Abstract
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders, which affects about 0.3% of the general population. As the population in the developed world ages, this creates an escalating burden on society both in economic terms and in quality of life for these patients and for the families that support them. Although currently available pharmacological or surgical treatments may significantly improve the quality of life of many patients with PD, these are symptomatic treatments that do not slow or stop the progressive course of the disease. Because motor impairments in PD largely result from loss of midbrain dopamine neurons in the substantia nigra pars compacta, PD has long been considered to be one of the most promising target diseases for cell-based therapy. Indeed, numerous clinical and preclinical studies using fetal cell transplantation have provided proof of concept that cell replacement therapy may be a viable therapeutic approach for PD. However, the use of human fetal cells as a standardized therapeutic regimen has been fraught with fundamental ethical, practical, and clinical issues, prompting scientists to explore alternative cell sources. Based on groundbreaking establishments of human embryonic stem cells and induced pluripotent stem cells, these human pluripotent stem cells have been the subject of extensive research, leading to tremendous advancement in our understanding of these novel classes of stem cells and promising great potential for regenerative medicine. In this review, we discuss the prospects and challenges of human pluripotent stem cell-based cell therapy for PD.
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease, and one of the most common movement disorders. The disease, characterized by both motor and non-motor symptoms, affects about 0.3% of the general population and ~1% of the population over the age of 60 (de Lau and Breteler, 2006). It is projected that this number will increase with the aging of the population in developed countries, and PD is currently considered to be a pandemic (Dorsey and Bloem, 2017). One of the hallmarks of PD is the loss of midbrain dopamine (mDA) neurons and, currently, dopamine (DA)-replacement therapy (e.g., L-dopa and/or DA agonists) is the gold standard and mainstay of medical therapy. Although pharmacological treatments can significantly improve the quality of life of many PD patients, the therapeutic window for achieving antiparkinsonian benefits without inducing unacceptable side effects, such as dyskinesia, shrinks over time in most patients (Kang and Fahn, 1988; Weiss et al., 1971).
Successful intervention for some symptoms can also be achieved surgically. In particular, deep brain stimulation has been well established as a nondestructive treatment (Miocinovic et al., 2013; Okun, 2012), but also carries surgical risk and is, like lesioning and pharmacological treatment, only palliative. Another potential non-ablative approach is the use of gene therapy, in which therapeutic genes (e.g., those encoding dopamine- or GABA-synthesizing enzymes or trophic factors) are stereotactically delivered into appropriate targets of patients’ brains using viral vectors so as to enter cells and produce the desired gene products locally and long-term (Bartus et al., 2014; Kaplitt et al., 1994; Kordower and Bjorklund, 2013; LeWitt et al., 2011). None of these established medical or surgical treatments, however, acts to prevent or replace the progressive loss of mDA neurons.
Despite extensive investigation, much of the pathological etiology of PD remains unknown, rendering the search for preventive and curative measures more difficult. There are two goals in the quest to move therapies for PD beyond purely symptomatic treatment: 1) early diagnosis and intervention to prevent or slow down the ongoing DA cell loss associated with the major motor symptoms of the disease, and 2) restoration and long-term recovery of this impaired motor function by replacing the missing cell population. Over the past several decades, research on both fronts has yielded substantial progress.
In 2006, Shinya Yamanaka and his colleagues reported their groundbreaking work on reprogramming terminally differentiated mouse somatic cells into early embryonic-like induced pluripotent stem cells (iPSCs) (Takahashi and Yamanaka, 2006). Subsequently, this and two additional groups demonstrated that human somatic tissues can be reprogrammed into human iPSCs (hiPSCs) using similar methods (Park et al., 2008; Takahashi et al., 2007; Yu et al., 2007). iPSC technology allows for efficient generation of patient- and disease-specific pluripotent stem cell lines which may then be differentiated into any cell type required. The iPSC approach can potentially address some of the major concerns of stem cell therapy, allowing production of sources of unlimited numbers of cells, reducing the immunogenicity of the implanted cells, and alleviating ethical concerns. It opens the door to the plausible use of iPSCs for personalized as well as generalized cell therapy.
The enormous progress in iPSC research over the last decade has been extensively discussed in a series of recent reviews (Hockemeyer and Jaenisch, 2016; Karagiannis and Eto, 2016; Li and Izpisua Belmonte, 2016; Li et al., 2015; Mertens et al., 2016; Scudellari, 2016; Takahashi and Yamanaka, 2016; Tapia and Scholer, 2016). However, as pointed out in a recent commentary (Scudellari, 2016), the major goals of most iPSC research have switched away from personalized cell therapy, and instead focus on mechanistic studies of human disease and development. In this review article, we will first discuss the conceptual background and the evidence for using cell replacement as a viable option to restore lost DA cell function. This discussion will be focused on the lessons that have been learned from clinical trials using fetal ventral mesencephalon (fVM) cell transplantation in PD patients, and on experimental studies using pluripotent stem cell (PSC) sources, including human embryonic stem cells (hESCs) and hiPSCs. We will address the concept of personalized cell replacement using iPSCs-derived DA cell populations as an autologous cell source for the treatment of PD (Figure 1). We will then outline the critical steps that must be taken to generate clinical grade pluripotent stem cells from these sources and, from them, to derive functional DA cell populations for transplantation. Finally, we will present a roadmap for personalized cell therapy by defining current obstacles that need to be overcome in order to bring this novel therapeutic approach from the lab bench to the clinic.
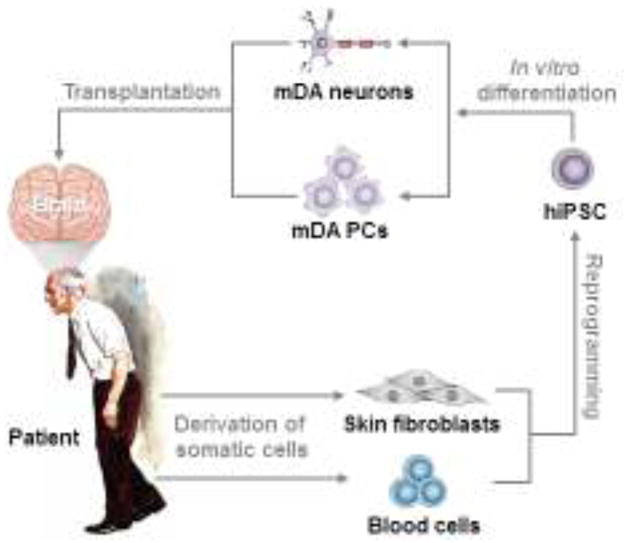
Figure 1.
Schematic overview of hiPSC-based personalized cell replacement therapy for PD. PD patient-derived somatic cells (e.g., skin fibroblasts or blood cells) are reprogrammed to autologous iPSCs, which are then differentiated to authentic mDA cell populations (e.g., mDA progenitor cells (PCs), mature mDA neurons, or their mixed populations) and transplanted to the PD patient’s brain.
2. PD as a promising target disease for cell therapy
The major symptoms of PD are resting tremor, rigidity, bradykinesia, and postural instability. The neuropathological hallmarks of PD are accumulation of Lewy bodies (Rocha et al., 2017; Wakabayashi et al., 2007) and the progressive loss of midbrain dopamine (mDA) neurons, primarily A9-type, in the substantia nigra pars compacta (SNpc) (Kalia and Lang, 2015). The resulting impairment of the nigrostriatal pathway is believed to be the direct cause of the major motor symptoms of the disease. However, PD is a multi-system disease that typically exhibits varying degrees of additional non-motor symptoms such as autonomic dysfunction, neuropsychiatric symptoms, and sleep disorders, which may precede the motor symptoms and some of which may be associated with degeneration and/or abnormalities beyond the loss of mDA neurons (Kalia and Lang, 2015). Clearly, transplantation of mDA cells is unlikely to ameliorate most of these non-motor symptoms and thus will not fully “cure” PD. Despite this limitation, PD remains one of the most promising target diseases for pluripotent stem cell-based therapy for the following reasons:
The selective loss of mDA neurons is by far the most prominent pathological feature of PD and is directly associated with all the major motor symptoms.
Many “proof of concept” studies have demonstrated that fVM cell transplantation carries the potential for significant and long-term recovery. Grafted cells re-innervate the striatum, restore DA neurotransmission and, in some patients, dramatically improve the motor behavioral defects associated with PD, effects lasting as long as two decades (Hagell et al., 1999; Kefalopoulou et al., 2014; Piccini et al., 1999).
There is some evidence suggesting that fVM transplantation can in fact improve some non-motor deficits in 6-OHDA lesioned rat models, including problems with visuospatial function and motivational processing, in addition to motor dysfunction (Lelos et al., 2012; Lelos et al., 2016).
Based on advancements in our understanding of developmental and molecular mechanisms of mDA neuronal differentiation, the generation of authentic, functional mDA neurons from hPSCs, including hESCs and hiPSCs, has been well established and has been extensively tested in diverse preclinical animal models.
Advances in reprogramming mechanisms and methodologies achieved over the last decade provide the tools to generate hiPSCs of the clinical grade required for human therapeutic use.
We will review these issues related to hPSC-based therapy for PD with an emphasis on the critical requirements of the clinical roadmap toward personalized cell therapy, in greater detail below.
3. Developing cell therapy approaches for PD
3.1. Fetal cell transplantation as early proof of concept studies - lessons and challenges
Over the past 40 years, a multitude of different cell populations has been used for cell transplantation in PD patients, including adrenal medullary, retinal, carotid body, and human and porcine fetal cells (Allan et al., 2010; Barker et al., 2015a; Bjorklund and Kordower, 2013; Lindvall, 2016). Among these efforts, fVM cell transplantation using aborted 6 to 9 weeks old human embryos evolved historically as the most promising approach (Figure 2). In fact, after the first pioneering clinical trial in Lund in 1987 (Lindvall et al., 1990; Mertens et al., 2016), multiple open-label studies worldwide (Sweden, England, Spain, USA, Mexico, Cuba, France, Belgium, Canada) showed dramatic improvement in some patients’ quality of life for up to two decades (Hagell et al., 1999; Kefalopoulou et al., 2014; Kordower et al., 2008; Li et al., 2008; Mendez et al., 2008; Piccini et al., 1999; Spencer et al., 1992). In addition, several studies have analyzed postmortem brains from transplanted patients and have shown engraftment of the fetal tissue with wide outgrowth and robust innervation of the host striatum by donor-derived DA neurons in the transplantation sites up to 24 years post treatment (Li et al., 2016). This demonstration of surviving and functioning cell grafts, together with the associated positive clinical outcomes, provided proof of concept for cell therapy as a viable option to treat PD. Based on these encouraging early results, two NIH-funded double blind, sham-controlled clinical trials were performed in the USA with 40 and 37 patients, respectively (Freed et al., 2001; Olanow et al., 2003). However, unlike the previous open-label trials, these sham-controlled studies did not show significant clinical benefit from fetal cell transplantation. These negative results may have been due to different clinical parameters (e.g., time point of grafting during disease stage), as well as diverse factors related to cell preparation and to the grafts themselves, such as low survival of transplanted cells, low percentage of DA neurons within the grafts, variable fetal cell preparation (i.e., fetus numbers and weeks post conception), non-uniform target sites (putamen or caudate, or both), and variable immune suppression regimens (Barker et al., 2015a; Bjorklund and Kordower, 2013; Freed et al., 2001; Lindvall, 2016; Olanow et al., 2003). Furthermore, 18 (32%) out of 56 total patients who received transplantation in these two studies developed graft-induced dyskinesia (Freed et al., 2001; Olanow et al., 2003). Though the concept of replacement of lost DA neurons by fVM was not invalidated, these unsatisfactory clinical outcomes significantly dampened the initial enthusiasm for this approach, resulting in a temporary moratorium on fVM transplantation in the early 2000s. To address the factors believed responsible for these problems, several funded teams and initiatives in Europe, USA, and Japan have since revived interest in this approach, and recently formed the G-FORCE PD consortium with the goal of developing a roadmap to clinical application (http://www.gforce-pd.com/) (Barker et al., 2015b). Within this effort, TRANSEURO is an ongoing European-funded initiative aiming to provide standardized protocols for fVM transplantation, including refined patient selection, optimized grafting, and a defined immunosuppression protocol. Results of this study are expected to be published in 2020 (http://www.transeuro.org.uk/) (Kirkeby et al., 2017b; Stoker et al., 2017). While this new trial may provide a standardized protocol for fVM transplantation with potentially better outcomes, the ethical and practical concerns attached to the use of human aborted tissue (usually 6 to 8 fetuses are required per patient) make this procedure unrealistic as a widely available clinical therapy (Bjorklund and Kordower, 2013; Brundin and Kordower, 2012; Buttery and Barker, 2014; Freed et al., 2001; Kirkeby et al., 2017b; Kordower et al., 1998; Kordower et al., 1996; Lindvall, 2013; Lindvall and Kokaia, 2010; Mendez et al., 2005; Olanow et al., 2003; Stoker et al., 2017).
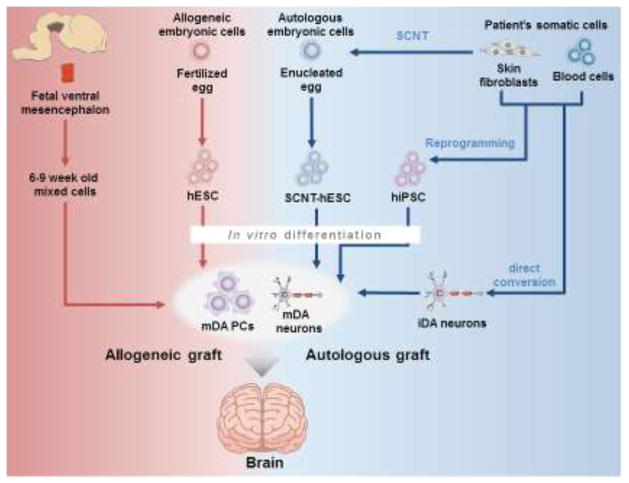
Figure 2.
Comparison of potential cell sources that can be used in cell replacement therapy for PD. The proof of concept of cell replacement therapy for PD was provided by implanting a heterogeneous population of fetal ventral mesencephalon (fVM) cells derived from 6 to 9 weeks old aborted embryos that consist of immature neural progenitors, neuroblasts, and neurons from which approximately 5% have the mDA phenotype. Their allogeneic characteristics makes the use of immune-suppressive regimen necessary. Pluripotent hESCs are derived from fertilized eggs or by somatic cell nuclear transfer (SCNT). In contrast, iPSCs can be derived from patient’s somatic tissues, allowing autologous cell transplantation after guided in vitro differentiation to mDA PCs and mDA neurons. Alternatively, autologous mDA cells can also be generated by direct conversion of somatic cells. These autologous cell grafts may avoid immunosuppression after transplantation. While autologous adult stem cells (see text for details) are potential cell sources, they are not included here because their potential to develop authentic mDA neurons are not clear. Autologous and allogeneic grafts are highlighted by blue and red lines, respectively.
An important discovery in postmortem tissue from patients that survived over two decades post fVM transplantation was the identification of α-synuclein-containing Lewy bodies in about 10%–15% of donor-derived DA neurons (Chu and Kordower, 2010; Kordower and Brundin, 2009; Kordower et al., 2008; Kurowska et al., 2011; Li et al., 2008; Li et al., 2010; Li et al., 2016), leading to the hypothesis that PD may be, at least in part, a prion-like disease with cell-to-cell transfer of α-synuclein (Angot et al., 2010; Chu and Kordower, 2015). Despite the development of this host pathological phenotype in some grafted cells, it appears that survival and function of the grafted cells is relatively unimpaired and that fetal grafts can indeed provide long-term therapeutic benefit.
Thus, past efforts to use cell therapy for PD have been associated with significant concerns and complications. In spite of this, lessons learned from these numerous fetal cell transplantation studies--in particular, the long-term and robust clinical improvements seen in some of the open-label studies, and the functional engraftment of the transplanted tissue--are sufficient to provide hope that improvements in cell replacement strategies for PD could yield tremendous positive impact on patients’ lives. Since limited and heterogeneous source tissue availability has been the most insurmountable obstacle in fetal cell transplantation, a standardized and unlimited cell source that is both safe and functionally efficacious would greatly facilitate cell therapy. Interestingly, a recent study has reported that human fetal VM cells can be expanded long-term using hypoxic conditions, although their clinical efficacy has not yet been tested (Moon et al., 2017).
One of the major concerns in cell transplantation for PD is the host immune response to the grafted tissue. Although the brain is often considered “immune-privileged”, there is in fact evidence that intracerebral immunologically-mediated graft rejection can and does occur (Barker and Widner, 2004; Sonntag, 2007). The use of an immunosuppressive regimen in experimental PD models is usually necessary to sustain long-term survival of allo- or xenografts. This issue, however, has not been systematically analyzed in any of the clinical trials to date (Wenker et al., 2016). Immune rejection in the brain has been a concern not only for graft survival, but also because of significant side effects of the immunosuppressive drugs used to suppress it. To circumvent immune rejection, some efforts have been undertaken to induce tolerance. For example, a recent study analyzed a role for graft-mediated immune repression through the T-cell modulator CTLA4-Ig in combination with systemic immunosuppression in an MPTP-induced monkey model of PD using porcine fVM from wild type or CTLA4-Ig transgenic animals (Aron Badin et al., 2016). The study concluded that, in this xenograft situation, graft CLTLA4-Ig-induced immune modulation alone could not prevent rejection, but that in combination with systemic immunosuppression it could protect the neural cell grafts. There have been other attempts to induce tolerance to tissue grafted into the brain, such as co-transplantation of porcine neuroblasts with syngeneic mesenchymal stem cells (MSCs) in the rat striatum (Leveque et al., 2015), or the use of immune-modulatory molecules (Uchida et al., 2001; Wennberg et al., 2001). The exact mechanisms of graft rejection in the brain are not well understood and may include both cellular and humoral immune responses (Cicchetti et al., 2003; Krystkowiak et al., 2007; Porfirio et al., 2015; Wenker et al., 2016). There is hope that strategies utilizing autologous cell sources, because they are derived from host tissue, will avoid the host immune response and, thus, obviate the need for immunosuppressive treatments altogether. Relevant to this concept are recent studies in primates showing that DA neurons derived from autologous iPSC, or from MHC-matched phenotypes, elicited only weak immune responses, while those from (fully) allogeneic iPSC caused a more significant immune reaction with diminished graft survival (Morizane et al., 2013; Morizane et al., 2017). In interpreting these results, caution is warranted (Kaneko and Yamanaka, 2013). In mouse models, several syngeneic iPSC-derived cell types, including endothelial cells, cardiomyocytes, and skin cells, are immunogenic to the adult autologous immune system and can be rejected when transplanted in sites that contain antigen-presenting cells (Liu et al., 2017). The immunogenicity of these iPSC-derived cells has been attributed to several possible factors, including residual epigenetic “memory” of the source cell type leading to the expression of abnormal or deregulated immunogenic proteins; somatic mutations; and fusion proteins with new immunogenic antigens. In terms of transplantation to the “immune-privileged” brain, one should keep in mind that immune privilege requires an intact blood brain barrier (BBB), which is locally disrupted by the surgical implantation of the graft. This disruption enables an interaction between the “sealed” environment of the brain and the immune system in the periphery, potentially changing the nature of the brain’s immune responses, at least in the immediate vicinity of the graft. Under these circumstances, it is plausible that autologous tissue sources that still carry some level of immunogenicity may trigger a rejection process, even after the BBB has been re-sealed (Barker and Widner, 2004; Sonntag, 2007).
3.2. ESC-derived DA cells as potential cell source
The problems attached to fVM cells and the critical lessons (both positive and negative) that have been learned from their clinical application have prompted investigators to explore alternative cell sources including diverse scalable stem cell types with the capacity to differentiate into multiple cell lineages.
The term “Stammzelle” (“stem cell”) was originally used in 1868 by a German physician and chemist, Ernst Haeckel, in describing the “ancestor unicellular organism from which all multicellular organisms evolve” (Ramalho-Santos and Willenbring, 2007). Our current usage is rather different, referring to progenitor cells from which the various fully differentiated tissue cells of a single mature multicellular organism develop. A wide range of such stem cell types has been identified and extensively characterized in vivo and in vitro. In particular, mouse ESCs (mESCs), which were first isolated and established in 1981 by Gail R. Martin, Martin J. Evans, and Matthew H. Kaufman, have revolutionized biological research and attracted particular interest due to their indefinite self-renewal and pluripotent differentiation potential, with the capability to generate any cell type of the body (Evans and Kaufman, 1981; Martin, 1981). Indeed, mESCs have been successfully used to generate unlimited cell populations with an A9-like mDA neuronal phenotype following optimized in vitro differentiation protocols. Two approaches have emerged as being most effective in accomplishing this goal: 1) a stepwise developmental protocol based on embryoid body (EB) formation followed by induction and expansion of neural progenitor cells (NPCs) and a final differentiation step to DA neurons (Lee et al., 2000); and 2) a co-culture based protocol, in which ESCs are differentiated on a layer of stromal cells with neural inductive properties, resulting in the generation of NPCs that can then be differentiated to mature DA neurons (Kawasaki et al., 2000). Over the years, these two prototype protocols have been used as a platform to test a multitude of factors (e.g., DA neuron-specific intrinsic transcription factors such as Nurr1 and Lmx1a, and extrinsic signals such as Sonic hedgehog (Shh) and Wnt1) in efforts to improve and refine DA neurogenesis (Bryja et al., 2006; Chung et al., 2009; Chung et al., 2002; Kim et al., 2006; Kim et al., 2002). Such studies have confirmed that the complex temporal and spatial sequences, as well as the regulation of factors and steps in early embryogenesis required to produce functional midbrain-specific DA neurons (Arenas et al., 2015; Tao and Zhang, 2016) can successfully be applied to in vitro differentiation of mESCs. Since mouse and human mDA neurons follow largely similar developmental steps and respond to similar factors, the knowledge gained from mESC differentiation studies can be readily translated to human PSCs, though some species-specific differences apply (Sonntag et al., 2005).
In 1998, James Thompson and colleagues isolated and established the first hESC lines (Thomson et al., 1998), opening a new era of PSC-based clinical regenerative medicine. The majority of knowledge obtained from mESCs was readily adapted to hESCs, leading to the development of several protocols for efficient production of mDA cell populations. Initially, the stromal co-culture method was widely used (Brederlau et al., 2006; Buytaert-Hoefen et al., 2004; Cooper et al., 2010; Iacovitti et al., 2007; Kim et al., 2010a; Ko et al., 2007; Morizane et al., 2010; Park et al., 2005; Perrier et al., 2004; Sonntag et al., 2007; Yan et al., 2005; Yang et al., 2008; Zeng et al., 2004), but other methods, including the production of neural cell suspensions, were also introduced (Ben-Hur et al., 2004; Cho et al., 2008; Doi et al., 2012; Schulz et al., 2004). As shown in Table 1, all these protocols utilized DA-specific morphogens and growth factors such as SHH, FGF8, BDNF, GDNF, and/or dbcAMP, and in addition to stromal cells, fetal midbrain astrocytes were also used in co-cultures to enhance mDA differentiation of hESCs (Roy et al., 2006; Song et al., 2017; Wagner et al., 1999). Though these studies successfully established multiple protocols for the production of mDA neurons, many issues remained, including inefficiency, inconsistent and incomplete DA differentiation, and occasional teratoma formation after transplantation into animal brains (Arenas, 2010). For these reasons, more recent efforts have focused on the identification of small molecules or peptides that can either activate or inhibit specific developmental pathways in order to achieve more optimal neural and/or mDA induction (Chambers et al., 2009; Fasano et al., 2010; Han et al., 2015; Hargus et al., 2010; Jaeger et al., 2011; Kirkeby et al., 2012; Li et al., 2011; Morizane et al., 2011). In particular, two regulatory loops, WNT1-LMX1A and SHH-FOXA2, have been shown to be critical in mDA neuron development (Chung et al., 2009; Joksimovic et al., 2009; Kittappa et al., 2007), and activation of these two pathways using morphogens or small molecules has proven critical for efficient generation of mDA neurons (Table 1).
Table 1.
Summary of differentiation and transplantation studies of hESCs
| Reference | Differentiation condition | In vitro Characteristics | In vivo Characteristics | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell line | Early stage | Maturation stage |
Cell fate | Functional analysis |
Animal model |
Graft innervation | TH+ neuron count/graft |
Behavior improvement |
|
| Perrier et al (2004) | H1, H9, HESC3 | Co-culture with stromal cell | B, AA, G, T, C | TH+/Tuj1+ (64~79%) | DA release Electrophysiology | N/A | N/A | N/A | N/A |
| Ben-Hur et al (2004) | HES1 | Noggin | Neurospheres | TH+/Tuj1+ (0.56%) | N/A | 6-OHDA rat | N/A | 389±83 | APO-IR; stepping and forelimb placing test |
| Buytaert-Hoefen et al (2004) | BG01 | Co-culture with astrocytes/PA6 | Co-culture with G | TH staining | N/A | N/A | N/A | N/A | N/A |
| Schulz et al (2004) | BG01, BG03 | EB, MedII, FGF2 | Neurobasal/B27 FCS, B, G |
TH+/Tuj1+ (75%) TH+/MAP2+ (64%) |
DA release Electrophysiology | 6-OHDA rat | N/A | N/A | N/A |
| Zeng et al (2004) | BG01, variant G01V | Co-culture with PA6 | Co-culture | TH+ (80~90%) | N/A | N/A | N/A | N/A | N/A |
| Park et al (2005) | SNU-hES3, HSF-6, Miz-hES-1 | Co-culture with PA6 and PA6-SHH/FGF8 | N2 or ITSA | TH+/Tuj1+ (41%) | DA release Electrophysiology | 6-OHDA rat | N/A | N/A | No improvement |
| Yan et al (2005) | H9, H1 | EB, SHH, FGF8 | B, AA, G, C | TH+ (30%) | DA release Electrophysiology | N/A | N/A | N/A | N/A |
| Roy et al (2006) | H9, H1 | EB, FGF2, FGF8, SHH, co-culture with hMAST | B, G, FBS | TH+ (54%) | DA release | 6-OHDA rat | N/A | 14,904 ~ 43,234/mm3 | AMP-IR, stepping test, cylinder test |
| Iacovitti et al (2007) | H9, BG01, HUES7, HUES8 | EB, Fibronectin, Noggin, bFGF | C | TH+/Tuj1+ (56~81%) | N/A | 6-OHDA rat | N/A | N/A | N/A |
| Ko et al (2007) | HSF-6, H9 | Co-culture with stromal cell | B, G, C | TH+/Tuj1+ (34~44%) | DA release | 6-OHDA rat | N/A | 201±86 | AMP-IR |
| Sonntag et al (2007) | H7, H9 | Co-culture with MS5-Wnt1, Noggin | B, AA, G, T, C | TH+ (10~25%) | N/A | 6-OHDA rat | N/A | 160±74 | AMP-IR |
| Cho et al (2008) | SNUhES1, SNUhES3, SNUhES16 | EB | Neurospheres, AA, SHH, FGF8 | TH+ (70~80%) En1+/TH+ (91%) DBH+/TH+ (4.1%) PMNT+/TH+ (4.4%) |
DA release Electrophysiology | 6-OHDA rat | N/A | 10,732±4,132 | APO-IR; AMP-IR; stepping test |
| Yang et al (2008) | H9 | SHH, FGF8 | B, AA, G, T, C | TH+/Tuj1+ (43%) | N/A | 6-OHDA rat | TH+/Synaptophysin+ fibers extended out of the graft. | 1,273±340 | AMP-IR |
| Chambers et al (2009) | WA-09 | Noggin and SB B, AA, SHH, FGF8 |
B, AA, G, T, C | TH+/Tuj1+ staining | N/A | N/A | N/A | N/A | N/A |
| Morizane et al (2010) | SA002 | Co-culture with PA6 | B, AA, G, C | TH+/Tuj1+ (17%) | DA release | N/A | N/A | N/A | N/A |
| Cooper et al (2010) | WA-09 | Noggin, RA, Wnt1, FGF8a, B, AA, SHH | B, AA, G, T, C | FOXA2+/TH+/Tuj1+ (5%) | N/A | N/A | N/A | N/A | N/A |
| Kim et al (2010) | H9, Miz-hES4, Miz-hES6, CHA-hES3, SNU-hES3 SNU-hES16 |
EB, SB, DM, SHH, FGF8 | B, AA, G | N/A | N/A | N/A | N/A | N/A | N/A |
| Kriks et al (2011) | H9, H1 | LDN, SB, SHH, FGF8, PMN, CHIR | B, AA, G, T, C, D | TH+ (70%) TH+/FOXA2+ (15~20%) |
DA release Electrophysiology | 6-OHDA mouse, 6-OHDA rat, MPTP monkey | hNCAM+ fibers extended to the non-transplanted brain side | >15,000 | AMP-IR, cylinder test, stepping test |
| Jaeger et al (2011) | H1, H7 | Smad inhibitor, PD0325901, FGF8b, SHH | B, AA, G, T, C | TH+ (25–60%) | Electrophysiology | N/A | N/A | N/A | N/A |
| Morizane et al (2011) | KhES1, 2, 3 | SB, DM, PMN, FGF8 | B, AA, G, C | TH+/Tuj1+ Staining | N/A | N/A | N/A | N/A | N/A |
| Doi et al (2012) | KhES1, 2 | Floating sphere with FGF2 (PA6 conditioned medium, SHH, FGF8) | B, G | TH+/Tuj1+ (12~35%) | DA release | MPTP monkey | N/A | 1.3–18.6×103 | Neurological scores, spontaneous movement |
| Xi et al (2012) | WA09 | SB, LDN, SHH, CHIR, FGF8b | B, AA, G, T, C | TH+ (44%) | Electrophysiology | N/A | N/A | N/A | N/A |
| Kirkeby et al (2012) | H9, SA121 | EB, Noggin, SB, SHH, CHIR | B, AA, G C or D |
FOXA2+/Lmx1a+ (80%) TH staining |
Electrophysiology | 6-OHDA rat | Graft innervated to CPu, Amy, Thal, SN, IC | N//A | AMP-IR, cylinder test |
| Sundberg et al. (2013) | H9 | SB, LDN, SHH, PMN, FGF8a, CHIR, Noggin, SAG, Wnt1, RA | B, AA, G, C, D | TH+ (>10~20%) | N/A | N/A | N/A | N/A | N/A |
| Grealish et al (2014) | H9 | EB, Noggin, SB, SHH and CHIR | B, AA, G, C, D | High co-expression of FOXA2+/Lmx1a+ | N/A | 6-OHDA rat | Graft innervated to CPu, FM, NAc, PL | >1000 | AMP-IR |
| Chen et al. (2016) | H9 | SB, DMH1, SHH, CHIR, SAG, FGF8b | B, AA, G, T, C, C-E | FOXA2+/Lmx1a+ (>80%) TH+ (60%) |
Electrophysiology | 6-OHDA mouse | hNCAM+ fibers innervated to CPu, NAc, Tu | 6,110±254 (61.1±1.0%) | AMP-IR, spontaneous rotation, cylinder test, rota-rod test |
| Kirkeby et al (2017a) | H9, RC17 | SB, Noggin, SHH, CHIR | B, AA, C, D, FGF8b | FOXA2+/Lmx1a+ (90.4±0.9%) | N/A | 6-OHDA rat | Graft innervated to CPu, PL cortex, midbrain | 3,716±1,026 per 100,000 cells grafted |
AMP-IR; cylinder test |
Abbreviations: AA, Ascorbic acid; AMP-IR, Amphetamine-induced rotation; Amy, Amygdala; APO-IR, Apomorphine-induced rotation; B, BDNF; C, Dibutyryl cAMP; C-E, Compound E; CHIR, CHIR99021; CPu, Caudate-putamen; D, DAPT; DM, Dorsomorphin; EB, Embryoid body; FM, Forceps minor; G, GDNF; hNCAM, Human neural cell adhesion molecule; IC, Internal capsule; ITSA, Insulin-Transferrin-Selenium-Sodium Pyruvate; LDN, LDN193189; N/A, Not available; NAc, Nucleus accumbens; PL, Prelimbic cortex; PMN, Purmorphamine; RA, Retinoic acid; SAG, Smo agonist; SB, SB431542; SHH, Sonic Hedgehog; SN, Substantia nigra; T, TGF-β3; Thal, Thalamus; Tu, Olfactory tubercle
Notes: A83-01, TGF β signaling inhibitor; C-E, Notch signaling inhibitor; CHIR, Wnt agonist; D, Notch signaling inhibitor; DM, BMP signaling inhibitor; DMH1, BMP signaling inhibitor; LDN, BMP signaling inhibitor; Noggin, BMP signaling inhibitor; PMN, Shh signaling activator; SB, TGF β signaling inhibitor; SAG, Shh signaling activator
An emerging theme from these recent studies has been that dual inhibition of SMADs (targeting BMP and TGFβ signaling), together with dual activation of WNT and SHH pathways, can facilitate robust mDA neuronal induction while suppressing alternate lineages. This results in highly efficient mDA differentiation protocols without the need for a co-culture system (Chen et al., 2016; Grealish et al., 2014; Kikuchi et al., 2017b; Kikuchi et al., 2011; Kirkeby et al., 2012; Kirkeby et al., 2017a; Kriks et al., 2011; Morizane et al., 2011; Samata et al., 2016; Samata et al., 2015; Sundberg et al., 2013; Xi et al., 2012). Some representative protocols are shown in Figure 3. These protocols successfully induce neurons that are positive for tyrosine hydroxylase (TH) (the rate-limiting enzyme for DA production) and that acquire typical mDA markers such as LMX1A, PITX3, FOXA2, NURR1, EN-1, as well as the DA transporter (DAT). Furthermore, many of these cells display a GIRK2-positive A9-type phenotype, although similar numbers of CALBINDIN-positive A10-type DA neurons are also produced. Importantly, these differentiated cells exhibit relevant electrophysiological properties and significantly improve motor behaviors in animal models of PD (Table 1). It is noteworthy that a recent study by Grealish et al. demonstrated that hESC-derived mDA neurons exhibit functional efficacy comparable to that of human fVM cells following transplantation into a rat model of PD, as evidenced by morphological, molecular, behavioral, and neuroimaging studies (Grealish et al., 2014). Taken together, this body of evidence provides strong preclinical support for the feasibility of using hESC-derived mDA neurons as a source for cell therapy in PD. However, there are two potential caveats limiting their application as an optimal therapeutic agent (Figure 2). First, the generation of hESCs requires fertilized eggs from donors and destruction of early embryos, which raises a plethora of ethical and legal concerns. Second, hESC-derived cell grafts are allogeneic to the recipient patients, making immune-suppressive regimens necessary. Despite these limitations, hESCs are currently the “gold standard” of hPSCs, and hESC-derived mDA cells are currently being developed for clinical trials in USA and Europe (Stoker et al., 2017; Tabar, 2016).
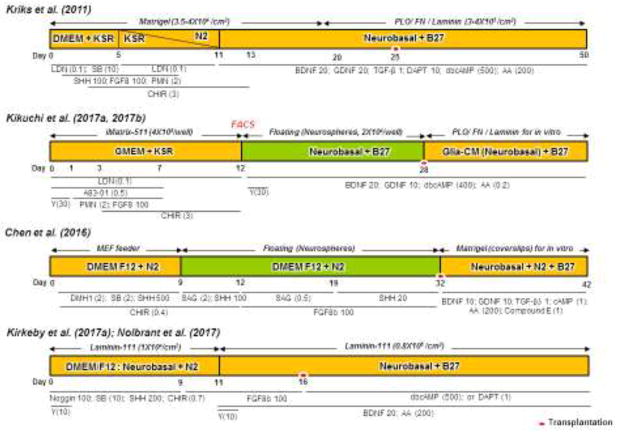
Figure 3.
Schematic diagrams of most representative in vitro differentiation protocols to generate mDA cells from hESCs and hiPSCs. Although these protocols show significant differences in specific chemicals, their concentrations, culture media and protocols, they commonly use chemicals/proteins for activation of the two regulatory loops (WNT1-LMX1A and SHH-FOXA2) and dual inhibition of SMADs (targeting BMP and TGFβ signaling). Kriks et al. induced direct differentiation of hESCs into mDA progenitors using dual-SMAD inhibitors (LDN and SB), SHH, FGF8, PMN and CHIR. On day 20, differentiating cells were dissociated by treatment with accutase and re-plated onto maturation media containing BDNF, GDNF, TGF-β3, DAPT, dbcAMP and AA (Kriks et al., 2011), in which cells were harvested at day 25 for transplantation. In contrast, Jun Takahashi and his colleagues used floating conditions, sorted cells at day 12 by Corin, and replated them in low cell adhesion 96 wells until Day28 for transplantation (Kikuchi et al., 2017a; Kikuchi et al., 2017b). Here, differentiation of hESCs/iPSCs was induced from day 1 to 12 using dual-SMAD inhibitors (LDN and A83-01), PMN, FGF8 and CHIR. The floating culture medium contained GDNF, BDNF, dbcAMP and AA. Next, Su-Chun Zhang and colleagues used direct differentiation at the starting point (D0-D8) and then cultured cells as floating aggregates until day 28 (Chen et al., 2016). Cells were cultured in neural induction condition with dual-SMAD inhibitors (DMH1 and SB), SHH and CHIR for induction of floor plate progenitors for 12 days. On day 8, cells were gently detached using a pipette and expanded as floating aggregates for 4 days in suspension condition. The spheres were triturated into smaller aggregates and differentiated in the presence of SAG, SHH and FGF8b until transplantation at day 32, when neurospheres were dissociated into single cells with accutase, and further differentiated in medium containing BDNF, GDNF, TGF-β3, cAMP, AA and compound E for in vitro study. Finally, Malin Parmar and colleagues induced hESCs differentiation using Noggin, SB, SHH and CHIR until day 9. Then, FGF8b was added until day 11 when cells were dissociated into single cells with accutase and replated with BDNF, FGF8b and AA until day 16 for transplantation. For terminal in vitro differentiation of the cells, dbcAMP and DAPT were added to medium from day 16 and onwards (Kirkeby et al., 2017a; Nolbrant et al., 2017). Numbers represent concentrations in ng/ml and those in parentheses represent concentrations in μM. Red dots represent the timing of cell transplantation. Yellow and green bars display adhesion and suspension (floating) culture conditions, respectively.
AA, Ascorbic acid; BDNF, Brain-derived neurotrophic factor; CHIR, CHIR99021; dbcAMP, Dibutyryl cyclic adenosine monophosphate; F, Fibronectin; FP, Floor plate; GDNF, Glial cell line-derived neurotrophic factor; KSR, knockout serum replacement; LDN, LDN193189; PLO, Polyornithine; PMN, Purmorphamine; SAG, Smo agonist; SB, SB431542; SHH, Sonic Hedgehog; TGF-β3, transforming growth factor beta 3; Y, Y-27632
One approach to circumventing the limitations of hESCs resulting from their allogenicity could be somatic cell nuclear transfer technology (SCNT), i.e., introduction of the nucleus of a somatic cell into an enucleated egg. This technology was developed in mammals in the late 1990s and became famous through the generation of the sheep “Dolly” (Campbell et al., 1996). Since then, the first SCNT-derived hESCs have been reported by Mitalipov and colleagues in 2013 (Tachibana et al., 2013), demonstrating another way to establish autologous hPSCs (Figure 2). However, despite its potential to generate therapeutic cells, such as mDA neurons (Amano et al., 2009; Wakayama et al., 2001), SCNT has also been associated with serious problems, mostly related to low efficiency (usually 1–5% of cloned embryos develop into normal animals) and abnormal phenotypes derived from incomplete nuclear reprogramming of the somatic cell nucleus by the oocyte (summarized in (Loi et al., 2016)). The significant practical and ethical issues associated with procurement of human oocytes for medical therapy would also undoubtedly create barriers similar to those for human embryonic sources.
3.3. iPSC-derived DA cells as potential cell source
Based on their groundbreaking study in 2006 showing that four transcription factors (i.e., Oct4, Sox2, Klf4 and c-Myc) could revert mouse fibroblasts into ESC-like iPSCs (Takahashi and Yamanaka, 2006), Shinya Yamanaka’s and two other groups subsequently reprogrammed human somatic tissues into hiPSCs using the same or similar sets of factors (Park et al., 2008; Takahashi et al., 2007; Yu et al., 2007). This iPSC technology offers the unprecedented possibility of generating disease- and patient-specific stem cells without destruction of early embryos. Thus, it revolutionized not only stem cell research aimed at studying human development and disease mechanisms, but also offered a new paradigm for personalized cell therapy by providing potentially unlimited autologous stem cell sources (Hockemeyer and Jaenisch, 2016; Karagiannis and Eto, 2016; Li and Izpisua Belmonte, 2016; Li et al., 2015; Mertens et al., 2016; Scudellari, 2016; Takahashi and Yamanaka, 2016; Tapia and Scholer, 2016; Trounson and DeWitt, 2016) (Figures 1 and 2). Indeed, patient-specific hiPSC-derived DA cells may circumvent many of the major issues associated with fetal cell transplantation: (1) ethical issues of using aborted tissue, (2) medical issues related to immune rejection and immunosuppression, and (3) practical issues of supply and availability. Despite this enormous potential, the iPSC technology is still in its early stages, and there are significant obstacles that must be overcome to make this new paradigm a clinical reality, as discussed below.
3.3.1. Identification and generation of clinical grade hiPSCs
The generation of hiPSCs involves two major processes: a.) delivery of vectors that encode reprogramming factors to target cells, and b.) forced expression of reprogramming factors. While the reprogramming factors have changed little over the years, there have been major developments in the delivery technologies. Initial reprogramming protocols used retroviral or lentiviral vectors, and while these virus-based hiPSCs have been useful for disease mechanism studies, they are considered less suitable for clinical application because integrated viral sequences result in multiple chromosomal disruptions, which could cause genetic dysfunction and/or tumor formation. Although there are currently no clinical data involving the use of virus-transduced iPSCs, a gene therapy approach to treat X-linked severe combined immunodeficiency with retrovirus-transduced hematopoietic cells resulted in leukemia through activation of the proto-oncogene LMO2 (Hacein-Bey-Abina et al., 2003a; Hacein-Bey-Abina et al., 2003b). In addition, reactivation or residual expression of reprogramming genes from integrated genomic sequences may trigger altered cell physiology and/or abnormal differentiation properties. For example, reactivation of c-Myc in iPSCs induced frequent tumor formation in chimeric mice (Okita et al., 2007). To avoid these fundamental problems, recent studies have attempted to generate hiPSCs using non-viral and/or non-integrating vectors such as adenovirus (Zhou and Freed, 2009), Sendai virus (Fusaki et al., 2009), temperature-sensitive Sendai virus (Ban et al., 2011), episomal vectors (Okita et al., 2011; Yu et al., 2009), RNAs (Warren et al., 2010), self-replicative RNAs (Yoshioka et al., 2013), miRNA (Miyoshi et al., 2011) or direct protein delivery methods (Kim et al., 2009). Of these, three methods--those using RNAs, Sendai virus, and episomal vectors--are among the most promising approaches, due to their reasonable efficiencies (Schlaeger et al., 2015). In addition to these “footprint-free” (i.e., without remaining genetic traces) reprogramming methods, recent studies have developed feeder-free and xeno-free culture conditions so that hiPSCs can be generated and maintained under good manufacturing practice (GMP)-compatible conditions that are required for clinical application (Oliveira et al., 2014; Silva et al., 2015; Wang et al., 2015). Taken together, progress made during the last decade has paved the way toward generating clinically viable hiPSCs. Nevertheless, the standardized definition of “clinical grade” hiPSCs (that is, of sufficient quality for FDA approval) remains ambiguous, and there are several remaining critical issues that need to be addressed to realize the full potential of hiPSCs in cell-based therapy, as described below.
3.3.2. Genomic stability and epigenetics
As hiPSCs-based therapy is a novel therapeutic paradigm for human diseases, it is critical to establish strict safety criteria for genomic integrity of the clinical cell product prior to clinical applications (Andrews et al., 2017; Daley, 2017; Peterson and Loring, 2014). It was due to problems in this regard that the second clinical trial of iPSC-based therapy in Japan was suspended after the identification of three copy-number variations (CNVs) and three single nucleotide variations (SNVs) (Garber, 2015; Mandai et al., 2017) although the first clinical trial turned out to be safe (Mandai et al., 2017). Mutations of various size – from karyotype abnormality to point mutations – have been found in hESCs and hiPSCs (reviewed in (Lund et al., 2012; Yoshihara et al., 2017b)). Putative factors contributing to mutations in hESCs and hiPSCs include: (1) pre-existing somatic mutations in parental cells (Abyzov et al., 2012; Cheng et al., 2012; Gore et al., 2011; Howden et al., 2011; Quinlan et al., 2011), (2) the reprogramming process itself (Gore et al., 2011; Ji et al., 2012; Sugiura et al., 2014; Yoshihara et al., 2017a), (3) passaging and cell culture (Gore et al., 2011; Liu et al., 2014), and (4) the differentiation process producing the target cell type. Several studies have reported that viral reprogramming methods cause significantly higher incidences of chromosomal variations than do non-viral methods (Kang et al., 2015; Park et al., 2014; Steichen et al., 2014). In contrast, another recent study suggested that the reprogramming process itself did not increase overall mutation rates (Kwon et al., 2017). Thus, a large-scale study using the most advanced methods is required to address these controversies and to compare systematically mutation rates across reprogramming methods. Different technologies have been used to screen genomic alterations of different sizes and their functional impacts. Whole genome and exome sequencing (WGS/WES) as well as RNA-seq using next-generation sequencing technology (NGS) provide the most comprehensive genome-wide coverage for diverse type of mutations and functional effects. For instance, using WGS, Loring and colleagues compared hiPSC lines prepared using three reprogramming methods: integrating retroviral vectors, non-integrating Sendai virus and synthetic mRNAs (Bhutani et al., 2016). Using sophisticated analytical approaches, 2 – 10 mutations per iPSC line were found in protein coding regions; however, the frequency of these mutations was not biased toward known cancer-causing mutations. Among the three reprogramming methods, there was no statistically significant difference in the number of mutations.
A critical question is whether such mutations have positive or negative effects on survival and expansion of hESCs and hiPSCs. Large CNVs often have negative impact on survival of cells harboring such genomic variants, thus late passage cells have fewer CNVs (Hussein et al., 2011). However, some mutations may have a positive effect on expansion leading to accumulated mutations in late passages (Gore et al., 2011; Laurent et al., 2011; Liu et al., 2014; Taapken et al., 2011). To this end, Eggan and colleagues screened 140 independent hESC lines, including 26 lines for potential clinical use, using WES to identify mutations (Merkle et al., 2017). Notably, five cell lines were found to carry six mosaic mutations in TP53 that may contribute to a selective advantage for survival and expansion, as allelic fraction increased with number of passages. More importantly, mutations in TP53 could result in functional effects such as coding changes in the DNA-binding domain of P53. As such a single copy deletion of chr17p13.1 harboring TP53 showed selective advantage to various stresses and differentiation capacity of hESC (Amir et al., 2017). Thus, screening for putative tumorigenic mutations at different stages of establishment, expansion, and differentiation of hESCs and hiPSCs will be mandatory for clinical usage (Trounson, 2017).
Finally, the epigenetic profile of iPSCs must be considered, because it can affect their transcriptional variation and differentiation into specific cell lineages. For example, it has been demonstrated that inactive X-chromosomes (Xi) in mouse somatic cells are reactivated during the reprogramming process (XCR). In contrast, hiPSCs appear to retain Xi during reprogramming and in their initial state, but exhibit XCR upon long-term culture, indicating a high incidence of epigenetic instability that may affect differentiation capacity (Andoh-Noda et al., 2017; Pasque and Plath, 2015). Many studies have demonstrated that iPSCs retain residual epigenetic signatures from their somatic tissue of origin, so-called epigenetic memory, which favors differentiation of iPSCs towards the same or similar cell lineages from which they are derived (Bar-Nur et al., 2011; Hargus et al., 2014; Hiler et al., 2015; Hu et al., 2010b; Kim et al., 2010b; Kim et al., 2011; Nazor et al., 2012; Ohi et al., 2011; Quattrocelli et al., 2015; Roost et al., 2017; Sanchez-Freire et al., 2014). In contrast, other studies have demonstrated that different genetic variations have a stronger influence on the differentiation properties of iPSCs than the donor cell types (Burrows et al., 2016; Kajiwara et al., 2012; Kyttala et al., 2016; Rouhani et al., 2014). In sum, the origin of the epigenetic variability and its influence on differentiation properties of iPSC lines remain controversial and appear to vary between the donor cell types and reprogramming methods (Ortmann and Vallier, 2017). Regardless of their origin, compromised genetic and/or epigenetic fidelity in hESCs and hiPSCs would constitute a significant risk factor(s) for cell therapy.
3.3.3. Generation of transplantable mDA progenitors from patient-derived hiPSCs
Despite the fact that hESCs are considered to be the “gold standard” for hPSCs-based cell differentiation, individual hESC lines are known to exhibit marked differences in differentiation properties (Osafune et al., 2008). Similarly, hiPSC lines have been shown to have substantial variability of differentiation potential (Ebert et al., 2009; Feng et al., 2010; Hu et al., 2010a). Thus, it is critical to understand whether hiPSC lines can be differentiated into functional mDA neurons as efficiently as well-characterized hESC lines (e.g., the H9 line), and whether the resulting mDA neurons exhibit the full potential to re-innervate the host brain and rescue motor behavior deficits in valid animal models of PD, as seen with fVM or hESC-derived cells. As summarized in Table 2, much research has addressed the in vitro differentiation of hiPSCs into mDA neurons, and results strongly indicate that hiPSC-derived mDA cells are functionally similar to those from hESCs (Cai et al., 2010; Chambers et al., 2009; Doi et al., 2014; Effenberg et al., 2015; Han et al., 2015; Hargus et al., 2010; Jiang et al., 2012; Kikuchi et al., 2017a; Kikuchi et al., 2017b; Kikuchi et al., 2011; Komatsu et al., 2015; Kriks et al., 2011; Ma et al., 2011; Morizane et al., 2011; Nolbrant et al., 2017; Rhee et al., 2011; Samata et al., 2016; Sanchez-Danes et al., 2012; Sundberg et al., 2013; Swistowski et al., 2010; Zhang et al., 2014). However, it should be pointed out that protocol development for hiPSC differentiation, and testing of the functional efficacy of these cells, is still an ongoing process. Although some protocols have been applied in clinical trials (discussed below), a fully optimized and standardized procedure for broader application has yet to be developed.
Table 2.
Summary of differentiation and transplantation studies of hiPSCs
| Reference | Differentiation condition | In vitro Characteristics | In vivo Characteristics | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell line | Early stage | Maturation stage |
Cell fate | Functional analysis |
Animal model |
Graft innervation |
TH+ neuron count/graft |
Behavior improvement |
|
| Chambers et al. (2009) | iPSC14, iPSC27 | Noggin, SB B, AA, SHH, FGF8 |
B, AA, G, T, C | TH+/Tuj1+ staining | N/A | N/A | N/A | N/A | N/A |
| Swistowski et al. (2010) | MR31, MMW2 | EB, SHH, FGF8 | B, AA, G, T, C | TH+ (30±5%) | N/A | 6-OHDA rat | N/A | 2,106±313/mm3 | AMP-IR |
| Cai et al. (2010) | IMR90-4 | EB, N2, B27 bFGF |
N2, B27, C | TH+ (6.5±1.4%) | DA release | 6-OHDA rat | N/A | N/A | No improvement |
| Hargus et al (2010) | A6, K1, S1, FF 17-5, FF 21-26 | Noggin, N2, SHH, FGF8, B, AA | B, AA, G, T, C | TH+ (3~9%) TH+/Tuj1+ (15~35%) |
N/A | 6-OHDA rat | Grafts send projections to the surrounding gray and white matter | 4,890±640 | AMP-IR |
| Kriks et al. (2011) | 2C6, SeV6 | LDN, SB, SHH, FGF8, PMN, CHIR | B, AA, G, T, C, D | TH+ (70%) TH+/FOXA2+ (15~20%) |
DA release Electrophysiology | 6-OHDA mouse 6-OHDA rat MPTP monkey |
hNCAM+ fibers extended to the non-transplanted brain side | >15,000 | AMP-IR, cylinder test, stepping test |
| Kikuchi et al. (2011) | 253G4 | DM, SB FGF8, SHH |
B, AA, G, C | TH+/Tuj1+ (85.46±3.13%) | DA release | MPTP monkey | N/A | 3.07×104 ~ 1.26×105 | Neurological score, time in movement |
| Morizane et al. (2011) | 201B6, 201B7, 253G1, 253G4 | SB, DM, PMN FGF8 |
B, AA, G, C | TH+/Tuj1+ Staining | N/A | N/A | N/A | N/A | N/A |
| Rhee et al. (2011) | IMR90-1, IMR90-4 Foreskin-1, SES8 Rv-hiPS |
EB, ITSA, bFGF, SHH, FGF8 | B, G, C | TH+/Tuj1+ (35~45%) | Electrophysiology DA release | 6-OHDA rat | N/A | 26,882±9089~54,418±23,660 | APO-IR |
| Jiang et al. (2012) | C001, C002, P001, P002 | B27, SB, bFGF, FGF8, SHH, AA, Wnt3a | FGF8, SHH, B, AA, G, T, C | TH+/FOXA2+ staining | Electrophysiology DA release | N/A | N/A | N/A | N/A |
| Sánchez-Danes et al. (2012) | Normal iPSC; PD iPSC; LRRK2 PD iPSC | EB, FGF2, N2/B27, FGF8, SHH | Co-culture with PA6, N2/B27 FGF8 and SHH | TH+/Tuj1+ (50~60%) | Electrophysiology DA release | N/A | N/A | N/A | N/A |
| Xi et al (2012) | IMR90-4 | SB, LDN, SHH, CHIR, FGF8b | B, AA, G, T, C | FOXA2+/TH+ (>90%) | Electrophysiology | N/A | N/A | N/A | N/A |
| Sundberg et al. (2013) | 2135 and 1815 | SB, LDN, SHH, PMN, FGF8a, CHIR, Noggin, SAG, Wnt1, RA | B, AA, G, C, D | TH+ (>10~20%) | N/A | N/A | N/A | N/A | N/A |
| Zhang et al. (2014) | Normal iPSC; PD iPSC | SB, LDN, SAG, FGF8b, PMN, CHIR | B, AA, G, T, C | TH+ staining | N/A | N/A | N/A | N/A | N/A |
| Doi et al. (2014) | 404C2, 836B3 | LDN, A83-01, PMN, FGF8, CHIR | B, AA, G, C | TH+ (20~45%) | DA release | 6-OHDA rat | N/A | 1,900±658 ~ 6,747±2,341 | AMP-IR |
| Han et al. (2015) | NCF-1, NCF-2, NCF-3, PD-1, PD-2 | Noggin, SB, SHH, B, A, FGF8 | B, AA, G, T, C | TH+ (33%) | N/A | 6-OHDA rat | N/A | N/A | APO-IR, rota-rod test |
| Komatsu et al. (2015) | 201B7, 253G1 | EB, SB, LDN, SHH, FGF8, PMN, CHIR | B, AA, G, T, C | TH+ (63±13%) | DA release | N/A | N/A | N/A | N/A |
| Effenberg et al. (2015) | hCBiPSCs | EB, FGF2, SHH, DM, SB, PMN, FGF8 | B, AA, G, T, C | TH+ (3.5~7.4%) | N/A | 6-OHDA rat | TH+ fiber outgrowth inside the grafts | 1,800±1,506 ~ 10,034±5,654 | N/A |
| Samata et al. (2016) | 1039A1 | A83-01, LDN, FGF8, PMN, CHIR | B, AA, G, C | TH+/FOXA2+ (>30%) after LRTM1+ sorting | DA release Electrophysiology | 6-OHDA rat | N/A | 44,777±72,03 | APO-IR, AMP-IR |
| MPTP monkey | Graft extended TH+ fibers into host brain | ~ 2.4 × 105 | N/A | ||||||
| Kikuchi et al. (2017a) | Normal iPSC PD iPSC |
A83-01, LDN, FGF8, PMN, CHIR | B, AA, G, C | FOXA2+/Tuj1+ (87.5±2.6%) Nurr1+ (16.4±3.8%) |
Electrophysiology | MPTP monkey | Graft extended TH+ fibers into host brain | 6.4±4.9 × 104 | Neurological score |
| Kikuchi et al. (2017b) | Normal iPSC PD iPSC |
A83-01, LDN, FGF8, PMN, CHIR | B, AA, G, C | FOXA2+ (95.3±1.6%) Nurr1+ (17.8±2.4%) |
Electrophysiology | 6-OHDA rat | Graft extended TH+ fibers into host brain | 227±94 ~ 267±51 | AMP-IR |
| Lehnen et al. (2017) | hFF-iPSC | EB, SB, LDN, CHIR, SHH, PMN | B, AA, G, C or D | FOXA2+>90% after IAP sorting | N/A | 6-OHDA rat | Graft extended TH+ fibers into host brain | 840~5200 | AMP-IR |
Abbreviations: AA, Ascorbic acid; AMP-IR, Amphetamine-induced rotation; APO-IR, Apomorphine-induced rotation; B, BDNF; C, Dibutyryl cAMP; C-E, Compound E; CHIR, CHIR99021; D, DAPT; DM, Dorsomorphin; EB, Embryoid body; G, GDNF; hNCAM, Human neural cell adhesion molecule; ITSA, Insulin-Transferrin-Selenium-Sodium Pyruvate; LDN, LDN193189; N/A, Not available; PMN, Purmorphamine; SAG, Smo agonist; SB, SB431542; SHH, Sonic Hedgehog; T, TGF-β3
To prepare a transplantable mDA cell source, it is critical to determine the optimal stage of in vitro differentiation at which cells should be harvested for implantation. This issue has been well-studied using mESC-derived mDA cells. While transplantation of small numbers of early stage (undifferentiated) mESCs resulted in spontaneous development of functional mDA neurons in vivo and significantly restored behavior in 6-OHDA lesioned rats, it could also cause teratoma formation (Bjorklund et al., 2002). In contrast, implantation of later stage, purified mDA neurons eliminated teratoma formation, but the grafts survived very poorly (Hedlund et al., 2008). This is consistent with several earlier studies also showing that fully differentiated mDA neurons generally survive poorly (Jonsson et al., 2009; Moon et al., 2013), and suggests that a “middle stage” (e.g., mDA progenitor cells) may represent a more optimal cell source than fully differentiated cells. In support of this notion, double fluorescence activated cell sorting (FACS) of mESC-differentiated mDA progenitors using the floor plate-specific surface marker CORIN and GFP gene expression driven by the Otx2 promoter provided a selected cell population that significantly rescued motor deficits in both rat and mouse models of PD without teratoma formation (Chung et al., 2011). As shown in Figure 3, recent representative studies have harvested hESCs/hiPSCs-derived mDA progenitor cells following in vitro differentiation for 16–32 days, when most of cell populations express mDA progenitor markers such as Lmx1a and FoxA2. Additionally, Takahashi and his colleagues recently showed that transplantation of hiPSC-derived mDA progenitors FACS-sorted by CORIN or LRTM1 significantly improved motor behavior in 6-OHDA lesioned rats, again without teratoma formation (Doi et al., 2014; Kikuchi et al., 2017a; Kikuchi et al., 2017b; Samata et al., 2016). Notably, CORIN-positive DA progenitors could be expanded in vitro to generate predominantly mDA neurons, with very few serotonergic neurons, making these cells an attractive cell source for potential clinical application. Indeed, this group’s recent studies demonstrated long term survival and function of CORIN-selected mDA progenitors derived from iPSCs from either healthy individuals or PD patients, and showed that these cells could ameliorate motor dysfunction both in a preclinical MPTP-lesioned monkey model (Kikuchi et al., 2017a) and in a 6-OHDA lesioned rat model (Kikuchi et al., 2017b), thereby further supporting the potential of iPSC-based autologous cell therapy. In addition, recent studies have demonstrated that FACS-sorting by surface markers can be used to select hPSC-derived neural cell populations for better expression of mDA markers after differentiation, and that these enriched populations can ameliorate motor function in rodent models of PD (Lehnen et al., 2017; Sundberg et al., 2013). Altogether, these studies suggest that FACS or magnetic-activated cell sorting (MACS) may be useful for the preparation of transplantable cell sources for clinical application.
Once a transplantable cell source is optimized and validated in animal models, technical issues related to cell preparation and handling, e.g., expandability, freeze/thaw stability, and preparation under GMP conditions prior to transplantation, must also be optimized. Notably, DA progenitors derived from both mouse and human pluripotent cells have been found to be expandable and able to undergo freezing and thawing without losing functionality (Chung et al., 2011; Wakeman et al., 2017).
3.3.4. Safety of hiPSC-derived cell grafts
Tumor (teratoma) formation is a feature of undifferentiated hPSCs and has been an issue for the safety of transplantation protocols. However, such tumors have not been reported in the hPSC-based protocols currently used in recent preclinical and clinical applications (e.g., see (Kikuchi et al., 2017a; Kikuchi et al., 2017b; Kirkeby et al., 2017a; Kriks et al., 2011; Mandai et al., 2017). Despite these encouraging results, regulatory bodies (like the FDA) still consider tumor formation a major concern that must be addressed prior to approval of protocols for clinical application. Therefore, for hiPSC-based cell therapy to become a clinically viable strategy, it is imperative to establish safe and effective mechanisms to remove from the transplantable cell population any residual undifferentiated cells that may retain the potential for teratoma formation. One possible approach is to use positive selection of authentic mDA cells by cell sorting, probably at the progenitor stage, as described above; but to completely avoid possible contamination with undifferentiated cells, other strategies may be necessary.
Toward this goal, two groups have identified small molecules that can selectively eliminate hPSCs while sparing differentiated cells. In the first approach, Ben-David et al. performed a high-throughput screen of >52,000 compounds and identified 15 that could selectively eliminate hPSCs while sparing most progenitors and differentiated cells (Ben-David et al., 2013). Interestingly, these compounds were found to inhibit oleic acid biosynthesis, showing the importance of lipid metabolism in hPSCs. Pre-treatment with one of these compounds prevented teratoma formation, suggesting that it could increase the safety of hPSC-based therapy. Using a different strategy, Lee et al. identified a unique profile of pro- and anti-apoptotic gene expression profiles in hPSCs and found that two anti-apoptotic genes, BIRC5 (encoding survivin) and BCL10, are selectively highly expressed in hPSCs (Lee et al., 2013). In addition, they identified small molecules (e.g., quercetin or YM155) that can selectively eliminate undifferentiated hPSCs by inhibiting survivin and thereby prevent tumor formation by inducing apoptosis of these cells (Lee et al., 2013). While effective against undifferentiated cells, quercetin and YM155 did not affect differentiated cell types including hiPSC-derived mDA neurons, allowing the possibility of direct application to hiPSC-based clinical cell therapy for PD. These small molecule-based methods may therefore be useful in meeting clinical safety standards established by the FDA and other regulatory agencies for hiPSC-based cell therapeutic approaches. In addition, a recent study reported that gamma irradiation reduces graft size and the number of hiPSC-derived early neural precursors, suggesting its potential preventive effect on tumor formation (Katsukawa et al., 2016).
Taken together, these findings suggest that tumor formation may be prevented by using the following approaches: 1) selection of high quality hiPSC line(s) with full pluripotent differentiation potential that do not contain any tumor-causing mutations; 2) removal of any undifferentiated cells by treatment with appropriate small molecules during in vitro differentiation; and/or 3) purification of functional mDA progenitors by sorting using appropriate surface marker(s).
3.3.5. hiPSC-derived DA cells from autologous versus allogeneic cell sources and other autologous cell sources
As described above, iPSC-based cell therapy for PD can address or circumvent many concerns that are attached to other stem cell-based approaches, including ethical, clinical, biological, and practical issues. Perhaps its greatest remaining obstacle, however, is the high cost incurred in generating hiPSC lines, validating the clones to be free of harmful mutation(s), and applying the rigorous requirements of GMP standards to the production of clinically transplantable mDA cells. This cost is especially high if personalized cell therapy requires an autologous cell source from each patient. To offset this economic burden, and to benefit a wider population of patients, the establishment of HLA-matching hESC or hiPSC line banks has been proposed (Bravery, 2015; Jacquet et al., 2013; Solomon et al., 2015). However, it still needs to be determined if the use of these major allogeneic HLA-matched hiPSCs can circumvent immune rejection sufficiently to avoid the need for long-term immunosuppression (Morizane et al., 2017). The number of such cell banks required will also vary with the HLA diversity of the potential patient population and may be more practical for certain countries and populations than for others.
As discussed above, a concern in allogeneic cell transplantation is the immune response to the grafted tissue, which could potentially be overcome with autologous grafts, as indicated by better survival of DA neurons derived from autologous iPSC when compared to allogeneic iPSC-generated neurons (Morizane et al., 2013). In addition to the potential of SCNT-ESCs as an autologous cell source for cell therapy, reprogramming technology has been further developed to convert somatic cells directly to different cell lineage(s) in vitro or even in vivo without the step of generating hiPSCs first, e.g., induced neurons (iNs) (Figure 2). Since the groundbreaking work of Vierbuchen et al. in 2010 (Vierbuchen et al., 2010), there has been a multitude of studies refining this technology and producing transdifferentiated autologous cells for transplantation, or directly reprogramming cells in the brain in situ (Tanabe et al., 2015). For example, using several combinations of reprogramming factors and defined culture conditions, direct conversion of human fibroblasts into human induced dopamine neurons (iDNs) that express typical mDA markers and improve motor behaviors in animal models of PD has been reported (Caiazzo et al., 2011; Dell’Anno et al., 2014). These cells can survive for several weeks to months once transplanted in rodent brains, exhibiting the capacity to integrate functionally into host neural circuits, and can support behavioral recovery in 6-OHDA treated animals. In addition, there has been recent progress in converting oligodendroglia, astrocytes, or transplanted fibroblasts in vivo directly into neurons (Grealish et al., 2016; Rivetti di Val Cervo et al., 2017; Yoo et al., 2017). However, while this direct conversion technology is intriguing and appears to have great potential, it is still in its infancy, and there are numerous obstacles that need to be overcome before its clinical potential might be realized. Among these are low conversion efficiencies, limited stability of the converted phenotypes, less potency and functionality when compared to cells that are derived from PSCs, and safety and efficacy of the reprogramming tools used in the conversion process both in vitro and in the human brain (Ang and Wernig, 2014; Kim, 2011; Mertens et al., 2016; Xu et al., 2015).
Finally, several attempts have been made to use autologous adult stem cells as a source for transplantation in PD, such as brain-derived progenitor cells (Bloch et al., 2014; Cave et al., 2014; Xu et al., 2013) or olfactory epithelial-derived progenitors (Wang et al., 2012), and MSCs. While brain progenitor cells can only be isolated through invasive brain surgery, MSCs are relatively easy to obtain, e.g., from muscle and bone marrow (BM) biopsies, or cord blood. These cells have been used in model systems of PD either in the naïve condition or after their modification, e.g., to deliver neurotrophic factors to support DA neuronal cell survival (Glavaski-Joksimovic et al., 2009; Glavaski-Joksimovic et al., 2010; Venkataramana et al., 2010), or after being first differentiated in vitro to DA neural cell populations (Bahat-Stroomza et al., 2009; Barzilay et al., 2009; Barzilay et al., 2008). While in some cases a beneficial effect on DA cell survival or functional rescue of motor deficits was observed, the overall success of translating adult stem cells to a cell therapy for PD has been limited. In fact, an open-labeled study to treat PD patients with autologous BM-derived MSCs did not demonstrate a conclusively beneficial therapeutic outcome (Venkataramana et al., 2010). In sum, research efforts on adult stem cell-based approaches for PD are ongoing, but there is currently no strong indication that these strategies hold clinically meaningful advantages over the use of hESC- or hiPSC-derived cells.
4. Clinical factors
If hPSC-based cell therapy is to become a feasible option for PD patients, much work will be needed to optimize this new therapy. Many of the factors that will determine the success of this approach will involve clinical considerations. The clinical protocols proposed up until this time have been based on fVM transplantation, and the focus has been to find alternative safe and effective tissue sources rather than to improve those protocols. Future work will need to consider the following issues:
PD is a disease with multiple etiologies and variable expression (Schapira and Jenner, 2011). Sporadic and familial (genetic) forms of the disease may differ in response to cell therapy (Schiesling et al., 2008). Design of initial clinical trials will therefore need to consider carefully the criteria of patient selection and how broadly applicable their results are intended to be. At present, the optimal age, stage and rate of progression of the disease for best clinical results from transplantation is unknown and needs to be further investigated (Piccini et al., 2005).
The degree to which the etiologic factors causing the disease may also affect the transplanted cells and the rate at which this may occur is not known. As discussed above, recent autopsy studies on clinically successful fVM-treated patients show that a significant proportion of the allogeneic graft showed the α-synuclein aggregation typical of the host disease (Li et al., 2016). A potential caveat of personalized cell therapy is that familial or sporadic PD patient-derived mDA cells may carry known and/or unknown genetic changes which will make the generated mDA cells more susceptible to the disease process.
The ideal implantation target has not yet been systematically determined. For fVM transplantation the primary chosen site was the striatum, which is the target of innervation of the A9 DA cells rather than their normal cell body location. This acknowledges the fact that the normal developmental environment in which fetal DA cells grow toward their targets is not present in adult tissue. It remains to be systematically studied whether hESC/hiPSC-derived cells may allow placement into locations such as the SNpc that could prove to be more effective.
Midbrain DA neurons include several different subtypes (for a recent review, see (Vogt Weisenhorn et al., 2016)). A9 DA cells are best correlated with, preferentially affected by, and lost in PD, and thus have been the obvious population to be replaced. A10 cells of the ventral tegmentum are, however, more resistant to degeneration and are largely spared in PD. At present, in vitro differentiation of hPSCs results in approximately equal ratios of A9 and A10 DA cells, even in the most optimized protocols (Figure 3). Accordingly, it is to be seen whether it is possible to purify A9 DA cells and whether such purified or enriched A9 DA cells may yield the better clinical result in transplantation.
Surgical technique and number of cells implanted may significantly affect clinical outcomes (Redmond et al., 2008). No single standardized technique currently exists for implantation based on fVM transplantation precedent, but avoidance of host tissue injury to decrease inflammatory and immune responses may be important (Wenker et al., 2016).
Of these issues, surgical aspects deserve some further consideration. It is somewhat remarkable that placing dopaminergic cell bodies into the striatum, where only their terminal processes are normally found, is effective at all. The choice of this target is related to its historical use in the days of adrenal medullary transplantation, where the transplanted tissue would not be expected to extend processes and where the volume of the implant was quite large (Lindvall et al., 1987). Some of these patients showed apparent clinical benefit (Goetz et al., 1990), enough to encourage the continued use of this target into the era of fetal tissue implantation, though the results of the adrenal work were later cast in doubt (Ahlskog et al., 1990). The normal location of the A9 DA cell bodies in the SNpc is a small, deep and surgically difficult target. Higher complication rates might be expected with surgical implantation into this region of cell volumes sufficient to show clinical efficacy, and the risk of damage to the remaining active DA cell population is a major concern. Additionally, it is not clear that in the adult brain, the necessary signals are present to guide outgrowth of axons from the transplanted cells to their basal gangliar targets. It is important, therefore, to recognize that the cells placed into the striatum are deprived of their normal environment and presynaptic input. How this may relate to limitations of this therapeutic strategy is not understood. Refinement of surgical technique to reduce trauma and minimize implanted volume may, in the future, allow a more physiological placement of the transplanted cells. Finally, the use of autologous iPSC-derived cells may eventually allow in vitro correction of genetic alterations that have led to the host disease state through gene editing approaches (e.g., CRISPR/Cas9), thus opening an entirely new approach to therapy (Yang et al., 2014). This approach will be particularly important for early-onset and more aggressive familial forms of PD.
5. Future road map to clinical transplantation
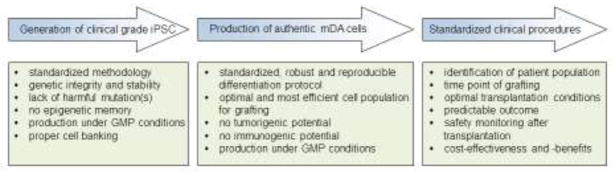
To make iPSC-based personalized cell therapy a clinical reality for PD, several goals must be reached (Figure 4):
Figure 4.
Steps of the clinical roadmap towards iPSC-based personalized cell replacement for PD. Detailed information is provided in the text.
Clinical grade iPSCs must be readily producible. This requirement is probably the most assured, as recent technical improvements have provided the necessary tools and methodologies to establish reproducibly such clinical grade iPSCs. However, use of hESCs, established hiPSC lines, or autologous cells from affected patients all hold advantages and disadvantages and there is currently no agreement on whether any of these approaches provides long term outcomes superior to the others. Nevertheless, it is noteworthy that non-viral, footprint-free reprogramming methods are generally less efficient than viral methods, and that individual hiPSC clones may exhibit incomplete reprogramming and, thus, significant clonal variations in their differentiation propensity. Therefore, it is critical to understand better the molecular and epigenetic mechanisms underlying human induced pluripotency for better reprogramming technologies (Ortmann and Vallier, 2017). For instance, it is now well established that hPSCs, like cancer cells, rely metabolically on glycolysis rather than on mitochondrial oxidative phosphorylation (Ito and Suda, 2014). Elucidation of mechanisms underlying this metabolic reprogramming (so-called Warburg-like effect) may lead to more efficient reprogramming methods to generate clinical grade iPSCs. Thus, identification of specific microRNAs governing metabolic reprogramming may be useful for better reprogramming methods (Cha et al., 2017). In addition, novel methodologies have been developed to characterize and select iPSC clones for their best quality and utility, e.g., the use of reference maps or programs aimed to determine the genome-wide DNA methylome and transcriptional profiles of isogeneic iPSC clones in reference to their parent cell type, so as to isolate those that are the least influenced by the reprogramming process and retain unbiased pluripotent differentiation properties (Bhutani et al., 2016; Bock et al., 2011; Ortmann and Vallier, 2017; Rouhani et al., 2014; Shutova et al., 2016; Tobin and Kim, 2012). Although PD is a chronic disease, generation and validation of clinical grade iPSCs with efficient differentiation potential to the mDA phenotype must be achieved in a reasonable time frame that is practical for clinical therapy.
The protocol for the production of authentic and functional mDA cells derived from hPSCs must be sufficiently streamlined so as to make such cells readily available for elective clinical use. Despite enormous progress on this issue, there is currently no standardized protocol available for broad application that meets FDA safety and regulatory criteria and fulfills all the requirements for translation to clinical use. Given that hiPSC lines are known to have more variable differentiation potential than do hESC lines (Ebert et al., 2009; Feng et al., 2010; Hu et al., 2010a), it will be critical to develop a robust and reproducible method that can be applied universally to diverse hiPSC lines to permit efficient mDA differentiation protocol(s), as examined by both in vitro properties (e.g., mDA marker expression and DA release) and in vivo function (e.g., axonal outgrowth, re-innervation to striatum, and robust behavioral recovery in animal models). At present, in vitro differentiation protocols have been developed mostly using hESC lines, such as the H9 line, which is currently being used in a study funded by the New York Stem Cell Institute in the laboratory of Dr. Lorenz Studer at Sloan Kettering Institute, NY. This study aims to produce transplantable mDA progenitors and has set a goal of applying for FDA approval and clinical testing in the near future (Tabar, 2016). In addition, several other groups in the US, Australia, and China, are either planning or currently performing clinical trials of transplantation of neural progenitor cells derived from hESCs, parthenogenetic hESCs, or hiPSCs (Barker et al., 2016; Barker et al., 2017; Cyranoski, 2017) (also see; https://www.nature.com/news/reprogrammed-cells-relieve-parkinson-s-symptoms-in-trials-1.22531). However, aside from public announcement in press releases or commentaries, and frequent discussion in social media, to date there is little to no information on these trials available in the scientific literature. Without such information, it is prudent to exercise caution in making any predictions based on these efforts and their implications for broader applicability (Barker et al., 2016).
As discussed above, the clinical outcomes of fVM transplantation have historically been variable and suboptimal, which may be related to inefficient generation and survival of mDA neurons in host brains, to their location or uneven anatomic distribution in the host striatum, or to other factors as yet not understood. A key problem appears to be survival of sufficient numbers of mDA neurons within the grafted tissue, as evidenced by postmortem brain analyses indicating unsurprisingly that better clinical outcome correlated with higher proportions of TH-positive neurons in the grafts. Thus, generation and survival of functional mDA neurons following transplantation requires further optimization. A recent report identifying a specific set of caudal midbrain markers correlated with high mDA yield following transplantation may be a promising approach (Kirkeby et al., 2017a).
A standardized clinical protocol for patient selection, screening, surgery and follow-up monitoring should be established. Initially, this protocol will be based on the clinical experience with human fVM transplantation. Although animal models can provide valid information, ultimate therapeutic success will only be measurable from clinical trials. Along this line, the TRANSEURO initiative is expected to provide a better and more advanced framework for future clinical use of iPSC-derived cells (http://www.transeuro.org.uk/) (Stoker et al., 2017).
Finally, a supportive political and socio-economical environment is of paramount importance. The expected high costs of developing cell replacement therapy for PD initially, and especially of a personalized approach using autologous cell sources, will require devotion of substantial resources. At present, development of the mDA cell differentiation protocol and its testing in animal models have been accomplished mainly in academic institutions and have relied heavily on federal or private funding. Allocation of public and commercial resources will be pivotal in order to move this work into the realm of clinical applicability. In this context, it is fortunate that based on the history of similar clinical endeavors in personalized medicine, the cost for autologous cell therapy will most likely continue to decrease. For instance, the costs for whole genome sequencing were initially millions of dollars per person, a figure now reduced to a few thousand dollars (https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/). This enormous speed of technological advancement not only instills hope for a similar trend in iPSC technology, but also directly influences the genome validation cost of iPSC clones and the costs for future automated cGMP-based cell production. Based on this, we are optimistic that the costs of hESC-, established hiPSC-, and autologous iPSC-based cell therapy will all become increasingly affordable for patients, thus rendering access to hiPSC-based personalized cell therapy for PD a clinical reality in the near future.
6. Perspective
In particular, is autologous iPSC-based, personalized cell replacement therapy for PD a feasible therapeutic option? As described in this review, we are cautiously optimistic that the answer is yes. While there are several potential paths toward this goal (Figure 2), we conclude based on technical robustness and efficacy, ethical and legal concerns, immunogenicity, feasibility and the current standing of technological development, that personalized autologous iPSC-based cell therapy holds particular promise for more effective treatment of this disease. Recently, Mandai et al. (Mandai et al., 2017) reported the first case in which a patient with age-related macular degeneration was treated by transplantation of a sheet of retinal pigment epithelial cells derived from autologous iPSCs. At 1 year after surgery, there was no sign of any adverse effect, and the patient’s acuity of the treated eye was stabilized, strongly supporting the feasibility of autologous iPSC-based cell therapy.
There remain uncertainties as to the clinical readiness, applicability and potential outcomes of this approach. Given that PD is a systemic and complex disease with a high degree of individual variability in pathology and progression, iPSC-based cell therapy may also best be thought of as one new and effective weapon in what is likely to remain a large armamentarium. Transplanted mDA cells can neither fully reconstruct the damaged or lost nigrostriatal circuit, nor be expected to ameliorate all of the non-motor symptoms associated with the disease. Yet we believe that it is reasonable to expect that iPSC-based cell therapy, by addressing the etiology underlying the major motor symptoms of the disease, will represent a milestone in therapy for Parkinson’s disease. In this regard, the collaborative work of the G-FORCE PD consortium is aimed at providing a roadmap for clinical application of these techniques in the near future, and several clinical trials are already ongoing or are planned to start before the end of this decade (Barker et al., 2017; Barker et al., 2015b). We envision a future in which a triad of approaches: symptomatic treatments including pharmaceuticals and deep brain stimulation; novel neuroprotective regimens that slow the course of the disease; and cell replacement therapy using hESC, established hiPSC, or autologous iPSC-derived mDA neurons, join together in the battle against this debilitating disease.
Highlights.
Discussing the prospects and challenges of human pluripotent stem cell-based cell therapy for PD.
Revisiting the concept of personalized cell replacement therapy for Parkinson’s disease using iPSCs-derived DA cell populations as an autologous cell source.
Lessons learned from clinical trials using fetal ventral mesencephalon cell transplantation.
Lessons learned from on-going pre-clinical studies using human embryonic stem cells.
Lessons learned from on-going pre-clinical studies using human induced pluripotent stem cells.
Outlining the critical steps required to generate clinical grade iPSCs and derivation of functional DA cell populations for transplantation.
Clinical roadmap for personalized cell therapy identifying obstacles that must be overcome.
Acknowledgments
The authors’ research was in part supported by NIH grants (NS084869 and NS070577). The authors are grateful to Dr. Olle Lindvall for his advice and suggestions. We also thank members of the Molecular Neurobiology Laboratory for their discussion and suggestions.
Abbreviations
- PD
Parkinson’s disease
- iPSCs
induced pluripotent stem cells
- hiPSCs
human induced pluripotent stem cells
- PSCs
pluripotent stem cells
- DA
dopamine
- mDA
midbrain dopamine
- fVM
fetal ventral mesencephalon
- hESCs
human embryonic stem cells
- SNpc
substantia nigra pars compacta
- EB
embryoid body
- NPCs
neural progenitor cells
- Shh
Sonic hedgehog
- SCNT
somatic cell nuclear transfer
- CNVs
copy-number variations
- SNVs
single nucleotide variations
- iNs
induced neurons
- iDNs
induced dopamine neurons
- PCs
progenitor cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abyzov A, Mariani J, Palejev D, Zhang Y, Haney MS, Tomasini L, Ferrandino AF, Rosenberg Belmaker LA, Szekely A, Wilson M, et al. Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature. 2012;492:438–442. doi: 10.1038/nature11629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlskog JE, Kelly PJ, van Heerden JA, Stoddard SL, Tyce GM, Windebank AJ, Bailey PA, Bell GN, Blexrud MD, Carmichael SW. Adrenal medullary transplantation into the brain for treatment of Parkinson’s disease: clinical outcome and neurochemical studies. Mayo Clin Proc. 1990;65:305–328. doi: 10.1016/s0025-6196(12)62532-4. [DOI] [PubMed] [Google Scholar]
- Allan LE, Petit GH, Brundin P. Cell transplantation in Parkinson’s disease: problems and perspectives. Curr Opin Neurol. 2010;23:426–432. doi: 10.1097/WCO.0b013e32833b1f62. [DOI] [PubMed] [Google Scholar]
- Amano T, Papanikolaou T, Sung LY, Lennington J, Conover J, Yang X. Nuclear transfer embryonic stem cells provide an in vitro culture model for Parkinson’s disease. Cloning Stem Cells. 2009;11:77–88. doi: 10.1089/clo.2008.0059. [DOI] [PubMed] [Google Scholar]
- Amir H, Touboul T, Sabatini K, Chhabra D, Garitaonandia I, Loring JF, Morey R, Laurent LC. Spontaneous Single-Copy Loss of TP53 in Human Embryonic Stem Cells Markedly Increases Cell Proliferation and Survival. Stem Cells. 2017;35:872–885. doi: 10.1002/stem.2550. [DOI] [PubMed] [Google Scholar]
- Andoh-Noda T, Akamatsu W, Miyake K, Kobayashi T, Ohyama M, Kurosawa H, Kubota T, Okano H. Differential X Chromosome Inactivation Patterns during the Propagation of Human Induced Pluripotent Stem Cells. Keio J Med. 2017;66:1–8. doi: 10.2302/kjm.2016-0015-OA. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Ben-David U, Benvenisty N, Coffey P, Eggan K, Knowles BB, Nagy A, Pera M, Reubinoff B, Rugg-Gunn PJ, et al. Assessing the Safety of Human Pluripotent Stem Cells and Their Derivatives for Clinical Applications. Stem Cell Reports. 2017;9:1–4. doi: 10.1016/j.stemcr.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang CE, Wernig M. Induced neuronal reprogramming. J Comp Neurol. 2014;522:2877–2886. doi: 10.1002/cne.23620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angot E, Steiner JA, Hansen C, Li JY, Brundin P. Are synucleinopathies prion-like disorders? Lancet Neurol. 2010;9:1128–1138. doi: 10.1016/S1474-4422(10)70213-1. [DOI] [PubMed] [Google Scholar]
- Arenas E. Towards stem cell replacement therapies for Parkinson’s disease. Biochem Biophys Res Commun. 2010;396:152–156. doi: 10.1016/j.bbrc.2010.04.037. [DOI] [PubMed] [Google Scholar]
- Arenas E, Denham M, Villaescusa JC. How to make a midbrain dopaminergic neuron. Development. 2015;142:1918–1936. doi: 10.1242/dev.097394. [DOI] [PubMed] [Google Scholar]
- Aron Badin R, Vadori M, Vanhove B, Nerriere-Daguin V, Naveilhan P, Neveu I, Jan C, Leveque X, Venturi E, Mermillod P, et al. Cell Therapy for Parkinson’s Disease: A Translational Approach to Assess the Role of Local and Systemic Immunosuppression. Am J Transplant. 2016;16:2016–2029. doi: 10.1111/ajt.13704. [DOI] [PubMed] [Google Scholar]
- Bahat-Stroomza M, Barhum Y, Levy YS, Karpov O, Bulvik S, Melamed E, Offen D. Induction of adult human bone marrow mesenchymal stromal cells into functional astrocyte-like cells: potential for restorative treatment in Parkinson’s disease. J Mol Neurosci. 2009;39:199–210. doi: 10.1007/s12031-008-9166-3. [DOI] [PubMed] [Google Scholar]
- Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, Shikamura M, Takada N, Inoue M, Hasegawa M, Kawamata S, et al. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc Natl Acad Sci U S A. 2011;108:14234–14239. doi: 10.1073/pnas.1103509108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Barker RA, Drouin-Ouellet J, Parmar M. Cell-based therapies for Parkinson disease-past insights and future potential. Nat Rev Neurol. 2015a;11:492–503. doi: 10.1038/nrneurol.2015.123. [DOI] [PubMed] [Google Scholar]
- Barker RA, Parmar M, Kirkeby A, Bjorklund A, Thompson L, Brundin P. Are Stem Cell-Based Therapies for Parkinson’s Disease Ready for the Clinic in 2016? J Parkinsons Dis. 2016;6:57–63. doi: 10.3233/JPD-160798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RA, Parmar M, Studer L, Takahashi J. Human Trials of Stem Cell-Derived Dopamine Neurons for Parkinson’s Disease: Dawn of a New Era. Cell Stem Cell. 2017;21:569–573. doi: 10.1016/j.stem.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Barker RA, Studer L, Cattaneo E, Takahashi J. G-Force PD: a global initiative in coordinating stem cell-based dopamine treatments for Parkinson’s disease. NPJ Parkinsons Dis. 2015b;1:15017. doi: 10.1038/npjparkd.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx. 2004;1:472–481. doi: 10.1602/neurorx.1.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Weinberg MS, Samulski RJ. Parkinson’s disease gene therapy: success by design meets failure by efficacy. Mol Ther. 2014;22:487–497. doi: 10.1038/mt.2013.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay R, Ben-Zur T, Bulvik S, Melamed E, Offen D. Lentiviral delivery of LMX1a enhances dopaminergic phenotype in differentiated human bone marrow mesenchymal stem cells. Stem Cells Dev. 2009;18:591–601. doi: 10.1089/scd.2008.0138. [DOI] [PubMed] [Google Scholar]
- Barzilay R, Kan I, Ben-Zur T, Bulvik S, Melamed E, Offen D. Induction of human mesenchymal stem cells into dopamine-producing cells with different differentiation protocols. Stem Cells Dev. 2008;17:547–554. doi: 10.1089/scd.2007.0172. [DOI] [PubMed] [Google Scholar]
- Ben-David U, Gan QF, Golan-Lev T, Arora P, Yanuka O, Oren YS, Leikin-Frenkel A, Graf M, Garippa R, Boehringer M, et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12:167–179. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, Reubinoff BE. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22:1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- Bhutani K, Nazor KL, Williams R, Tran H, Dai H, Dzakula Z, Cho EH, Pang AW, Rao M, Cao H, et al. Whole-genome mutational burden analysis of three pluripotency induction methods. Nat Commun. 2016;7:10536. doi: 10.1038/ncomms10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Kordower JH. Cell therapy for Parkinson’s disease: what next? Mov Disord. 2013;28:110–115. doi: 10.1002/mds.25343. [DOI] [PubMed] [Google Scholar]
- Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch J, Brunet JF, McEntire CR, Redmond DE. Primate adult brain cell autotransplantation produces behavioral and biological recovery in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonian St. Kitts monkeys. J Comp Neurol. 2014;522:2729–2740. doi: 10.1002/cne.23579. [DOI] [PubMed] [Google Scholar]
- Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD, Ziller M, Croft GF, Amoroso MW, Oakley DH, et al. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144:439–452. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravery CA. Do human leukocyte antigen-typed cellular therapeutics based on induced pluripotent stem cells make commercial sense? Stem Cells Dev. 2015;24:1–10. doi: 10.1089/scd.2014.0136. [DOI] [PubMed] [Google Scholar]
- Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- Brundin P, Kordower JH. Neuropathology in transplants in Parkinson’s disease: implications for disease pathogenesis and the future of cell therapy. Prog Brain Res. 2012;200:221–241. doi: 10.1016/B978-0-444-59575-1.00010-7. [DOI] [PubMed] [Google Scholar]
- Bryja V, Bonilla S, Cajanek L, Parish CL, Schwartz CM, Luo Y, Rao MS, Arenas E. An efficient method for the derivation of mouse embryonic stem cells. Stem Cells. 2006;24:844–849. doi: 10.1634/stemcells.2005-0444. [DOI] [PubMed] [Google Scholar]
- Burrows CK, Banovich NE, Pavlovic BJ, Patterson K, Gallego Romero I, Pritchard JK, Gilad Y. Genetic Variation, Not Cell Type of Origin, Underlies the Majority of Identifiable Regulatory Differences in iPSCs. PLoS Genet. 2016;12:e1005793. doi: 10.1371/journal.pgen.1005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery PC, Barker RA. Treating Parkinson’s disease in the 21st century: can stem cell transplantation compete? J Comp Neurol. 2014;522:2802–2816. doi: 10.1002/cne.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buytaert-Hoefen KA, Alvarez E, Freed CR. Generation of tyrosine hydroxylase positive neurons from human embryonic stem cells after coculture with cellular substrates and exposure to GDNF. Stem Cells. 2004;22:669–674. doi: 10.1634/stemcells.22-5-669. [DOI] [PubMed] [Google Scholar]
- Cai J, Yang M, Poremsky E, Kidd S, Schneider JS, Iacovitti L. Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6-OHDA-lesioned rats. Stem Cells Dev. 2010;19:1017–1023. doi: 10.1089/scd.2009.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- Cave JW, Wang M, Baker H. Adult subventricular zone neural stem cells as a potential source of dopaminergic replacement neurons. Front Neurosci. 2014;8:16. doi: 10.3389/fnins.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha Y, Han MJ, Cha HJ, Zoldan J, Burkart A, Jung JH, Jang Y, Kim CH, Jeong HC, Kim BG, et al. Metabolic control of primed human pluripotent stem cell fate and function by the miR-200c-SIRT2 axis. Nat Cell Biol. 2017;19:445–456. doi: 10.1038/ncb3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Xiong M, Dong Y, Haberman A, Cao J, Liu H, Zhou W, Zhang SC. Chemical Control of Grafted Human PSC-Derived Neurons in a Mouse Model of Parkinson’s Disease. Cell Stem Cell. 2016;18:817–826. doi: 10.1016/j.stem.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Hansen NF, Zhao L, Du Y, Zou C, Donovan FX, Chou BK, Zhou G, Li S, Dowey SN, et al. Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression. Cell Stem Cell. 2012;10:337–344. doi: 10.1016/j.stem.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim MH, Kim JH, et al. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Lewy body pathology in fetal grafts. Ann N Y Acad Sci. 2010;1184:55–67. doi: 10.1111/j.1749-6632.2009.05229.x. [DOI] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. The prion hypothesis of Parkinson’s disease. Curr Neurol Neurosci Rep. 2015;15:28. doi: 10.1007/s11910-015-0549-x. [DOI] [PubMed] [Google Scholar]
- Chung S, Leung A, Han BS, Chang MY, Moon JI, Kim CH, Hong S, Pruszak J, Isacson O, Kim KS. Wnt1-lmx1a forms a novel autoregulatory loop and controls midbrain dopaminergic differentiation synergistically with the SHH-FoxA2 pathway. Cell Stem Cell. 2009;5:646–658. doi: 10.1016/j.stem.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Moon JI, Leung A, Aldrich D, Lukianov S, Kitayama Y, Park S, Li Y, Bolshakov VY, Lamonerie T, et al. ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proc Natl Acad Sci U S A. 2011;108:9703–9708. doi: 10.1073/pnas.1016443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Sonntag KC, Andersson T, Bjorklund LM, Park JJ, Kim DW, Kang UJ, Isacson O, Kim KS. Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur J Neurosci. 2002;16:1829–1838. doi: 10.1046/j.1460-9568.2002.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F, Fodor W, Deacon TW, van Horne C, Rollins S, Burton W, Costantini LC, Isacson O. Immune parameters relevant to neural xenograft survival in the primate brain. Xenotransplantation. 2003;10:41–49. doi: 10.1034/j.1399-3089.2003.01130.x. [DOI] [PubMed] [Google Scholar]
- Cooper O, Hargus G, Deleidi M, Blak A, Osborn T, Marlow E, Lee K, Levy A, Perez-Torres E, Yow A, et al. Differentiation of human ES and Parkinson’s disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci. 2010;45:258–266. doi: 10.1016/j.mcn.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranoski D. Trials of embryonic stem cells to launch in China. Nature. 2017;546:15–16. doi: 10.1038/546015a. [DOI] [PubMed] [Google Scholar]
- Daley GQ. Polar Extremes in the Clinical Use of Stem Cells. N Engl J Med. 2017;376:1075–1077. doi: 10.1056/NEJMe1701379. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- Dell’Anno MT, Caiazzo M, Leo D, Dvoretskova E, Medrihan L, Colasante G, Giannelli S, Theka I, Russo G, Mus L, et al. Remote control of induced dopaminergic neurons in parkinsonian rats. J Clin Invest. 2014;124:3215–3229. doi: 10.1172/JCI74664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi D, Morizane A, Kikuchi T, Onoe H, Hayashi T, Kawasaki T, Motono M, Sasai Y, Saiki H, Gomi M, et al. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson’s disease. Stem Cells. 2012;30:935–945. doi: 10.1002/stem.1060. [DOI] [PubMed] [Google Scholar]
- Doi D, Samata B, Katsukawa M, Kikuchi T, Morizane A, Ono Y, Sekiguchi K, Nakagawa M, Parmar M, Takahashi J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Reports. 2014;2:337–350. doi: 10.1016/j.stemcr.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey ER, Bloem BR. The Parkinson Pandemic-A Call to Action. JAMA Neurol. 2017 doi: 10.1001/jamaneurol.2017.3299. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effenberg A, Stanslowsky N, Klein A, Wesemann M, Haase A, Martin U, Dengler R, Grothe C, Ratzka A, Wegner F. Striatal Transplantation of Human Dopaminergic Neurons Differentiated From Induced Pluripotent Stem Cells Derived From Umbilical Cord Blood Using Lentiviral Reprogramming. Cell Transplant. 2015;24:2099–2112. doi: 10.3727/096368914X685591. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fasano CA, Chambers SM, Lee G, Tomishima MJ, Studer L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell. 2010;6:336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Feng Q, Lu SJ, Klimanskaya I, Gomes I, Kim D, Chung Y, Honig GR, Kim KS, Lanza R. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 2010;28:704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol. 2015;33:890–891. doi: 10.1038/nbt0915-890. [DOI] [PubMed] [Google Scholar]
- Glavaski-Joksimovic A, Virag T, Chang QA, West NC, Mangatu TA, McGrogan MP, Dugich-Djordjevic M, Bohn MC. Reversal of dopaminergic degeneration in a parkinsonian rat following micrografting of human bone marrow-derived neural progenitors. Cell Transplant. 2009;18:801–814. doi: 10.3727/096368909X470801. [DOI] [PubMed] [Google Scholar]
- Glavaski-Joksimovic A, Virag T, Mangatu TA, McGrogan M, Wang XS, Bohn MC. Glial cell line-derived neurotrophic factor-secreting genetically modified human bone marrow-derived mesenchymal stem cells promote recovery in a rat model of Parkinson’s disease. J Neurosci Res. 2010;88:2669–2681. doi: 10.1002/jnr.22435. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tanner CM, Penn RD, Stebbins GT, 3rd, Gilley DW, Shannon KM, Klawans HL, Comella CL, Wilson RS, Witt T. Adrenal medullary transplant to the striatum of patients with advanced Parkinson’s disease: 1-year motor and psychomotor data. Neurology. 1990;40:273–276. doi: 10.1212/wnl.40.2.273. [DOI] [PubMed] [Google Scholar]
- Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, Bramoulle Y, Van Camp N, Perrier AL, Hantraye P, Bjorklund A, et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15:653–665. doi: 10.1016/j.stem.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grealish S, Drouin-Ouellet J, Parmar M. Brain repair and reprogramming: the route to clinical translation. J Intern Med. 2016;280:265–275. doi: 10.1111/joim.12475. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval JL, Fraser CC, Cavazzana-Calvo M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003a;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003b;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hagell P, Schrag A, Piccini P, Jahanshahi M, Brown R, Rehncrona S, Widner H, Brundin P, Rothwell JC, Odin P, et al. Sequential bilateral transplantation in Parkinson’s disease: effects of the second graft. Brain. 1999;122(Pt 6):1121–1132. doi: 10.1093/brain/122.6.1121. [DOI] [PubMed] [Google Scholar]
- Han F, Wang W, Chen B, Chen C, Li S, Lu X, Duan J, Zhang Y, Zhang YA, Guo W, et al. Human induced pluripotent stem cell-derived neurons improve motor asymmetry in a 6-hydroxydopamine-induced rat model of Parkinson’s disease. Cytotherapy. 2015;17:665–679. doi: 10.1016/j.jcyt.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Hargus G, Cooper O, Deleidi M, Levy A, Lee K, Marlow E, Yow A, Soldner F, Hockemeyer D, Hallett PJ, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci U S A. 2010;107:15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargus G, Ehrlich M, Arauzo-Bravo MJ, Hemmer K, Hallmann AL, Reinhardt P, Kim KP, Adachi K, Santourlidis S, Ghanjati F, et al. Origin-dependent neural cell identities in differentiated human iPSCs in vitro and after transplantation into the mouse brain. Cell Rep. 2014;8:1697–1703. doi: 10.1016/j.celrep.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Hedlund E, Pruszak J, Lardaro T, Ludwig W, Vinuela A, Kim KS, Isacson O. Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson’s disease. Stem Cells. 2008;26:1526–1536. doi: 10.1634/stemcells.2007-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiler D, Chen X, Hazen J, Kupriyanov S, Carroll PA, Qu C, Xu B, Johnson D, Griffiths L, Frase S, et al. Quantification of Retinogenesis in 3D Cultures Reveals Epigenetic Memory and Higher Efficiency in iPSCs Derived from Rod Photoreceptors. Cell Stem Cell. 2015;17:101–115. doi: 10.1016/j.stem.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Jaenisch R. Induced Pluripotent Stem Cells Meet Genome Editing. Cell Stem Cell. 2016;18:573–586. doi: 10.1016/j.stem.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden SE, Gore A, Li Z, Fung HL, Nisler BS, Nie J, Chen G, McIntosh BE, Gulbranson DR, Diol NR, et al. Genetic correction and analysis of induced pluripotent stem cells from a patient with gyrate atrophy. Proc Natl Acad Sci U S A. 2011;108:6537–6542. doi: 10.1073/pnas.1103388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010a;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Friedrich AM, Johnson LV, Clegg DO. Memory in induced pluripotent stem cells: reprogrammed human retinal-pigmented epithelial cells show tendency for spontaneous redifferentiation. Stem Cells. 2010b;28:1981–1991. doi: 10.1002/stem.531. [DOI] [PubMed] [Google Scholar]
- Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Narva E, Ng S, Sourour M, Hamalainen R, Olsson C, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–U67. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- Iacovitti L, Donaldson AE, Marshall CE, Suon S, Yang M. A protocol for the differentiation of human embryonic stem cells into dopaminergic neurons using only chemically defined human additives: Studies in vitro and in vivo. Brain Res. 2007;1127:19–25. doi: 10.1016/j.brainres.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet L, Stephenson E, Collins R, Patel H, Trussler J, Al-Bedaery R, Renwick P, Ogilvie C, Vaughan R, Ilic D. Strategy for the creation of clinical grade hESC line banks that HLA-match a target population. EMBO Mol Med. 2013;5:10–17. doi: 10.1002/emmm.201201973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger I, Arber C, Risner-Janiczek JR, Kuechler J, Pritzsche D, Chen IC, Naveenan T, Ungless MA, Li M. Temporally controlled modulation of FGF/ERK signaling directs midbrain dopaminergic neural progenitor fate in mouse and human pluripotent stem cells. Development. 2011;138:4363–4374. doi: 10.1242/dev.066746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Ng SH, Sharma V, Neculai D, Hussein S, Sam M, Quang T, Church GM, McPherson JD, Nagy A, et al. Elevated Coding Mutation Rate During the Reprogramming of Human Somatic Cells into Induced Pluripotent Stem Cells. Stem Cells. 2012;30:435–440. doi: 10.1002/stem.1011. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ren Y, Yuen EY, Zhong P, Ghaedi M, Hu Z, Azabdaftari G, Nakaso K, Yan Z, Feng J. Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun. 2012;3:668. doi: 10.1038/ncomms1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksimovic M, Yun BA, Kittappa R, Anderegg AM, Chang WW, Taketo MM, McKay RD, Awatramani RB. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat Neurosci. 2009;12:125–131. doi: 10.1038/nn.2243. [DOI] [PubMed] [Google Scholar]
- Jonsson ME, Ono Y, Bjorklund A, Thompson LH. Identification of transplantable dopamine neuron precursors at different stages of midbrain neurogenesis. Exp Neurol. 2009;219:341–354. doi: 10.1016/j.expneurol.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Kajiwara M, Aoi T, Okita K, Takahashi R, Inoue H, Takayama N, Endo H, Eto K, Toguchida J, Uemoto S, et al. Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:12538–12543. doi: 10.1073/pnas.1209979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Yamanaka S. To be immunogenic, or not to be: that’s the iPSC question. Cell Stem Cell. 2013;12:385–386. doi: 10.1016/j.stem.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Kang UJ, Fahn S. Management of tardive dyskinesia. Ration Drug Ther. 1988;22:1–7. [PubMed] [Google Scholar]
- Kang X, Yu Q, Huang Y, Song B, Chen Y, Gao X, He W, Sun X, Fan Y. Effects of Integrating and Non-Integrating Reprogramming Methods on Copy Number Variation and Genomic Stability of Human Induced Pluripotent Stem Cells. PLoS One. 2015;10:e0131128. doi: 10.1371/journal.pone.0131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O’Malley KL, During MJ. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- Karagiannis P, Eto K. Ten years of induced pluripotency: from basic mechanisms to therapeutic applications. Development. 2016;143:2039–2043. doi: 10.1242/dev.138172. [DOI] [PubMed] [Google Scholar]
- Katsukawa M, Nakajima Y, Fukumoto A, Doi D, Takahashi J. Fail-Safe Therapy by Gamma-Ray Irradiation Against Tumor Formation by Human-Induced Pluripotent Stem Cell-Derived Neural Progenitors. Stem Cells Dev. 2016;25:815–825. doi: 10.1089/scd.2015.0394. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Kefalopoulou Z, Politis M, Piccini P, Mencacci N, Bhatia K, Jahanshahi M, Widner H, Rehncrona S, Brundin P, Bjorklund A, et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014;71:83–87. doi: 10.1001/jamaneurol.2013.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, Mizuma H, Takara S, Takahashi R, Inoue H, et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 2017a;548:592–596. doi: 10.1038/nature23664. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Morizane A, Doi D, Okita K, Nakagawa M, Yamakado H, Inoue H, Takahashi R, Takahashi J. Idiopathic Parkinson’s disease patient-derived induced pluripotent stem cells function as midbrain dopaminergic neurons in rodent brains. J Neurosci Res. 2017b;95:1829–1837. doi: 10.1002/jnr.24014. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Morizane A, Doi D, Onoe H, Hayashi T, Kawasaki T, Saiki H, Miyamoto S, Takahashi J. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson’s disease. J Parkinsons Dis. 2011;1:395–412. doi: 10.3233/JPD-2011-11070. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Lee JS, Leem JW, Huh YJ, Kim JY, Kim HS, Park IH, Daley GQ, Hwang DY, Kim DW. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev. 2010a;6:270–281. doi: 10.1007/s12015-010-9138-1. [DOI] [PubMed] [Google Scholar]
- Kim DW, Chung S, Hwang M, Ferree A, Tsai HC, Park JJ, Nam TS, Kang UJ, Isacson O, Kim KS. Stromal cell-derived inducing activity, Nurr1, and signaling molecules synergistically induce dopaminergic neurons from mouse embryonic stem cells. Stem Cells. 2006;24:557–567. doi: 10.1634/stemcells.2005-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010b;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Huo H, Loh YH, Aryee MJ, Lensch MW, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS. Converting human skin cells to neurons: a new tool to study and treat brain disorders? Cell Stem Cell. 2011;9:179–181. doi: 10.1016/j.stem.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Kirkeby A, Nolbrant S, Tiklova K, Heuer A, Kee N, Cardoso T, Ottosson DR, Lelos MJ, Rifes P, Dunnett SB, et al. Predictive Markers Guide Differentiation to Improve Graft Outcome in Clinical Translation of hESC-Based Therapy for Parkinson’s Disease. Cell Stem Cell. 2017a;20:135–148. doi: 10.1016/j.stem.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeby A, Parmar M, Barker RA. Strategies for bringing stem cell-derived dopamine neurons to the clinic: A European approach STEM-PD. Prog Brain Res. 2017b;230:165–190. doi: 10.1016/bs.pbr.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Kittappa R, Chang WW, Awatramani RB, McKay RD. The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol. 2007;5:e325. doi: 10.1371/journal.pbio.0050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JY, Park CH, Koh HC, Cho YH, Kyhm JH, Kim YS, Lee I, Lee YS, Lee SH. Human embryonic stem cell-derived neural precursors as a continuous, stable, and on-demand source for human dopamine neurons. J Neurochem. 2007;103:1417–1429. doi: 10.1111/j.1471-4159.2007.04898.x. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Konagaya S, Egawa EY, Iwata H. Maturation of human iPS cell-derived dopamine neuron precursors in alginate-Ca2+ hydrogel. Biochim Biophys Acta. 2015;1850:1669–1675. doi: 10.1016/j.bbagen.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Bjorklund A. Trophic factor gene therapy for Parkinson’s disease. Mov Disord. 2013;28:96–109. doi: 10.1002/mds.25344. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Brundin P. Lewy body pathology in long-term fetal nigral transplants: is Parkinson’s disease transmitted from one neural system to another? Neuropsychopharmacology. 2009;34:254. doi: 10.1038/npp.2008.161. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Freeman TB, Chen EY, Mufson EJ, Sanberg PR, Hauser RA, Snow B, Olanow CW. Fetal nigral grafts survive and mediate clinical benefit in a patient with Parkinson’s disease. Mov Disord. 1998;13:383–393. doi: 10.1002/mds.870130303. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Rosenstein JM, Collier TJ, Burke MA, Chen EY, Li JM, Martel L, Levey AE, Mufson EJ, Freeman TB, et al. Functional fetal nigral grafts in a patient with Parkinson’s disease: chemoanatomic, ultrastructural, and metabolic studies. J Comp Neurol. 1996;370:203–230. doi: 10.1002/(SICI)1096-9861(19960624)370:2<203::AID-CNE6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystkowiak P, Gaura V, Labalette M, Rialland A, Remy P, Peschanski M, Bachoud-Levi AC. Alloimmunisation to donor antigens and immune rejection following foetal neural grafts to the brain in patients with Huntington’s disease. PLoS One. 2007;2:e166. doi: 10.1371/journal.pone.0000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska Z, Englund E, Widner H, Lindvall O, Li JY, Brundin P. Signs of degeneration in 12–22-year old grafts of mesencephalic dopamine neurons in patients with Parkinson’s disease. J Parkinsons Dis. 2011;1:83–92. doi: 10.3233/JPD-2011-11004. [DOI] [PubMed] [Google Scholar]
- Kwon EM, Connelly JP, Hansen NF, Donovan FX, Winkler T, Davis BW, Alkadi H, Chandrasekharappa SC, Dunbar CE, Mullikin JC, et al. iPSCs and fibroblast subclones from the same fibroblast population contain comparable levels of sequence variations. Proc Natl Acad Sci U S A. 2017;114:1964–1969. doi: 10.1073/pnas.1616035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyttala A, Moraghebi R, Valensisi C, Kettunen J, Andrus C, Pasumarthy KK, Nakanishi M, Nishimura K, Ohtaka M, Weltner J, et al. Genetic Variability Overrides the Impact of Parental Cell Type and Determines iPSC Differentiation Potential. Stem Cell Reports. 2016;6:200–212. doi: 10.1016/j.stemcr.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R, Lynch C, Harness JV, Lee S, Barrero MJ, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8:106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MO, Moon SH, Jeong HC, Yi JY, Lee TH, Shim SH, Rhee YH, Lee SH, Oh SJ, Lee MY, et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci U S A. 2013;110:E3281–3290. doi: 10.1073/pnas.1303669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- Lehnen D, Barral S, Cardoso T, Grealish S, Heuer A, Smiyakin A, Kirkeby A, Kollet J, Cremer H, Parmar M, et al. IAP-Based Cell Sorting Results in Homogeneous Transplantable Dopaminergic Precursor Cells Derived from Human Pluripotent Stem Cells. Stem Cell Reports. 2017;9:1207–1220. doi: 10.1016/j.stemcr.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelos MJ, Dowd E, Dunnett SB. Nigral grafts in animal models of Parkinson’s disease. Is recovery beyond motor function possible? Prog Brain Res. 2012;200:113–142. doi: 10.1016/B978-0-444-59575-1.00006-5. [DOI] [PubMed] [Google Scholar]
- Lelos MJ, Morgan RJ, Kelly CM, Torres EM, Rosser AE, Dunnett SB. Amelioration of non-motor dysfunctions after transplantation of human dopamine neurons in a model of Parkinson’s disease. Exp Neurol. 2016;278:54–61. doi: 10.1016/j.expneurol.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveque X, Mathieux E, Nerriere-Daguin V, Thinard R, Kermarrec L, Durand T, Haudebourg T, Vanhove B, Lescaudron L, Neveu I, et al. Local control of the host immune response performed with mesenchymal stem cells: perspectives for functional intracerebral xenotransplantation. J Cell Mol Med. 2015;19:124–134. doi: 10.1111/jcmm.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, Sarkar A, Siddiqui MS, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Widner H, Rehncrona S, Bjorklund A, Lindvall O, Brundin P. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov Disord. 2010;25:1091–1096. doi: 10.1002/mds.23012. [DOI] [PubMed] [Google Scholar]
- Li M, Izpisua Belmonte JC. Looking to the future following 10 years of induced pluripotent stem cell technologies. Nat Protoc. 2016;11:1579–1585. doi: 10.1038/nprot.2016.108. [DOI] [PubMed] [Google Scholar]
- Li W, Chen S, Li JY. Human induced pluripotent stem cells in Parkinson’s disease: A novel cell source of cell therapy and disease modeling. Prog Neurobiol. 2015;134:161–177. doi: 10.1016/j.pneurobio.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Li W, Englund E, Widner H, Mattsson B, van Westen D, Latt J, Rehncrona S, Brundin P, Bjorklund A, Lindvall O, et al. Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain. Proc Natl Acad Sci U S A. 2016;113:6544–6549. doi: 10.1073/pnas.1605245113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Sun W, Zhang Y, Wei W, Ambasudhan R, Xia P, Talantova M, Lin T, Kim J, Wang X, et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci U S A. 2011;108:8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O. Developing dopaminergic cell therapy for Parkinson’s disease--give up or move forward? Mov Disord. 2013;28:268–273. doi: 10.1002/mds.25378. [DOI] [PubMed] [Google Scholar]
- Lindvall O. Clinical translation of stem cell transplantation in Parkinson’s disease. J Intern Med. 2016;279:30–40. doi: 10.1111/joim.12415. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Backlund EO, Farde L, Sedvall G, Freedman R, Hoffer B, Nobin A, Seiger A, Olson L. Transplantation in Parkinson’s disease: two cases of adrenal medullary grafts to the putamen. Ann Neurol. 1987;22:457–468. doi: 10.1002/ana.410220403. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, Leenders KL, Sawle G, Rothwell JC, Marsden CD, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science. 1990;247:574–577. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Kaplan A, Yuan B, Hanna JH, Lupski JR, Reiner O. Passage number is a major contributor to genomic structural variations in mouse iPSCs. Stem Cells. 2014;32:2657–2667. doi: 10.1002/stem.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li W, Fu X, Xu Y. The Immunogenicity and Immune Tolerance of Pluripotent Stem Cell Derivatives. Front Immunol. 2017;8:645. doi: 10.3389/fimmu.2017.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi P, Iuso D, Czernik M, Ogura A. A New, Dynamic Era for Somatic Cell Nuclear Transfer? Trends Biotechnol. 2016;34:791–797. doi: 10.1016/j.tibtech.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Lund RJ, Narva E, Lahesmaa R. Genetic and epigenetic stability of human pluripotent stem cells. Nat Rev Genet. 2012;13:732–744. doi: 10.1038/nrg3271. [DOI] [PubMed] [Google Scholar]
- Ma L, Liu Y, Zhang SC. Directed differentiation of dopamine neurons from human pluripotent stem cells. Methods Mol Biol. 2011;767:411–418. doi: 10.1007/978-1-61779-201-4_30. [DOI] [PubMed] [Google Scholar]
- Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N Engl J Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L, Dagher A, Isacson O. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Ghosh S, Kamitaki N, Mitchell J, Avior Y, Mello C, Kashin S, Mekhoubad S, Ilic D, Charlton M, et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature. 2017;545:229–233. doi: 10.1038/nature22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens J, Marchetto MC, Bardy C, Gage FH. Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat Rev Neurosci. 2016;17:424–437. doi: 10.1038/nrn.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013;70:163–171. doi: 10.1001/2013.jamaneurol.45. [DOI] [PubMed] [Google Scholar]
- Miyoshi N, Ishii H, Nagano H, Haraguchi N, Dewi DL, Kano Y, Nishikawa S, Tanemura M, Mimori K, Tanaka F, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633–638. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Moon J, Lee HS, Kang JM, Park J, Leung A, Hong S, Chung S, Kim KS. Stem cell grafting improves both motor and cognitive impairments in a genetic model of Parkinson’s disease, the aphakia ak mouse. Cell Transplant. 2013;22:1263–1279. doi: 10.3727/096368912X657242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, Schwarz SC, Lee HS, Kang JM, Lee YE, Kim B, Sung MY, Hoglinger G, Wegner F, Kim JS, et al. Preclinical Analysis of Fetal Human Mesencephalic Neural Progenitor Cell Lines: Characterization and Safety In Vitro and In Vivo. Stem Cells Transl Med. 2017;6:576–588. doi: 10.5966/sctm.2015-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane A, Darsalia V, Guloglu MO, Hjalt T, Carta M, Li JY, Brundin P. A simple method for large-scale generation of dopamine neurons from human embryonic stem cells. J Neurosci Res. 2010;88:3467–3478. doi: 10.1002/jnr.22515. [DOI] [PubMed] [Google Scholar]
- Morizane A, Doi D, Kikuchi T, Nishimura K, Takahashi J. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J Neurosci Res. 2011;89:117–126. doi: 10.1002/jnr.22547. [DOI] [PubMed] [Google Scholar]
- Morizane A, Doi D, Kikuchi T, Okita K, Hotta A, Kawasaki T, Hayashi T, Onoe H, Shiina T, Yamanaka S, et al. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a non-human primate. Stem Cell Reports. 2013;1:283–292. doi: 10.1016/j.stemcr.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane A, Kikuchi T, Hayashi T, Mizuma H, Takara S, Doi H, Mawatari A, Glasser MF, Shiina T, Ishigaki H, et al. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat Commun. 2017;8:385. doi: 10.1038/s41467-017-00926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazor KL, Altun G, Lynch C, Tran H, Harness JV, Slavin I, Garitaonandia I, Muller FJ, Wang YC, Boscolo FS, et al. Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell Stem Cell. 2012;10:620–634. doi: 10.1016/j.stem.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolbrant S, Heuer A, Parmar M, Kirkeby A. Generation of high-purity human ventral midbrain dopaminergic progenitors for in vitro maturation and intracerebral transplantation. Nat Protoc. 2017;12:1962–1979. doi: 10.1038/nprot.2017.078. [DOI] [PubMed] [Google Scholar]
- Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat Cell Biol. 2011;13:541–549. doi: 10.1038/ncb2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Okun MS. Deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2012;367:1529–1538. doi: 10.1056/NEJMct1208070. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Oliveira PH, da Silva CL, Cabral JM. Concise review: Genomic instability in human stem cells: current status and future challenges. Stem Cells. 2014;32:2824–2832. doi: 10.1002/stem.1796. [DOI] [PubMed] [Google Scholar]
- Ortmann D, Vallier L. Variability of human pluripotent stem cell lines. Curr Opin Genet Dev. 2017;46:179–185. doi: 10.1016/j.gde.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- Park CH, Minn YK, Lee JY, Choi DH, Chang MY, Shim JW, Ko JY, Koh HC, Kang MJ, Kang JS, et al. In vitro and in vivo analyses of human embryonic stem cell-derived dopamine neurons. J Neurochem. 2005;92:1265–1276. doi: 10.1111/j.1471-4159.2004.03006.x. [DOI] [PubMed] [Google Scholar]
- Park H, Kim D, Kim CH, Mills RE, Chang MY, Iskow RC, Ko S, Moon JI, Choi HW, Man Yoo PS, et al. Increased genomic integrity of an improved protein-based mouse induced pluripotent stem cell method compared with current viral-induced strategies. Stem Cells Transl Med. 2014;3:599–609. doi: 10.5966/sctm.2013-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Pasque V, Plath K. X chromosome reactivation in reprogramming and in development. Curr Opin Cell Biol. 2015;37:75–83. doi: 10.1016/j.ceb.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SE, Loring JF. Genomic instability in pluripotent stem cells: implications for clinical applications. J Biol Chem. 2014;289:4578–4584. doi: 10.1074/jbc.R113.516419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini P, Brooks DJ, Bjorklund A, Gunn RN, Grasby PM, Rimoldi O, Brundin P, Hagell P, Rehncrona S, Widner H, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat Neurosci. 1999;2:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- Piccini P, Pavese N, Hagell P, Reimer J, Bjorklund A, Oertel WH, Quinn NP, Brooks DJ, Lindvall O. Factors affecting the clinical outcome after neural transplantation in Parkinson’s disease. Brain. 2005;128:2977–2986. doi: 10.1093/brain/awh649. [DOI] [PubMed] [Google Scholar]
- Porfirio B, Paganini M, Mazzanti B, Bagnoli S, Bucciantini S, Ghelli E, Nacmias B, Putignano AL, Rombola G, Saccardi R, et al. Donor-Specific Anti-HLA Antibodies in Huntington’s Disease Recipients of Human Fetal Striatal Grafts. Cell Transplant. 2015;24:811–817. doi: 10.3727/096368913X676222. [DOI] [PubMed] [Google Scholar]
- Quattrocelli M, Swinnen M, Giacomazzi G, Camps J, Barthelemy I, Ceccarelli G, Caluwe E, Grosemans H, Thorrez L, Pelizzo G, et al. Mesodermal iPSC-derived progenitor cells functionally regenerate cardiac and skeletal muscle. J Clin Invest. 2015;125:4463–4482. doi: 10.1172/JCI82735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Boland MJ, Leibowitz ML, Shumilina S, Pehrson SM, Baldwin KK, Hall IM. Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell. 2011;9:366–373. doi: 10.1016/j.stem.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M, Willenbring H. On the origin of the term “stem cell”. Cell Stem Cell. 2007;1:35–38. doi: 10.1016/j.stem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr, Vinuela A, Kordower JH, Isacson O. Influence of cell preparation and target location on the behavioral recovery after striatal transplantation of fetal dopaminergic neurons in a primate model of Parkinson’s disease. Neurobiol Dis. 2008;29:103–116. doi: 10.1016/j.nbd.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee YH, Ko JY, Chang MY, Yi SH, Kim D, Kim CH, Shim JW, Jo AY, Kim BW, Lee H, et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J Clin Invest. 2011;121:2326–2335. doi: 10.1172/JCI45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivetti di Val Cervo P, Romanov RA, Spigolon G, Masini D, Martin-Montanez E, Toledo EM, La Manno G, Feyder M, Pifl C, Ng YH, et al. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model. Nat Biotechnol. 2017;35:444–452. doi: 10.1038/nbt.3835. [DOI] [PubMed] [Google Scholar]
- Rocha EM, De Miranda B, Sanders LH. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol Dis. 2017 doi: 10.1016/j.nbd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Roost MS, Slieker RC, Bialecka M, van Iperen L, Gomes Fernandes MM, He N, Suchiman HED, Szuhai K, Carlotti F, de Koning EJP, et al. DNA methylation and transcriptional trajectories during human development and reprogramming of isogenic pluripotent stem cells. Nat Commun. 2017;8:908. doi: 10.1038/s41467-017-01077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhani F, Kumasaka N, de Brito MC, Bradley A, Vallier L, Gaffney D. Genetic background drives transcriptional variation in human induced pluripotent stem cells. PLoS Genet. 2014;10:e1004432. doi: 10.1371/journal.pgen.1004432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Samata B, Doi D, Nishimura K, Kikuchi T, Watanabe A, Sakamoto Y, Kakuta J, Ono Y, Takahashi J. Purification of functional human ES and iPSC-derived midbrain dopaminergic progenitors using LRTM1. Nat Commun. 2016;7:13097. doi: 10.1038/ncomms13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samata B, Kikuchi T, Miyawaki Y, Morizane A, Mashimo T, Nakagawa M, Okita K, Takahashi J. X-linked severe combined immunodeficiency (X-SCID) rats for xeno-transplantation and behavioral evaluation. J Neurosci Methods. 2015;243:68–77. doi: 10.1016/j.jneumeth.2015.01.027. [DOI] [PubMed] [Google Scholar]
- Sanchez-Danes A, Richaud-Patin Y, Carballo-Carbajal I, Jimenez-Delgado S, Caig C, Mora S, Di Guglielmo C, Ezquerra M, Patel B, Giralt A, et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med. 2012;4:380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Freire V, Lee AS, Hu S, Abilez OJ, Liang P, Lan F, Huber BC, Ong SG, Hong WX, Huang M, et al. Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J Am Coll Cardiol. 2014;64:436–448. doi: 10.1016/j.jacc.2014.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov Disord. 2011;26:1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- Schiesling C, Kieper N, Seidel K, Kruger R. Review: Familial Parkinson’s disease--genetics, clinical phenotype and neuropathology in relation to the common sporadic form of the disease. Neuropathol Appl Neurobiol. 2008;34:255–271. doi: 10.1111/j.1365-2990.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, DeVine A, Ettenger A, Fitzgerald K, Godfrey M, et al. A comparison of non-integrating reprogramming methods. Nat Biotechnol. 2015;33:58–63. doi: 10.1038/nbt.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TC, Noggle SA, Palmarini GM, Weiler DA, Lyons IG, Pensa KA, Meedeniya AC, Davidson BP, Lambert NA, Condie BG. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22:1218–1238. doi: 10.1634/stemcells.2004-0114. [DOI] [PubMed] [Google Scholar]
- Scudellari M. How iPS cells changed the world. Nature. 2016;534:310–312. doi: 10.1038/534310a. [DOI] [PubMed] [Google Scholar]
- Shutova MV, Surdina AV, Ischenko DS, Naumov VA, Bogomazova AN, Vassina EM, Alekseev DG, Lagarkova MA, Kiselev SL. An integrative analysis of reprogramming in human isogenic system identified a clone selection criterion. Cell Cycle. 2016;15:986–997. doi: 10.1080/15384101.2016.1152425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M, Daheron L, Hurley H, Bure K, Barker R, Carr AJ, Williams D, Kim HW, French A, Coffey PJ, et al. Generating iPSCs: translating cell reprogramming science into scalable and robust biomanufacturing strategies. Cell Stem Cell. 2015;16:13–17. doi: 10.1016/j.stem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Solomon S, Pitossi F, Rao MS. Banking on iPSC--is it doable and is it worthwhile. Stem Cell Rev. 2015;11:1–10. doi: 10.1007/s12015-014-9574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Oh SM, Kwon OC, Wulnansari N, Lee HS, Chang MY, Lee E, Sun W, Lee SE, Chang S, et al. Cografting astrocytes improves cell therapeutic outcomes in a Parkinson’s disease model. J Clin Invest. 2017 doi: 10.1172/JCI93924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag K-C. Cellular Transplantation. Elsevier; 2007. Immunological Considerations in CNS Transplants; pp. 305–326. [DOI] [Google Scholar]
- Sonntag KC, Pruszak J, Yoshizaki T, van Arensbergen J, Sanchez-Pernaute R, Isacson O. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells. 2007;25:411–418. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag KC, Simantov R, Isacson O. Stem cells may reshape the prospect of Parkinson’s disease therapy. Brain Res Mol Brain Res. 2005;134:34–51. doi: 10.1016/j.molbrainres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Spencer DD, Robbins RJ, Naftolin F, Marek KL, Vollmer T, Leranth C, Roth RH, Price LH, Gjedde A, Bunney BS, et al. Unilateral transplantation of human fetal mesencephalic tissue into the caudate nucleus of patients with Parkinson’s disease. N Engl J Med. 1992;327:1541–1548. doi: 10.1056/NEJM199211263272201. [DOI] [PubMed] [Google Scholar]
- Steichen C, Luce E, Maluenda J, Tosca L, Moreno-Gimeno I, Desterke C, Dianat N, Goulinet-Mainot S, Awan-Toor S, Burks D, et al. Messenger RNA- versus retrovirus-based induced pluripotent stem cell reprogramming strategies: analysis of genomic integrity. Stem Cells Transl Med. 2014;3:686–691. doi: 10.5966/sctm.2013-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker TB, Blair NF, Barker RA. Neural grafting for Parkinson’s disease: challenges and prospects. Neural Regen Res. 2017;12:389–392. doi: 10.4103/1673-5374.202935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Kasama Y, Araki R, Hoki Y, Sunayama M, Uda M, Nakamura M, Ando S, Abe M. Induced Pluripotent Stem Cell Generation-Associated Point Mutations Arise during the Initial Stages of the Conversion of These Cells. Stem Cell Reports. 2014;2:52–63. doi: 10.1016/j.stemcr.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg M, Bogetofte H, Lawson T, Jansson J, Smith G, Astradsson A, Moore M, Osborn T, Cooper O, Spealman R, et al. Improved cell therapy protocols for Parkinson’s disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells. 2013;31:1548–1562. doi: 10.1002/stem.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L, Zeng X. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells. 2010;28:1893–1904. doi: 10.1002/stem.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taapken SM, Nisler BS, Newton MA, Sampsell-Barron TL, Leonhard KA, McIntire EM, Montgomery KD. Karotypic abnormalities in human induced pluripotent stem cells and embryonic stem cells. Nat Biotechnol. 2011;29:313–314. doi: 10.1038/nbt.1835. [DOI] [PubMed] [Google Scholar]
- Tabar VS. 133 The Development of Human Embryonic Stem Cell-Derived Dopamine Neurons for Clinical Use in Parkinson Disease. Neurosurgery. 2016;63(Suppl 1):154–155. [Google Scholar]
- Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat Rev Mol Cell Biol. 2016;17:183–193. doi: 10.1038/nrm.2016.8. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Haag D, Wernig M. Direct somatic lineage conversion. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140368. doi: 10.1098/rstb.2014.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Zhang SC. Neural Subtype Specification from Human Pluripotent Stem Cells. Cell Stem Cell. 2016;19:573–586. doi: 10.1016/j.stem.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia N, Scholer HR. Molecular Obstacles to Clinical Translation of iPSCs. Cell Stem Cell. 2016;19:298–309. doi: 10.1016/j.stem.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tobin SC, Kim K. Generating pluripotent stem cells: differential epigenetic changes during cellular reprogramming. FEBS Lett. 2012;586:2874–2881. doi: 10.1016/j.febslet.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trounson A. Potential Pitfall of Pluripotent Stem Cells. N Engl J Med. 2017;377:490–491. doi: 10.1056/NEJMcibr1706906. [DOI] [PubMed] [Google Scholar]
- Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol. 2016;17:194–200. doi: 10.1038/nrm.2016.10. [DOI] [PubMed] [Google Scholar]
- Uchida T, Kajiwara K, Ideguchi M, Yoshikawa K, Morioka J, Suzuki M. Co-administration of adenovirus vector expressing CTLA4-Ig prolongs transgene expression in the brain of mice sensitized with adenovirus. Brain Res. 2001;898:272–280. doi: 10.1016/s0006-8993(01)02194-1. [DOI] [PubMed] [Google Scholar]
- Venkataramana NK, Kumar SK, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, Rao DK, Das M, Jan M, Gupta PK, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155:62–70. doi: 10.1016/j.trsl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt Weisenhorn DM, Giesert F, Wurst W. Diversity matters - heterogeneity of dopaminergic neurons in the ventral mesencephalon and its relation to Parkinson’s Disease. J Neurochem. 2016;139(Suppl 1):8–26. doi: 10.1111/jnc.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Akerud P, Castro DS, Holm PC, Canals JM, Snyder EY, Perlmann T, Arenas E. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nat Biotechnol. 1999;17:653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Tanji K, Mori F, Takahashi H. The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology. 2007;27:494–506. doi: 10.1111/j.1440-1789.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Tabar V, Rodriguez I, Perry AC, Studer L, Mombaerts P. Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer. Science. 2001;292:740–743. doi: 10.1126/science.1059399. [DOI] [PubMed] [Google Scholar]
- Wakeman DR, Hiller BM, Marmion DJ, McMahon CW, Corbett GT, Mangan KP, Ma J, Little LE, Xie Z, Perez-Rosello T, et al. Cryopreservation Maintains Functionality of Human iPSC Dopamine Neurons and Rescues Parkinsonian Phenotypes In Vivo. Stem Cell Reports. 2017;9:149–161. doi: 10.1016/j.stemcr.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hao J, Bai D, Gu Q, Han W, Wang L, Tan Y, Li X, Xue K, Han P, et al. Generation of clinical-grade human induced pluripotent stem cells in Xeno-free conditions. Stem Cell Res Ther. 2015;6:223. doi: 10.1186/s13287-015-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Lu C, Roisen F. Adult human olfactory epithelial-derived progenitors: a potential autologous source for cell-based treatment for Parkinson’s disease. Stem Cells Transl Med. 2012;1:492–502. doi: 10.5966/sctm.2012-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JL, Ng LK, Chase TN. Long-lasting dyskinesia induced by levodopa. Lancet. 1971;1:1016–1017. doi: 10.1016/s0140-6736(71)91410-3. [DOI] [PubMed] [Google Scholar]
- Wenker SD, Leal MC, Farias MI, Zeng X, Pitossi FJ. Cell therapy for Parkinson’s disease: Functional role of the host immune response on survival and differentiation of dopaminergic neuroblasts. Brain Res. 2016;1638:15–29. doi: 10.1016/j.brainres.2015.06.054. [DOI] [PubMed] [Google Scholar]
- Wennberg L, Czech KA, Larsson LC, Mirza B, Bennet W, Song Z, Widner H. Effects of immunosuppressive treatment on host responses against intracerebral porcine neural tissue xenografts in rats. Transplantation. 2001;71:1797–1806. doi: 10.1097/00007890-200106270-00016. [DOI] [PubMed] [Google Scholar]
- Xi J, Liu Y, Liu H, Chen H, Emborg ME, Zhang SC. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells. 2012;30:1655–1663. doi: 10.1002/stem.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Belkacemi L, Jog M, Parrent A, Hebb MO. Neurotrophic factor expression in expandable cell populations from brain samples in living patients with Parkinson’s disease. FASEB J. 2013;27:4157–4168. doi: 10.1096/fj.12-226555. [DOI] [PubMed] [Google Scholar]
- Xu J, Du Y, Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell. 2015;16:119–134. doi: 10.1016/j.stem.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang ZJ, Oldenburg M, Ayala M, Zhang SC. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells. 2008;26:55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Yang JL, Byrne S, Pan J, Church GM. CRISPR/Cas9-Directed Genome Editing of Cultured Cells. Curr Protoc Mol Biol. 2014;107:17. doi: 10.1002/0471142727.mb3101s107. [DOI] [PubMed] [Google Scholar]
- Yoo J, Lee E, Kim HY, Youn DH, Jung J, Kim H, Chang Y, Lee W, Shin J, Baek S, et al. Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson’s disease therapy. Nat Nanotechnol. 2017 doi: 10.1038/nnano.2017.133. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Araki R, Kasama Y, Sunayama M, Abe M, Nishida K, Kawaji H, Hayashizaki Y, Murakawa Y. Hotspots of De Novo Point Mutations in Induced Pluripotent Stem Cells. Cell Rep. 2017a;21:308–315. doi: 10.1016/j.celrep.2017.09.060. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Hayashizaki Y, Murakawa Y. Genomic Instability of iPSCs: Challenges Towards Their Clinical Applications. Stem Cell Rev. 2017b;13:7–16. doi: 10.1007/s12015-016-9680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka N, Gros E, Li HR, Kumar S, Deacon DC, Maron C, Muotri AR, Chi NC, Fu XD, Yu BD, et al. Efficient generation of human iPSCs by a synthetic self-replicative RNA. Cell Stem Cell. 2013;13:246–254. doi: 10.1016/j.stem.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zeng X, Cai J, Chen J, Luo Y, You ZB, Fotter E, Wang Y, Harvey B, Miura T, Backman C, et al. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004;22:925–940. doi: 10.1634/stemcells.22-6-925. [DOI] [PubMed] [Google Scholar]
- Zhang P, Xia N, Reijo Pera RA. Directed dopaminergic neuron differentiation from human pluripotent stem cells. J Vis Exp. 2014:51737. doi: 10.3791/51737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:2667–2674. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]