Posterior reversible encephalopathy syndrome (PRES) refers to a clinicoradiologic entity with characteristic features on neuroimaging and nonspecific symptoms comprising headache, confusion, visual disturbances and seizures.1,2 This syndrome may occur in diverse situations, including hypertensive encephalopathy, eclampsia, and the use of cytotoxic and immunosuppressive drugs. The lesions in PRES are thought to be due to vasogenic edema, predominate in the posterior cerebral hemispheres, and are reversible with appropriate management. We present the case of a young male with sickle cell disease (SCD) who had recurrent episodes of this syndrome in association with repeated priapism, sickle cell nephropathy and hypertension. Though the association of SCD, priapism, exchange transfusion and neurological events has been described previously,3 the occurrence of recurrent PRES in SCD preceded by episodes of priapism has not been reported before.
CASE
A 20-year-old male with SCD presented to our accident and emergency department with sudden onset of severe headache, blurred vision and confusion. There was no fever, vomiting, jaundice, rash or chest symptoms. He had had multiple episodes of priapism in the recent past and had been treated with exchange transfusion and intracorporal adrenaline. On examination, the patient was afebrile but drowsy. His pulse was 90/minute and blood pressure 220/110 mm Hg. He perceived moving objects at 2 to 3 feet and his pupils were normally reactive. He did not have papilledema or obvious cranial nerve palsies and was moving all his limbs. His deep tendon reflexes were normal, plantars were flexor and he had no meningeal signs. Laboratory investigations were normal except for (normal values in parenthesis) low serum albumin 20 g/L (35–50 g/L), serum creatinine 148 μmol/L (62–140 μmol/L) and blood urea 7.5 mmol/L (3–7 mmol/L). His hemoglobin was 11.1 g/dL (13–15.5 g/dL), platelet count 339 (140–440×109) and serum lactic dehydrogenase [LDH] was 152 u/L (135–225 u/L). The hemoglobin pattern by electrophoresis showed HbA 69.1%, HbA2 3.5%, HbS 17.7% and HbF 2.0%.
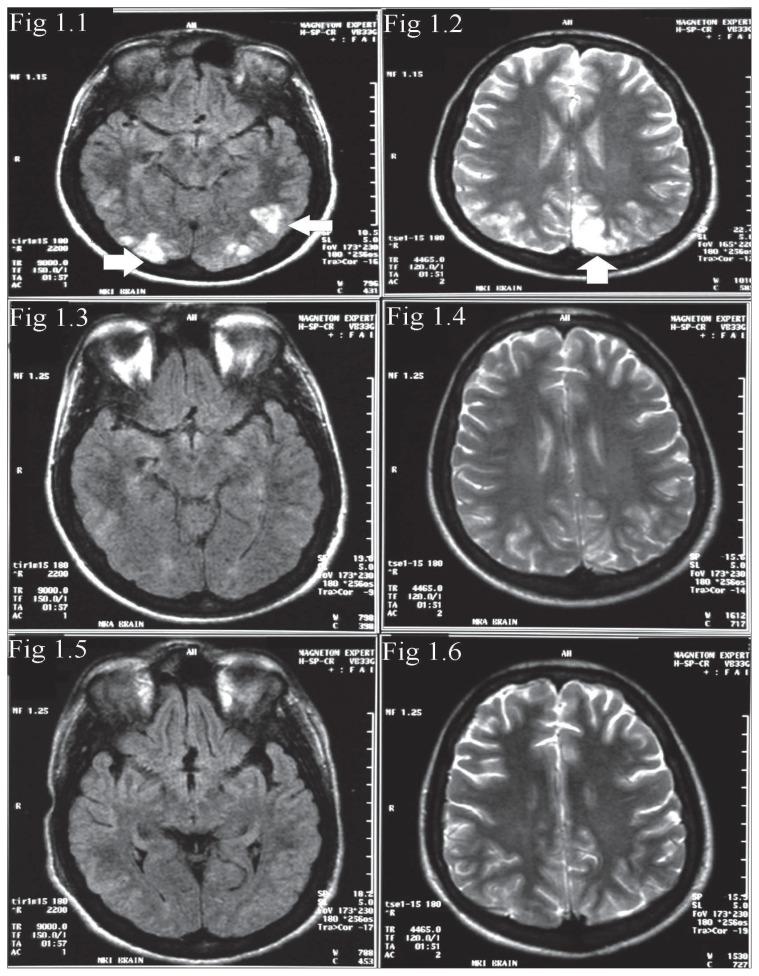
A plain CT scan of the brain showed small poorly defined hypodense areas in both occipital lobes involving the grey and white matter. On account of his sickle disease status and hypertension, a differential diagnosis of infarcts or PRES was entertained and MRI of the brain including MR venography [MRV] was done about six hours after the CT scan. The T2 and fluid attenuated inversion recovery [FLAIR] sequences showed multiple hyperintense lesions in the parietal and occipital lobes involving both grey and white matter (Figures 1.1 and 1.2). The rest of brain including the MRV was normal. Diffusion weighted imaging [DWI] was not available. A diagnosis of possible PRES was made. The patient was managed with lowering of blood pressure with captopril and nitroglycerin infusion. Investigation of his hypertension revealed that the patient had gross proteinuria. Urinary creatinine was 4042 μmol/L with serum creatinine 170 μmol/L (62–140 μmol/L), urine osmolality 358 mosm/kg (850–1000 mosm/kg), creatinine clearance 0.63 mL/sec (1.62–2.8 mL/sec) and 24-hour urine protein of 9.9 g/day (0.05–0.1 g/day). Ultrasound of the abdomen showed normal-sized kidneys with mildly echogenic cortex, suggesting renal parenchymal disease. The rest of abdomen was normal with no other cause for hypertension. The patient underwent renal biopsy and histopathology showed a membranoproliferative pattern of sickle cell nephropathy with marked tubulo-interstitial changes and normal blood vessels.
Figure 1.
FLAIR (1.1) and T2 (1.2) images of initial MRI study showing hyperintense lesions in the posterior temporal and occipital lobes. Follow-up MRI 2 weeks later (1.3 and 1.4) shows partial resolution of the lesions. Total resolution of the abnormalities is seen on MRI 6 weeks after the onset (1.5 and 1.6).
MRI of the brain repeated about two weeks after admission showed significant resolution of the previously identified lesions (Figures 1.3 and 1.4); at this time, the patient was much better and his hypertension was fully controlled on oral antihypertensives. He was not given exchange transfusion as his HbS was only 17.7% and he had received an exchange transfusion in the week preceding his admission for priapism. His nephropathy was managed conservatively. While in the hospital, the patient continued to have episodes of severe headache with periods of sudden loss of vision that recovered completely in a few hours. He also developed isolated episodes of generalized seizures, which were managed with phenytoin and phenobarbitone. At discharge, the patient was free of headaches and his vision was normal and he was advised to continue captopril, phenytoin and phenobarbitone.
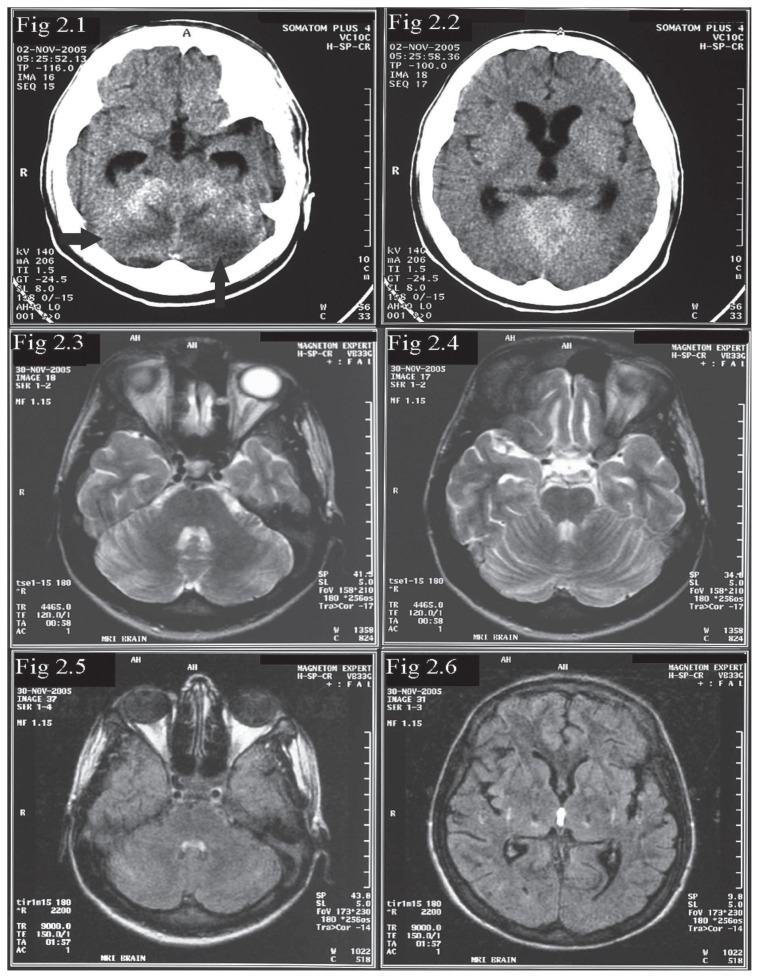
Six weeks after his first admission, the patient presented with status epilepticus and had to be intubated and treated with general anesthesia. BP at this presentation was 160/113 mm Hg. A CT of the brain showed new small areas of subtle hypodensity in cerebellum and left parietal region (images not shown). MRI of the brain was obtained a few days later, after treatment with multiple antihypertensives including intravenous labetolol infusion. This MRI showed total resolution of the lesions in the parietal and occipital lobes that were seen in the first MR study (Figures 1.5 and 1.6). The new lesions noted in the CT study were only seen as subtle, small hyperintensities in the left frontal and right cerebellar hemisphere (image not shown). The disappearance of the lesions in the parietal and occipital lobes was fully in keeping with a diagnosis of PRES. The patient had priapism twice while in the hospital and both episodes subsided spontaneously. About two weeks into his admission to the hospital, the patient had a sharp elevation of BP (224/112mm of Hg) and became very drowsy. A brain CT showed extensive cerebellar hypodensity with a mass effect, resulting in effacement of the fourth ventricle (Figures 2.1 and 2.2). He improved with lowering of BP. MRI of the brain obtained four weeks after the CT scan was normal (Figures 2.3 and 2.4). The disappearance of the cerebellar lesions with control of BP was once again consistent with PRES. This patient thus had recurrent PRES, with the first episode typically sited in the parietal and occipital lobes, the second episode presenting as status epilepticus with less pronounced changes in the cerebellum on imaging and the third episode producing marked edema in the cerebellum, causing hydrocephalus. All episodes responded to lowering of BP. At last review, BP was controlled, but the patient had mild cerebellar ataxia and could walk with support.
Figure 2.
Non-contrast CT (2.1 and 2.2) during third episode of PRES showing hypodensities in cerebellum and mild hydrocephalus due to mass effect on fourth ventricle. FLAIR and T2-weighted images of MRI 4 weeks later show resolution of the lesions and the hydrocephalus (Figure 2.3–2.6).
DISCUSSION
The terms posterior reversible leukoencephalopathy, reversible posterior cerebral edema syndrome, and posterior reversible encephalopathy syndrome refer to a clinicoradiologic entity characterized by headaches, altered mentation, visual disturbances, seizures, and transient changes in posterior cerebral hemispheres on neuroimaging. 1,2 This syndrome was first reported by Hinchey et al in 1996,1 who referred to the condition as reversible posterior leukoencephalopathy [RPLE] syndrome. Casey et al2 thought the term leukoencephalopathy was misleading, as there is usually no accompanying destructive process of the white matter. He proposed that the condition be referred to as PRES. Though the changes in a typical case of PRES are reversible, it can progress to ischemia, cerebral infarction and even death.4
PRES can occur in various situations. In patients with hypertensive encephalopathy and in pregnant patients with eclampsia, PRES is thought to occur after a subacute elevation in blood pressure.1,5 In the setting of uremia, hemolytic-uremic syndrome, and thrombotic thrombocytopenia purpura, endothelial toxins are thought to play a role in the development of PRES.1,4,6 Cyclosporine and tacrolimus are the drugs most frequently associated with the development of PRES, others being cisplatin, gemcitabine, and combination chemotherapy regimens.7 PRES has also been described with Mycobacterium avium intracellulare-related hypercalcemia in AIDS.8
The underlying pathophysiologic mechanism in uncomplicated PRES is thought to be vasogenic edema due to cerebrovascular autoregulatory dysfunction.1,2,9 The predilection for involvement of the posterior circulation territories results from the relatively sparse sympathetic innervation of the vertebrobasilar circulation. 4,10 In a healthy subject, brain perfusion is maintained by both myogenic and neurogenic mechanisms.5 In patients with PRES, the myogenic response is blunted by passive overdistention of the vessel due to elevations in BP or direct toxic effects on the endothelium.4,9 The cerebral autoregulatory mechanisms then become more dependent on the neurogenic response. The more poorly innervated areas in the posterior circulation are therefore more vulnerable to the leakage of fluid into the interstitium and vasogenic edema.4,5
In contrast to the nonspecific clinical findings of PRES, the MRI pattern is often characteristic and represents an essential component of the diagnosis.11 Typically, the lesions of PRES predominate in the parieto-occipital white matter, with some involvement of the overlying cortex and are hyperintense on T2-weighted and FLAIR images. In diffusion-weighted images, they are usually hypointense or isointense with an increase of the apparent diffusion coefficient [ADC], indicating vasogenic edema.2,10 Follow-up MRI after appropriate therapy for the cause of PRES shows resolution of the lesions, unless the condition progresses to infarction or hemorrhage. PRES lesions involving the occipital lobe spare the calcarine and paramedian occipital lobe. This feature, along with the predominant involvement of white matter helps to distinguish this syndrome from bilateral infarctions of the posterior cerebral artery.1 Though the lesions in PRES predominate in the territories supplied by the posterior circulation, other areas of the brain like the frontal and temporal lobes, cerebellum, thalamus and brainstem may also be involved.1,2,4,12 When regions of the brain other than the parieto-occipital lobes are predominantly involved, the syndrome can be called atypical. In such cases, a diffusion-weighted MRI with ADC mapping shows increased ADC values representing vasogenic edema in these areas, thus differentiating atypical PRES from other brain disorders that affect the same sites, causing cytotoxic edema with reduced ADC values.13 As we could not obtain a diffusion-weighted image, the diagnosis of PRES could only be confirmed in our patient by resolution of the changes on follow-up imaging.
Though SCD is common, the incidence of PRES is apparently low. A case series of children with SCD presenting with acute chest syndrome had three children with PRES, all of whom had high blood pressure recordings.14 To the best of our knowledge, the occurrence of recurrent PRES in SCD in conjunction with recurrent priapism and sickle glomerulopathy has not been described before. It lends credence to the observation of the clustering of neurological events in patients with SCD with priapism treated with exchange transfusion. ASPEN syndrome, an eponym for association of SCD, priapism, exchange transfusion and neurological events has been described previously. The neurological events are thought to be related to cerebral ischemia after an acute increase in total hemoglobin percentage, with a concomitant decrease in percent hemoglobin S and subsequent release of vasoactive substances during penile detumescence.3 About 5% to 18% of patients with SCD develop renal failure.15 Sickle cell glomerulopathy shows a picture of glomerular hypertrophy and focal glomerulosclerosis.16 Basement membrane lucencies and areas of apparent duplication are seen, which present a membranoproliferative glomerulonephritis (MPGN) like picture.17 There is an increased risk of multiorgan failure, including kidney failure in patients with SCD who have episodes of priapism, especially in those with postpubertal presentation.18 Our patient had repeated admissions for priapism prior to developing PRES. We also found that he had high blood pressure recordings during each of his presentations with priapism. Kato et al have noted that steady-state LDH elevation identifies a subset of patients with SCD at risk for pulmonary hypertension, cutaneous leg ulceration, priapism and early death.19 Our patient had normal LDH throughout his episodes of PRES and also during his admissions for priapism.
In summary, we have presented the case of a young male with SCD who developed recurrent PRES following frequent episodes of priapism and acute hypertension secondary to sickle nephropathy. This case highlights the need to expect neurological events in patients with SCD who present with recurrent priapism. It is imperative that the syndrome of PRES is correctly recognized on neuroimaging, as the condition is reversible and potential complications like cerebral infarction and death can be avoided with appropriate therapy.
REFERENCES
- 1.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 2.Casey SO, Sampaio RC, Michel E, et al. Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. Am J Neuroradiol. 2000;21:1199–1206. [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel JF, Rich MA, Brock WA. Association of sickle cell disease, priapism, exchange transfusion and neurological events: ASPEN syndrome. J Urol. 1993 Nov;150(5 Pt 1):1480–1482. doi: 10.1016/s0022-5347(17)35817-2. [DOI] [PubMed] [Google Scholar]
- 4.Covarrubias DJ, Luetmer PH, Campeau NG. Posterior Reversible Encephalopathy Syndrome: Prognostic Utility of Quantitative Diffusion-Weighted MR Images. Am J Neuroradiol. 2002;23:1038–1048. [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz RB, Feske SK, Polak JF, et al. Preeclampsia-eclampsia: clinical and neuroradiologic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217:317–376. doi: 10.1148/radiology.217.2.r00nv44371. [DOI] [PubMed] [Google Scholar]
- 6.Bakshi R, Shaikh ZA, Bates VE, Kinkel PR. Thrombotic thrombocytopenic purpura: brain CT and MR findings in 12 patients. Neurology. 1999;52:1285–1288. doi: 10.1212/wnl.52.6.1285. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert RM. Neurologic Complications. In: Abeloff, editor. Clinical Oncology. 3rd edition. Churchill Livingstone; 2004. p. 1224. [Google Scholar]
- 8.Choudhary M, Rose F. Posterior reversible encephalopathic syndrome due to severe hypercalcemia in AIDS. Scand J Infect Dis. 2005;37(6–7):524–526. doi: 10.1080/00365540510037984. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee P, McKinstry RC. Reversible posterior leukoencephalopathy syndrome: evaluation with diffusion-tensor MR imaging. Radiology. 2001;219:756–765. doi: 10.1148/radiology.219.3.r01jn48756. [DOI] [PubMed] [Google Scholar]
- 10.Edvinson L, Owman C, Sjoberg NO. Autonomic nerves, mast cells, and amine receptors in human brain vessels: a histochemical and pharmacological study. Brain Res. 1976;115:337–393. doi: 10.1016/0006-8993(76)90356-5. [DOI] [PubMed] [Google Scholar]
- 11.Lamy C, Oppenheim C, Méder JF, Mas JL. Neuroimaging in Posterior Reversible Encephalopathy Syndrome. J Neuroimaging. 2004;14(2):89–96. [PubMed] [Google Scholar]
- 12.Thambisetty M, Biousse V, Newman NJ. Hypertensive brainstem encephalopathy: clinical and radiographic features. J Neurol Sci. 2003;208(1–2):93–99. doi: 10.1016/s0022-510x(02)00379-9. [DOI] [PubMed] [Google Scholar]
- 13.Ahn KJ, You WJ, Jeong SL, et al. Atypical manifestations of reversible posterior leukoencephalopathy syndrome: findings on diffusion imaging and ADC mapping. Neuroradiology. 2004;46(12):978–983. doi: 10.1007/s00234-004-1276-1. [DOI] [PubMed] [Google Scholar]
- 14.Henderson JN, Noetzel MJ, McKinstry RC, White DA, Armstrong M, DeBaun MR. Reversible posterior leukoencephalopathy syndrome and silent cerebral infarcts are associated with severe acute chest syndrome in children with sickle cell disease. Blood. 2003;101(2):415–419. doi: 10.1182/blood-2002-04-1183. [DOI] [PubMed] [Google Scholar]
- 15.Scheinman JI. Sickle cell nephropathy. In: Holliday M, Barratt TM, Avner ED, editors. Pediatric Nephrology. Baltimore: Williams & Wilkins; 1994. pp. 908–919. [Google Scholar]
- 16.Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette C. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med. 1992;326:910–915. doi: 10.1056/NEJM199204023261402. [DOI] [PubMed] [Google Scholar]
- 17.Bakir AA, Hathiwala SC, Ainis H, Hryhorczuk, Rhee HL, Levy PS, Dunea G. Prognosis of nephrotic syndrome in sickle glomerulopathy. Am J Nephrol. 1987;7:110–115. doi: 10.1159/000167444. [DOI] [PubMed] [Google Scholar]
- 18.Sharpsteen JR, Powars D, Johnson C, Rogers ZR, Williams WD, Posch RJ. Multisystem damage associated with tricorporal priapism in sickle cell disease. Am J Med. 1993;94:289–295. doi: 10.1016/0002-9343(93)90061-s. [DOI] [PubMed] [Google Scholar]
- 19.Kato GJ, McGowan V, Machado RF, Little JA, et al. Lactate dehydrogenase as a biomarker of hemolysis - associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension and death in patients with sickle cell disease. Blood. 2006;107:2279–85. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]