shown that targeting whole structures, opposed to specific target points, results in improved safety metrics when compared to manually generated plans.
c. External validation of computer assisted planning
To provide external validation of the CAP planned trajectories 116 paired manual and CAP electrode plans for 13 patients were rated by neurosurgeons with expertise in SEEG from external institutions. The manual plans presented to the raters had already been implanted and no haemorrhages (clinically or non-clinically significant) occurred so by definition can be considered feasible. Of interest,69.4% of manual implantations were rated as feasible by the external raters reflecting the variation in individual surgeon practices and preferences depending on the implantation method used. Raters were asked to rate the feasibility of the trajectories based on their individual practices and whether they would be prepared to implant the trajectories themselves. It would be expected therefore that raters use different safety margins and heuristics, such as crossing of sulci, when assessing trajectories. CAP trajectories were deemed feasible in 62.2% and was able to generate feasible electrodes in 19.2% of cases where manual plans were considered infeasible. CAP is able to generate clinically feasible electrodes which are no less feasible than manually planned electrodes when externally rated. To our knowledge this is the first study in which both manual and CAP electrodes have been rated by blinded external raters to provide a more methodologically robust comparison between the implantation methods.
d. Limitations of the study
Methodologically the main limitation of the study is that it is retrospective in nature. Retrospective comparisons provide the potential for bias when generating the comparison dataset. Given that CAP data were generated in an automated fashion many months after the manual plans the impact of bias is likely to be minimal but cannot be completely excluded. A prospective validation study is currently underway.
MRV vessel segmentations were used to generate both the CAP and the manual electrode trajectories. The Gadolinium enhanced T1 sequences were used as the reference image against which raters assessed feasibility. Gadolinium enhanced T1 sequences highlight a number of vessels that are not possible to segment from the MRV. As such one would expect this to favour manual planning over the CAP generated electrodes. There is significant heterogeneity between the vessel segmentation methods used within European and North American epilepsy surgery centres. Although DSA is regarded by many as the gold-standard, it in itself is an invasive investigation that carries risk and radiation exposure. Given that DSA was not the standard of care in our institution at the time of manual electrode implantation we were unable to assess the impact of DSA on CAP trajectories. A potential future improvement of CAP would be to plan using DSA or multi-modal MR vessel segmentations.
Sulcal models used for CAP electrode generation are based on brain parcellation and the ability to segment CSF. The presence of CSF below the level of the gyrus is then taken to be within a sulcus and this is used as a region for exclusion during CAP electrode generation. CSF based sulcal models are not optimal in young patients, as the majority of sulci do not have visible CSF within them and the sulci are ‘potential’ as opposed to actual spaces. Further improvement in sulcal model generation is likely to lead to improved CAP electrode safety.
EpiNav™ has an integrated export function to allow planned trajectories to be seamlessly exported to the S7 stealth station (Medtronic Inc). Currently the software does not seamlessly export to other neuronavigation systems and this could potentially reduce the number of potential users of the software especially in the developing world. The software runs on most Windows PCs that contain a suitable NVidia graphics card. EpiNav was developed at University College London and is not commercial software. We are disseminating it for use at collaborating centres following appropriate local research ethics committee approval free of charge.
5. Conclusion
Here we provide a retrospective validation study of CAP for the placement of SEEG electrodes in patients with drug resistant focal epilepsy. CAP electrodes overall had an improved risk profile, increased minimum distance from vessels, shorter intracranial length, increased GM sampling, and lower drilling angles to the skull. CAP electrode were assessed by blinded external raters as feasible in 62.2% of cases compared to 69.4% of manually generated trajectories and were also found to be feasible when manually planned electrodes were infeasible in 19.4% of cases. CAP electrode planning is a valuable tool that can be used as a first-line method of electrode trajectory generation. The electrodes can then be reviewed by the operating surgeon with the ability to iterate through CAP-generated alternative trajectories or to re-plan electrodes manually when CAP electrodes are deemed infeasible. Given that CAP electrodes can be generated in a fraction of the time compared to manual electrodes this is likely to reduce the planning burden whilst ensuring improved safety metrics.
Table 3.
External blinding ratings of electrode feasibility
| Manual feasible | |||
|---|---|---|---|
| CAP feasible | Yes | No | Total |
| Yes | 42.8% (42/98) | 19.4% (19/98) | 62.2% (62/98) |
| No | 26.5% (26/98) | 11.2% (11/98) | 37.8% (37/98) |
| Total | 69.4% (68/98) | 30.6% (30/98) | 100% (98/98) |
Funding sources
This work was supported by the Wellcome Trust [Innovation Grant 106882] and the National Institute for Health Research University College London Hospitals Biomedical Research Centre. GPW was supported by an MRC Clinician Scientist Fellowship (MR/M00841X/1).
Footnotes
Conflicts of Interest: The authors report no conflict of interests
References
- 1.Beriault S, Al Subaie F, Mok K, Sadikot AF, Pike GB. Automatic trajectory planning of DBS neurosurgery from multi-modal MRI datasets. Med Image Comput Comput Assist Interv. 2011;14:259–266. doi: 10.1007/978-3-642-23623-5_33. [DOI] [PubMed] [Google Scholar]
- 2.Beriault S, Al Subaie F, Collins DL, Sadikot AF, Pike GB. A multi-modal approach to computer-assisted deep brain stimulation trajectory planning. Int J Comput Assist Radiol Surg. 2012;7:687–704. doi: 10.1007/s11548-012-0768-4. [DOI] [PubMed] [Google Scholar]
- 3.Brunenberg EJL, Vilanova A, Visser-Vandewalle V, Temel Y, Ackermans L, Platel B, et al. Automatic trajectory planning for deep brain stimulation: a feasibility study. Med Image Comput Comput Assist Interv. 2007;10:584–592. doi: 10.1007/978-3-540-75757-3_71. [DOI] [PubMed] [Google Scholar]
- 4.Cardinale F, Cossu M, Castana L, Casaceli G, Schiariti MP, Miserocchi A, et al. Stereoelectroencephalography: Surgical methodology, safety, and stereotactic application accuracy in 500 procedures. Neurosurgery. 2013;72:353–366. doi: 10.1227/NEU.0b013e31827d1161. [DOI] [PubMed] [Google Scholar]
- 5.Cardinale F, Pero G, Quilici L, Piano M, Colombo P, Moscato A, et al. Cerebral Angiography for Multimodal Surgical Planning in Epilepsy Surgery: Description of a New Three-Dimensional Technique and Literature Review. World Neurosurg. 2015;84:358–367. doi: 10.1016/j.wneu.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso MJ, Modat M, Wolz R, Melbourne A, Cash D, Rueckert D, et al. Geodesic Information Flows: Spatially-Variant Graphs and Their Application to Segmentation and Fusion. IEEE Trans Med Imaging. 2015;34:1976–1988. doi: 10.1109/TMI.2015.2418298. [DOI] [PubMed] [Google Scholar]
- 7.Davis DH, Kelly PJ, Marsh WR, Kall BA, Goerss SJ. Computer-assisted stereotactic biopsy of intracranial lesions in pediatric patients. Pediatr Neurosci. 1988;14:31–36. doi: 10.1159/000120359. [DOI] [PubMed] [Google Scholar]
- 8.De Momi E, Caborni C, Cardinale F, Casaceli G, Castana L, Cossu M, et al. Multi-trajectories automatic planner for StereoElectroEncephaloGraphy (SEEG) Int J Comput Assist Radiol Surg. 2014;9:1087–1097. doi: 10.1007/s11548-014-1004-1. [DOI] [PubMed] [Google Scholar]
- 9.Elias WJ, Sansur CA, Frysinger RC. Sulcal and ventricular trajectories in stereotactic surgery. J Neurosurg. 2009;110:201–207. doi: 10.3171/2008.7.17625. [DOI] [PubMed] [Google Scholar]
- 10.Prados Ferran, Cardoso M Jorge, Burgos Ninon, Wheeler-Kingshott Claudia AM, O S. NiftyWeb: web based platform for image processing on the cloud. Int Soc Magn Reson Med 24th Sci Meet Exhib; Singapore. 2016. [Google Scholar]
- 11.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 12.Giorgi C, Broggi G, Casolino D, Franzini A, Pluchino F. Computer assisted analysis of neuroradiological data in planning neurosurgical procedures. J Neurosurg Sci. 1989;33:19–22. [PubMed] [Google Scholar]
- 13.Giorgi C, Casolino SD, Franzini A, Servello D, Passerini A, Broggi G, et al. Computer-assisted planning of stereotactic neurosurgical procedures. Childs Nerv Syst. 1989;5:299–302. doi: 10.1007/BF00274517. [DOI] [PubMed] [Google Scholar]
- 14.Guo T, Parrent AG, Peters TM. Automatic target and trajectory identification for deep brain stimulation (DBS) procedures. Med Image Comput Comput Assist Interv. 2007;10:483–490. doi: 10.1007/978-3-540-75757-3_59. [DOI] [PubMed] [Google Scholar]
- 15.Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: Current concepts and future perspectives. Lancet Neurol. 2016;15:106–115. doi: 10.1016/S1474-4422(15)00225-2. [DOI] [PubMed] [Google Scholar]
- 16.Mullin JP, Shriver M, Alomar S, Najm I, Bulacio J, Chauvel P, et al. Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. Epilepsia. 2016;57:386–401. doi: 10.1111/epi.13298. [DOI] [PubMed] [Google Scholar]
- 17.Nowell M, Rodionov R, Zombori G, Sparks R, Rizzi M, Ourselin S, et al. A Pipeline for 3D Multimodality Image Integration and Computer-assisted Planning in Epilepsy Surgery. J Vis Exp. 2016 doi: 10.3791/53450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowell M, Rodionov R, Zombori G, Sparks R, Winston G, Kinghorn J, et al. Utility of 3D multimodality imaging in the implantation of intracranial electrodes in epilepsy. Epilepsia. 2015;56:403–413. doi: 10.1111/epi.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowell M, Sparks R, Zombori G. Europe PMC Funders Group Comparison of computer-assisted planning and manual planning for depth electrode implantations in epilepsy. 2016;124:1820–1828. doi: 10.3171/2015.6.JNS15487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowell M, Sparks R, Zombori G, Miserocchi A, Rodionov R, Diehl B, et al. Comparison of computer-assisted planning and manual planning for depth electrode implantations in epilepsy. J Neurosurg. 2015:1–3. doi: 10.3171/2015.6.JNS15487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowinski WL, Yang GL, Yeo TT. Computer-aided stereotactic functional neurosurgery enhanced by the use of the multiple brain atlas database. IEEE Trans Med Imaging. 2000;19:62–69. doi: 10.1109/42.832961. [DOI] [PubMed] [Google Scholar]
- 22.Shamir RR, Tamir I, Dabool E, Joskowicz L, Shoshan Y. A method for planning safe trajectories in image-guided keyhole neurosurgery. Med Image Comput Comput Assist Interv. 2010;13:457–464. doi: 10.1007/978-3-642-15711-0_57. [DOI] [PubMed] [Google Scholar]
- 23.Shenai MB, Ross DA, Sagher O. The use of multiplanar trajectory planning in the stereotactic placement of depth electrodes. Neurosurgery. 2007;60:272–6. doi: 10.1227/01.NEU.0000255390.92785.A4. discussion 276. [DOI] [PubMed] [Google Scholar]
- 24.Sparks R, Vakharia V, Rodionov R, Vos SB, Diehl B, Wehner T, et al. Anatomy-driven multiple trajectory planning (ADMTP) of intracranial electrodes for epilepsy surgery. Int J Comput Assist Radiol Surg. 2017 doi: 10.1007/s11548-017-1628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sparks R, Zombori G, Rodionov R, Nowell M, Vos SB, Zuluaga MA, et al. Automated multiple trajectory planning algorithm for the placement of stereo-electroencephalography (SEEG) electrodes in epilepsy treatment. Int J Comput Assist Radiol Surg. 2016:1–14. doi: 10.1007/s11548-016-1452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Téllez-Zenteno JF, Hernández-Ronquillo L, Buckley S, Zahagun R, Rizvi S. A validation of the new definition of drug-resistant epilepsy by the International League Against Epilepsy. Epilepsia. 2014;55:829–834. doi: 10.1111/epi.12633. [DOI] [PubMed] [Google Scholar]
- 27.Trope M, Shamir RR, Joskowicz L, Medress Z, Rosenthal G, Mayer A, et al. The role of automatic computer-aided surgical trajectory planning in improving the expected safety of stereotactic neurosurgery. Int J Comput Assist Radiol Surg. 2015;10:1127–1140. doi: 10.1007/s11548-014-1126-5. [DOI] [PubMed] [Google Scholar]
- 28.Vaillant M, Davatzikos C, Taylor RH, Bryan RN. A path-planning algorithm for image-guided neurosurgery. In: Troccaz J, Grimson E, Mösges R, editors. CVRMed-MRCAS’97: First Joint Conference Computer Vision, Virtual Reality and Robotics in Medicine and Medical Robotics and Computer-Assisted Surgery Grenoble, France, March 19--22, 1997 Proceedings; Berlin, Heidelberg: Springer Berlin Heidelberg; 1997. pp. 467–476. [Google Scholar]
- 29.Vakharia VN, Sparks R, O’Keeffe AG, Rodionov R, Miserocchi A, McEvoy A, et al. Accuracy of intracranial electrode placement for stereoencephalography: A systematic review and meta-analysis. Epilepsia. 2017:1–12. doi: 10.1111/epi.13713. 13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zamorano L, Jiang Z, Kadi AM. Computer-assisted neurosurgery system: Wayne State University hardware and software configuration. Comput Med Imaging Graph. 1994;18:257–271. doi: 10.1016/0895-6111(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 31.Zuluaga MA, Rodionov R, Nowell M, Achhala S, Zombori G, Mendelson AF, et al. Stability, structure and scale: improvements in multi-modal vessel extraction for SEEG trajectory planning. Int J Comput Assist Radiol Surg. 2015;10:1227–1237. doi: 10.1007/s11548-015-1174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]