Tracheobronchopathia osteochondroplastica (TO) is a benign disease, first described in 1855 and rather rarely since. Only a few hundred cases have been reported. Osseocartilaginous submucosal nodules projecting into the lumen of the trachea and bronchi characterize the disease.1 The mechanism of its occurrence remains controversial. Most cases are asymptomatic and frequently diagnosed incidentally during intubation, endoscopy or autopsy.2,3 The incidence of TO appears to be underestimated in the literature in view of the fact that it is usually benign. However, a more accurate estimate of its true prevalence may become available through the use of bronchoscopy and computerized tomographic scanning.
CASE
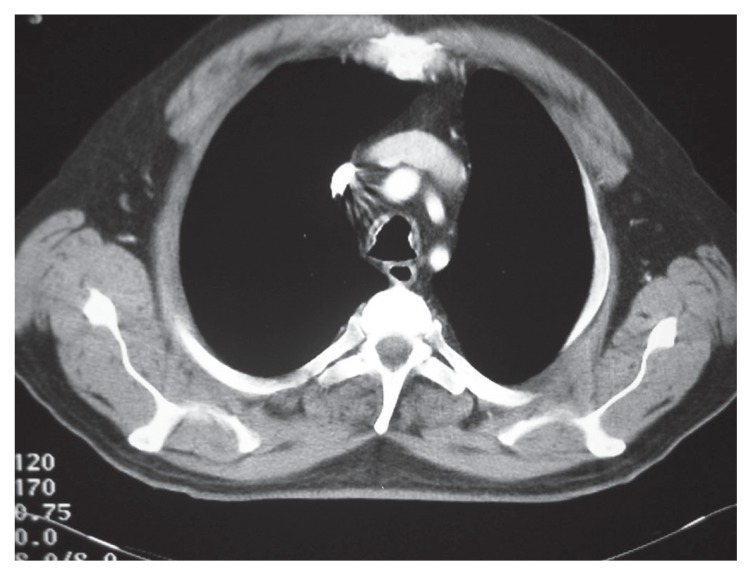
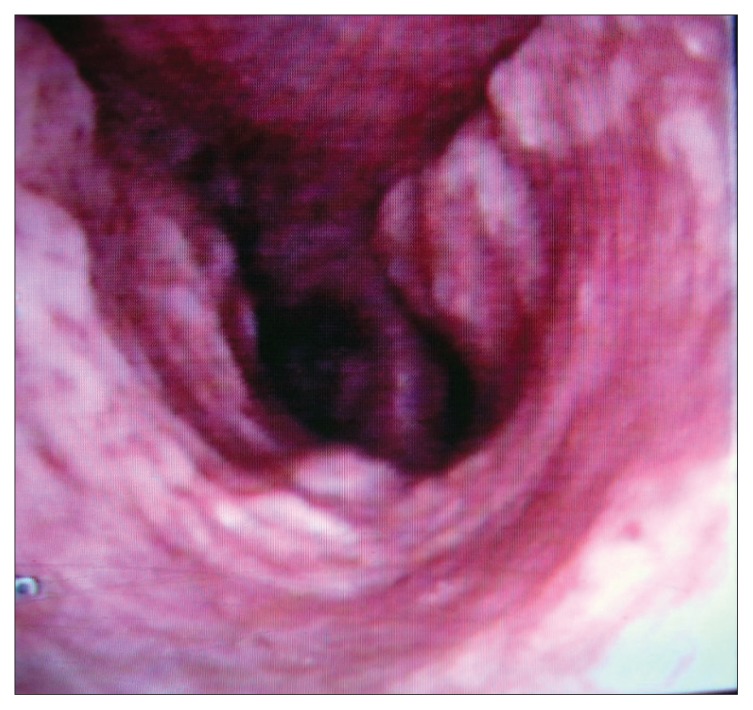
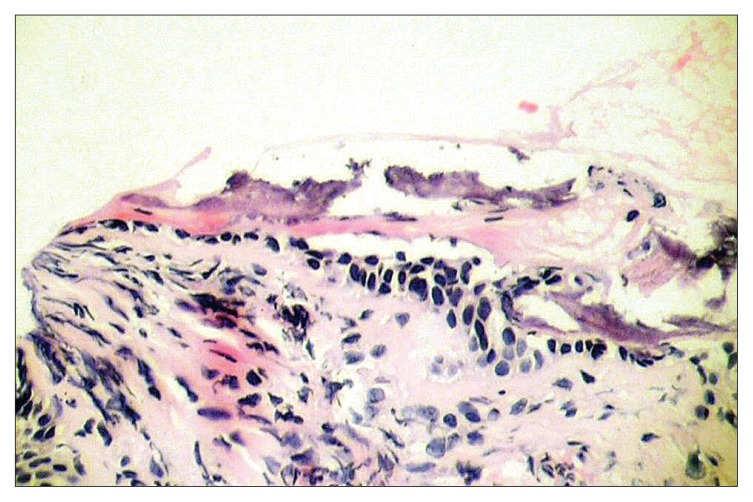
A 66-year-old non-smoking male patient was referred to respiratory medicine at King Hussein Medical Center, Amman in July 2005 with a 6-year history of recurrent attacks of mild hemoptysis. He was assessed by different general practitioners over the years and had been diagnosed as having recurrent attacks of acute bronchitis. Several chest X-rays were done during this period, which had been reported as normal. Two weeks prior to his admission he had a mild fever sensation with excessive sweats, mainly at night. He had diabetes mellitus for 15 years and was on an oral hypoglycemic agent, which was shifted to insulin in 2002 because of poor control. Past surgical history was positive for an appendectomy 10 years ago and surgical repair of a diaphragmatic hernia 3 years later with no endotracheal intubation problems or any postoperative pulmonary complications. He was a retired phosphate mine guard but had never been in close contact with phosphate. There was no history of cigarette smoking or contacts with domestic animals or birds. There was no history to suggest contact with any case of tuberculosis. Upon admission he had a temperature of 38°C, a pulse of 80 beats/minute and a respiratory rate of 16/minute His chest auscultations revealed only a few scattered expiratory wheezes. A chest X-ray showed a left lower zone homogenous opacity obscuring the left cardiac border. Laboratory tests including a complete blood count, liver and kidney functions, and arterial blood gases were normal. Pulmonary function tests including a flow volume loop and diffusion tests were normal. His sputum cytology was negative for both malignant cells and acid-fast bacilli stain and culture. A high-resolution chest CT scan demonstrated small nodules with calcifications at the trachea wall with mild tracheal narrowing but without any deformity (Figure 1). A diagnostic fiber optic bronchoscopy showed multiple yellow-white, hard, papilla-like numerous nodules on the anterior and lateral walls of the trachea sparing its membranous part extending from just below the vocal cords to the proximal part of both main bronchi (Figure 2). Histopathological examination of the biopsy samples from these lesions showed osteocartilaginous tissue, typical of TO (Figure 3). Bronchial wash stains for bacteria, TB and fungi were negative. During his stay he was seen by an ophthalmologist and an ENT surgeon in order to rule out signs of systemic diseases, moderate diabetic retinal changes were the only abnormalities demonstrated. He was treated as a case of chest infection and had a very good clinical recovery with resolution of his chest X-ray inflammatory changes. On his 6-week follow up visit the patient showed no symptoms of chest infection but continued to complain from recurrent attacks of mild hemoptysis. His sputum and bronchial washings TB cultures that were requested during his stay in hospital proved to be negative.
Figure 1.
A computerized tomography scan of the chest showing nodules and calcifications of the trachea.
Figure 2.
Fiber optic bronchoscopy showing multiple hard numerous nodules on the anterior and lateral walls of the trachea.
Figure 3.
Microscopic examination of bronchial biopsy sample showing the abnormal osteocartilaginous tissue.
DISCUSSION
Tracheopathia osteochondroplastica is an uncommon abnormality of the cartilaginous tracheal wall, characterized by the presence of osteocartilaginous calcified nodules within the submucosa, with a variable degree of diffuse tracheal narrowing. It is characterized by the presence of polyps in the trachea and main bronchi, which consist of osseous tissue. Involvement may extend to lobar or segmental bronchi. The nodules originate in the airway cartilages and thus typically spare the posterior membranous wall of the airways. The disorder is more common in men and the diagnosis is usually made in the fourth through sixth decades of life. There is no relationship to smoking or other systemic disorders. During the last sixteen-year of bronchoscopic experience at KHMC, our pulmonologists encountered only this patient with TO among more than 5000 performed bronchoscopies. This case presentation emphasizes the importance of bronchoscopic examination in the diagnosis of such cases and clearly underlines the significance of the early referral of all cases with recurrent hemoptysis to a pulmonologist.
The etiology of TO remains unknown. There are reported cases of TO with a concomitant silicosis and non-Hodgkin pulmonary lymphoma4,5 but it seems that these cases occurred by coincidence. Our patient worked in a phosphate mine for a long period but his work did not involve direct or close contact with the raw silica. His radiological and pulmonary function tests did not suggest such a clinical diagnosis. The majority of patients remain asymptomatic, but a small number develop severe airway stenosis. Other pulmonary symptoms include dyspnea and hemoptysis. One of the complications is the increased incidence of recurrent respiratory infections. The pulmonary symptoms and complications are the consequence of narrowing and thickening of the airway walls. Our patient had described several chest infective episodes most probably because of the mucosal wall irregularity that he had. A long-standing diabetes history probably had an influence on the recurrences of these attacks. Plain chest X-ray films are often unremarkable but may demonstrate atelectasis, consolidation, tracheal modularity, or narrowing. CT reveals tracheal modularity with calcification and narrowing.6,7 The diagnosis is usually made on the basis of endoscopy. Differential diagnosis of nodular excrescences includes tracheobronchial amyloidosis, endobronchial sarcoidosis, calcificating lesions of tuberculosis, papilomatosis, tracheobronchial calcinosis and relapsing polychondritis. Our patient didn’t have any of the systemic symptoms that usually point out to the above diagnoses nor he had their histopathological finding on his bronchial biopsy, which was rather diagnostic of TO. Bronchoscopy is the most definitive diagnostic test. The bronchoscopic appearance alone is diagnostic of the disease. Although there is no specific therapy for this disorder, management of TO includes bronchodilators, prompt treatment of pulmonary infections, and bronchoscopic dilatation when indicated. However, in severe cases, bronchoscopic removal of obstructing excrescences and surgery has been performed with therapeutic effect.8 Treatments attempted included cryotherapy, laser excision, external beam irradiation, and bronchoscopic removal of the obstructing lesions. The prognosis is usually favorable. Clinicians should include this disease in the list of differential diagnoses when confronted with symptoms like persistent and often productive cough, haemoptysis, dyspnoea and wheeze. Awareness of the condition as a differential diagnosis to neoplasm is important, to avoid unnecessary intervention or treatment.
REFERENCES
- 1.Nienhuis DM, Prakash UB, Edell ES. Tracheobronchopathia osteochondroplastica. Ann Otol Rhinol Laryngol. 1990 Sep;99(9 Pt 1):689–94. doi: 10.1177/000348949009900903. [DOI] [PubMed] [Google Scholar]
- 2.Mohan K, Owen S, Yeong C. Tracheobronchopathia osteochondroplastica as an incidental finding. Asian Cardiovasc Thorac Ann. 2004 Sep;12(3):280–1. doi: 10.1177/021849230401200324. [DOI] [PubMed] [Google Scholar]
- 3.Coetmeur D, Bovyn G, Leroux P, Niel-Duriez M. Tracheobronchopathia osteochondroplastica presenting at the time of a difficult intubation. Respir Med. 1997 Sep;91(8):496–8. doi: 10.1016/s0954-6111(97)90116-5. [DOI] [PubMed] [Google Scholar]
- 4.Pinheiro GA, Antao VC, Muller NL. Tracheobronchopathia osteochondroplastica in a patient with silicosis: CT, bronchoscopy, and pathology findings. J Comput Assist Tomogr. 2004 Nov-Dec;28(6):801–3. doi: 10.1097/00004728-200411000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Karlikaya C, Yuksel M, Kilicli S, Candan L. Tracheobronchopathia osteochondroplastica. Respirology. 2000 Dec;5(4):377–80. [PubMed] [Google Scholar]
- 6.Morita S, Yokoyama N, Yamashita S, Izumi M, Kanda T, Nagataki S. Tracheopathia osteochondroplastica complicated with thyroid cancer: case report and review of the literature in Japan. Jpn J Med. 1990 Nov-Dec;29(6):637–41. doi: 10.2169/internalmedicine1962.29.637. [DOI] [PubMed] [Google Scholar]
- 7.Restrepo S, Pandit M, Villamil MA, Rojas IC, Perez JM, Gascue A. Tracheobronchopathia osteochondroplastica: helical CT findings in 4 cases. J Thorac Imaging. 2004 Apr;19(2):112–6. doi: 10.1097/00005382-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Kutlu CA, Yeginsu A, Ozalp T, Baran R. Modified slide tracheoplasty for the management of tracheobroncopathia osteochondroplastica. Eur J Cardiothorac Surg. 2002 Jan;21(1):140–2. doi: 10.1016/s1010-7940(01)01080-6. [DOI] [PubMed] [Google Scholar]