Abstract
BACKGROUND
Access to glycosylated hemoglobin (HbA1c) assays in clinical practice remains limited. We investigated the relationship of fasting plasma glucose and HbA1c to determine optimal glucose levels for predicting HbA1c.
PATIENTS AND METHODS
We retrospectively analyzed data on 2888 patients with type 2 diabetes mellitus aged ≥20 years using a linear regression of HbA1c against fasting plasma glucose. A receiver-operating characteristic analysis was used to determine optimal cut-points for fasting glucose in relation to HbA1c, area under the curve, sensitivity and specificity, and 95% confidence intervals (CI) for each cut-point.
RESULTS
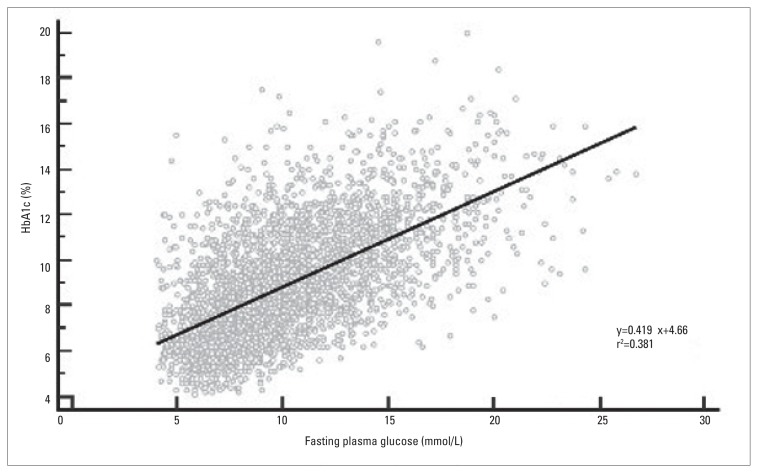
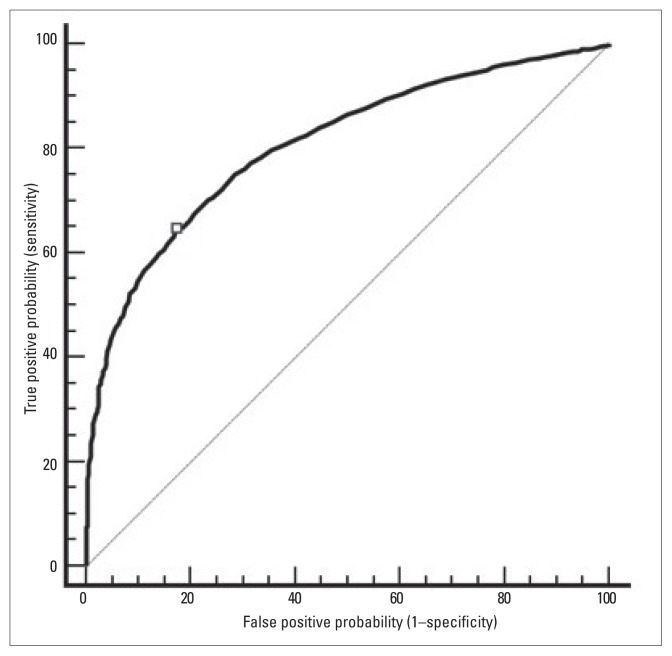
The mean (standard deviation) for the age of patients was 52±11.6 years. The average HbA1c was 8.9±2.46% and mean fasting plasma glucose was 10.1±3.62 mmol/L. The prevalence of HbA1c ≥7.0% and >6.5% was 76% and 82%, respectively. Overall, fasting plasma glucose and HbA1c were linearly correlated (r=0.62, P=0.001). A fasting plasma glucose of >9.0 mmol/L predicted HbA1c ≥7.0% with an area under the curve = 0.807 (95% CI, 0. 0.794 to 0.821), while fasting plasma glucose >8.2 mmol/L predicted HbA1c >6.5%, with an area under the curve = 0.805 (95% CI, 0.791 to 0.818). The sensitivity of both cut-points was 64.5% and 70.7%, the specificity was 82.7% and 76.4%, the positive likelihood ratio was 3.73 and 2.99, and the positive predictive value was 92.2% and 93.2%, respectively.
CONCLUSION
When HbA1c determination is not available, fasting plasma glucose levels may be used to identify patients with uncontrolled type 2 diabetes and initiate timely intensification of therapy to avoid long-term complications of diabetes.
Both the Diabetes Control and Complications Trial (DCCT)1 and the United Kingdom Prospective Diabetes Study (UKPDS)2 have demonstrated unequivocally that tight control of blood glucose can significantly reduce progression of complications in both type 1 and type 2 diabetes mellitus. Glycosylated hemoglobin (HbA1c) has been used as a “gold standard” for mean glycemia and as a measure of risk for the development of diabetes-related complications.3,4 The American Diabetes Association (ADA) suggested HbA1c levels less than 7% as a goal for optimal glycemic control,5 while the American Association of Clinical Endocrinology (AACE)6 and the International Diabetes Federation (IDF) set a lower target (6.5% or less).7
The ADA recommends that HbA1c be tested 2 to 4 times annually, based on the patient’s glycemic control. However, several barriers to HbA1c testing have been identified at the primary and managed care level,8–10 including physicians’ and patients’ awareness and the relative cost of the test. In this study we aimed to determine the relationship of fasting plasma glucose (FPG) measurements obtained routinely in primary health care outpatient clinics to simultaneously measured HbA1c levels. We also investigated optimal FPG levels which best predict HbA1c ≥7.0% or >6.5% to guide physicians in intensifying antidiabetic therapy.
SUBJECTS AND METHODS
We retrospectively analyzed the data of 2888 patients with type 2 diabetes mellitus, aged 20 years or older, attending mini-diabetes clinics at 21 of 24 primary health care centers, in the capital, Muscat (Oman). These centers cover a population of over 600 000 and nearly 8000 diabetic patients are on their National Diabetes Registers. About 14% of patients had their HbA1c tested more than once during the study period, and were included in the analysis, giving the total number of 3359. Patients with missing data were excluded from the analysis. The study included patients attending over the period from July 2003 to June 2004.
Venous blood samples were collected in tubes containing ethylenediaminetetraacetic acid (EDTA), labeled and transferred in cold boxes filled with ice to a tertiary hospital laboratory. There, samples were stored at 2 to 8ºC as recommended by the manufacturer for analysis on every Monday of the week. Samples were analyzed using Roche/Hitachi 902 instruments (Boehringer Mannheim, Tokyo, Japan). According to the manufacturer, HbA1c determination in this instrument is based on turbid metric inhibition immunoassay for hemolyzed whole blood and uses the detergent tetradecyltrimetylammonium bromide (TTAB) as the hemolysing reagent to eliminate interference from leukocytes. All hemoglobin variants that are glycated at the N-terminal of the B-chain and have antibody recognizable regions identical to that of HbA1c are determined by the assay. Reagents and calibration kits were supplied by the same manufacturer. Prior to analysis of every batch, the instrument was checked for calibration.
Plasma-calibrated fasting blood glucose was determined in primary health centers using One Touch II ( Johnson & Johnson) glucose meters. On every request for analysis of HbA1c, primary care physicians were required to state the fasting capillary blood glucose level that was determined on the same day of venous blood collection for HbA1c.
A linear regression analysis was performed to study the relationship between FPG and HbA1c using Stata (version 9.1, Stata Corporation, TX, USA). Receiver-operating characteristic (ROC) curves were generated to determine optimal FPG cut-points predicting HbA1c ≥7.0% and >6.5%, using MedCalc (version9), which also calculates the area under the curve (AUC), sensitivity, specificity, likelihood ratio and positive predictive values with 95% confidence intervals (CI). The larger the AUC the more accurate the test; an associated P-value <0.05 was considered statistically significant. Unlike predictive values, likelihood ratios are not affected by the prevalence of the attribute and can be used to summarize how many times a subject with a specified FPG cut-point is more (or less) likely to have HbA1c ≥7.0% (or >6.5%) than a subject without that FPG value. For any test, likelihood ratios above 1 increase the probability that the “disorder” is present (in this case HbA1c ≥7.0% or >6.5%), and likelihood ratios <1 decrease the probability that the “disorder” is present.11 Permission to conduct the study was obtained from the research and ethical committee within the Ministry of Health.
RESULTS
The mean age (SD) of the 2888 patients was 52 (11.6) years. The average HbA1c was 8.9% (2.46%) (normal range, 4% to 6%) and the mean FPG was 10.1 (3.62) mmol/L. Over 76% (95% CI, 74.5% to 77.5%) of patients had HbA1c ≥7% and nearly 82% (95% CI, 80.6% to 83.3%) had HbA1c>6.5%. Figure 1 shows that FPG was significantly correlated with HbA1c (r=0.62, P=0.001). An ROC curve analysis revealed that FPG was a significant predictor for HbA1c ≥7.0% and >6.5%, with a similar AUC: 0.807 (95% CI, 0.794 to 0.821) and 0.805 (95% CI, 0.791 to 0.818), respectively. An FPG >9.0 mmol/L was depicted as the best cut-point to predict HbA1c levels ≥7.0% (Figure 2) and FPG >8.2 mmol/L (not shown) for HbA1c >6.5%. Both cut-points had a sensitivity of 64.5% and 70.7%, specificity of 82.7% and 76.4%, positive likelihood ratios of 3.73 and 2.99, and positive predictive values of 92.2% and 93.2%, respectively (Table 1).
Figure 1.
Relationship between fasting plasma glucose and HbA1c, among 3359 Omani patients with type 2 diabetes mellitus.
Figure 2.
Receiver-operating characteristic curve showing the performance of fasting plasma glucose in predicting glycosylated hemoglobin (HbA1c) in 3359 individuals with type 2 diabetes mellitus in Oman. The diagonal interrupted reference line (AUC=0.50) defines points where a test is no better than chance in identifying individuals with diabetes.
Table 1.
Optimal cut-points predicting glycosylated hemoglobin (HbA1c) levels using fasting plasma glucose (FPG) with associated sensitivity, specificity, positive likelihood ratio, positive predictive value and area under the ROC curves.
| Optimal FPG | Sensitivity (95% CI) | Specificity (95% CI) | +LR | PPV (%) | AUC (95% CI) | |
|---|---|---|---|---|---|---|
| HbA1c ≥ 7.0% | > 9.0 mmol/l | 64.5 (62.6 to 66.3) | 82.7 (79.9 to 85.3) | 3.73 | 92.2 | 0.807 (0.794 to 0.821) |
| HbA1c > 6.5% | > 8.2 mmol/l | 70.7 (69.0 to 72.4) | 76.4 (72.8 to 79.7) | 2.99 | 93.2 | 0.805 (0.791 to 0.818) |
ROC, receiver-operating characteristic; CI, confidence interval; +LR, positive likelihood ratio; PPV, positive predictive value; AUC, area under the curve.
DISCUSSION
This study highlights the relation between FPG and glycated hemoglobin used to monitor long-term glycemic control among Omani patients with type 2 diabetes mellitus. Multi-center prospective studies have demonstrated that intensive treatment and improved control of diabetes can prevent or slow the development of its complications. The DCCT results suggest that a 10% decrease in HbA1c (from 8.0% to 7.2%) can result in up to a 40% to 50% reduction in microvascular complications in individuals with type 1 diabetes.12 Similarly, the United Kingdom Prospective Diabetes Study determined that an 11% reduction in HbA1c will lower the risks of microvascular complications by 25% and myocardial infarction by 16% in patients with type 2 diabetes.2
In many diabetes clinics in developing countries, HbA1c may not always be available for the clinician to permit therapy adjustment. For example, in two university teaching hospitals, only 40% and 50% of patients had HbA1c registered on their medical records within the previous year.13,14 On the other hand, FPG is often routinely measured in diabetes clinics for all patients prior to consultation with the physician. Thus the utility of FPG would increase if the physician can use this value to predict the glycemic index of the patient with diabetes in an outpatient setting. Our study provides specific FPG cut-points that may be used to guide clinical decisions to intensify antidiabetic therapy in the absence of HbA1c or home glucose monitoring values. Nearly two-thirds (64.5%) of our patients with FPG >9.0 mmol/L were correctly identified to have HbA1c ≥7.0% while FPG >8.2 mmol/L identified nearly three-quarter (72%) of those with HbA1c >6.5%.
Rohlfing et al, in their analysis of glucose profiles and HbA1c of the DCCT, found that an FPG of 9.5 mmol/ L corresponded to an HbA1c of 7%, and 7.5 mmol/L corresponded to 6%.15 El-Kebbi et al16 reported that an FPG >9.2 mmol/L best predicted HbA1c >8.0% in a population of African Americans, with a sensitivity of 80% and specificity of 83%. When casual postprandial plasma glucose was used, a lower cut-point (8.3 mmol/L) was reported to predict both HbA1c >6.5% or ≥7.0%.17
Although many studies have used statistical methods to predict HbA1c from FPG, the validity of this approach in assessing glycemic control in patients with diabetes has been questioned.18 Bouma et al19 showed in 1020 patients with type 2 diabetes that HbA1c is difficult to predict from FPG values: only 66% of the patients with HbA1c <7.0% were identified by FPG values <7.8 mmol/L. They concluded that predicting HbA1c changes from FPG changes is even more difficult. Similarly, Avignon et al,20 demonstrated in 66 type 2 diabetic patients that early post-lunch (2:00 pm) and extended post-lunch (5:00 pm) plasma glucose correlated significantly and independently with HbA1c, but that of pre-breakfast and pre-lunch plasma glucose did not. Nonetheless, the ADA states that “FPG is some-what better than post-prandial glucose in predicting HbA1c, especially in type 2 diabetes”.21 Other studies have shown that, in such patients, fasting blood glucose determinations (at intervals of weeks to months) provide a better measure of long-term glycemia than in patients with type 1 diabetes.22,23
In addition to the statistical methods used to estimate long-term glycemic control, standard methods such as self-monitoring of blood glucose, venous blood glucose and HbA1c remain the main stay of diabetes care to achieve a specific level of glycemic control and to prevent hypoglycemia. Physicians ought to use standard methods of investigation when providing diabetes care and encouraging patients to perform self-monitoring blood glucose measurements as needed.
This study documents for the first time that the majority of patients (76%) with type 2 diabetes in primary care in Oman exhibit poor glycemic control (HbA1c ≥7.0%) according to the ADA guidelines.5 The prevalence of poor control increases to 82% if the AACE target is applied (HbA1c >6.5%). These results are consistent with two studies from Saudi Arabia and one from Lebanon where poor control was prevalent among 73%, 77%, and 72% of patients with diabetes, respectively. 13,14,24 In comparison, two National Health and Nutrition Examination Surveys, conducted between the periods 1988–1994 and 1999–2000 in the US, reported that 56% and 63% patients had poor glycemic control, respectively.25 Higher rates of poor control were reported from other studies among African Americans (77%) and Finnish people (75%).17,26 This clearly high-lights the urgent need for intervention to improve diabetes care in developing and developed countries.
Our study has some limitations. First, adopting an FPG cut-point of >9.0 mmol/L as a predictor of HbA1c ≥7.0% would result in about 35.5% of individuals being “missed” as they will be considered to have good glycemic control when in fact they do not (false negative). Similarly, 17.3% of people may be at risk of hypoglycemia as they may be subjected to more intense anti-diabetic therapy when in fact having good glycemic control (false positive). Nonetheless, hypoglycemia tends to be uncommon and mild in patients with type 2 diabetes, even in settings in which intensive diabetes therapy is guided by treatment algorithms based on glucose levels obtained during office visits.27 In addition, in the presence of high levels of poor control (as is the case in many settings), hypoglycemia may not be a common complication.
The above relation between FPG and HbA1c was derived without adjustment for other confounders like age, gender, body mass index, smoking status, type and duration of diabetes. Including such variables may result in more accurate cut-points, yet it would have compromised the objective of this study, which was to increase the utility of FPG in clinical practice by making it simple.
Finally, the accuracy of glucose measurement instruments used in primary care during the study period was not evaluated by simultaneous matched measurement of venous blood samples. However, several studies have demonstrated a high degree of accuracy of such instruments when compared with the laboratory reference over a broad range of glucose concentrations.28
In conclusion, we have identified a cut-point for FPG that may be used as an indicator of glycemic control in patients with type 2 diabetes when current HbA1c levels or home blood glucose monitoring records are not available.
Acknowledgment
We would like to thank Dr. Shalini Nooyi for her critical review of the draft manuscript and Mr. Khalid Saleem for his technical support during this study.
REFERENCES
- 1.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 3.Jeppsson JO, Jerntorp P, Almer LO, Persson R, Ekberg G, Sundkvist G. Capillary blood on filter paper for determination of HbA1c by ion exchange chromatography. Diabetes Care. 1996;19:142–5. doi: 10.2337/diacare.19.2.142. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27:1761–73. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2006;29(Suppl 1):S4–42. [PubMed] [Google Scholar]
- 6.The American Association of Clinical Endocrinologists Medical Guidelines for the Management of Diabetes Mellitus: the AACE system of intensive diabetes self-management--2000 update. Endocr Pract. 2000;6:43–84. [PubMed] [Google Scholar]
- 7.International Diabetes Federation. Global Guideline for Type 2. Diabetes. [Accessed 12 July 2006]. http://www.idf.org/webdata/docs/IDF%20GGT2D.pdf.
- 8.Delaronde S. Barriers to A1C Testing Among a Managed Care Population. The Diabetes Educator. 2005;31:235–239. doi: 10.1177/0145721705275328. %R 101177/0145721705275328. [DOI] [PubMed] [Google Scholar]
- 9.Chin MH, Cook S, Jin L, et al. Barriers to providing diabetes care in community health centers. Diabetes Care. 2001;24:268–74. doi: 10.2337/diacare.24.2.268. [DOI] [PubMed] [Google Scholar]
- 10.Islam N, Akhter J, Kayani N, Khan MA. Fructosamine: an alternative assessment of past glycaemic control in developing countries. J Pak Med Assoc. 1993;43:238–40. [PubMed] [Google Scholar]
- 11.Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. 2004;329:168–9. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968–83. [PubMed] [Google Scholar]
- 13.Al-Ghamdi AA. Role of HbA1c in management of diabetes mellitus. Saudi Med J. 2004;25:342–5. [PubMed] [Google Scholar]
- 14.Akel M, Hamadeh G. Quality of diabetes care in a university health center in Lebanon. Int J Qual Health Care. 1999;11:517–21. doi: 10.1093/intqhc/11.6.517. [DOI] [PubMed] [Google Scholar]
- 15.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275–8. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 16.el-Kebbi IM, Ziemer DC, Gallina DL, Phillips LS. Diabetes in urban African-Americans. VI. Utility of fasting or random glucose in identifying poor glycemic control. Diabetes Care. 1998;21:501–5. doi: 10.2337/diacare.21.4.501. [DOI] [PubMed] [Google Scholar]
- 17.El-Kebbi IM, Ziemer DC, Cook CB, Gallina DL, Barnes CS, Phillips LS. Utility of casual postprandial glucose levels in type 2 diabetes management. Diabetes Care. 2004;27:335–9. doi: 10.2337/diacare.27.2.335. [DOI] [PubMed] [Google Scholar]
- 18.Caputo S, Pitocco D, Ruotolo V, Ghirlanda G. What Is the Real Contribution of Fasting Plasma Glucose and Postprandial Glucose in Predicting HbA1c and Overall Blood Glucose Control? Diabetes Care. 2001;24 doi: 10.2337/diacare.24.11.2011. 2011- [DOI] [PubMed] [Google Scholar]
- 19.Bouma M, Dekker JH, de Sonnaville JJ, et al. How valid is fasting plasma glucose as a parameter of glycemic control in non-insulin-using patients with type 2 diabetes? Diabetes Care. 1999;22:904–7. doi: 10.2337/diacare.22.6.904. [DOI] [PubMed] [Google Scholar]
- 20.Avignon A, Radauceanu A, Monnier L. Non-fasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care. 1997;20:1822–1826. doi: 10.2337/diacare.20.12.1822. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Postprandial Blood Glucose. Diabetes Care. 2001;24:775–778. doi: 10.2337/diacare.24.4.775. [DOI] [PubMed] [Google Scholar]
- 22.Singer DE, Coley CM, Samet JH, Nathan DM. Tests of glycemia in diabetes mellitus. Their use in establishing a diagnosis and in treatment. Ann Intern Med. 1989;110:125–37. doi: 10.7326/0003-4819-110-2-125. [DOI] [PubMed] [Google Scholar]
- 23.Howe-Davies S, Simpson RW, Turner RC. Control of maturity-onset diabetes by monitoring fasting blood glucose and body weight. Diabetes Care. 1980;3:607–10. doi: 10.2337/diacare.3.5.607. [DOI] [PubMed] [Google Scholar]
- 24.Akbar DH. Low rates of diabetic patients reaching good control targets. East Mediterr Health J. 2001;7:671–8. [PubMed] [Google Scholar]
- 25.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–42. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 26.Valle T, Koivisto VA, Reunanen A, Kangas T, Rissanen A. Glycemic control in patients with diabetes in Finland. Diabetes Care. 1999;22:575–9. doi: 10.2337/diacare.22.4.575. [DOI] [PubMed] [Google Scholar]
- 27.Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-Kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med. 2001;161:1653–9. doi: 10.1001/archinte.161.13.1653. [DOI] [PubMed] [Google Scholar]
- 28.Urdang M, Ansede-Luna G, Muller B, Newson R, Lacy-Pettit A, O’Shea D. An independent piolot study into the accuracy and reliability of home blood glucose monitors. Lancet. 1999;353:1065–6. doi: 10.1016/S0140-6736(99)00326-8. [DOI] [PubMed] [Google Scholar]