Abstract
Bronchoscopic lung volume reduction (BLVR) procedure has expanded the treatment spectrum of patients with end-stage emphysema. These treatments include valve, coil, thermal vapor ablation, bio-lung volume reduction, targeted lung denervation, and airway bypass stent. This short review provides an up-to-date information on BLVR treatments, their clinical benefits, and an overview of complications. BLVR treatments generally affect dyspnea by reducing hyperinflation and residual volume (RV). Benefits of treatment are associated with improvement in lung function parameters (forced expiration volume in the first second, total lung capacity, RV, and 6-minute walking test) and quality of life. Serious potential pulmonary complications, such as pneumothorax, pneumonia, respiratory failure, and chronic obstructive pulmonary disease exacerbation, may also occur after BLVR treatment. In addition to these, low-cost BLVR methods, such as autologous blood and fibrin glue, are in the developmental stage. Bronchoscopic lung volume reduction treatments are a promising method with positive results for patients with severe emphysema. The widespread use of these techniques, inadequate selection of patients, and non-critical and, therefore, unsuccessful use of BLVR in non-specialist centers lead to a false negative impression of the effectiveness of these techniques. In addition to these considerations, it is obvious that these treatments, which are quite expensive, are burdening social health systems. The reduction of costs or the development of lower-cost treatment methods is important for the future and for the availability of treatments.
Keywords: Emphysema, bronchoscopic, lung volume reduction, valve, procedures, coil
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterized by irreversible airway obstruction with persistent respiratory symptoms and is a relatively treatable disease. Emphysema is one of the most common chronic and progressive conditions associated with COPD. Pathophysiologically, it includes the destruction of the lung parenchyma via chronic inflammation, followed by the permanent enlargement of the terminal bronchioles, dynamic hyperinflation, and loss of elastic recoil, air trapping, and reduced exercise capacity [1]. In addition, there is an increase in residual volume (RV) due to thoracic compression and dysfunction of the respiratory muscles in patients with COPD [2]. Smoking is still the most common cause, and dust exposure and alpha-1 antitrypsin deficiency are also known to be associated with its pathogenesis [3].
At present, standard treatment options include smoking cessation, bronchodilation, mucolytic agents, phosphodiesterase 4 inhibitors, respiratory rehabilitation, and nutritional support, vaccination against influenza and pneumococcus, and long-term oxygen therapy [4]. These treatments help to increase the exercise capacity of patients and decrease symptom exacerbation. However, they do not cure the pathophysiology of the disease or prevent disease progression. In the last decade, in addition to lung volume reduction surgeries (LVRSs), bronchoscopic lung volume reduction (BLVR) has expanded the treatment spectrum of patients with end-stage emphysema [5,6]. These treatments include valve, coil, thermal vapor ablation (TVA), bio-lung volume reduction (BioLVR), targeted lung denervation (TLD), and airway bypass stent (ABS).
Lung volume reduction surgeries is based on the principle of resecting damaged lung parenchyma and reducing hyperinflation in selected patients with heterogeneous emphysema with upper lobe predominance. Patients are often selected for surgery in accordance with the National Emphysema Treatment Trial (NETT) criteria [5]. Patients with hypercapnic respiratory failure, which is defined as an arterial partial pressure of carbon dioxide (PaCO2) >60 mmHg, and oxygen therapy requirement during rest are not recommended for surgery. LVRS can reportedly improve exercise capacity, lung function, and quality of life, especially in patients with upper lobe emphysema [5]. However, patient selection is limited due to high postoperative non-fatal pulmonary complications and short-term mortality of 6.9% [5]. In addition to medical treatment and surgery, BLVR treatments, especially in specific types of patients, have reportedly yielded promising short- to medium-term results [6–10]. After these results, the BLVR treatment methods (valve and coil) were introduced into the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 guideline [4]. The guideline states that LVRS or BLVR treatments can be considered only in a refractory situation to optimal medical treatment. However, it has been reported that patients who are not eligible for both treatment methods may be candidates for lung transplantation if their forced expiration volume in the first second (FEV1) values are <25% [4].
This short review provides an up-to-date information on BLVR treatments, their clinical benefits, and an overview of complications. All bronchoscopic approaches are presented here.
BRONCHOSCOPIC APPROACH TO LUNG VOLUME REDUCTION
Owing to the high complication rate and risk of mortality observed during the NETT trial, in recent years, less invasive emphysema treatment procedures and bronchoscopic approaches have been developed [5]. These methods have been proven effective and safe in the short- to medium-term clinical trials [6–10]. Studies investigating long-term results are ongoing. The indications, effect mechanisms, outcomes, and complication rates of these BLVR treatment modalities are discussed further below.
COMMON INDICATIONS
Patients tend to exhibit the same typical indications, with some variation in minor details. In general, they include evidence of emphysema diagnosed via computed tomography, post-bronchodilator FEV1 <15%–50% predicted, RV >175%, total lung capacity (TLC) >100%, 6-minute walking test (6MWT) >150 m, pulmonary hypertension not present, PaCO2 <50–55 mmHg, and cessation of smoking >2 months prior [6]. Table 1 shows the generally accepted indications pertaining to all BLVR techniques.
Table 1.
General inclusion criteria in the literature
| Methods | FEV1 % pred | RV % pred | TLC % pred | PAB, mmHg | PaCO2 mmHg | 6MWT m | mMRC p | DLCO % | Smoking cessation |
|---|---|---|---|---|---|---|---|---|---|
| Valves13–20 | 15–50 | >175 | >100 | <50 | <60 | 100–500 | >2 | - | >6 Months |
| Coils24–33 | 15–45 | >175 | >100 | <50 | <55 | 150–450 | - | - | >8Weeks |
| TVA37–39 | 20–45 | >150 >200* |
>100 | <35 | <55 | >140 | - | >20 | >4 Months |
| BioLVR40,41 | <50 | >150 | >100 | <25 | <65 | >150 | - | 20–60 | >4 Months |
| TLD42,43 | 30–60 | - | - | <25 | <60 | - | - | - | >6 Months |
| ABS44–45 | <50 | >180 | - | <25 | <50 | - | >2 | >15 | >8 Weeks |
TVA: thermal vapor ablation; LVR: lung volume reduction; TLD: targeted lung denervation; ABS: airway bypass stent; FEV1: forced expiration volume in the 1 s; RV: residual volume; TLC: total lung capacity; PAB: pulmonary arterial pressure; PaCO2: arterial partial pressure of carbon dioxide; 6MWT: 6-minute walking test; mMRC: Modified British Medical Research Council; DLCO: diffusing capacity for carbon monoxide;
preferably
MECHANISM
BLVR treatments generally affect dyspnea by reducing hyperinflation and RV. Individually, they have different mechanisms of action and are not suitable for every patient. Endobronchial valves (EBVs) reduce air trapping, coils improve the elastic recoil of the lungs, BioLVR and TVA create a local inflammatory reaction, ABS blocks the airway, and TLD reduces bronchoconstriction through innervation (Table 2) [6].
Table 2.
Mechanism of the BLVR procedures
| Valve13–20 | Coil24–33 | TVA37–39 | ABS42,43 | TLD44,45 | |
|---|---|---|---|---|---|
| Mechanism | Reducing airway Trapping | Improvement of elastic recoil | Local inflammatory reactions | Blocking Airway | Reduction of bronchoconstriction |
| Reversibility | Yes | Partial | No | Partial | No |
| Dependence of collateral ventilation | Yes | No | No | Yes | No |
| Type of Emphysema | Heterogeneous | Heterogeneous or homogeneous | Heterogeneous, only upper lobe | Homogeneous | Heterogeneous or homogeneous |
| Possible relevant complications | Pneumothorax, Dislocation, or migration of Valve | COPD Exacerbation, Pneumonia | Local and systemic inflammatory reactions, Pneumonia or Pneumonitis | Exacerbation, pneumothorax, hemoptysis | Exacerbation, Procedure related |
BLVR: bronchoscopic lung volume reduction; TVA: thermal vapor ablation; LVR: lung volume reduction; TLD: targeted lung denervation; ABS: airway bypass stent
Endobronchial Valves Implantation
Endobronchial valves (Spiration, Inc., Redmond, WA, USA) are inserted bronchoscopically into the targeted segmental or subsegmental bronchi. With regard to blocking, they only allow a unidirectional flow of air during expiration, so that the treated lung section is vented, and the following atelectasis formation achieves the desired volume-reducing effect. The procedure can be performed under mild sedation as a standard bronchoscopic intervention. Patients with collateral ventilation or parenchymal connections in the relevant lung lobe are not suitable for this procedure. If collateral ventilation (short-circuit connection) is present, the desired atelectasis does not occur in the targeted lobe after valve insertion. With planned valve therapy, the likelihood of a good response to therapy by computed tomographic analysis may indicate completeness of interlobar fissures, and/or the direct determination of collateral ventilation with the Chartis system can be estimated during bronchoscopy [11,12].
Bronchoscopic valve application aims to achieve targeted lobar volume reduction (TLVR) in patients with severe emphysema and is considered an efficacy parameter. In this respect, a quantitative multidetector computed tomography scan is used to calculate and analyze efficacy, and currently, a 350 mL reduction is considered indicative of a favorable response [13]. Nevertheless, the importance of TLVR after valve implantation is unclear.
Several randomized controlled trials have confirmed the efficacy of EBV treatment [13–20]. Efficacy has been assessed via changes in lung function parameters, exercise tests, and health-related quality of life questionnaires, such as the St. George’s Respiratory Questionnaire (SGRQ) [20], and significant improvements have been reported. Studies conducted from 2010 to 2017 collectively indicate that the mean FEV1 increases to 77.5 mL (34.5–140 mL) and 13.7% (4.3%–20.7%), the mean RV decreases to 440 mL (200–680 mL), and the mean 6MWT increases to 40.8 m (9.3–91.0 m) (Table 3) [13–20].
Table 3.
Overview of principal BLVR-Valve Studies
| Study/Author | Year | n | Follow-up time, M | Δ FEV1 | Δ RV L | Δ 6MWT m | Δ SGRQ point | TLVR mL |
|---|---|---|---|---|---|---|---|---|
| Sciurba et al. [14] | 2010 | 220 | 6 | +34.5 mL, +4.3% | * | +9.3 | −2.8 | * |
| Herth et al. [15] | 2012 | 111 | 12 | +15.0% | * | +22% | −5.0 | * |
| Davey et al. [16] | 2015 | 25 | 6 | +60 mL, +8.7% | −0.26 | +25.0 | −4.4 | * |
| Valipour et al. [17] | 2016 | 43 | 3 | +100 mL | −0.42 | +22.6 | −8.6 | −1195 |
| Klooster et al. [18] | 2017 | 64 | 12 | +17.0% | −0.68 | +61.0 | −11.0 | * |
| Fiorelli et al. [19] | 2017 | 33 | 60 | +17.0% | −39% | +91.0 | −17.0 | * |
| Kemp et al. [20] | 2017 | 65 | 6 | +140 mL, +20.7% | −0.66 | +36.2 | −7.2 | * |
| Welling et al. [13] | 2018 | 318 | 6 | +53.4 mL | −0.20 | * | −4.3 | −463 |
|
| ||||||||
| Overview of Complications | ||||||||
| Study/Author | Year | n | Follow-up, M | Pneumonia | COPD Exc. | PNX | Valve EMR | Death |
|
| ||||||||
| Sciurba et al. [14] | 2010 | 220 | 6 | 3.2% | 9.3% | 4.2% | * | 0.9% |
| Herth et al. [15] | 2012 | 111 | 12 | 11.7% | 42.3% | 8.0% | * | 5.4% |
| Davey et al. [16] | 2015 | 25 | 6 | 8.0% | 64.0% | 8.0% | 20.0% | 8.0% |
| Valipour et al. [17] | 2016 | 43 | 3 | 0 | 16.3% | 25.6% | 7.0% | 0 |
| Klooster et al. [18] | 2017 | 64 | 12 | 8.0% | 14.0% | 22.0% | 17.0% | 3.0% |
| Fiorelli et al. [19] | 2017 | 33 | 60 | 3.0% | * | 6.0% | 5.0% | 0 |
| Kemp et al. [20] | 2017 | 65 | 6 | 4.6% | 4.6% | 29.2% | 1.5% | 1.5% |
n: patient population; M: month; TLVR: target lobar volume reduction; SGRQ: St George’s Respiratory Questionnaire total score; COPD: chronic obstructive pulmonary disease; Exc: Exacerbation; PNX: pneumothorax; EMR: Expectoration, migration, and replacement; FEV1: forced expiration volume in the 1 s; Δ: change;
no data
In the previous studies, the mean survival after EBV treatment was 51 (46.5–56.3) months, and the death rate was 27% (9/33 patients) in the longest follow-up study. The causes of death reported were cancer, myocardial infarction, and end-stage respiratory failure [15]. Another interesting study has reported the 10-year survival results of patients with EBV treatment between 2002 and 2004. In that study, 19 patients were examined, and 40% of patients with atelectasis and 14% of patients without atelectasis were alive. In addition, attention has been drawn to the future development of collateral ventilation in patients and, if necessary, the replacement of EBVs [21].
Notably, however, EBV treatment is also associated with some risk. Data related to complications in published studies include COPD exacerbation rates of 9.3%–64.0%, pneumonia rates of up to 11.7%, pneumothorax rates of 4.2%–29.2%, valve migration and replacement rates of 1.5%–20.0%, and mortality rates of up to 8%. Another less frequent and important complication is granulation formation around the valves and associated EBV dislocation (Table 3) [13–20].
Finally, the EBV procedure is relatively expensive. Depending on the relevant agreements with local commercial and social health systems, the cost of the Chartis catheter and the delivery system for a single procedure range from €8,200 to €9,200. This does not include any costs associated with the actual performance of the operation itself or the management of any complications should they arise. It is a current challenge for both healthcare systems and valve manufacturing companies to make this treatment more accessible in developing countries [22].
Endobronchial Coil Implantation
Coil treatment (PneumRx, Inc., Mountain View, CA, USA) has been developed for patients with interlobar collateral ventilation in the years following EBV therapy. A pilot study of 22 BLVR operations published by Herth et al. [23] in 2010 was successful, and the treatment was reported to be more useful in heterogeneous emphysema.
Over the years, there have been improvements in procedures and techniques, and it is generally considered acceptable practice for a patient to undergo bronchoscopic placement of 8–14 coils (mean number of 10 coils) in one lung under general anesthesia and fluoroscopy [24,25]. Depending on the anatomical length of the subsegment, 100, 125, and 150 mm coils are preferred. In general, each subsegment has 1 coil implant, with an average of 10 coils per lobe. Although the middle lobe and lingual segment are generally not preferred for treatment, it is necessary for the patient to have intact parenchymal tissue in unintended lung areas. Emphysema in patients is confirmed via high-resolution computed tomography (HRCT), with the required lung function parameters of FEV1 15%–45%, RV >175%, TLC >100%, and 6MWT 150–450 m. The treatment method is not recommended for patients who are active smokers or patients with paraseptal emphysema, pulmonary arterial pressure (PAP) >50 mmHg, bronchiectasis, lung cancer, or bullous lesions >4 cm. It is also not recommended for patients undergoing anticoagulation therapy, though in such cases, the treatment may be feasible in conjunction with the implantation of left atrial appendage occlusion and subsequent anticoagulation reduction.
The principle of the coils is simple. The implanted wire spirals collect the diseased lung tissue, creating more space for less diseased tissue in the surrounding region. This enhances the capacity for respiratory function, the natural elasticity of the lung improves, and the lungs can stretch and contract better during the breathing process. As a result, this alleviates respiratory distress in patients. Studies conducted from 2012 to 2018 indicate that the average FEV1 increases to 130 mL (90–200 mL) and 12.1% (7%–14%), the average RV decreases to 420 mL (310–510 mL) and 16.5% (14.5%–22.0%), and the mean 6MWT increases to 47 m (14.6–84.0 m) (Table 4) [24–33].
Table 4.
Overview of principal BLVR-Coil Studies
| Author | Year | n | Follow-up, M | Δ FEV1 | Δ RV | Δ 6MWT | Δ SGRQ |
|---|---|---|---|---|---|---|---|
| Slebos et al. [24] | 2012 | 16 | 6 | +14.9% | −11.4% | +84.4 m | −14.9 |
| Shah et al. [25] | 2013 | 23 | 3 | +14.1% | −0.51 L | +51.1 m | −8.6 |
| Klooster et al. [26] | 2014 | 10 | 6 | +0.11 L | −22.0% | +61.0 m | −15.0 |
| Deslee et al. [27] | 2014 | 34 | 12 | +16.0% | −13.75% | +51.4 m | −11.1 |
| Hartman et al. [28] | 2014 | 35 | 12 | +0.20 L | −21% | +31.0 m | −4.2 |
| 27 | 24 | − 0.05 L | −10% | −12.0 m | −8.0 | ||
| 22 | 36 | − 0.04 L | −2% | −31.5 m | −7.2 | ||
| Zoumot et al. [29] | 2015 | 35 | 12 | + 8.9% | −0.32 L | +34.1 m | −6.1 |
| Deslee et al. [30] | 2016 | 50 | 12 | + 0.09 L | −0.52 L | +9% | −11.1 |
| Sciurba et al. [31] | 2016 | 158 | 12 | + 7.0% | −0.31 L | +14.6 m | −8.9 |
| Gülsen et al. [32] | 2017 | 40 | 6 | +0.15 L | −14.5% | +48.0 m | −10.4 |
| Simon et al. [33] | 2018 | 33 | 3 | +0.10 L | −0.44 L | +48.0 m | * |
|
| |||||||
| Overview of Complications | |||||||
| Author | Year | n | Follow-up, M | Pnx | COPD Exc. | Pneumonia | Death |
|
| |||||||
| Slebos et al. [24] | 2012 | 16 | 6 | 0 | 87.0% | 18.7% | 0 |
| Shah et al. [25] | 2013 | 23 | 3 | 8.6% | 17.0% | 0 | 0 |
| Klooster et al. [26] | 2014 | 10 | 6 | 10% | 70.0% | 0 | 0 |
| Deslee et al. [27] | 2014 | 34 | 12 | 11.6% | 33.0% | 23.3% | 0 |
| Hartman et al. [28] | 2014 | 35 | 12 | 6.0% | 51.0% | 46.0% | 3% |
| 27 | 24 | 0 | 37.0% | 7.0% | 8% | ||
| 22 | 36 | 0 | 36.0% | 5.0% | 6% | ||
| Zoumot et al. [29] | 2015 | 84 | 12 | 9.6% | 10.8% | 2.4% | - |
| Deslee et al. [30] | 2016 | 50 | 12 | 2.0% | 26.0% | 18.0% | 8% |
| Sciurba et al. [31] | 2016 | 158 | 12 | 9.7% | 27.7% | 20.0% | 6.5% |
| Gülsen et al. [32] | 2017 | 40 | 6 | 0 | 41.4% | 17.0% | 2% |
| Simon et al. [33] | 2018 | 33 | 3 | 0 | 46.3% | 5.6% | 0 |
n: patient population; COPD: Chronic obstructive pulmonary disease; FEV1: forced expiration volume in the 1 s; Δ: change; M: month; SGRQ: St George’s Respiratory Questionnaire total score
Complications may also ensue immediately or after treatment using this treatment method. The complication rates in published studies include COPD exacerbation of 41.0% (10.8%–87.0%), pneumonia of 14.8% (0%–46.0%), pneumothorax of 5.7% (0%–11.6%), and mortality of 3.3% (0%–8%). Another less frequent complication is pleuritic chest pain and hemoptysis after treatment (Table 4) [24–33].
The total costs for 1 year are $53,521 for BLVR coil treatment and $5912 for follow-up patients only [30]. Based on one preliminary study (REVOLENS) of patients with severe emphysema followed up for 6 months, the initial cost of BLVR coil treatment appears to be high, but it seems that large-scale studies are needed to determine its long-term cost and effectiveness [30].
Thermal Vapor Ablation
In addition to coils and EBVs, other endoscopic options for reducing lung volume have been developed. Bronchoscopic TVA (Uptake Medical Corporation, Seattle, WA, USA) is one of the techniques. It delivers thermal energy directly to the airways via a bronchoscope through heated water vapor. It induces an inflammatory reaction by instilling water vapor into the destroyed lung parenchyma. The local inflammatory reaction leads to fibrosis and scarring after approximately 8–12 weeks and thus to the desired lung volume reduction. This irreversible procedure is only preferred in patients with upper lobe emphysema, regardless of collateral ventilation [34,35]. The instillation of a hot water vapor at 75°C occurs via a special balloon catheter, with which the lung area to be treated is occluded. The vapor dose is calculated based on the mass of the lung tissue to be treated, and the average vapor dose is 10 cal/g alveolar tissue. This makes the targeted treatment of the area selected based on the HRCT possible. The heterogeneity index (HI) was identified as a predictor of an excellent outcome following bronchoscopic TVA. Patients with heterogeneous disease with an HI >1.2 and patients with COPD GOLD IV had greater improvements in FEV1 and 6MWT in recent studies [34,35].
The criteria in defining patients who can be treated in principle are (1) upper lobe dominant emphysema determined via HRCT; (2) ages between 40 and 75 years; (3) FEV1 between 15% and 45% predicted; (4) RV >150% predicted, ly >200; (5) TLC >100% predicted; (6) diffusing capacity of the lungs for carbon monoxide (DLCO) >20% predicted; (7) 6MWT >140 m; (8) PaCO2 <55 mmHg and partial arterial oxygen pressure >45 mm Hg; and (9) non-smoker for ≥4 months. Bronchoscopic TVA treatment is not recommended for patients with (1) known α-1-antitrypsin deficiency, asthma, or bronchiectasis; (2) bulla >1/3 lobe of the lung; (3) history of thoracotomy; (4) left ventricular ejection fraction ≤40%; or (5) pulmonary hypertension (mean pulmonary artery pressure ≥35 mmHg) [36].
There are fewer published TVA studies than published studies pertaining to coils and EBVs. Two major TVA studies conducted from 2012 to 2016 showed reduced hyperinflation and dyspnea and improvement of exercise tolerance and health-related quality of life. The first study was an uncontrolled follow-up study in which unilateral bronchoscopic thermoablation with a vapor dose of 10 cal/g was performed in 44 patients with an HI of >1.2. After 6 months of treatment, there was a 48% decrease in lobar lung volume and a significant improvement in lung function parameters and quality of life. There were also significant improvements in FEV1 (0.14±0.16 L), RV (−0.40±0.71 L), 6MWT (46.5±67.1 m), and SGRQ (−14±15.1 points) at a 6-month interval. A GOLD IV subgroup shows greater improvements than GOLD III [37]. The second study was the first randomized controlled trial of TVA treatment (n=46) versus medical management (n=24) and reported mean relative improvements of 14.7% (7.8%–21.5%) and 0.13 L (0.063–0.198 L) in FEV1, −9.7 points (−15.7 to −3.7 points) in SGRQ, and 30.5 m (1.5–62.4 m) in 6MWT at a 6-month follow-up [38].
The most common complication in the first few weeks was an excessive inflammatory response characterized by respiratory symptoms, such as dyspnea, cough, fever, and mild hemoptysis [39]. The most common complications in the following period were pneumonia (18%–23%) and COPD exacerbation (9%–24%) (Table 5). Therefore, consistent inpatient follow-up and concomitant prophylactic antibiotic and anti-inflammatory therapy are required. The severity of the local inflammatory response correlates with the good response to thermoablation and better result [34]. More effective TVA is associated with more pronounced inflammation. In addition, there appears to be a correlation between the volume of the treated lung lobe and the extent of the inflammatory response. Patients with a treated lung volume of >1700 mL had to be re-hospitalized more frequently during the course of the disease due to a pronounced local inflammatory response. However, 6 months after TVA, this patient population exhibited the greatest benefit from TVA.
Table 5.
Overview of principal Termal Vapor Ablation Studies
| Author | Year | n | Follow-up, M | ΔFEV1 mL | Δ RV mL | Δ 6MWT m | Δ SGRQ point | TLVR mL |
|---|---|---|---|---|---|---|---|---|
| Herth et al. [37] | 2012 | 22 | 12 | +64£ | −270£ | +10.9£ | 9.4£ | −735£ |
| 22 | 12 | +108† | −335† | +25.6† | −12.7† | −772† | ||
| Gompelmann et al. [35] | 2014 | 44 | 6 | +141 | −406 | * | * | −716 |
| Gompelmann et al. [39] | 2016 | 35 | 12 | +65 | −108 | 6.2 | −12.1 | * |
| Herth et al. [38] | 2016 | 45 | 6 | +130 | −302 | 30.5 | −9.7 | * |
|
| ||||||||
| Overview of Complications | ||||||||
| Author | Year | n | Follow-up, M | Pneumonia or pneumonitis | COPD Exc. | PNX | SAEs | Death |
|
| ||||||||
| Gompelmann et al. [39] | 2016 | 35 | 12 | 23.0% | 9.0% | 3.0% | 26.0% | 3.0% |
| Herth et al. [38] | 2016 | 45 | 6 | 18.0% | 24.0% | 2.0% | 36.0% | 2.0% |
SAEs: serious adverse events;
COPD Gold III patients;
COPD Gold III patients;
COPD GOLD IV patients;
n: patient population; M: month; TLVR: target lobar volume reduction; FEV1: forced expiration volume in the 1 s; Δ: change; 6MWT: 6-minute walking test
Bio-Lung Volume Reduction
BioLVR or polymeric LVR is a method of bronchoscopic instillation of hydrogel into the target lobe that is intended to block lung tissue in patients with advanced emphysema. AeriSeal (Aeris Therapeutics, Inc., Woburn, MA, USA) is a foam-like liquid medium used in bronchioles and alveoli with 10 mL (low dose) or 20 mL (high dose) for each part. The application of the polymer foam is an irreversible BLVR procedure. Bronchoscopically applied polymer resin causes fibrosis in the targeted pulmonary parenchyma. A subsequent inflammatory reaction functions to decrease the tissue in the target regions and reduce volume. In the first study, which included 14 patients and was published in 2011, a positive therapeutic effect was apparent but so were complications, mainly due to inflammatory processes [40]. The results reported included an FEV1 increase of +15.9% predicted, a 6MWT increase of 28.7 m, RV/TLC change of −7.4% predicted, forced vital capacity (FVC) increase of 24.1% predicted, DLCO change of +9.3%, and SGRQ score change of −9.9 points [40].
In the “ASPIRE” study conducted in 2014, BioLVR using hydrogel was compared with medical therapy and has since been abandoned due to increased adverse events and an unacceptable complication rate resulting in patients with BioLVR requiring more hospitalization [41]. The clinical method it entails is currently not recommended.
Targeted Lung Denervation
Targeted Lung Denervation is the most recently developed novel therapeutic COPD treatment. Technically, it is not a volume reduction procedure; rather, its aims are ablation of parasympathetic nerves innervating the basal lung and the reduction of bronchoconstriction. Its effects are similar to those of anticholinergic drugs. It is performed via a double-cooled radiofrequency catheter (Holaira, Minneapolis, MN, USA). Radiofrequency (15–20 W) is applied to the target lung and 8 points. The cooler is also used in order to prevent the resulting heat from affecting the inner surface of the airway. It is usually applied bilaterally and step by step. Slebos et al. [42] conducted an initial pilot study in 2015 and reported that 20 W is more successful than 15 W. Patients receiving TLD incorporating 20 W reported an increase of 11.6% in FEV1, an increase of 6.8 min in submaximal cycle endurance, and a decrease of 11.1 points in SGRQ score. The most frequent adverse event at a 1-year follow-up was COPD exacerbation (59%). Gastritis (18%), respiratory inflammation (18%), and anaphylactic reaction (5%) were also observed. Non-serious complications, such as bronchial perforation, ulceration, and stenosis, may also rarely occur depending on the procedure. In another study, the anti-inflammatory effects of TLD were reported, and it was asserted that further research is needed [43].
The major criteria in defining patients who can be treated in principle are (1) age ≥40 years; (2) COPD with FEV1/FVC <70% and FEV1 between 30% and 60% predicted; (3) positive relative change in FEV1 >15% following inhalation of ipratropium; (4) non-smoking for ≥6 months; (5) smoking history of at least 10 pack-years; and (6) PaCO2 <60 mmHg, PaO2 >55 mmHg, and PAP <25 mmHg [42]. The first prospective randomized controlled trial of lung denervation (“AIRFLOW-1”, NCT02058459) is still ongoing. TLD is not yet used in routine clinical practice.
Airway Bypass Stent
ABS (Broncus Technologies, Inc., Mountain View, CA, USA) is only recommended for patients with severe homogeneous emphysema. In this method, extra-anatomical transitions are made from the airway walls to the lung parenchyma to release the trapped air. A small drug (paclitaxel)-eluting stent is inserted into each drilled hole so that bypass occlusion is avoided. The intention is to bypass small collapsed airways. However, studies and data on this treatment method are limited, and this BLVR technique is still in the trial phase.
In a small study reported by Choong et al. [44] in 2009, ABS improved pulmonary function parameters and reduced respiratory distress. However, no statistically significant results were obtained. In 2011, the “EASE” study involving 315 patients with severe homogeneous emphysema was reported [45]. Patients were randomly assigned to the ABS group (n=208) or the control group (n=107), followed by 1, 3, 6, and 12 months of respiratory function parameter assessment, 6MWT, and SGRQ. There was no statistically significant difference between the two groups detected in their study. Compared with baseline, there was an increase of 12% in FEV1 and 1 point in the modified British Medical Research Council scale 6 months after the procedure. Therefore, it was reported that ABS is effective in the short term. Loss of efficacy over time is thought to be due to factors involving mucus plugs and granuloma formation. For this reason, it has been stated that further research is required in order to optimize the treatment method, and it is currently not one of the preferred BLVR treatments in practice.
Bronchoscopic Lung Volume Reduction Using Autologous Blood and Fibrin Glue
A pilot study reported in 2016 assessed the efficacy and safety of BLVR using low-cost agents including autologous blood (n=7) and fibrin glue (n=8) [46]. On week 12 after the procedure, the autologous blood group exhibited an 8% FEV1 increase and a 16 m 6MWT increase, whereas the fibrin glue group exhibited a 17% FEV1 increase and a 96 m 6MWT increase. One patient in the autologous blood group had pneumonia in the first 10 days after the procedure, and one patient in the fibrin glue group had exhibited COPD exacerbation at 12 weeks follow-up, and mortality was not reported. The method was shown to constitute an effective and safe treatment, with good cost effectiveness for patients with severe emphysema. This biological lung volume reduction treatment is very promising in terms of its low-cost. Notably, however, the very small sample size and short follow-up detract from the power of the study. There is a need for long-term studies of cost, effectiveness, and safety in relation to this method.
REVERSIBILITY OF ALL BRONCHOSCOPIC LUNG VOLUME REDUCTION PROCEDURES
Reversibility of treatments and informing patients in this regard is a very important issue. EBV treatments are generally considered reversible, whereas TLD and TVA are irreversible. The coil procedure is regarded as partially reversible. Only three studies in the literature describe the removal of the coils. In a small case series (n=3) of patients with pleuritic pain, the coils could be removed within 4 weeks after implantation [47]. In another study, persistent thoracic pain was reported after treatment, and two coils were removed after 10 months [48]. Gülşen et al. [32] removed and replaced the coil in 10% of the patients during the procedure. Thus, the literature suggests that the removal of the coils is generally only possible during the procedure and in the following few months, and that it is not possible to remove all coils.
BENEFITS OF BRONCHOSCOPIC LUNG VOLUME REDUCTION METHODS
With regard to the benefits of treatment, EBV is associated with average increases of 77.5 mL (34.5–140.0 mL) in FEV1 and 40.8 m (9.3–91.0 m) in 6MWT and an average reduction of 440 mL (200–680 mL) in RV. Coil therapy reportedly results in average increases of 130 mL (90–200 mL) in FEV1 and 47.0 m (14.6–84.0 m) in 6MWT and an average reduction of 420 mL (310–510 mL) in RV. TVA treatment results in average increases of 101 mL (64–141 mL) in FEV1 and 18.3 m (6.2–30.5 m) in 6MWT and an average reduction of 284 mL (108–406 mL) in RV (Table 6).
Table 6.
Overview of benefits of all BLVR techniques in the literature
| Technique | Δ FEV mL | Δ FEV % pred. | Δ RV mL | Δ RV % pred. | Δ 6MWT m |
|---|---|---|---|---|---|
| Valve | 77.5 (34.5–140) | 13.7 (4.3–20.7) | 440 (200–680) | - | 40.8 (9.3–91.0) |
| Coil | 130 (90.0–200) | 12.1 (7.0–14.0) | 420 (310–510) | 16.5 (14.5–22.0%) | 47.0 (14.6–84.0) |
| TVA | 101 (64.0–141) | - | 284 (108–406) | - | 18.3 (6.2–30.5) |
|
| |||||
| Overview of all mean complication rates | |||||
| Technique | Pneumonia | COPD Exacerbation | PNX | Migration and replacement | Death |
|
| |||||
| Valve | 6.4 (0–11.7) | 25.0 (4.6–64.0) | 14.7 (4.2–29.2) | 10.1 (1.5–20.0) | 2.6 (0–8.6) |
| Coil | 14.8 (0–46.0) | 41.0 (10.8–87.0) | 5.7 (0–11.6) | - | 3.3 (0–8.0) |
| TVA | 21.0 (18–23.0) | 16.5 (9.0–24.0) | 2.5 (2–3.0) | - | 2.5 (2–3.0) |
Data of benefits are shown mean (min–max)/Data of complications are shown mean % (min–max)
COPD: chronic obstructive pulmonary disease; FEV: forced expiration volume; Δ: change; M: month; PNX: pneumothorax; 6MWT: 6-minute walking test
COMPLICATIONS OF BRONCHOSCOPIC LUNG VOLUME REDUCTION TREATMENT AT A GLANCE
Serious potential pulmonary complications are associated with BLVR treatment methods. The complications of EBV treatments most commonly reported in the literature are COPD exacerbation (25.0%; 4.6%–64.0%) and pneumothorax (14.7%; 4.2%–29.2%). In this treatment method, valve migration is reportedly seen in approximately 10.1% (1.5%–20.0%) of the patients. Coil treatments are most commonly associated with COPD exacerbation (41.0%; 10.8%–87.0%), pneumonia (14.8%; 0%–46.0%), and pneumothorax (5.7%; 0%–11.6%). In TVA treatments, the most common complications are pneumonia (21.0%; 18.0%–23%) and COPD exacerbation (16.5%; 9.0%–24.0%). The mortality rates are reportedly 2.6%, 3.3%, and 2.5%, respectively (Table 6).
POST-TREATMENT OF ACUTE INFLAMMATORY RESPONSE
Bronchoscopic Lung Volume Reduction treatments generally induce an acute inflammatory response (fever, dyspnea, cough, chest pain, and/or elevated inflammatory markers) after treatment. The authors generally recommend and administer steroid and prophylactic antibiotic therapy for 7 days to reduce inflammation. In addition, it is advisable to monitor patients in the hospital for at least 1 night and administer prophylactic non-steroidal anti-inflammatory drugs and stress ulcer prophylaxis for 3 days after treatment [49,50]. Although there is no general consensus on the application of prophylactic antibiotics, they are commonly used by the authors due to the high risk of COPD exacerbation and pneumonia. Pneumococcal and influenza vaccines are an overlooked but absolutely necessary consideration in these patients.
ONGOING AND FUTURE STUDIES
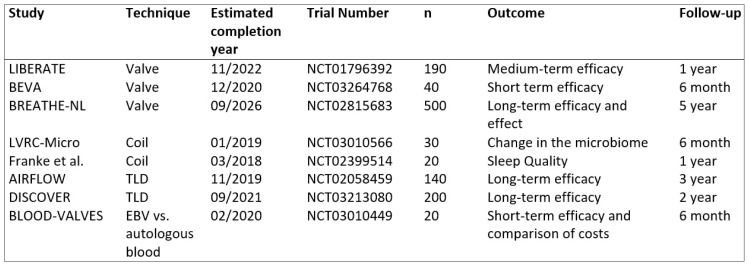
There are many future studies of BLVR techniques planned. The most interesting are the “BREATHE-NL” study in which a 5-year efficacy and benefit of EBV treatments was planned, the “LVRC-Micro Study” in which changes in the microbiome of the lungs will be observed after BLVR coil treatment, the study of sleep quality in coil therapy, the 3-year effects of TLD treatment, the “AIRFLOW-1 Study” to investigate the 3-year effects and benefits of TLD treatment, and the “BLOOD-VALVES Study” to compare EBV with low-cost autologous blood (Figure 1).
Figure 1.
Ongoing randomized clinical trials of bronchoscopic lung volume reduction therapies
CONCLUSION
BLVR treatments are a promising method with positive results for patients with severe emphysema. Optimal patient and method selection for BLVR treatments and implementation in experienced centers are very important. However, it is necessary to be prepared for potential complications. The widespread use of these techniques, inadequate selection of patients, and non-critical and therefore, unsuccessful use of BLVR in non-specialist centers lead to a false negative impression of the effectiveness of these techniques. In addition to these considerations, it is obvious that these treatments, which are quite expensive, are burdening social health systems. The reduction of costs or the development of lower-cost treatment methods is important for the future and for the availability of treatments. Therefore, BLVR in patients with severe emphysema will be a treatment of choice in the near future once clinical trials show the ongoing long-term effectiveness of the procedures.
Footnotes
Peer-review: Externally peer-reviewed.
Conflict of Interest: The author have no conflicts of interest to declare.
Financial Disclosure: The author declared that this study has received no financial support.
REFERENCES
- 1.Holloway RA, Donnelly LE. Immunopathogenesis of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2013;19:95–102. doi: 10.1097/MCP.0b013e32835cfff5. [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell DE, Webb KA. The major limitation to exercise performance in COPD is dynamic hyperinflation. J Appl Physiol (1985) 2008;105:753–5. doi: 10.1152/japplphysiol.90336.2008b. [DOI] [PubMed] [Google Scholar]
- 3.Kemp SV, Polkey MI, Shah PL. The epidemiology, etiology, clinical features, and natural history of emphysema. Thorac Surg Clin. 2009;19:149–58. doi: 10.1016/j.thorsurg.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2017. Access: http://www.goldcopd.org/
- 5.Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med. 2011;184:881–93. doi: 10.1164/rccm.201103-0454CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herth FJ, Slebos DJ, Criner GJ, et al. Endoscopic Lung Volume Reduction: An Expert Panel Recommendation - Update 2017. Respiration. 2017;94:380–8. doi: 10.1159/000479379. [DOI] [PubMed] [Google Scholar]
- 7.Ramaswamy A, Puchalski J. Bronchoscopic lung volume reduction: recent updates. J Thorac Dis. 2018;10(4):2519–27. doi: 10.21037/jtd.2018.02.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi M, Lee WS, Lee M, Jeon K, Sheen S, Jheon S, Kim YS. Effectiveness of bronchoscopic lung volume reduction using unilateral endobronchial valve: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:703–10. doi: 10.2147/COPD.S75314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snell G, Herth FJ, Hopkins P, Baker KM, Witt C, Gotfried MH, Valipour A, Wagner M, Stanzel F, Egan JJ, Kesten S, Ernst A. Bronchoscopic thermal vapour ablation therapy in the management of heterogeneous emphysema. Eur Respir J. 2012;39(6):1326–33. doi: 10.1183/09031936.00092411. [DOI] [PubMed] [Google Scholar]
- 10.Gompelmann D, Eberhardt R, Herth FJ. Novel Endoscopic Approaches to Treating Chronic Obstructive Pulmonary Disease and Emphysema. Semin Respir Crit Care Med. 2015;36(4):609–15. doi: 10.1055/s-0035-1555614. [DOI] [PubMed] [Google Scholar]
- 11.Gompelmann D, Eberhardt R, Slebos DJ, et al. Diagnostic performance comparison of the Chartis System and high-resolution computerized tomography fissure analysis for planning endoscopic lung volume reduction. Respirology. 2014;19:524–30. doi: 10.1111/resp.12253. [DOI] [PubMed] [Google Scholar]
- 12.Reymond E, Jankowski A, Pison C, et al. Prediction of lobar collateral ventilation in 25 patients with severe emphysema by fissure analysis with CT. AJR Am J Roentgenol. 2013;201:571–5. doi: 10.2214/AJR.12.9843. [DOI] [PubMed] [Google Scholar]
- 13.Welling JBA, Hartman JE, van Rikxoort EM, et al. Minimal important difference of target lobar volume reduction after endobronchial valve treatment for emphysema. Respirology. 2018;23:306–10. doi: 10.1111/resp.13178. [DOI] [PubMed] [Google Scholar]
- 14.Sciurba FC, Ernst A, Herth FJ, et al. VENT Study Research Group A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233–44. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 15.Herth FJ, Noppen M, Valipour A, et al. International VENT Study Group. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012;39:1334–42. doi: 10.1183/09031936.00161611. [DOI] [PubMed] [Google Scholar]
- 16.Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR- HIFi study): a randomised controlled trial. Lancet. 2015;386:1066–73. doi: 10.1016/S0140-6736(15)60001-0. [DOI] [PubMed] [Google Scholar]
- 17.Valipour A, Slebos DJ, Herth F, et al. IMPACT Study Team Endo-bronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med. 2016;194:1073–82. doi: 10.1164/rccm.201607-1383OC. [DOI] [PubMed] [Google Scholar]
- 18.Klooster K, Hartman JE, Ten Hacken NH, et al. One-Year Follow-Up after Endobronchial Valve Treatment in Patients with Emphysema without Collateral Ventilation Treated in the STELVIO Trial. Respiration. 2017;93:112–21. doi: 10.1159/000453529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiorelli A, Santoriello C, De Felice A, et al. Bronchoscopic lung volume reduction with endobronchial valves for heterogeneous emphysema: long-term results. J Vis Surg. 2017;3:170. doi: 10.21037/jovs.2017.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemp SV, Slebos DJ, Kirk A, et al. TRANSFORM Study Team. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (TRANSFORM) Am J Respir Crit Care Med. 2017;196:1535–43. doi: 10.1164/rccm.201707-1327OC. [DOI] [PubMed] [Google Scholar]
- 21.Garner J, Kemp SV, Toma TP, et al. Survival after Endobronchial Valve Placement for Emphysema: A 10-Year Follow-up Study. Am J Respir Crit Care Med. 2016;194:519–21. doi: 10.1164/rccm.201604-0852LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarad N. Clinical review: Endobronchial valve treatment for emphysema. Chron Respir Dis. 2016;13:173–88. doi: 10.1177/1479972316631139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herth FJ, Eberhard R, Gompelmann D, et al. Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Ther Adv Respir Dis. 2010;4:225–31. doi: 10.1177/1753465810368553. [DOI] [PubMed] [Google Scholar]
- 24.Slebos DJ, Klooster K, Ernst A, et al. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest. 2012;142:574–82. doi: 10.1378/chest.11-0730. [DOI] [PubMed] [Google Scholar]
- 25.Shah PL, Zoumot Z, Singh S, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med. 2013;1:233–40. doi: 10.1016/S2213-2600(13)70047-X. [DOI] [PubMed] [Google Scholar]
- 26.Klooster K, Ten Hacken NH, Franz I, et al. Lung volume reduction coil treatment in chronic obstructive pulmonary disease patients with homogeneous emphysema: a prospective feasibility trial. Respiration. 2014;88:116–25. doi: 10.1159/000362522. [DOI] [PubMed] [Google Scholar]
- 27.Deslee G, Klooster K, Hetzel M, et al. Lung volume reduction coil treatment for patients with severe emphysema: a European multicentre trial. Thorax. 2014;69:980–6. doi: 10.1136/thoraxjnl-2014-205221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartman JE, Klooster K, Gortzak K, et al. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology. 2015;20:319–26. doi: 10.1111/resp.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zoumot Z, Kemp SV, Singh S, et al. Endobronchial coils for severe emphysema are effective up to 12 months following treatment: medium term and cross-over results from a randomized controlled trial. PLoS One. 2015;10:e0122656. doi: 10.1371/journal.pone.0122656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deslée G, Mal H, Dutau H, et al. REVOLENS Study Group. Lung Volume Reduction Coil Treatment vs Usual Care in Patients With Severe Emphysema: The REVOLENS Randomized Clinical Trial. JAMA. 2016;315:175–84. doi: 10.1001/jama.2015.17821. [DOI] [PubMed] [Google Scholar]
- 31.Sciurba FC, Criner GJ, Strange C, et al. RENEW Study Research Group. Effect of Endobronchial Coils vs Usual Care on Exercise Tolerance in Patients With Severe Emphysema: The RENEW Randomized Clinical Trial. JAMA. 2016;315:2178–89. doi: 10.1001/jama.2016.6261. [DOI] [PubMed] [Google Scholar]
- 32.Gulsen A, Sever F, Girgin P, et al. Evaluation of bronchoscopic lung volume reduction coil treatment results in patients with severe emphysema. Clin Respir J. 2017;11:585–92. doi: 10.1111/crj.12387. [DOI] [PubMed] [Google Scholar]
- 33.Simon M, Harbaum L, Oqueka T, et al. Endoscopic lung volume reduction coil treatment in patients with very low FEV1: an observational study. Ther Adv Respir Dis. 2018;12:1753466618760133. doi: 10.1177/1753466618760133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gompelmann D, Eberhardt R, Ernst A, et al. The localized inflammatory response to bronchoscopic thermal vapor ablation in patients with emphysema. Respiration. 2013;86:324–31. doi: 10.1159/000354175. [DOI] [PubMed] [Google Scholar]
- 35.Gompelmann D, Eberhardt R, Herth FJ. Technology update: bronchoscopic thermal vapor ablation for managing severe emphysema. Med Devices (Auckl) 2014;7:335–41. doi: 10.2147/MDER.S49369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snell GI, Hopkins P, Westall G, et al. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg. 2009;88:1993–8. doi: 10.1016/j.athoracsur.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 37.Herth FJ, Ernst A, Baker KM, et al. Characterization of outcomes 1 year after endoscopic thermal vapor ablation for patients with heterogeneous emphysema. Int J Chron Obstruct Pulmon Dis. 2012;7:397–405. doi: 10.2147/COPD.S31082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herth FJ, Valipour A, Shah PL, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicentre, parallel-group, open-label, randomised controlled STEP-UP trial. Lancet Respir Med. 2016;4:185–93. doi: 10.1016/S2213-2600(16)00045-X. [DOI] [PubMed] [Google Scholar]
- 39.Gompelmann D, Eberhardt R, Schuhmann M, et al. Lung Volume Reduction with Vapor Ablation in the Presence of Incomplete Fissures: 12-Month Results from the STEP-UP Randomized Controlled Study. Respiration. 2016;92:397–403. doi: 10.1159/000452424. [DOI] [PubMed] [Google Scholar]
- 40.Herth FJ, Gompelmann D, Stanzel F, et al. Treatment of advanced emphysema with emphysematous lung sealant (AeriSeal®) Respiration. 2011;82:36–45. doi: 10.1159/000322649. [DOI] [PubMed] [Google Scholar]
- 41.Come C, Abu-Hijleh M, Berkowitz D, et al. Results of the ASPIRE endoscopic lung volume reduction trial at study termination. Eur Respir J. 2014;44:3717. [Google Scholar]
- 42.Slebos DJ, Klooster K, Koegelenberg CF, et al. Targeted lung denervation for moderate to severe COPD: a pilot study. Thorax. 2015;70:411–9. doi: 10.1136/thoraxjnl-2014-206146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kistemaker LE, Slebos DJ, Meurs H, et al. Anti-inflammatory effects of targeted lung denervation in patients with COPD. Eur Respir J. 2015;46:1489–92. doi: 10.1183/13993003.00413-2015. [DOI] [PubMed] [Google Scholar]
- 44.Choong CK, Cardoso PF, Sybrecht GW, et al. Airway bypass treatment of severe homogeneous emphysema: taking advantage of collateral ventilation. Thorac Surg Clin. 2009;19:239–45. doi: 10.1016/j.thorsurg.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Shah PL, Slebos DJ, Cardoso PF, et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema(EASE trial): randomised, sham-controlled, multicentre trial. Lancet. 2011;378:997–1005. doi: 10.1016/S0140-6736(11)61050-7. [DOI] [PubMed] [Google Scholar]
- 46.Bakeer M, Abdelgawad TT, El-Metwaly R, et al. Low cost biological lung volume reduction therapy for advanced emphysema. Int J Chron Obstruct Pulmon Dis. 2016;11:1793–800. doi: 10.2147/COPD.S112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hetzel M, Bartscher E, Merk T, et al. Reversibilitat der Implantation von RePneu Coils. Poster session (67/P314) presented at the 54th Annual meeting of the Pneumologie; Hannover, Germany. 2013. [Google Scholar]
- 48.Dutau H, Bourru D, Guinde J, et al. Successful Late Removal of Endobronchial Coils. Chest. 2016;150:143–5. doi: 10.1016/j.chest.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 49.Herth FJ, Eberhardt R, Ingenito EP, et al. Assessment of a novel lung sealant for performing endoscopic volume reduction therapy in patients with advanced emphysema. Expert Rev Med Devices. 2011;8:307–12. doi: 10.1586/erd.11.10. [DOI] [PubMed] [Google Scholar]
- 50.Kramer MR, Refaely Y, Maimon N, et al. Bilateral endoscopic sealant lung volume reduction therapy for advanced emphysema. Chest. 2012;142:1111–7. doi: 10.1378/chest.12-0421. [DOI] [PubMed] [Google Scholar]