Abstract
BACKGROUND
Conventional methods of radiographic examination are often unsatisfactory for identifying worms in the biliary tract. Ultrasonography is a non-invasive, quick and safe procedure known to have diagnostic accuracy. We studied the ultrasonographic appearances of biliary ascariasis and the role of ultrasonography in diagnosis and management.
METHODS
In a prospective 5-year study, a sonographic diagnosis of biliary ascariasis was made on 46 Yemeni patients. The diagnosis was based mainly on sonographic appearances supported by clinical and laboratory results and proved by outcome of either surgical or medical management or spontaneous exit of worms. Follow-up ultrasound was performed for all patients to confirm the diagnosis and to monitor management.
RESULTS
Parasites were present in the dilated main bile duct in 23 patients, in the gallbladder in 12 patients, in the intrahepatic ducts in 6 patients, in the main pancreatic duct in 4 patients and as an intrahepatic abscess in one patient. The characteristic appearance of Ascaris lumbricoides was as single or multiple echogenic non-shadowing linear or curved strips with or without echoic tubular central lines that represent the digestive tracts of the worm. A spaghetti-like appearance was seen in 9 patients and amorphous fragments were seen in 2 patients. Sixteen patients underwent surgery, 20 patients were treated medically (including spontaneous exit of the worm in 7 patients without treatment) and in 10 patients worms were extracted by endoscopic retrograde cholangiopancreatography.
CONCLUSIONS
Follow-up ultrasound was found to be effective in confirming the diagnosis and monitoring management.
In endemic areas, which include Yemen, infestation with Ascaris lumbricoides is very common. It is a frequent cause of biliary tract diseases, which can be a serious public health problem in this part of the world.1,2,3 Thus, early detection and treatment of this disease is of prime importance. The adult worm can migrate through the extra-hepatic biliary tree and may reach intra-hepatic ducts and the gallbladder causing biliary colic, obstruction, acute cholecystitis, acute cholangitis and hepatic abscess. Ascaris is also an etiological factor for acute pancreatitis and the overall mortality rate is 3%.4 Conventional methods of radiographic examination are often unsatisfactory for identifying worms in the biliary tract. Ultrasonography, as a non-invasive, quick and safe procedure, has been known to have diagnostic accuracy in the work-up of biliary ascariasis.5–12 Our study addressed the ultrasound appearances of biliary ascariasis and the value of ultrasound in confirming the diagnosis and assessing management outcome in a relatively large number of Yemeni patients.
METHODS
The study, which included 46 patients (30 female and 16 male), was conducted between May 2000 and June 2005 in the Kuwait University Hospital (Sana’a) and Sana’a Radiology Center. General abdominal sonography focusing on the liver and biliary system was performed for all patients referred with acute right hypochondrial or epigastric pain using Siemens Sonoline 450 and Logic 400 machines and a 3.5 MHz probe. The liver and biliary systems as well as the pancreas were scanned in longitudinal, transverse and oblique projections while patients were in the supine and lateral positions. Inclusion criteria were based on ultrasound findings suggestive of biliary ascariasis: straight or curved non-shadowing echoic cord-like structures with or without centrally hypoechoic tubes, the “spaghetti” sign, intrahepatic hyperechoic bundles, or a rounded hyperechoic pseudotumorous mass (Table 1). In addition, a sonographic appearance of sinuous motility or change of configuration of non-shadowing tube-like intrabiliary structures was an important inclusion diagnostic criteria.
Table 1.
Distribution and appearance of biliary ascariasis based on ultrasound findings.
| No. of patients | % | |
|---|---|---|
| Distribution | ||
| Main bile duct | 23 | 50.0 |
| Gall bladder | 12 | 26.1 |
| Common bile duct | 6 | 13.0 |
| Main pancreatic duct | 4 | 8.7 |
| With right hepatic lobe abscess | 1 | 2.2 |
| Ultrasound appearance | ||
| Straight or curved non-shadowing echogenic strips (strips sign) with or without tube sign | 34 | 73.9 |
| Spaghetti sign (aggregates of multiple worms) | 9 | 19.6 |
| Intrahepatic hyperechoic bundles | 1 | 2.2 |
| Rounded hyperechoic pseudotumorous mass | 2 | 4.4 |
Follow-up ultrasound was performed at 3 successive intervals: one week after initial ultrasound diagnosis for all patients, 2 weeks after initial treatment for 39 patients and after 2 weeks after repeated medical treatment for 3 patients. The clinical laboratory and management data as well as ultrasound follow-up outcome were recorded. The final diagnosis was based on established sonographic criteria and supported by the medical, surgical and endoscopic outcome as well as clinical and laboratory findings.
RESULTS
The ages of the 46 patients included in this study were between 6 to 67 years (mean and median age, 18 years). There were 30 females (63%) and 16 males (37%), including 4 women who were pregnant (3, 5, 6 and 7 months). About 60% of the patients resided in rural areas. The laboratory results showed raised serum alkaline phosphatase in 40% of patients, abnormal alanine aminotransferase in 30%, raised serum amylase in 12%, and leukocytosis in 90%. Ova or adult forms of Ascaris lumbricoides were seen in the stool of 91% of patients. Each case of biliary ascariasis was clinically acute. The diagnosis of biliary ascariasis was established incorrectly in one patient owing to excessive post-sphincterotomy pneumobilia. In 46 cases where biliary ascariasis was seen on ultrasound there were 23 cases with worms in the main bile duct and 12 cases with worms in the gallbladder. There were 6 cases with worms in the common bile ducts as well as in intrahepatic ducts, 2 in the right lobe and 4 in the left lobe (Table 2). In 4 cases worms were seen in the main pancreatic duct and in one case an intrahepatic complicated abscess cavity was seen. Ultrasound demonstrated common bile duct dilatation, which measured 9 to 20 mm, in 35 cases. In 2 patients parasites were present in fusiform, dilated extrahepatic ducts that were proved at surgery to be choledochal cysts (Figures 1, 2) with stones.
Table 2.
Clinical presentation of biliary ascariasis.
| Signs | No. of patients | % |
|---|---|---|
| Recurrent biliary colic | 26 | 57 |
| Acute cholingitis | 8 | 18 |
| Acute cholecystitis | 7 | 15 |
| Acute pancreatitis | 4 | 8 |
| Liver abscess | 1 | 2 |
Associated nausea or vomiting was seen in 90% and jaundice in 40% of patients.
Figure 1.
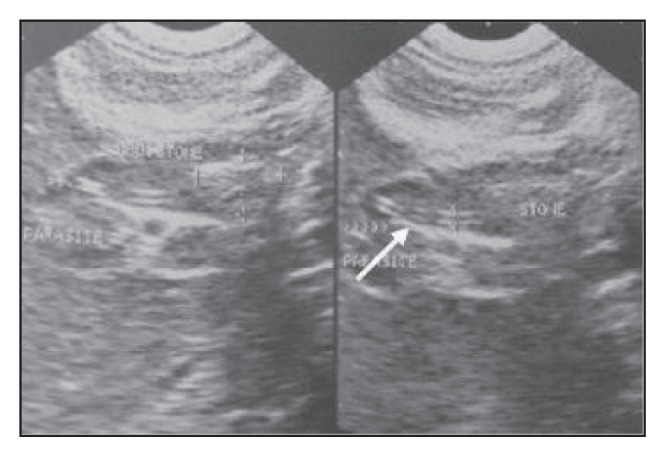
An oblique ultrasound scan of a 25-year-old female patient demonstrating fusiform expansion of the common bile duct (at surgery proved to be a choledochal cyst), which contains a rather large shadowing stone with an associated echogenic non-shadowing tubular structure (with anechoic linear center) proximal to the stone (arrow) and extending to the common hepatic duct.
Figure 2.
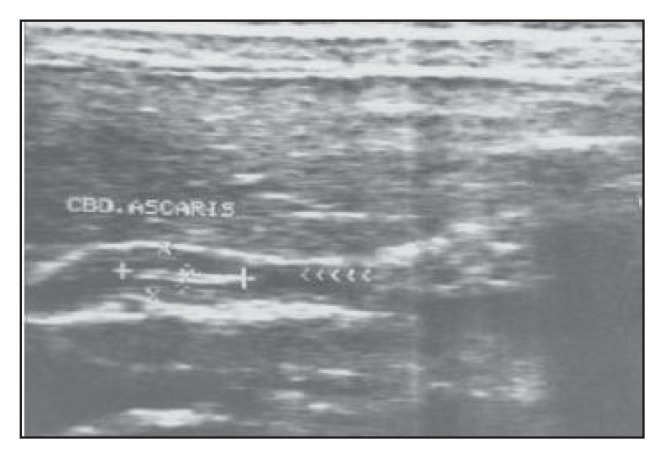
Another female patient aged 16 years with fusiform expansion of the common bile duct containing a non-shadowing, echogenic tubular structure with an anechoic linear center that showed active movement during examination.
In 34 out of 46 patients, the ultrasound showed a usual appearance of one or more non-shadowing tubular structures that were either straight or coiled or appeared as a thick echogenic strip with a central anechoic tube representing the digestive tract of the worm (Figures 3, 4, 5, 7, 8). In 9 patients the bile duct was so solidly packed with worms that no lumen was visible, with a spaghetti-like appearance of multiple worms seen side by side (Figure 6). In one patient ultrasound showed intrahepatic hyperechoic bundles (Figure 9a) and in two patients a hyperechoic, round pseudotumorous mass was seen (Figure 9b, 10). Follow-up ultrasound showed that these bundles were changing in size and appearance. Table 1 shows the distribution and appearance of ultrasound findings.
Figure 3a.
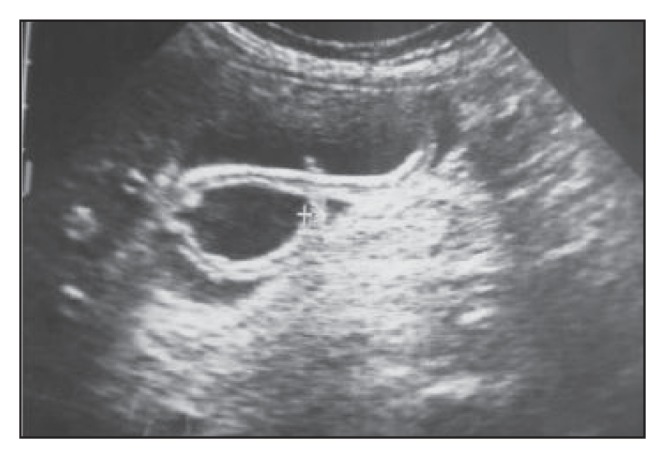
Longitudinal ultrasound section of a relatively distended gallbladder containing a long echogenic, non-shadowing intraluminal tubular structure with a central anechoic linear area (the “inner tube” sign), representing the digestive tract of the worm.
Figure 4a.
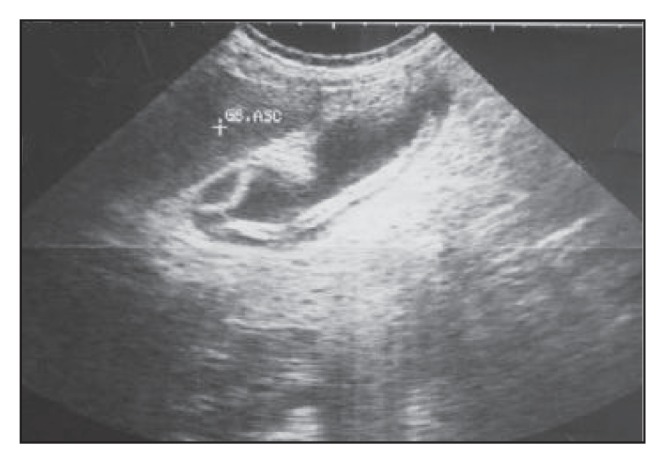
Ultrasound scan of a gallbladder of another patient showing two intralumenal worms.
Figure 5a.
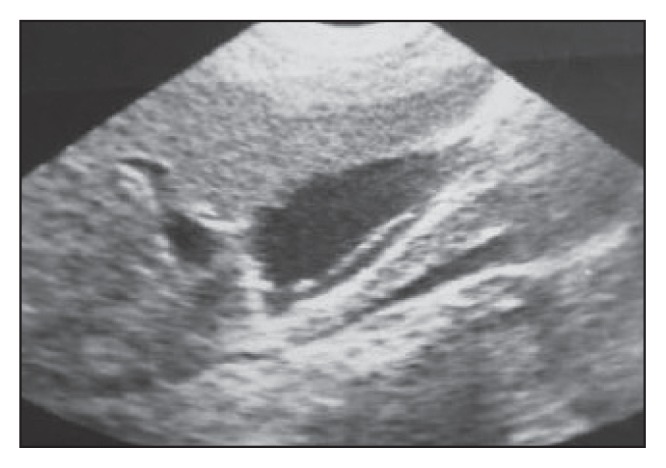
A longitudinal section of gallbladder with the worm represented as an echogenic, non-shadowing tube-like structure (strip sign).
Figure 7a.
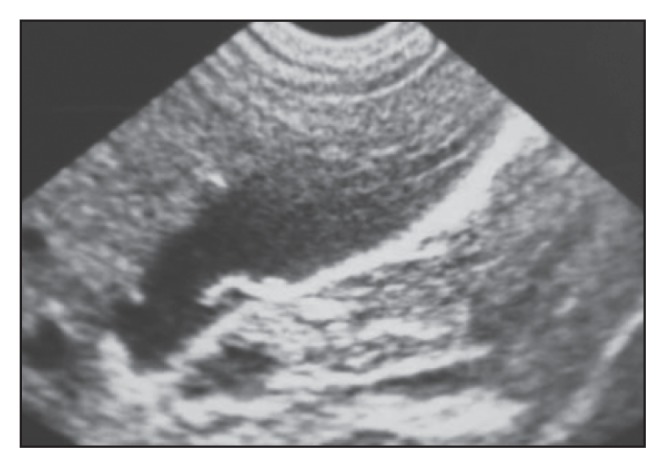
In this patient, the gallbladder shows short non-shadowing hyperchoic curved strips that become ring-like in Figure 7b.
Figure 8.
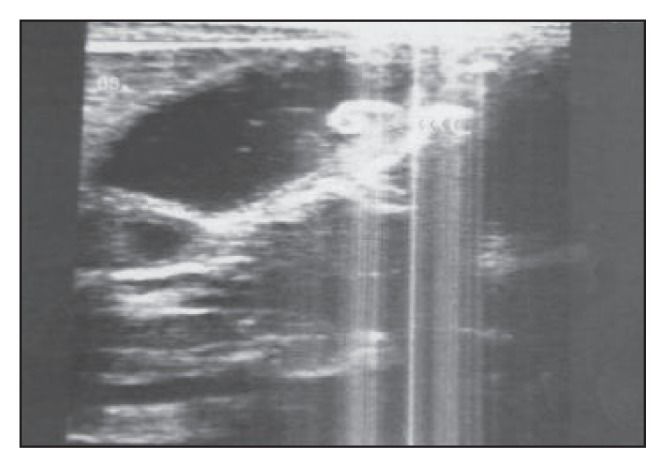
A longitudinal section of the gallbladder of a 15-year-old female patient shows a comma-shaped worm that exhibits active movement during ultrasound examination.
Figure 6.
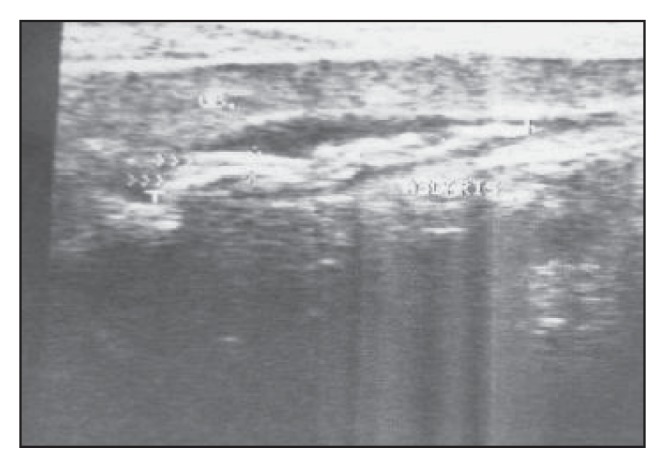
A longitudinal scan of a relatively contracted gallbladder showing several non-shadowing, overlapped, tube-like worms (“spaghetti” sign).
Figure 9a.
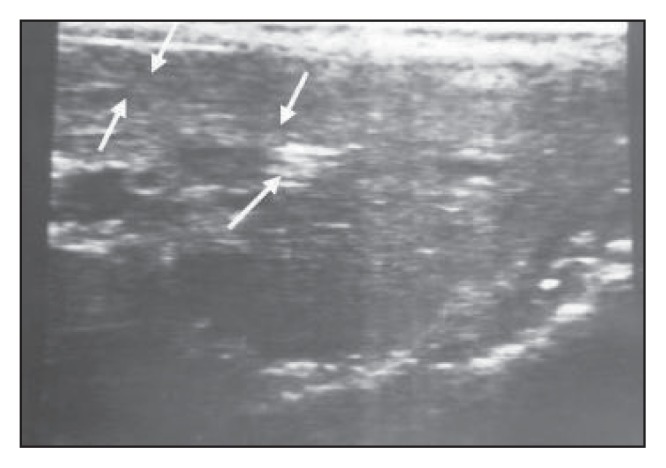
A transverse scan through the left hepatic lobe shows a dilated intrahepatic duct that is packed with worms, producing an amorphous appearance and some worms in the form of a bolus.
Figure 9b.
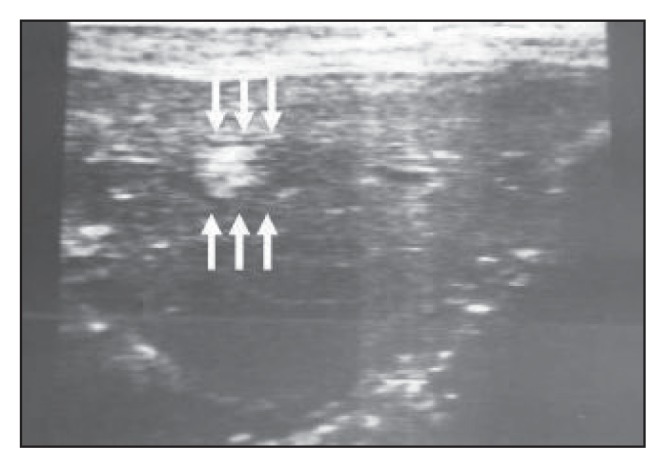
Another transverse scan through the left hepatic lobe showing a dilated intrahepatic duct packed with worms in the form of a bolus.
Figure 10.
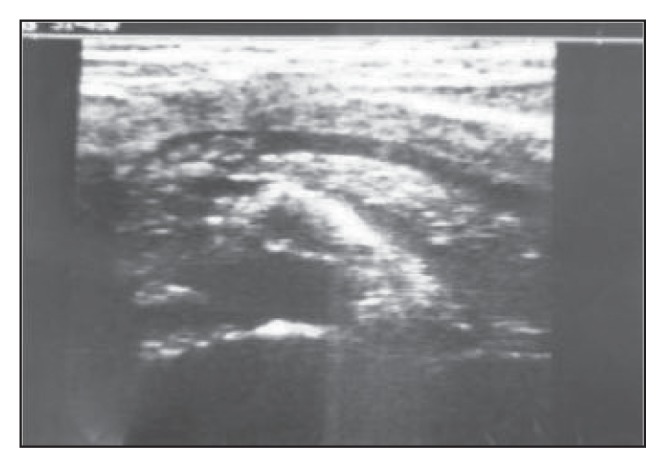
An ultrasound scan through the pancreas showing a worm bolus in the proximal segment of the dilated pancreatic duct.
Serial follow-up ultrasound was performed to confirm the diagnosis and to evaluate management outcome. The first follow-up ultrasound performed for all patients one week after initial ultrasound diagnosis revealed changes in the configuration of parasites, confirming diagnosis in 32 patients. In 7 patients, there was spontaneous exits of worms from the biliary tree without treatment. The other 39 patients underwent different types of management. Thirteen patients were treated by anthelminitic drugs and 16 patients by surgery. Worms had to be removed from the biliary system by endoscopic retrograde cholangiopancreatography in 10 patients. A second follow-up ultrasound performed 2 weeks after treatment for all patients showed a failure of management in 3 patients who underwent repeated medical management. In those patients a third followup ultrasound was normal 2 weeks later.
DISCUSSION
Ascaris lumbricoides is the largest intestinal roundworm and the most frequent of human helminthic parasites. It is endemic in third world countries, including Yemen, where poor health standards, low socioeconomic status and geoclimatic conditions influence the parasite prevalence in these parts of the world.1,2,3
The commonest extraintestinal manifestation of the worm is the biliary system, as it can easily gain entry into the common bile duct via the ampulla of Vater, which usually provokes biliary colic, fever, acute cholangitis and obstructive jaundice although asymptomatic cases are described.4,5 Trans-abdominal ultrasound is a reliable modality for the diagnosis and post-therapeutic surveillance of biliary ascariasis. Several studies have discussed the sonographic findings of the roundworm in the biliary tracts and gall bladder.6–13 Common findings have been described by Khuroo et al.6 Linear or curvilinear thick, echogenic, non-shadowing structures with central anechoic longitudinal tubes are typical of ascariasis. The “inner tube” or the “double tube sign” implies the visualization of the hypoechoic alimentary canal of the worm. The worms are often seen as one or more non-shadowing, tube-like echoic structures, which may be straight or coiled (strip sign). Overlapping coiled or aggregates of worms may have an appearance like spaghetti. Dilatation of the common bile duct with or without a distended gallbladder is the next most common finding. However, when the lumen is packed solid with worms, especially if the solidity appears amorphous, a dilated bile duct could be missed. Intrahepatic duct boluses and bundles give a pseudotumorous appearance, 14,15 but acute clinical features, age and socioeconomic status should give a clue to the correct diagnosis. In addition, boluses should shrink. Worms in the gallbladder lumen are less common,16,17 but in our study they were relatively common findings. The end-on appearance of the intraductal worm on transverse view is useful to solve any doubt of diagnosis on longitudinal view.18 In our study young women were predominantly affected, although young children are most affected by Ascaris infestation with less biliary incidence, which may be due to the narrowed ampulla and lumen of biliary channels in children. One patient was false positive due to extensive biliary gaseous shadowing as post-sphincterotomy status. Pregnant patients may present with biliary parasites as seen in four cases in our study, and ultrasound was very helpful in their management. In addition, worms can complicate existing biliary duct anomalies as seen in our study of two incidentally found fusiform choledochal cysts (proved at surgery). About one fourth of our patients had associated intraductal or gallbladder stones, but there is evidence that biliary ascariasis causes intrahepatic stones.12 Serial follow-up ultrasound demonstrated a change in the configuration of the worms in the majority of our patients and was a confirmative diagnostic factor. In addition, follow-up ultrasound showed complete disappearance of the worms from the biliary system of all our cases at the end of management. Thus, serial follow-up ultrasound was found to be very effective in confirming the diagnosis of biliary worms and has an important role in assessing their management outcome.
Figure 3b.
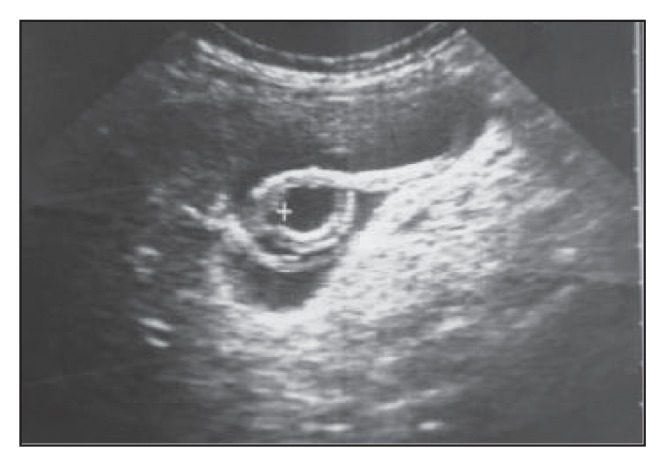
Follow-up of Figure 3a shows change in configuration of the previously seen worm with the appearance of an additional smaller one posterior to it.
Figure 4b.
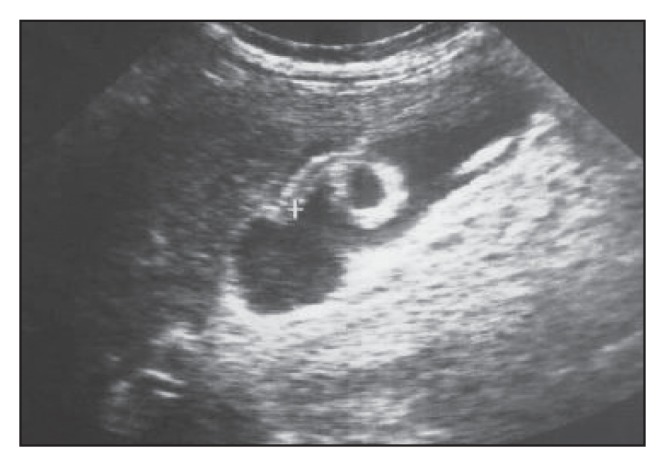
Change in the configuration and positioning of the worm seen in Figure 4a on the control ultrasound, confirming the diagnosis.
Figure 5b.
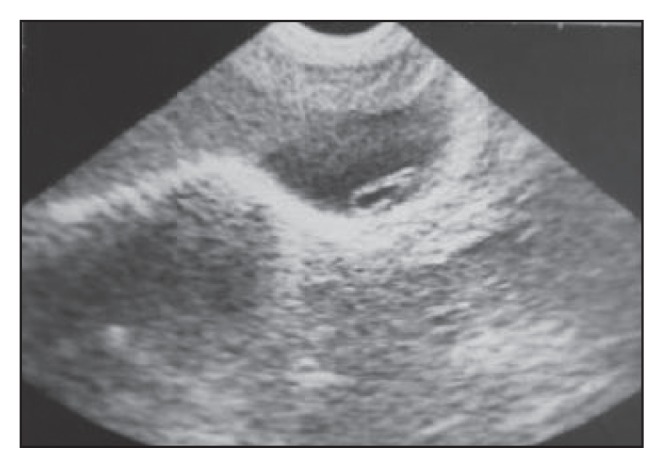
Change in configuration of the worm in Figure 5a. with the worm folded on itself on the control scan, confirming the diagnosis.
Figure 7b.
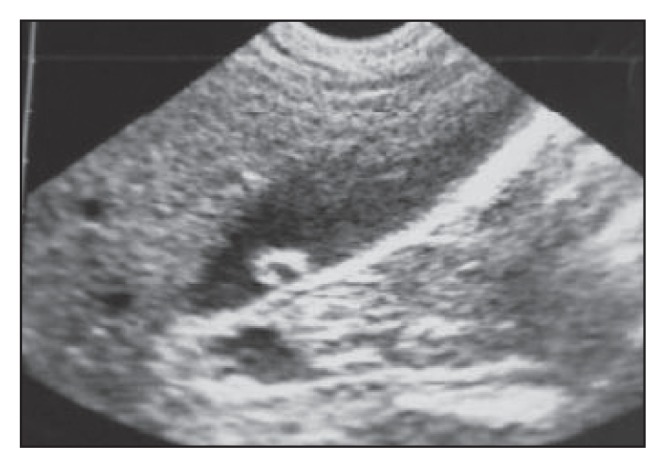
The curved strips in Figure 7a have become ring-like.
REFERENCES
- 1.Gabaldon A, Mofidi C, Morishita K, et al. Control of Ascariasis. Report of WHO expert Committee. World Health Organ. Tech, Report Ser. 1967;379:6–7. [Google Scholar]
- 2.Crompton DWT. The Prevalence of Ascaris. Parasitol Today. 1988;4:162–8. doi: 10.1016/0169-4758(88)90152-4. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Ascariasis (editorial) Lancet. 1989;1:997–998. [PubMed] [Google Scholar]
- 4.Khuroo MS, Zarger SA, Yattoo GN, et al. Ascariasis Indicated Acute Pancreatitis. Br J Surg. 1992;79:1335–8. doi: 10.1002/bjs.1800791231. [DOI] [PubMed] [Google Scholar]
- 5.Gomez NA, Leon CJ, Ortiz O. Ultrasound in the Diagnosis of Round Worms in Gallbladder and Common Bile Duct. Surg Endosc. 1993 Jul-Aug;7(4):339–42. doi: 10.1007/BF00725953. [DOI] [PubMed] [Google Scholar]
- 6.Khuroo MS, Zargar SA, Mahajan R, et al. Sonographic Appearances in Biliary Ascariasis. Gastroenterol. 1987;93:267–272. doi: 10.1016/0016-5085(87)91013-4. [DOI] [PubMed] [Google Scholar]
- 7.Schulmon A, Loxton TA, Heydenrych JJ, et al. Sonographic Diagnosis of Biliary Ascariasis. AJR. 1982;139:485–489. doi: 10.2214/ajr.139.3.485. [DOI] [PubMed] [Google Scholar]
- 8.Cerri GG, Leite GJ, Simoes JB, et al. Ultrasonographic Evaluation of Ascaris in Biliary tract. Radiology. 1983;146:753–754. doi: 10.1148/radiology.146.3.6828691. [DOI] [PubMed] [Google Scholar]
- 9.Kubaska 5M, Chew FS. Biliary Ascariasis. AJR. 1997;169:492. doi: 10.2214/ajr.169.2.9242760. [DOI] [PubMed] [Google Scholar]
- 10.Dasai Shobha. Biliary Ascariasis Sonographic Findings. AJR. 1995;164:767–768. doi: 10.2214/ajr.164.3.7863917. [DOI] [PubMed] [Google Scholar]
- 11.Sur A, Bhatia M, Chander BN, et al. Hepato Biliary Ascariasis. Ind j Radiolo image. 2002;2(2):221–225. [Google Scholar]
- 12.Khuroo MS, Zargar SA. Biliary ascariasis. Gastroenterology. 1985;88:418–423. [PubMed] [Google Scholar]
- 13.Khuroo MS. Ascariasis. Gastroenterol Clin North Am. 1996;25:553–577. doi: 10.1016/s0889-8553(05)70263-6. [DOI] [PubMed] [Google Scholar]
- 14.Schulmon A. Us Appearances of Intra and ExtraHepatic Biliary Ascariasis. Abdominal imaging. 1998;23:60–66. doi: 10.1007/s002619900286. [DOI] [PubMed] [Google Scholar]
- 15.Hamaloglu E. Biliary Ascariasis in 15 patients. Int Surg. 1992;77:77–79. [PubMed] [Google Scholar]
- 16.Filice C, Marchi L, Meloni C, et al. Ultra-sound in the Diagnosis of Gallbladder Ascariasis. Abdom Imaging. 1995;20:320–322. doi: 10.1007/BF00203363. [DOI] [PubMed] [Google Scholar]
- 17.Khuroo MS, Zargar SA, Yattoo GN, et al. Sonographic Findings in Gallbladder Ascariasis. J Clin Ultrasound. 1992;20:587–591. doi: 10.1002/jcu.1870200904. [DOI] [PubMed] [Google Scholar]
- 18.Aslam M, Dore SP, Verbanck JJ, et al. Ultrasonographic Diagnosis of Hepatobiliary Ascariasis. J Ultrasound Med. 1993;12:573–576. doi: 10.7863/jum.1993.12.10.573. [DOI] [PubMed] [Google Scholar]


