Abstract
The neurotransmitter dopamine (DA) plays a key role in several biological processes including reward, mood, motor activity and attention. Synaptic DA homeostasis is controlled by the dopamine transporter (DAT) which transports extracellular DA into the presynaptic neuron after release and regulates its availability to receptors. Many neurological disorders such as schizophrenia, bipolar disorder, Parkinson disease and attention-deficit hyperactivity disorder are associated with imbalances in DA homeostasis that may be related to DAT dysfunction. DAT is also a target of psychostimulant and therapeutic drugs that inhibit DA reuptake and lead to elevated dopaminergic neurotransmission. We have recently demonstrated the acute and chronic modulation of DA reuptake activity and DAT stability through S-palmitoylation, the linkage of a 16-carbon palmitate group to cysteine via a thioester bond. This review summarizes the properties and regulation of DAT palmitoylation and describes how it serves to affect various transporter functions. Better understanding of the role of palmitoylation in regulation of DAT function may lead to identification of therapeutic targets for modulation of DA homeostasis in the treatment of dopaminergic disorders.
Keywords: posttranslational modification, palmitoyl acyl transferase, acyl protein thioesterase, phosphorylation, protein trafficking, 2 bromopalmitate
1. Introduction
Dopamine (DA) is a neurotransmitter that controls numerous functions including attention, mood, cognition, reward, and movement (Iversen and Iversen, 2007), and altered DA homeostasis is associated with many neurological diseases including depression, bipolar disorder (BD), schizophrenia, attention deficit hyperactivity disorder (ADHD), Parkinson disease and substance abuse, but the underlying mechanisms remain largely unknown (Bannon et al., 1998; Kristensen et al., 2011; Pramod et al., 2013). Dopamine tone is modulated by the dopamine transporter (DAT), an integral membrane protein that removes neurotransmitter from the extracellular space and thus regulates signaling at pre- and postsynaptic DA receptors (Giros et al., 1996). DAT is a target of numerous psychoactive drugs that suppress reuptake and induce elevated synaptic DA levels, including addictive compounds such as cocaine, amphetamine (AMPH), and methamphetamine (METH), and therapeutic drugs such as Adderall® (AMPH), Ritalin® (methylphenidate) and Wellbutrin® (bupropion) prescribed for dopamine related disorders including ADHD and depression.
DA reuptake activity undergoes acute and chronic regulation in response to signaling molecules and drug exposure, leading to impacts on DA tone that may contribute to DA imbalances in disease (Ramamoorthy et al., 2011; Schmitt and Reith, 2010). These responses can occur by up- or down-regulation of transporter cell surface numbers or by kinetic modification of transport velocity. DAT is subject to several post-translational modifications that mediate these processes (Bermingham and Blakely, 2016; Kristensen et al., 2011; Vaughan and Foster, 2013) and in this review we discuss the role of palmitoylation in control of reuptake and other DAT functions.
2. Transport Mechanism
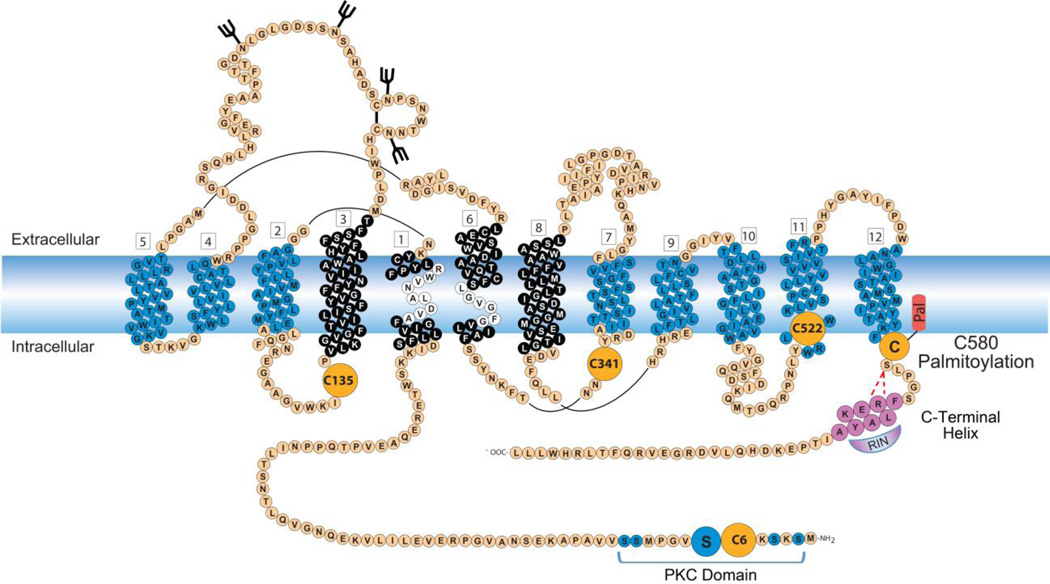
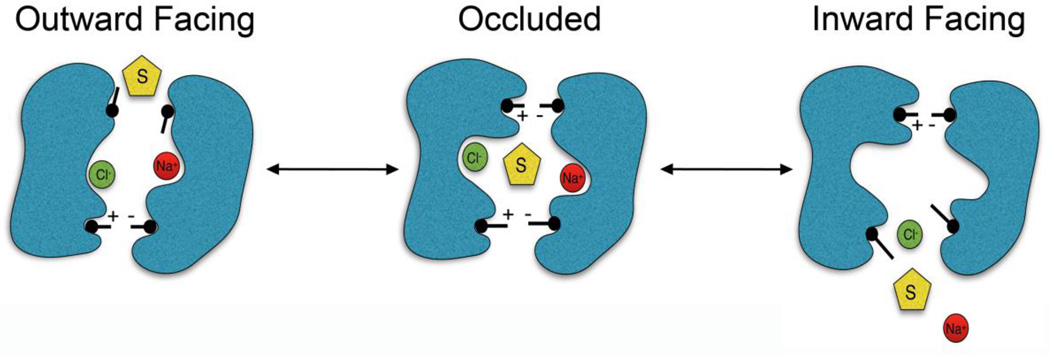
DAT belongs to the SLC6 family of Na+-Cl− dependent transporters and is composed of 12 transmembrane spanning domains (TMDs) arranged in two antiparallel aligned pentahelical bundles with cytoplasmically oriented N- and C-termini (Kristensen et al., 2011; Pramod et al., 2013) (Fig. 1). DAT structural and transport mechanism details have been elucidated by homology to the crystallized bacterial leucine transporter (LeuT) and the Drosophila (d)DAT, which facilitated the identification of the helix packing arrangement, substrate active site and translocation pathway, and extracellular and intracellular gating networks that dictate substrate movement (Beuming et al., 2006; Dahal et al., 2014; Wang et al., 2015). The inner core that forms the permeation pathway consists primarily of TMD1, TMD3, TMD6, and TMD8 (Fig. 1) with the DA binding site generated by critical residues contained within unwound segments in the middle of TMDs 1 and 6 (Fig. 1). Substrate translocation occurs through a series of conformational changes in which substrate is bound and released on opposite sides of the membrane (Forrest et al., 2008; Kristensen et al., 2011), a process known as the alternating access mechanism. Substrate binds to the outward facing conformation the transporter adopts when the extracellular gate is open and the intracellular gate is closed, sealing off the cytoplasmic side of the pathway from the extracellular environment (Fig. 2, Outward Facing). Once the substrate is bound, the transporter transitions into an occluded state in which both the extracellular and intracellular gates are closed (Fig. 2, Occluded), and then transitions into the inward facing conformation where the intracellular gate opens, releasing substrate to the cell interior (Fig. 2, Inward Facing). After substrate has been released the intracellular gate is reestablished and the empty transporter returns to the outward facing conformation, allowing the next molecule of extracellular substrate to bind. The overall kinetic capacity of the protein is thus dictated by the rate of these transitions.
Fig. 1.
Schematic diagram of the expanded membrane topology of DAT with 12 TMDs and intracellularly facing N- and C-termini. Vertical stacks of amino acids (blue, black and white) represent α-helical membrane spanning domains arranged in two antiparallel aligned pentahelical bundles (TMDs 1–5 and 6–10) with TMDs 11 and 12 located peripheral to these bundles. The DA binding site is positioned within the inner core (black) with critical residues contained within unwound segments found in the middle of TMD1 and TMD6 (white). The known sites of glycosylation (black branched sticks), phosphorylation within the PKC-domain containing Ser7 (blue circles) and palmitoylation (orange circle with red appendage) are shown. Potential additional sites of palmitoylation are shown as large orange circles. The C-terminal helix (mauve, FREKLAYA) is also shown with Ras-like GTPase Rin 1 bound (Rin, mauve) and red dashed lines depicting hydrogen bonding between Arg 587 and Ser 581.
Fig. 2.
Illustration of the alternating access mechanism of DA transport. Substrate (S) and ions (Na+ and Cl−) access the binding site of the outward facing transporter via the open outer gate, the outer gate closes yielding the occluded state, which then transitions to the inward facing state releasing substrate and ions to the cytosol. The transporter then rectifies to the outward facing structure. Black sticks represent the gating network of amino acid side chains.
In addition, mammalian DATs have large N- and C-termini that extend into the cytoplasm (Fig. 1). These domains receive regulatory input via post-translational modifications and interactions with binding partners, and transmit this information to the functional core. The N-terminal domain is a site for input from protein kinases including PKC and ERK (reviewed in Foster and Vaughan, 2016 this issue), and we have recently determined that modification by S-palmitoylation occurs near the C-terminal domain (Foster and Vaughan, 2011) (Fig. 1). This review will describe the characterization of palmitoylation and its linkage to changes in transport activity and other functions.
3. Protein S-palmitoylation
S-palmitoylation of proteins is the thioesterification of palmitate, a 16-carbon fatty acid, to a cysteine residue. For integral membrane proteins this modification regulates a variety of properties including functional activity, trafficking, turnover, membrane raft targeting, and cholesterol binding (el-Husseini Ael and Bredt, 2002; Fang et al., 2006; Greaves and Chamberlain, 2011; Huang and El-Husseini, 2005; Huber et al., 2006; Lai and Linder, 2013). The thioester bond between the fatty acid and the protein is labile and reversible, allowing for dynamically regulated cycles of palmitoylation and depalmitoylation to occur throughout the protein's lifetime (Linder and Deschenes, 2007) in a manner analogous to phosphorylation and dephosphorylation (Fukata and Fukata, 2010; Smotrys and Linder, 2004).
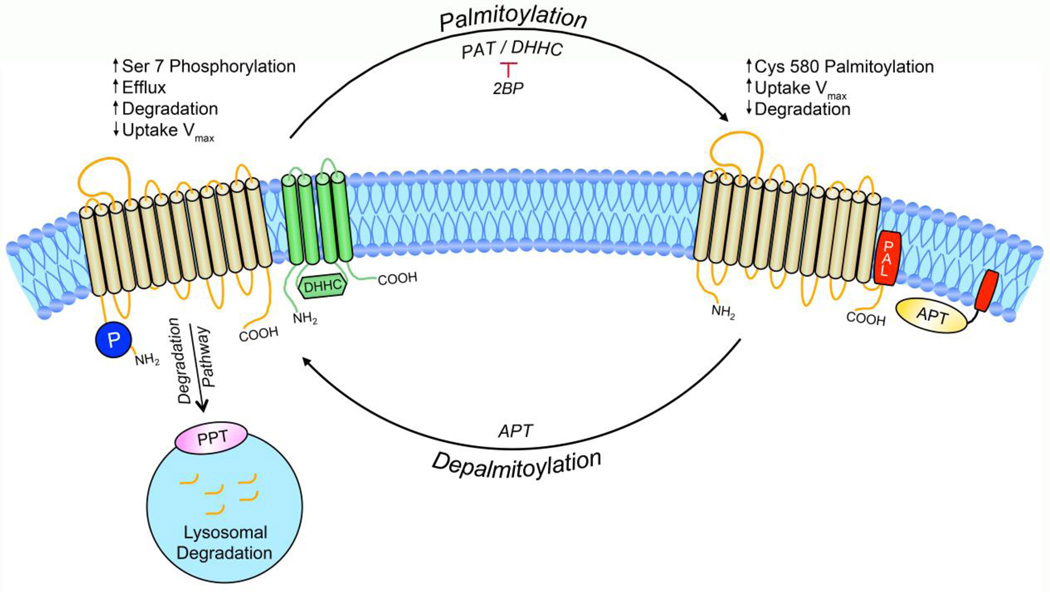
Catalysis of palmitoylation is driven by palmitoyl acyltransferases (PATs) (Fig. 3). The human genome contains 23 PAT enzymes (Fang et al., 2006; Fukata et al., 2004; Fukata et al., 2006; Roth et al., 2002), that are also known as DHHC enzymes in reference to the conserved amino acid sequence Asp-His-His-Cys in the active site (Roth et al., 2002). The enzymes have varied tissue distributions, including several that are highly expressed in the brain, and intracellular localizations, with most being localized to the Golgi apparatus, endoplasmic reticulum, or plasma membrane (Korycka et al., 2012; Ohno et al., 2006). PATs are polytopic membrane proteins that typically contain 4 TMDs with the catalytic domain present in the intracellular loop between TMDs 2 and 3 (Fig. 3) (Fukata et al., 2004; Mitchell et al., 2006), suggesting that palmitoylation reactions with integral proteins generally occur near the cytosolmembrane interface (Salaun et al., 2010). Little is known regarding mechanisms of substrate specificity or enzyme regulation, but the large number of PATs and their specific subcellular localization suggests that palmitoylation machinery is tightly regulated. Currently there are few specific pharmacological PAT activators or inhibitors, limiting the experimental approaches available for examining functional properties. However, functional consequences of modification can be probed by reducing palmitoylation with the compound 2-bromopalmitate (2BP) a nonspecific irreversible PAT inhibitor (Jennings et al., 2009), or by increasing palmitoylation by overexpression of PAT enzymes (Fukata et al., 2004; Moritz et al., 2015).
Fig. 3.
Functional consequences of DAT palmitoylation. The cycle of DAT palmitoylation and depalmitoylation by palmitoyl acyltransferases (green, PAT / DHHC) and acyl protein thioesterases (yellow, APT), respectively, is shown. Identified palmitoylation site Cys580 (red, PAL) and the reciprocally regulated Ser 7 phosphorylation site (dark blue, P) are also shown. DAT destined for degradation is directed toward the lysosome via an unknown sorting mechanism which could include depalmitoylation by lysosomal localized palmitoyl-protein thioesterase (PPT, pink).
Protein depalmitoylation is catalyzed by acyl protein thioesterases (APTs), enzymes that belong to a superfamily of serine hydrolases. There are multiple forms of these proteins with various subcellular distribution and potential functions. Enzymes named APT1, APT2, and APT1-like proteins remove palmitate from cytosolically accessible sites of membrane proteins (Linder and Deschenes, 2007), and are thought to function in regulated turnover of palmitate. Palmitoyl protein thioesterase 1 and 2 (PPT1, PPT2) are associated with lysosomes and are linked with control of protein stability by catalyzing protein depalmitoylation prior to lysosomal degradation (Fukata and Fukata, 2010; Vesa et al., 1995). Recently members of a subfamily of α/β-hydrolase domain-containing serine hydrolases not belonging to either of these families were found to depalmitoylate the neuronal protein PSD-95 (Yokoi et al., 2016), suggesting that additional proteins involved in depalmitoylation remain to be discovered.
4. DAT palmitoylation characteristics
Our labs have demonstrated the modification of rat (r), mouse (m), and human (h) DATs by S-palmitoylation in both rodent brain tissue and heterologous cell systems using metabolic labeling of DAT with [3H]palmitic acid in living cells or tissue, and acyl biotinyl exchange (ABE), an in vitro assay for post-hoc detection of endogenous palmitoylation (Foster et al., 2016; Foster and Vaughan, 2011). Both methods have yielded comparable results in numerous analyses, indicating their interchangeability. Metabolic labeling of DAT with [3H]palmitic acid is easily detected within 1h, indicative of a rapid rate of palmitate turnover, and ABE analysis also indicates the presence of robust tonic palmitoylation levels. Palmitoylation of DAT is inhibited by 2BP, consistent with catalysis driven by PATs, and is reversible in vitro with hydroxylamine, which cleaves thioester bonds, confirming that the modification occurs via a thioester linkage.
Rodent and human DATs contain five conserved cysteine residues, Cys6, Cys135, Cys341/342, Cys522/523, and Cys580/581, that are predicted to be exposed to the cytoplasm and thus represent potential S-palmitoylation sites (Fig. 1). To identify the site(s) modified, we mutated each of these residues individually to alanine (A) and assessed the proteins for [3H]palmitic acid labeling (Foster and Vaughan, 2011). Analysis of rDAT Cys→Ala mutants showed that incorporation was reduced by ~60% in C580A DAT, indicating this residue as a major palmitoylation site (Fig. 1), with a simlar reduction of labeling obtained for hDAT C581A (Shetty and Foster, unpublished results). This cysteine is located at the membrane-cytoplasm interface of TMD12, the most C-terminal membrane spanning helix, a common palmitoylation site for membrane proteins (Salaun et al., 2010). However, a substantial portion of [3H]palmitate labeling still remains on C580/581A DATs, demonstrating that palmitoylation is occurring on one or more additional site(s). None of the other Cys→Ala mutants in these studies displayed decreased labeling, suggesting that an unexamined site is modified, that multiple sites are modified at low stoichioimetries that are difficult to detect by this method, or that the mutations induced compensatory palmitoylation on another site, possibly Cys580. Our studies to date have focused on properties of Cys580/581, but identification of the additional site(s) is an important issue as each modification site may confer distinct regulatory effects (Hayashi et al., 2005).
To examine the functional ramifications of palmitoylation in the WT protein we utilized the PAT inhibitor 2BP (Foster and Vaughan, 2011). In rat striatal synaptosomes, significant inhibition of DAT palmitoylation was detectable within 30 min of 2BP treatment, with further decreases occurring between 45 and 60 min. In contrast, inhibition of DAT [3H]palmitate labeling in LLCPK1 cells was less pronounced and required treatment times of 6–18h indicating major differences in palmitoylation kinetics between these systems. As a consequence, we have developed rDAT-expressing N2a neuroblastoma cells and immortalized rat dopaminergic midbrain neurons (N27), as neuronal cells lines for palmitoylation analyses. In these cells DAT palmitoylation is considerably more sensitive to 2BP (Rastedt, Stanislowski, Vaughan and Foster, unpublished results), indicating a greater similarity of this property to brain tissue.
Using 2BP to examine functional ramifications of DAT palmitoylation we have identified multiple effects that can be broadly categorized as rapid or slow changes. In synaptosomes (Foster and Vaughan, 2011) and in rDAT-N2a and rDAT-N27 cell lines (Rastedt, Stanislowski, Vaughan, and Foster, unpublished results), low dose and short-term 2BP treatments cause up to ~50% reductions in DA transport Vmax, with no detectable change in total or surface transporter levels. The acute decreases in the kinetic efficiency of transport with reduced palmitoylation indicates that palmitoylation function to enhance the kinetic capacity of the transporter. Consistent with these findings transport Vmax of palmitoylation-deficient C580A DAT is strongly reduced relative to the WT protein (Moritz et al., 2015). PMA-induced downregulation is also increased in 2BP-treated and C580A DATs, indicating that the enhanced activity derived from palmitoylation functions to oppose PKC-induced down-regulation (Foster and Vaughan, 2011). These findings thus identify a novel mechanism for control of DAT transport activity and implicate Cys580 palmitoylation in enhancement of the kinetic capacity of the transporter.
Longer-term 2BP treatments in both rDAT-LLCPK1 cells and synaptosomes were also associated with further losses of transport that were accompanied by loss of DAT protein and appearance of low Mr transporter fragments. This indicates that prolonged suppression of palmitoylation drives DAT into the degradation pathway (Foster and Vaughan, 2011). Consistent with this finding, C580A DAT displays increased turnover assessed via [35S] methionine pulse-chase analysis (Moritz, Vaughan, and Foster, unpublished results), indicating that palmitoylation supports the metabolic stability of DAT and functions as a mechanism for long-term control of total transporter levels.
More recently we have been able to elevate DAT palmitoylation by overexpressing DHHC enzymes in rDAT cell systems (Moritz et al., 2015). Our findings indicate that DAT palmitoylation can be catalyzed by a subset of neuronally-expressed PATs, although many PATs were without effect, indicative of substrate specificity. Increased DAT palmitoylation driven by DHHC enzyme co-expression led to increased DA uptake Vmax without changes in transporter surface expression or KM, further supporting the contention that DAT palmitoylation increases the rate of transport (Rastedt et al., 2014). Together these findings are consistent with the mechanism shown in Fig. 3 in which conditions that enhance palmitoylation lead to acute increases in transport Vmax driven by a kinetic mechanism, while sustained suppression of palmitoylation leads to targeting of DAT for degradation.
5. Reciprocal regulation of DAT palmitoylation and phosphorylation
The enhancement of transport by palmitoylation is thus opposite to the reduction of transport imparted by phosphorylation, prompting us to examine the relationship of these modifications in greater detail. DAT undergoes protein kinase C (PKC)-dependent phosphorylation in a serine cluster on the distal end of the N-terminus (Fig. 1), with phosphorylation of Ser7 within this domain a primary determinate in PKC-induced transport down-regulation (reviewed in Foster and Vaughan, 2016 this issue). We have now shown that not only do Cys580 palmitoylation and Ser7 phosphorylation exert opposing effects on transport activity, but that they are mechanistically linked in a reciprocal manner (Moritz et al., 2015). Palmitoylation status affects phosphorylation, as C580A mutation and 2BP treatment, conditions that reduce DAT palmitoylation, lead to enhanced basal and PKC-stimulated DAT phosphorylation, which in some cases was localized to Ser7, and elevation of palmitoylation with DHHC 2 overexpression leads to reduced transporter 32PO4 labeling (Moritz et al., 2015). Conversely, phosphorylation conditions affect palmitoylation, as activation of PKC, which elevates Ser7 phosphorylation, reduces DAT palmitoylation, and conditions that reduce Ser7 phosphorylation (S7A mutation, PKC inhibitor) enhance DAT palmitoylation. Similar reciprocity of palmitoylation and phosphorylation have been shown for other proteins (Charych et al., 2010; Dorfleutner and Ruf, 2003; Lin et al., 2009; Moffett et al., 1996), although in most cases the modified sites are close in primary sequence and the reciprocal relationship of the modifications has been attributed to steric hindrance. As the modified sites in DAT are far apart in primary sequence, the underlying mechanism for the reciprocal regulation remains unknown. Importantly, transporter activity follows these modifications, as decreased transport Vmax is driven by increased phosphorylation/decreased palmitoylation and increased transport Vmax is driven by decreased phosphorylation/increased palmitoylation supporting the concerted regulation of transport by the balance between these modifications (Fig 3).
6. Possible palmitoylation mechanisms in transport kinetics
In the studies described above altered transport levels were not associated with changes in DAT plasma membrane levels detected by surface biotinylation indicating that regulation of uptake occurred via a kinetic mechanism. Kinetic regulation of transport by palmitoylation implies that the modification is affecting the rate at which the protein transitions through the transport cycle. As Cys580 lies outside the core TM bundles that function in substrate binding and permeation, it is likely that palmitoylation affects transport capacity indirectly through effects on gating networks, permeation pathway conformational changes or binding partner interactions.
Palmitoylation can affect integral membrane proteins in numerous ways. At the level of protein - lipid bilayer interaction, transmembrane domains that are longer than the width of the membrane bilayer create a situation of hydrophobic mismatch that is energetically unfavorable (van Duyl et al., 2002) and can affect protein function. Palmitoylation may assist in reducing mismatch by promoting changes in helix orientation or tilting (Strandberg and Killian, 2003), a possibility for DAT that is consistent with the site of palmitoylation at the base of TMD12. Partitioning of proteins into membrane raft domains, which possess greater membrane thickness than non-raft regions may also reduce mismatch (Andersen and Koeppe, 2007) or promote interactions with cholesterol or raft binding partners that are impacted by palmitoylation. DAT is associated with membrane raft microdomains (Cremona et al., 2011; Foster et al., 2008; Gabriel et al., 2013; Hong and Amara, 2006; Navaroli et al., 2011; Sakrikar et al., 2012) and phosphorylation of DAT is enriched at these sites (Foster et al., 2008), suggesting this as a possible mechanism for the concerted regulation of phosphorylation and palmitoylation. It is also possible that palmitoylation of DAT at Cys580 may affect its ability to form a dimer, as the LeuT crystal structure showed a dimer interface at TMD12 (Yamashita et al., 2005). Dimer and monomer forms of DAT at the cell surface may exhibit unique transport characteristic or subcellular localization (Foster et al., 2008; Hong and Amara, 2006; Hong and Amara, 2010; Jones et al., 2012; Seidel et al., 2005; Zhen et al., 2015) that affect DAT transport kinetics.
A novel attribute revealed by the dDAT crystal structure is the presence of a motif formed by a hairpin loop between the base of TMD12 and an adjacent helix formed by residues 586–595. This helix lies parallel to the plane of the membrane and interacts with intracellular loop 3 (IL3) to stabilize the intracellular gate and indirectly impact the ability of TMD1 to undergo conformational changes needed for transport (Penmatsa et al., 2015). The interaction between the helix and IL3 is stabilized by hydrogen bonding between Thr582 and Arg589 which are conserved in rDAT as Ser581 and Arg587 (Fig. 1), strongly suggesting that structural impacts on TMD12 by palmitoylation at Cys580 could affect the ability of Ser581 to form this H-bond, providing a potential mechanistic explanation for its ability to modulate transport activity. This helix was originally identified as an endocytosis motif that mediates PKC-dependent endocytosis (Holton et al., 2005; Navaroli et al., 2011). Current surface biotinylation findings indicate that palmitoylation-mediated regulation of transport occurs independently of plasma membrane changes, but further examination of the effect of these modifications on trafficking may be warranted due to the proximity of the palmitoylation site to the helix.
7. Palmitoylation in disease
Many proteins with essential functions in neurotransmission and synaptic plasticity are palmitoylated (Duncan and Gilman, 1998; el-Husseini Ael and Bredt, 2002; Fang et al., 2006; Fukata et al., 2004; Hayashi et al., 2005; Keller et al., 2004), and dysregulation of palmitoylation is associated with multiple neurological disorders, including intellectual disability, Huntington disease, Alzheimer disease, schizophrenia, and the lysosomal storage disease infantile neuronal ceroid lipofuscinosis (reviewed in Cho and Park, 2016). In many cases these diseases are caused or associated with dysregulation of the acyl transferases or thioesterases that cause inappropriate palmitoylation of target proteins, suggesting that similar dysregulation of palmitoylation inputs into DAT could have strong impacts on its associated functions. In particular, reduced palmitoylation of glial glutamate transporter-1 results in decreased glutamate uptake, that has been suggested as a cause of excitotoxicity (Huang et al., 2010).
Several DAT SNPs associated with disease states have now been identified, including A559V, identified from ADHD and BD patients (Mazei-Robison et al., 2005). This residue is found at the extracellular end of TMD12, and induces significant effects on transport functions including reverse transport (Mazei-Robison et al., 2008), which is driven by DAT hyperphosphorylation (Khoshbouei et al., 2004; Wang et al., 2016). This mutant displays reduced palmitoylation, which could mechanistically link it to altered phosphorylation and efflux, resulting in hyperdopaminergia that is associated with ADHD and BD (Shetty et al., 2015), and supporting the concept that DA imbalances could follow from dysregulated palmitoylation.
8. Summary
The findings described here highlight the importance of palmitoylation in the functionality and regulation of DAT. Changes in transporter functionality driven by palmitoylation thus suggest this modification as a potential therapeutic target for modulation of DA reuptake in disease states through modulation of the palmitoyl transferases or thioesterases that regulate the modification. Mutations in the palmitoyl transferases and APT/PPT enzymes have also resulted in disease states, indicating that the palmitoylation status of a protein is relevant to disease processes. Understanding the mechanisms of these posttranslational modifications and the effect they have on DAT, along with elucidation of the N- and C-terminal domain structures, is critical in understanding the regulatory processes that occur in disease states and potential treatment via these specific therapeutic targets.
Highlights.
DAT is reversibly modified with a 16-carbon palmitate group through a thioester bond.
Enhanced DAT palmitoylation leads to acute increases in transport Vmax driven by a kinetic mechanism.
Sustained suppression of palmitoylation leads to targeting of DAT for degradation.
DAT is regulated in a reciprocal manner by palmitoylation and phosphorylation.
Acknowledgments
Funding: This work was supported by National Institutes of Health grants DA 031991 (JDF), DA13147 and 5P20-104360 (RAV), P30-GM103329 (UND), P20-GM12345 (UND) and ND EPSCoR Doctoral Dissertation Fellowship (DER)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen OS, Koeppe RE., 2nd Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Sacchetti P, Granneman JG. The dopamine transporter: potential involvement in neuropsychiatric disorders. Philadelphia: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- Bermingham DP, Blakely RD. Kinase-dependent Regulation of Monoamine Neurotransmitter Transporters. Pharmacol Rev. 2016;68:888–953. doi: 10.1124/pr.115.012260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuming T, Shi L, Javitch JA, Weinstein H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2006;70:1630–1642. doi: 10.1124/mol.106.026120. [DOI] [PubMed] [Google Scholar]
- Charych EI, Jiang LX, Lo F, Sullivan K, Brandon NJ. Interplay of palmitoylation and phosphorylation in the trafficking and localization of phosphodiesterase 10A: implications for the treatment of schizophrenia. J Neurosci. 2010;30:9027–9037. doi: 10.1523/JNEUROSCI.1635-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, Park M. Palmitoylation in Alzheimer's disease and other neurodegenerative diseases. Pharmacol Res. 2016;111:133–151. doi: 10.1016/j.phrs.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, Rothman JE, Galli A, Javitch JA, Yamamoto A. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nature neuroscience. 2011;14:469–477. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal RA, Pramod AB, Sharma B, Krout D, Foster JD, Cha JH, Cao J, Newman AH, Lever JR, Vaughan RA, Henry LK. Computational and biochemical docking of the irreversible cocaine analog RTI 82 directly demonstrates ligand positioning in the dopamine transporter central substrate-binding site. J Biol Chem. 2014;289:29712–29727. doi: 10.1074/jbc.M114.571521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfleutner A, Ruf W. Regulation of tissue factor cytoplasmic domain phosphorylation by palmitoylation. Blood. 2003;102:3998–4005. doi: 10.1182/blood-2003-04-1149. [DOI] [PubMed] [Google Scholar]
- Duncan JA, Gilman AG. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS) J Biol Chem. 1998;273:15830–15837. doi: 10.1074/jbc.273.25.15830. [DOI] [PubMed] [Google Scholar]
- el-Husseini Ael D, Bredt DS. Protein palmitoylation: a regulator of neuronal development and function. Nat Rev Neurosci. 2002;3:791–802. doi: 10.1038/nrn940. [DOI] [PubMed] [Google Scholar]
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci. 2006;26:12758–12768. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest LR, Zhang YW, Jacobs MT, Gesmonde J, Xie L, Honig BH, Rudnick G. Mechanism for alternating access in neurotransmitter transporters. Proc Natl Acad Sci U S A. 2008;105:10338–10343. doi: 10.1073/pnas.0804659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Rastedt DE, ChallaSivaKanaka S, Vaughan RA. Analysis of Neurotransmitter Transporter Posttranslational Modification: Phosphorylation and Palmitoylation. In: Bönisch H, Sitte HH, editors. Neurotransmitter Transporters Investigative Methods. New York: Springer; 2016. [Google Scholar]
- Foster JD, Vaughan RA. Palmitoylation controls dopamine transporter kinetics, degradation, and protein kinase C-dependent regulation. J Biol Chem. 2011;286:5175–5186. doi: 10.1074/jbc.M110.187872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Vaughan RA. Phosphorylation mechanisms in dopamine transporter regulation. J Chem Neuroanat. 2016 doi: 10.1016/j.jchemneu.2016.10.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nature reviews. Neuroscience. 2010;11:161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Iwanaga T, Fukata M. Systematic screening for palmitoyl transferase activity of the DHHC protein family in mammalian cells. Methods. 2006;40:177–182. doi: 10.1016/j.ymeth.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Gabriel LR, Wu S, Kearney P, Bellve KD, Standley C, Fogarty KE, Melikian HE. Dopamine transporter endocytic trafficking in striatal dopaminergic neurons: differential dependence on dynamin and the actin cytoskeleton. J Neurosci. 2013;33:17836–17846. doi: 10.1523/JNEUROSCI.3284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Greaves J, Chamberlain LH. DHHC palmitoyl transferases: substrate interactions and (patho)physiology. Trends Biochem Sci. 2011;36:245–253. doi: 10.1016/j.tibs.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Rumbaugh G, Huganir RL. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47:709–723. doi: 10.1016/j.neuron.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Holton KL, Loder MK, Melikian HE. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat Neurosci. 2005;8:881–888. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Amara SG. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience Program No. 532.4; 2006. Modulation of dopamine transporter function by membrane cholesterol. [Google Scholar]
- Hong WC, Amara SG. Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. J Biol Chem. 2010;285:32616–32626. doi: 10.1074/jbc.M110.150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, El-Husseini A. Modulation of neuronal protein trafficking and function by palmitoylation. Curr Opin Neurobiol. 2005;15:527–535. doi: 10.1016/j.conb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Huang K, Kang MH, Askew C, Kang R, Sanders SS, Wan J, Davis NG, Hayden MR. Palmitoylation and function of glial glutamate transporter-1 is reduced in the YAC128 mouse model of Huntington disease. Neurobiol Dis. 2010;40:207–215. doi: 10.1016/j.nbd.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Huber TB, Schermer B, Muller RU, Hohne M, Bartram M, Calixto A, Hagmann H, Reinhardt M, Koos F, Kunzelmann K, Shirokova E, Krautwurst D, Harteneck C, Simons M, Pavenstadt H, Kerjaschki D, Thiele C, Walz G, Chalfie M, Benzing T. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci U S A. 2006;103:17079–17086. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30:188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Jennings BC, Nadolski MJ, Ling Y, Baker MB, Harrison ML, Deschenes RJ, Linder ME. 2-Bromopalmitate and 2-(2-hydroxy-5-nitro-benzylidene)-benzo[b]thiophen-3-one inhibit DHHC-mediated palmitoylation in vitro. J Lipid Res. 2009;50:233–242. doi: 10.1194/jlr.M800270-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Zhen J, Reith ME. Importance of cholesterol in dopamine transporter function. Journal of neurochemistry. 2012;123:700–715. doi: 10.1111/jnc.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoe-Pognetto M, Luscher B. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korycka J, Lach A, Heger E, Boguslawska DM, Wolny M, Toporkiewicz M, Augoff K, Korzeniewski J, Sikorski AF. Human DHHC proteins: a spotlight on the hidden player of palmitoylation. Eur J Cell Biol. 2012;91:107–117. doi: 10.1016/j.ejcb.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Lai J, Linder ME. Oligomerization of DHHC protein S-acyltransferases. J Biol Chem. 2013;288:22862–22870. doi: 10.1074/jbc.M113.458794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–887. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, Galli A, Blakely RD. Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci. 2008;28:7040–7046. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robison MS, Couch RS, Shelton RC, Stein MA, Blakely RD. Sequence variation in the human dopamine transporter gene in children with attention deficit hyperactivity disorder. Neuropharmacology. 2005;49:724–736. doi: 10.1016/j.neuropharm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res. 2006;47:1118–1127. doi: 10.1194/jlr.R600007-JLR200. [DOI] [PubMed] [Google Scholar]
- Moffett S, Adam L, Bonin H, Loisel TP, Bouvier M, Mouillac B. Palmitoylated cysteine 341 modulates phosphorylation of the beta2-adrenergic receptor by the cAMP-dependent protein kinase. J Biol Chem. 1996;271:21490–21497. doi: 10.1074/jbc.271.35.21490. [DOI] [PubMed] [Google Scholar]
- Moritz AE, Rastedt DE, Stanislowski DJ, Shetty M, Smith MA, Vaughan RA, Foster JD. Reciprocal Phosphorylation and Palmitoylation Control Dopamine Transporter Kinetics. J Biol Chem. 2015;290:29095–29105. doi: 10.1074/jbc.M115.667055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaroli DM, Stevens ZH, Uzelac Z, Gabriel L, King MJ, Lifshitz LM, Sitte HH, Melikian HE. The plasma membrane-associated GTPase Rin interacts with the dopamine transporter and is required for protein kinase C-regulated dopamine transporter trafficking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:13758–13770. doi: 10.1523/JNEUROSCI.2649-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y, Kihara A, Sano T, Igarashi Y. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta. 2006;1761:474–483. doi: 10.1016/j.bbalip.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Penmatsa A, Wang KH, Gouaux E. X-ray structures of Drosophila dopamine transporter in complex with nisoxetine and reboxetine. Nat Struct Mol Biol. 2015;22:506–508. doi: 10.1038/nsmb.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramod AB, Foster JD, Carvelli L, Henry LK. SLC6 transporters: Structure, function, regulation, disease association and therapeutics. Mol Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastedt DE, Foster JD, Vaughan RA. Multiple palmitoyl acyltransferases modify DAT palmitoylation. FASEB J. 2014;28 803.805. [Google Scholar]
- Roth AF, Feng Y, Chen L, Davis NG. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol. 2002;159:23–28. doi: 10.1083/jcb.200206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakrikar D, Mazei-Robison MS, Mergy MA, Richtand NW, Han Q, Hamilton PJ, Bowton E, Galli A, Veenstra-Vanderweele J, Gill M, Blakely RD. Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:5385–5397. doi: 10.1523/JNEUROSCI.6033-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun C, Greaves J, Chamberlain LH. The intracellular dynamic of protein palmitoylation. J Cell Biol. 2010;191:1229–1238. doi: 10.1083/jcb.201008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann NY Acad Sci. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- Seidel S, Singer EA, Just H, Farhan H, Scholze P, Kudlacek O, Holy M, Koppatz K, Krivanek P, Freissmuth M, Sitte HH. Amphetamines take two to tango: an oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol Pharmacol. 2005;67:140–151. doi: 10.1124/mol.67.1.. [DOI] [PubMed] [Google Scholar]
- Shetty M, Morrell J, Hovland M, Grove B, Vaughan RA, Foster JD. Membrane properties of attention–deficit hyperactivity disorder associated dopamine transporter coding variant (A559V) linked with altered palmitoylation and phosphorylation. Washington, DC: Society for Neuroscience; 2015. [Google Scholar]
- Smotrys JE, Linder ME. Palmitoylation of intracellular signaling proteins: regulation and function. Annual review of biochemistry. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- Strandberg E, Killian JA. Snorkeling of lysine side chains in transmembrane helices: how easy can it get? FEBS Lett. 2003;544:69–73. doi: 10.1016/s0014-5793(03)00475-7. [DOI] [PubMed] [Google Scholar]
- van Duyl BY, Rijkers DT, de Kruijff B, Killian JA. Influence of hydrophobic mismatch and palmitoylation on the association of transmembrane alpha-helical peptides with detergent-resistant membranes. FEBS Lett. 2002;523:79–84. doi: 10.1016/s0014-5793(02)02939-3. [DOI] [PubMed] [Google Scholar]
- Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharm Sci. 2013;34:489–496. doi: 10.1016/j.tips.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesa J, Hellsten E, Verkruyse LA, Camp LA, Rapola J, Santavuori P, Hofmann SL, Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376:584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- Wang KH, Penmatsa A, Gouaux E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature. 2015;521:322–327. doi: 10.1038/nature14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Bubula N, Brown J, Wang Y, Kondev V, Vezina P. PKC phosphorylates residues in the N-terminal of the DA transporter to regulate amphetamine-induced DA efflux. Neurosci Lett. 2016;622:78–82. doi: 10.1016/j.neulet.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Yokoi N, Fukata Y, Sekiya A, Murakami T, Kobayashi K, Fukata M. Identification of PSD-95 Depalmitoylating Enzymes. J Neurosci. 2016;36:6431–6444. doi: 10.1523/JNEUROSCI.0419-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen J, Antonio T, Cheng SY, Ali S, Jones KT, Reith ME. Dopamine transporter oligomerization: impact of combining protomers with differential cocaine analog binding affinities. J Neurochem. 2015;133:167–173. doi: 10.1111/jnc.13025. [DOI] [PMC free article] [PubMed] [Google Scholar]