Abstract
Background
Gait alterations were documented in diabetic patients. However, the effect of diabetes on cortical control of gait has not been reported. We evaluated the effect of diabetes on prefrontal cortex (PFC) Oxygenated Hemoglobin (HbO2) levels during active walking in older adults.
Methods
Of the total sample (n=315; mean age=76.84±6.71ys; %female=56.5) 43 participants (13.7%) had diabetes. The experimental paradigm consisted of two single tasks: Normal-Walk (NW); and Cognitive Interference (Alpha); and one dual-task condition consisting of the two single tasks, Walk-While-Talk (WWT). Functional Near-Infrared-Spectroscopy (fNIRS) was used to quantify PFC HbO2 levels.
Results
Older adults without diabetes showed higher PFC HbO2 levels in WWT compared to both NW and Alpha. HbO2 levels during NW were not different between the two groups. Consistent with Neural Inefficiency, older adults with diabetes exhibited higher HbO2 levels during Alpha while performing significantly worse than those without diabetes. Moreover, the presence of diabetes was associated with attenuated HbO2 levels during WWT. This pattern is consistent with Capacity Limitations suggesting a failure to recruit brain resources vis-à-vis the more cognitively challenging WWT condition.
Conclusions
A distinct functional neural signature of diabetes was established during active and attention demanding walking among older adults without overt neurological disease.
Keywords: Diabetes, Locomotion, Prefrontal Cortex, Aging, fNIRS, Executive Functions
1. INTRODUCTION
Diabetes is common in the general population, notably among older adults (CDC, 2014). A recent cohort study reported a 9.3% prevalence of confirmed diabetes among individuals age 72 years and older (Palta et al., 2017). The presence of diabetes is associated with numerous adverse health outcomes (Nolan, Damm, & Prentki, 2011). While the literature concerning the effect of diabetes on locomotion is relatively limited, a few studies observed the presence of gait alterations in diabetic patients (Allet et al., 2008). A population study of middle aged and older adults revealed that individuals with diabetes performed worse on quantitative gait parameters compared to those without diabetes (Maksimovic et al., 2016). A large cohort study of older Catholic clergymen and women who were free of dementia and Parkinson disease at baseline, found that the presence of diabetes was associated with decline in gait and worsening rigidity (Arvanitakis et al., 2004). A few investigations began to shed light on factors that might explain the negative effect of diabetes on mobility outcomes (Rucker, McDowd, & Kluding, 2012). For instance, diabetes was associated with reduced gait speed, shorter step length, wider and longer stance, in a sample of ambulatory older adults who participated in the Cardiovascular Health study (Brach, Talkowski, Strotmeyer, & Newman, 2008). Notably, this association was explained, in part, by performance on measures of executive functions as well as measures of global cognition, depressive symptoms and demographic variables. Gait alterations were observed in patients with diabetes compared to controls irrespective of the presence of polyneuropathy (Sawacha et al., 2009). However, in the same study, reduced ranges of motion were recorded among patients with diabetes who also had polyneuropathy. Finally, a recent review pointed to associations between diabetic neuropathy and mobility outcomes including gait and falls (Alam et al., 2017). The ability to ambulate effectively is critical for functional independence and wellbeing. Among older adults, slower walking speed predicts increased risk of incident disability, morbidity and mortality (Studenski et al., 2011). Hence, identifying factors that influence gait decline and impairment among older adults with diabetes has important clinical and public health implications.
There is unequivocal evidence that locomotion and cognition, executive functions in particular, and their underlying brain substrates are interrelated in older adults (Holtzer, Verghese, Xue, & Lipton, 2006; Holtzer, Wang, & Verghese, 2014; Rosso et al., 2013). Dual-task methodology provides a framework for evaluating the effect of divided attention, a facet of executive functions (Miyake et al., 2000), on walking. Specifically, the decline in gait speed in dual compared to single walking task conditions is causally linked to attention resources (Holtzer, Wang, et al., 2014). Executive functions (Koechlin, Ody, & Kouneiher, 2003) and dual-tasking (Filmer, Mattingley, & Dux, 2013) are subserved by the prefrontal cortex (PFC). However, relatively little is known about the functional brain correlates of locomotion because active walking cannot be assessed in MRI and PET scanners. A review of neuroimaging studies of locomotion in aging emphasized the paucity of research in this area but identified the PFC and related circuits as key brain regions involved in higher order control of locomotion, especially under attention-demanding conditions (Holtzer, Epstein, Mahoney, Izzetoglu, & Blumen, 2014). Further, a recent review suggested that fNIRS provides a promising approach to identifying neural activity during locomotion in normal and disease populations (Gramigna et al., 2017). Indeed, studies using fNIRS provided converging evidence that PFC oxygenation levels, assessed during active walking, increased in dual compared to single task conditions in older adults (Holtzer et al., 2015; Holtzer, Schoen, et al., 2016; Holtzer, Verghese, et al., 2016; Holtzer, Yuan, et al., 2016; Mirelman et al., 2017). This effect was also observed in patients with Multiple Sclerosis (Hernandez et al., 2016) and Parkinson’s disease (Maidan, Bernad-Elazari, Giladi, Hausdorff, & Mirelman, 2017; Maidan et al., 2016). Dual-task walking is designed to better approximate real world conditions and may thus have greater ecological validity. For example, poor dual-task walking performance predicted increased risk of incident falls (Ayers, Tow, Holtzer, & Verghese, 2014) as well as frailty, disability and mortality in older adults (Verghese, Holtzer, Lipton, & Wang, 2012). Moreover, differences in PFC activation levels, as assessed with fNIRS during dual-task walking, predicted incident falls among healthy older adults (Verghese, Wang, Ayers, Izzetoglu, & Holtzer, 2016).
In contrast to the limited literature concerning the relationship between diabetes and locomotion, evidence for the effect of the former on cognition and brain structure and function is robust. A recent meta-analytic study revealed poor performance in executive functions and memory in patients with diabetes compared to controls (Sadanand, Balachandar, & Bharath, 2016). Cross-sectional studies (Brundel, van den Heuvel, de Bresser, Kappelle, & Biessels, 2010; den Heijer et al., 2003; Manschot et al., 2007; Moran et al., 2013) reported greater gray matter atrophy and white matter loss in temporal and frontal regions in patients with type 2 diabetes compared to controls. Longitudinal studies (de Bresser et al., 2010; Kooistra et al., 2013; van Elderen et al., 2010) found that the presence of type 2 diabetes was associated with accelerated brain atrophy and white matter loss. The association between brain function and structure and cognitive performance in individuals with diabetes, however, remains equivocal (Moheet, Mangia, & Seaquist, 2015). Critically, the effect of diabetes on higher order control of locomotion and its underlying brain substrates has not been reported, notably when gait is evaluated under cognitively demanding conditions.
1.1. Current Study
The current study was designed to evaluate the effect of diabetes on cortical control of locomotion. We used fNIRS to measure PFC HbO2 levels during active walking under Normal-Walk (NW), cognitive interference task (Alpha) and Walk-While-Talk (WWT) conditions in a cohort of community residing non-demented older adults. The focus on the PFC as a region of interest was based on previous literature, in particular studies that involved gait and fNIRS (for review see (Gramigna et al., 2017)). We hypothesized that, among older adults, the presence of diabetes would have a distinct effect on brain activation patterns during walking. Specifically, two models provided a conceptual framework for evaluating the effect of diabetes on brain function, operationalized as PFC HbO2 levels, during two single tasks and one dual-task walking conditions. The Capacity Limitations hypothesis (Reuter-Lorenz et al., 2000) proposes that in the context of cognitively challenging tasks or when comparing tasks that increase in terms of difficulty or complexity the presence of brain pathology would be associated with attenuated brain activation patterns. Neural Inefficiency, (Rypma, Berger, & D’Esposito, 2002; Zarahn, Rakitin, Abela, Flynn, & Stern, 2007) on the other hand, exists when higher brain activations are associated with equivalent or worse task performance. This latter model suggests that due to compromised structure and/or function the brain does not efficiently allocate resources to support cognitive task demands. It is noteworthy that the two models are not mutually exclusive as differences in activation patterns across groups may vary as a function of task type and complexity. We also evaluated the effect of diabetes on stride velocity and cognition during single and dual-task conditions.
2. METHODS
2.1. Participants
Participants were community residing older adults (age≥65yrs) enrolled in “Central Control of Mobility in Aging” (CCMA), a cohort study designed to determine cognitive and brain predictors of mobility as previously described (Holtzer, Mahoney, & Verghese, 2014). Briefly, structured telephone interviews were administered to obtain verbal assent and determine initial eligibility. Individuals deemed study eligible based on the telephone interview were invited to two annual study visits during which trained research assistants administered comprehensive neuropsychological, psychological, and mobility assessments. The fNIRS dual-task walking paradigm was administered during the first day of testing. The study physician conducted structured neurological examinations, which included review of medical history. Cognitive status was determined via established consensus diagnostic case conference procedures (Holtzer, Verghese, Wang, Hall, & Lipton, 2008). Exclusion criteria were: current or history of severe neurological or psychiatric disorders, inability to ambulate independently, significant loss of vision and/or hearing that threatened the validity of the testing procedures, and recent or anticipated medical procedures that may affect ambulation. A total of 315 participants who completed the two-day in-person baseline annual assessments between June of 2011 and January of 2014 were included in this study. The Review Board of Albert Einstein College of Medicine approved this study. Written informed consents were obtained in-person from all participants.
2.2. Diabetic Status
Study physicians determined the presence of diabetes during a structured in-person interview assessing medical history and current medications usage. Confirmation of diabetes required a positive self-report by study participants as well as evidence based on current medications known to be prescribed to patients with diabetes only, specifically, oral hypoglycemic agents or insulin. Based on the medical history, age and medications, participants were diagnosed with type 2 diabetes, though glucose blood levels were not available to confirm the diagnosis and type of diabetes. Neuropathy, in general, or diabetic neuropathy did not serve as exclusion criteria but clinical gait subtyping, which included neuropathic gait served as a covariate (see covariates section for details).
2.3. Quantitative Assessment of Gait
Zenometrics. A 4 × 14 foot Zeno electronic walkway using ProtoKinetics Movement Analysis Software (PKMAS) was utilized to assess quantitative measures of gait (Zenometrics, LLC; Peekskill, NY). Gait measures were based on the location and mathematical parameters between footfalls on the instrumented walkway. Split-half intra-class correlations (ICC) for stride velocity in NW and WWT were greater than 0.95 revealing excellent internal consistency (Holtzer et al., 2015).
2.4 Walking Paradigm
The walking paradigm has been validated in numerous studies (Holtzer et al., 2006; Holtzer, Wang, et al., 2014). There were two single task conditions: 1) Normal Pace Walk (NW) and 2) Cognitive (Alpha). In NW participants were asked to walk around the electronic walkway at their “normal pace” for three consecutive loops. In Alpha, participants were required to stand still while reciting alternate letters of the alphabet for 30-sec out loud. In Walk-While-Talk (WWT) participants were instructed to walk around the walkway for three consecutive loops at their normal pace while reciting alternate letters of the alphabet. Participants were instructed to pay equal attention to both tasks. Stride velocity was calculated over the entire length of walking the three-loop course, which consisted of six consecutive straight walks and five turns (Holtzer et al., 2015).
2.5. fNIRS System
The walking paradigm and fNIRS procedures were described in detail in our previous publications (Holtzer et al., 2015; Holtzer, Verghese, et al., 2016; Holtzer, Yuan, et al., 2016). Validation of this fNIRS system has also been established in other studies (Izzetoglu et al., 2005). fNIRS measures changes in cortical oxygenated hemoglobin (HbO2) levels using light–tissue interaction properties of light within the near infrared range. fNIRS has been validated against traditional neuroimaging methods and is better able to handle motion artifacts (Cooper et al., 2012). Changes in hemodynamic activity in the PFC were assessed using fNIRS Imager 1100 (fNIRS Devices, LLC, Potomac, MD). The system collects data at a sampling rate of 2Hz. The fNIRS sensor consists of 4 LED light sources and 10 photodetectors, which cover the forehead using 16 voxels, with a source-detector separation of 2.5 cm. The light sources on the sensor (Epitex Inc. type L4X730/4X805/4X850-40Q96-I) contain three built-in LEDs having peak wavelengths at 730, 805, and 850 nm, with an overall outer diameter of 9.2 ± 0.2 mm. The photodetectors (Bur Brown, type OPT101) are monolithic photodiodes with a single supply transimpedance amplifier. We implemented a standard sensor placement procedure. The fNIRS was placed on the forehead so that the horizontal symmetry axis central (y-axis) coincides with symmetry axis of the head, (i.e. in between the eyes). On the vertical axis, the sensor is positioned right above the eyebrows in relation to the international 10–20 system so that FP1 and FP2 marker locations are approximately positioned on the bottom channel row level (Ayaz et al., 2006). Given the sensitivity of the fNIRS recording device, the lighting in the test room was reduced such that the mean illumination of the forehead was approximately 150 lux. A recent MRI fNIRS co-registration study provided further validation for the spatial resolution of the current fNIRS device in older adults (Chen, Blumen, Izzetoglu, & Holtzer, 2017).
2.5.1. Preprocessing and hemodynamic signal extraction
Raw data at 730 and 850nm wavelengths were inspected for excessive noise, saturation or dark current conditions. The A/D converter in the fNIRs system has an anti-aliasing filter with cut-off frequency set to eliminate the signal components that can cause aliasing based on the Nyquist theorem for 2Hz sampling rate. In addition, we used a FIR (finite impulse response) filter to remove signal artifacts. We performed low-pass filtering to the intensity measurements (at 730 and 850nm) during the overall recording period with a cut-off frequency set to 0.14Hz to eliminate possible respiration signal and other higher frequency noise that may exist beyond the cognitive activity related hemodynamic signal components, which is usually taken to be up-to 0.1Hz (Izzetoglu, Chitrapu, Bunce, & Onaral, 2010). Saturation or dark current conditions were excluded (4% of the data). Oxygenated hemoglobin (HbO2), deoxygenated hemoglobin (Hb), oxygenation or oxygen index (HbO2-Hb) and total hemoglobin (HbO2+Hb) signals can be calculated from the artifact-removed raw intensity measurements at 730 and 850 nm using modified Beer-Lambert law (Izzetoglu et al., 2010). HbO2 is more reliable and sensitive to locomotion-related changes in cerebral oxygenation (Harada, Miyai, Suzuki, & Kubota, 2009) and was thus used as the primary outcome in the current study. Consistent with our prior studies (Holtzer et al., 2015; Holtzer, Schoen, et al., 2016; Holtzer, Verghese, et al., 2016; Holtzer, Yuan, et al., 2016) separate proximal 10-second baselines, administered prior to each experimental condition, were used to determine relative changes HbO2 concentrations in NW, Alpha and WWT. During the baselines participants were asked to remain still, fixate on the wall directly in front of them, and count silently in their head from 1 to 10 at a rate of one number per second.
2.5.2. Epoch and feature extraction
Mean HbO2 data were extracted separately for NW and WWT. For Alpha, mean HbO2 values, which are based on the entire 30 sec task duration, were used for extraction and comparison. We implemented additional steps that were designed to optimize the acquisition of task related HbO2 by synchronizing fNIRS and gait events. A central “hub” computer with E-Prime 2.0 software sent synchronized triggers to both the fNIRS system and PKMAS. The fNIRS acquisition software received triggers from E-prime that were each indicative of a unique condition representing the beginning or end of either a baseline or test condition. A second level post-processing time synchronization was then implemented using the first recorded foot contact with the walkway as a time stamp. The recording of fNIRS was terminated at the end of the 6th and final straight walk. This end point was determined algorithmically by PKMAS (Holtzer et al., 2015). Internal consistency of HbO2 measurements, determined by split-half intra-class correlations within each task, was excellent for NW (0.830), Alpha (0.864) and WWT (0.849) (Holtzer et al., 2015). We used a block study design. The three test conditions were presented in a counterbalanced order using a Latin-square design to eliminate the effect of test order.
2.6. Covariates
Covariates were determined based on existing relevant literature (Holzer et al., 2015, 2016, 2017) and included the following: general level of cognitive function was assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Duff et al., 2008). Age, gender and education were determined via self-report during a structured clinical interview. Study physicians assessed medical history. Dichotomous rating (presence or absence) of diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, Parkinson’s disease, chronic obstructive lung disease, angina, and myocardial infarction was used to calculate a disease comorbidity summary score (range 0–10) in previous publications (Holtzer et al., 2008). For the purpose of the current study the disease comorbidity summary score was recalculated excluding diabetes (range=0–9). Structured and validated clinical assessment of gait was conducted by the study physician as part of the neurological examination independently of the experimental walking protocol (Holtzer, Verghese, et al., 2016). Accordingly, gait status included the following four categories: 1) Normal gait was defined by the absence of neurological or non-neurological gait abnormalities; 2) Neuropathic gait, also referred to as peripheral neurological gait abnormality, was defined by unilateral or bilateral foot drop such as a “stocking”-pattern sensory loss, and an absence of deep-tendon reflexes in the lower limbs; 3) Central neurological gait abnormalities (NGA) status was defined by the presence of gait abnormalities due to brain causes, and included frontal, ataxic, hemiparetic, unsteady, spastic, Parkinsonian or unsteady subtypes; 4) The presence of non-neurological gait abnormalities was determined based on causes such as hip and knee osteoarthritis. The Geriatric Depression Scale (GDS) was used to assess self-reported depressive symptoms (Yesavage et al., 1982).
2.7. Statistical analysis
Linear Mixed Effects (LME) and Generalized Estimating Equations (GEE) Poisson models were used to assess the main and moderating effects of diabetes on the change in gait velocity and rate of letter generation in WWT compared to NW and Alpha, respectively. A linear mixed effect model was utilized to assess the main and moderating effects of diabetes on HbO2 levels in WWT compared to NW and Alpha, allowing different variations in HbO2 levels among fNIRS channels. Moderation effects were determined by 2-way interactions of diabetic status x task. Both LME and GEE models handle missing data without imputation. Analyses controlled for age, gender, education, RBANS total index score, disease comorbidity summary score, BMI, and clinical gait status. Two-sided tests were used and statistical significance was set at 0.05. Statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, N.C.).
3. Results
3.1. Sample characteristics
The sample consisted of 315 non-demented participants (mean age=76.84±SD=6.71ys; mean education=14.43± SD=3.02ys; %female=56.5) who completed the two-day in-person baseline annual assessments. Of the 43 participants with diabetes (13.7%) 37 received oral hypoglycemic medications and 6 were on insulin therapy. The mean RBANS Index score (91.26± SD=11.92) was indicative of average overall cognitive function. Mean scores on the GDS (4.81± SD=3.97) and the modified disease comorbidity index (1.44± SD=1.00) were both low. Descriptive statistics of demographic, behavioral and fNIRS data were further stratified by diabetic group status (Table 1). Unadjusted group comparisons revealed that the presence of diabetes was associated with significantly higher BMI, slower stride velocity during NW, lower rate of correct letter generation during Alpha and WWT, higher error rate of letter generation during WWT and higher HbO2 levels during Alpha. There was no statistically significant difference in clinical gait status between the two groups.
Table 1.
Summary of sample characteristics stratified by diabetic status
| Total sample | W/out Diabetes | With Diabetes | P* | |
|---|---|---|---|---|
| Number, (%) | ||||
| Participants: | 315, (100) | 272, (86.3) | 43, (13.7) | |
| Women: | 178, (56.5) | 159, (58.5) | 19, (44.2) | 0.079 |
| Gait Status: | 0.506 | |||
| Normal | 163, (51.8) | 144, (52.9) | 19, (44.2) | |
| NN GA | 86, (27.3) | 74, (27.2) | 12, (27.9) | |
| Central-NGA | 28, (8.9) | 24, (8.8) | 4, (9.3) | |
| Neuropathic GA | 38, (12) | 30, (11) | 8, (18.6) | |
| Mean(SD) | ||||
| Age, years: | 76.84(6.7) | 76.71(6.7) | 77.68(6.8) | 0.334 |
| Education, years: | 14.43(3.0) | 14.53(3.1) | 13.84(2.7) | 0.153 |
| GDS total score: | 4.81(3.9) | 4.81(4.0) | 4.84(3.7) | 0.700 |
| BMI: | 29.24(6.6) | 28.82(6.4) | 31.90(6.9) | 0.002 |
| Disease comorbidity: | 1.44(1.0) | 1.44(1.0) | 1.47(.91) | 0.848 |
| RBANS (total Index score): | 91.26(11.9) | 91.25(11.9) | 91.28(12.1) | 0.913 |
| Stride Velocity NW (cm/sec): | 79.89(17.5) | 80.67(17.5) | 74.91(16.9) | 0.059 |
| Stride Velocity WWT (cm/sec): | 64.91(18.8) | 65.1(18.8) | 63.69(18.9) | 0.637 |
| Alpha: rate of letter generation (correct/per minute) | 33.44(11.8) | 34.21(11.5) | 28.56(12.2) | 0.007 |
| WWT: rate of letter generation (correct/per minute) | 34.18(14.8) | 35.06(14.9) | 28.14(12.1) | 0.008 |
| NW: HbO2 levels | 0.11(0.64) | 0.10(0.64) | 0.15(0.64) | 0.054 |
| Alpha: HbO2 levels | 0.68(0.55) | 0.66(0.56) | 0.85(0.51) | <0.001 |
| WWT: HbO2 levels | 0.70(0.88) | 0.71(0.89) | 0.62(0.78) | 0.016 |
P=group comparisons between participants with and without diabetes
Note: RBANS Repeatable Battery for the Assessment of Neuropsychological Status; NW=Normal Walk; WWT=Walk While Talk; GDS=Geriatric Depression Scale; Normal=Normal Gait; NNGA=Gait Abnormalities Due to Non-neurological Causes; Central NGA=Neurological Gait Abnormalities Due to Central Causes; Neuropathic GA=Gait Abnormalities Due to Neuropathy; BMI=Body Mass Index
3.2. Effects of diabetes on stride velocity
Linear mixed effects model revealed that stride velocity was significantly reduced in WWT compared to NW among participants without diabetes (estimate=−15.537; p<0.001). The two-way interaction of diabetes x task was significant (estimate=−4.327; p=0.023). Specifically, the effect of dual tasking on stride velocity among participants with diabetes was significant (estimate=−11.214; p<0.001) but smaller compared to those without diabetes. Summary of the unadjusted and adjusted linear mixed effect models examining the effects of task, diabetes and their interaction on stride velocity is presented in Table 2.
Table 2.
Linear mixed effects models: effect of diabetes on stride velocity (cm/sec)
| Unadjusted model
| ||||
|---|---|---|---|---|
| Variables | Estimate | SE | 95% CI | P |
| NW vs. WWT | −15.541 | 0.695 | −16.909 to −14.173 | <0.001 |
| Diabetes (NW) | −5.63 | 3.043 | −11.751 to −0.224 | 0.059 |
| Diabetes (WWT) | −1.436 | 3.043 | −7.424 to 4.552 | 0.637 |
| Diabetes x (NW vs. WWT) | 4.327 | 1.893 | 0.601 to 8.053 | 0.023 |
|
| ||||
| Adjusted model | ||||
|
| ||||
| NW vs. WWT | −15.537 | 0.695 | −16.905 to −14.179 | <0.001 |
| Diabetes (NW) | −2.983 | 2.671 | −8.241 to −2.273 | 0.265 |
| Diabetes (WWT) | 1.339 | 2.671 | −3.917 to 6.597 | 0.616 |
| Diabetes x (NW vs. WWT) | 4.323 | 1.893 | 0.597 to 8.049 | 0.023 |
| Gender | 0.906 | 1.754 | −2.546 to 4.358 | 0.605 |
| BMI | −0.009 | 0.133 | −0.273 to 0.254 | 0.942 |
| Gait: normal vs. NN | −6.555 | 2.180 | −10.846 to 2.264 | 0.002 |
| Gait: normal vs. Central NGA | −4.516 | 3.148 | −10.713 to 1.679 | 0.152 |
| Gait: normal vs. Neuropathic GA | −9.437 | 2.778 | −14.905 to −3.969 | <0.001 |
| Age | −0.903 | 0.135 | −1.169 to −0.636 | <0.001 |
| Education | 0.238 | 0.293 | −0.340 to 0.816 | 0.417 |
| Disease comorbidity index | −2.580 | 0.862 | −4.278 to −0.882 | 0.003 |
| RBANS Total Index Score | 0.316 | 0.070 | 0.177 to 0.455 | <0.001 |
| GDS total Score | −0.350 | 0.220 | −0.783 to 0.083 | 0.11 |
Note: RBANS Repeatable Battery for the Assessment of Neuropsychological Status; NW=Normal Walk; WWT=Walk While Talk; GDS=Geriatric Depression Scale; Normal=Normal Gait; NNGA=Gait Abnormalities Due to Non-neurological Causes; Central NGA=Neurological Gait Abnormalities Due to Central Causes; Neuropathic GA=Gait Abnormalities Due to Neuropathy; BMI=Body Mass Index; N=315; all estimates represent the effect of condition, group status, interactions and covariates on the dependent measure, stride velocity (cm/sec).
3.3. Effects of diabetes on alpha
GEE analysis revealed that the rate of correct letter generation was not significantly reduced in WWT compared to Alpha among participants without diabetes (estimate of log of rate ratio=−0.021 (p=0.422) or among participants with diabetes (estimate of log of rate ratio=−0.007, p=0.856). The effect of diabetes on the rate of correct letter generation was significant during Alpha (estimate of log of rate ratio=−0.131; p=0.016) and marginally significant during WWT (estimate of log of rate ratio=−0.117; p=0.061). The interaction of diabetes x task was not significant (estimate of difference in log of rate ratio =−0.014; p=0.789). Summary of the unadjusted and adjusted GEE models examining the effects of task, diabetes and their interaction on the rate of correct letter generation is presented in Table 3.
Table 3.
General estimating equations: effect of diabetes on the rate of correct letter generation
| Unadjusted model
| ||||
|---|---|---|---|---|
| Variables | Estimate | SE | 95% CI | P |
| Alpha vs. WWT (among non- diabetics) | −0.033 | 0.026 | −0.085 to 0.018 | 0.204 |
| Diabetes (Alpha) | −0.180 | 0.067 | −0.313 to −0.048 | 0.007 |
| Diabetes (WWT) | −0.190 | 0.071 | −0.331 to −0.049 | 0.008 |
| Diabetes x (Alpha vs. WWT) | −0.009 | 0.056 | −0.119 to 0.100 | 0.863 |
|
| ||||
| Adjusted model | ||||
|
| ||||
| alpha vs. WWT | −0.021 | 0.026 | −0.072 to 0.030 | 0.422 |
| Diabetes (Alpha) | −0.131 | 0.055 | −0.239 to −0.023 | 0.016 |
| Diabetes (WWT) | −0.117 | 0.062 | −0.240 to 0.005 | 0.061 |
| Diabetes x (Alpha vs. WWT) | 0.014 | 0.052 | −0.088 to 0.116 | 0.789 |
| Gender | 0.066 | 0.039 | −0.011 to 0.144 | 0.094 |
| BMI | −0.004 | 0.003 | −0.010 to 0.001 | 0.158 |
| Gait: normal vs. NNGA | 0.027 | 0.045 | −0.062 to 0.117 | 0.549 |
| Gait: normal vs. Central NGA | 0.017 | 0.061 | −0.102 to 0.138 | 0.773 |
| Gait: normal vs. Neuropathic GA | −0.071 | 0.069 | −0.208 to 0.065 | 0.305 |
| Age | 0.001 | 0.003 | −0.004 to 0.008 | 0.568 |
| Education | 0.029 | 0.007 | 0.014 to 0.044 | <0.001 |
| Disease comorbidity index | −0.011 | 0.018 | −0.048 to 0.025 | 0.535 |
| RBANS Total Index Score | 0.013 | 0.001 | 0.009 to 0.016 | <0.001 |
| GDS total Score | 0.007 | 0.005 | −0.002 to 0.017 | 0.123 |
Note: RBANS Repeatable Battery for the Assessment of Neuropsychological Status; NW=Normal Walk; WWT=Walk While Talk; GDS=Geriatric Depression Scale; Normal=Normal Gait; NNGA=Gait Abnormalities Due to Non-neurological Causes; Central NGA=Neurological Gait Abnormalities Due to Central Causes; Neuropathic GA=Gait Abnormalities Due to Neuropathy; BMI=Body Mass Index; N=315; all estimates represent the effect of condition, group status, interactions and covariates on the dependent measure, rate of correct letter generation (correct/minute).
3.4. Effects of diabetes on task-related changes in HbO2 levels
Results of the linear mixed effects models revealed significant task effects where HbO2 levels were increased in WWT compared to NW (estimate=−0.577; p<0.001) and Alpha (estimate=−0.085; p<0.001) among participants without diabetes. Diabetes moderated the effect of task on changes in HbO2 levels. Two-way interactions revealed that the presence of diabetes was associated with an attenuated increase in HbO2 levels from NW to WWT (estimate=0.173; p= p<0.001) and with a differential effect on the change in HbO2 levels from Alpha to WWT (estimate=0.236; p<0.001). Specifically, there was a decline in HbO2 levels from Alpha to WWT among participants with diabetes (estimate=0.151, p=0.001).
Summary of the results of the unadjusted and adjusted models is presented in Table 4. The two-way interactions of diabetes x task are also depicted in Figure 1.
Table 4.
Linear mixed effects models: Effect of diabetes on HbO2 levels during walking
| Unadjusted model
| ||||
|---|---|---|---|---|
| Variables | Estimate | SE | 95% CI | P |
| NW vs. WWT | −0.577 | 0.018 | −0.613 to −0.540 | <0.001 |
| Alpha vs. WWT | −0.085 | 0.018 | −0.121 to −0.048 | <0.001 |
| Diabetes (NW) | 0.077 | 0.040 | −0.001 to 0.156 | 0.054 |
| Diabetes (Alpha) | 0.139 | 0.040 | 0.061 to 0.217 | <0.001 |
| Diabetes (WWT) | −0.095 | 0.039 | −0.173 to −0.017 | 0.016 |
| Diabetes x NW vs. WWT | 0.172 | 0.050 | 0.074 to 0.271 | <0.001 |
| Diabetes x Alpha vs. WWT | 0.235 | 0.050 | 0.136 to 0.333 | <0.001 |
|
| ||||
| Adjusted model | ||||
|
| ||||
| NW vs. WWT | −0.577 | 0.018 | −0.613 to −0.540 | <0.001 |
| Alpha vs. WWT | −0.085 | 0.018 | −0.121 to −0.048 | <0.001 |
| Diabetes (NW) | 0.039 | 0.040 | −0.039 to 0.118 | 0.327 |
| Diabetes (Alpha) | 0.102 | 0.039 | 0.023 to 0.180 | 0.010 |
| Diabetes (WWT) | −0.134 | 0.039 | −0.212 to −0.056 | <0.001 |
| Diabetes x NW vs. WWT | 0.173 | 0.050 | 0.074 to 0.272 | <0.001 |
| Diabetes x Alpha vs. WWT | 0.236 | 0.050 | 0.138 to 0.335 | <0.001 |
| Gender | −0.269 | 0.019 | −0.307 to −0.231 | <0.001 |
| BMI | −0.002 | 0.001 | −0.005 to 0.002 | 0.068 |
| Gait: normal vs. NNGA | 0.046 | 0.024 | −0.001 to 0.093 | 0.054 |
| Gait: normal vs. Central NGA | −0.040 | 0.035 | −0.109 to 0.029 | 0.257 |
| Gait: normal vs. Neuropathic GA | 0.072 | 0.031 | 0.011 to 0.133 | 0.019 |
| Age | −0.001 | 0.001 | −0.003 to 0.002 | 0.559 |
| Education | −0.007 | 0.003 | −0.013 to −0.001 | 0.027 |
| Disease comorbidity index | −0.011 | 0.009 | −0.030 to 0.006 | 0.211 |
| RBANS Total Index Score | −0.001 | 0.001 | −0.002 to 0.001 | 0.515 |
| GDS total Score | 0.003 | 0.002 | −0.001 to 0.008 | 0.160 |
Note: RBANS Repeatable Battery for the Assessment of Neuropsychological Status; NW=Normal Walk; WWT=Walk While Talk; GDS=Geriatric Depression Scale; Normal=Normal Gait; NNGA=Gait Abnormalities Due to Non-neurological Causes; Central NGA=Neurological Gait Abnormalities Due to Central Causes; Neuropathic GA=Gait Abnormalities Due to Neuropathy; BMI=Body Mass Index; N=315; all estimates represent the effect of condition, group status, interactions and covariates on the dependent measure, HbO2 levels (expressed in micromolar units).
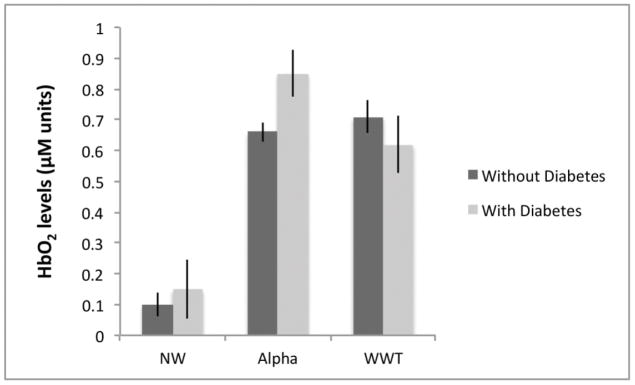
Figure 1.
Changes in HbO2 levels, (Y-axis - expressed in micromolar units), from Normal Walk (NW) and Alpha to Walk-While-Talk (WWT) as a function of diabetic status.
Diabetic status significantly moderated the change in HbO2 levels from NW to WWT (p<0.001) and from Alpha to WWT (p<0.001). Errors bars are based on the standard errors obtained from the linear mixed effects model.
3.5. Sensitivity analyses
Several analyses were conducted to further clarify the findings reported above. Laterality: Laterality effects were not significant in NW (p=0.310) or Alpha (p=0.535). Laterality was significant in WWT, with higher HbO2 levels observed in left compared to right channels (p=0.007). To determine whether laterality impacted the reported findings separate linear mixed effects models were conducted using HbO2 levels in the left (1–8) and right (9–16) channels as the outcome measures. Higher HbO2 levels in WWT were observed compared to NW (estimate=−0.655; SE=0.030; p<0.001) and Alpha (estimate=−0.089; SE=0.030; p=0.004) on the left side. Higher HbO2 levels were observed in WWT compared to NW (estimate=−0.595; SE=0.030; p=<0.001) but not compared to Alpha (estimate=−0.025; SE=0.030; 95%CI=−0.084 to 0.034; p=0.403) on the right side. The two way interactions of diabetes status x task remained significant and in the same direction in the left (WWT vs NW; estimate=0.082; SE=0.041; p=0.044; WWT vs. Alpha; estimate=0.114; SE=0.041; p=0.005) and right (WWT vs NW; estimate=0.079; SE=0.040; p=0.050; WWT vs. Alpha; estimate=0.169; SE=0.040; p<0.001) side models suggesting that laterality did not influence the effect of diabetes on the study outcomes. (p=0.007). Deoxygenated Hemoglobin (Hb): to further validate the reported task-related changes in HbO2 levels we examined the pattern of change in deoxygenated hemoglobin (HB). We found a reciprocal decline (mean Hb levels: NW=−0.129, Alpha=−0.086, WWT=−0.340). Results from the linear mixed effect model confirmed a significantly greater reciprocal task related decline in Hb levels in WWT compared to NW (estimate=0.283; SE=0.051; p<0.001) and Alpha (estimate=0.312; SE=0.051; p<0.001).
4. Discussion
The current study was designed to determine the effect of diabetes on cortical control of locomotion. We found that, among non-demented older adults, the presence of diabetes was associated with differential activation patterns across task conditions. Specifically, persons with diabetes had higher PFC HbO2 levels during Alpha compared to those without diabetes. In contrast, during WWT, a condition that experimentally increases cognitive demands of locomotion, the presence of diabetes was associated with lower PFC HbO2 levels. In addition, diabetic status moderated the change in PFC HbO2 levels in WWT compared to NW and Alpha. These findings and their implications are discussed below.
The PFC is a key region implicated in cortical control of walking, notably under attention-demanding dual-task conditions, in older adults (Holtzer et al., 2015; Holtzer, Schoen, et al., 2016; Holtzer, Verghese, et al., 2016; Holtzer, Yuan, et al., 2016; Mirelman et al., 2017). Recent reviews provided converging evidence that changes in brain architecture and function (Moran et al., 2017) as well as neurodegeneration (Bharadwaj et al., 2017) exist in older adults with diabetes beyond what would be expected in normal aging. Hence, the role of the PFC in cortical control of locomotion may become altered in older adults with diabetes. In the current study PFC oxygenation levels during NW were not different between the two groups. But older adults with diabetes exhibited higher HbO2 levels during Alpha while performing significantly worse compared to those without diabetes. These results are consistent with Neural Inefficiency (Rypma et al., 2002; Zarahn et al., 2007) wherein higher brain activations are associated with equivalent or worse task performance suggesting that the brain does not efficiently allocate resources to support cognitive task demands. As reviewed earlier, the Capacity Limitations hypothesis (Reuter-Lorenz et al., 2000) proposes that in disease populations with existing brain pathologies brain activation patterns would be attenuated during cognitive tasks that impose greater demands in terms difficulty and complexity. Whereas older adults without diabetes demonstrated increased PFC HbO2 levels in WWT compared to Alpha individuals with diabetes showed the opposite trajectory. This pattern suggests that, among participants with diabetes, brain resources may have already been maximized and inefficiently utilized during the cognitive interference task. In contrast, older adults with diabetes exhibited a failure to recruit adequate brain resources vis-à-vis the more challenging WWT condition. Importantly, the presence of diabetes was associated with worse letter generation performance during WWT. To our knowledge and consistent with Capacity Limitations the current investigation provided first evidence for reduced HbO2 levels in the PFC during attention-demanding locomotion in older adults with diabetes.
As expected, the dual-task manipulation resulted in slower stride velocity in WWT compared to NW among older adults with or without diabetes. It is noteworthy, however, that older adults with diabetes demonstrated smaller dual-task cost in stride velocity compared to older adults without diabetes (see table 2). While intuitively contradictory, there are two possible explanations to this finding. First, older adults with diabetes were significantly slower in NW compared to those without diabetes and thus had less to lose in terms of gait speed under WWT. Indeed, analysis examining the proportionality of dual-task costs (i.e., dual-task cost in velocity divided by STW velocity) supports this argument revealing insignificant differences in proportional dual-task costs between older adults with and without diabetes (p=0.192). Second, the posture first hypothesis suggests that due to evolutionary reasons, specifically safety and long-term survival, older adults prioritize walking over cognitive interference tasks (Lindenberger, Marsiske, & Baltes, 2000). We have recently refined the posture first hypothesis indicting that it was evident only among older adults with known locomotive limitations (Holtzer, Verghese, et al., 2016). A recent report found that the presence of peripheral diabetic neuropathy was associated with worse perception of steadiness and poorer walking performance (Reeves, Brown, Petrovic, Boulton, & Vileikyte, 2017). Hence, due to their already slower gait and possible concerns about stability and safety older adults with diabetes may have prioritized gait over the cognitive interference task, which resulted in reduced dual-task costs compared to participants without diabetes.
4.1. Clinical implications
Dual-task walking, a robust predictor of critical health and functional outcomes (Ayers et al., 2014; Verghese et al., 2012), can be measured relatively quickly without sophisticated equipment in normal and diseased populations. While it remains to be determined whether or not inclusion of this measure in risk assessment of older adults with diabetes will improve prediction accuracy of falls and other clinical and functional outcomes, reinforcement of the importance of exercise and institution of multifactorial intervention aimed at fall prevention could be helpful. It is also noteworthy that computerized cognitive remediation (Verghese, Mahoney, Ambrose, Wang, & Holtzer, 2010) and training in dual-task walking (Schwenk, Zieschang, Oster, & Hauer, 2010) resulted in improved dual-task walking performance. In light of documented impairments in executive functions in patients with diabetes it remains to be evaluated whether improving dual-task walking via cognitive and or locomotive interventions may improve brain efficiency and also reduce the incidence of adverse health outcomes in this patient population.
4.2. Limitations, strengths and future directions
Strengths of this investigation include the novelty and reliability of our experimental procedures, careful clinical characterization of the participants and multivariate analyses accounting for a range of possible confounders including neuropathic gait. Glucose blood levels were not available to confirm the diagnosis and type of diabetes. But the presence of diabetes was determined in a structured clinical interview and validated against medications prescribed only to diabetic patients. Measures of treatment adherence and glycemic control were not available. Future studies should examine whether the type of diabetes, disease duration and severity including HbA1c, are related to dual-task walking and its underlying brain substrates. While the analyses controlled for a number of possible confounders including general level of cognitive function, future studies comprising larger and more diverse samples of diabetic patients should examine whether or not executive functions and possibly other cognitive domains are related to performance and the functional neural underpinning of dual-task walking. We did not exclude participants with diabetic neuropathy or distinguish unilateral from bilateral gait abnormalities. But analyses controlled for the presence of different types of gait abnormalities including neuropathic gait, which were all assessed clinically and independently of the quantitative gait fNIRS protocol. It is also possible the small vessel disease may have contributed to the reduced blood flow observed in participants with diabetes during dual-task walking. The study design did not include traditional imaging in all participants that would have enabled further insights into underlying brain substrates that might influence the effect of diabetes on the behavioral and functional PFC correlates of locomotion. The current fNIRS system offers significant advantages in terms of portability and capability to assess cortical activation during active walking. While the system’s limitations in terms of depth of penetration and spatial resolution should be acknowledged numerous studies provided unequivocal evidence of it’s utility in measuring changes in PFC HbO2 levels in response to cognitive challenges. We have provided evidence of excellent reliability of the fNIRS signal across task conditions (Holtzer et al., 2015) and have recently reported on MRI fNIRs co-registration study further validating the use of this system among older adults (Chen et al., 2017). The laterality analysis was indicative of task specificity effects consistent with our (Holtzer et al., 2015) and previous (Dux, Ivanoff, Asplund, & Marois, 2006; Filmer, Mattingley, & Dux, 2013; Filmer, Mattingley, Marois, & Dux, 2013) studies implicating a left lateralized brain pattern in dual-tasking. Importantly, the moderating effect of diabetes on changes in HbO2 levels across task conditions did not change as a function of laterality. Future studies should utilize multi-modal neuroimaging to provide whole brain structural and functional context to the effect of diabetes on the fNIRS findings reported herein. HbO2 is more reliable and sensitive to locomotion-related changes in cerebral oxygenation compared to deoxygenated hemoglobin (Harada et al., 2009) and was thus used as the primary outcome in the current study. However, sensitivity analysis revealed a reciprocal decline in Hb levels across task conditions. While the reciprocal decline in Hb levels is not always observed (for review see (Hoshi, 2016)) its presence in the current study both complemented and provided further validation to our findings. Methodological issues that are concerned with the use of fNIRS including but not limited to skull thickness and skin response have been previously discussed (Erdogan, Yucel, & Akin, 2014) and should be considered in any investigation that uses this imaging technique. It is critical to emphasize that such limitations were not likely to influence the moderating effects of diabetes on task-related changes in PFC oxygenation levels given that experimental conditions were administered in a random order and had the same walking environment and physical requirements.
4.3. Conclusion
A distinct functional neural signature of diabetes was established during active and attention demanding walking. The potential clinical utility of dual-task walking and its underlying brain response for assessment and interventions of mobility limitations and falls risk in older adults with diabetes should be examined in future studies.
Highlights.
We evaluated the effect of diabetes on cortical control of gait in older adults.
Diabetes was associated with poorer walking performance.
Diabetes attenuated brain responses during active dual-task walking.
Findings may inform mobility risk assessments in older adults with diabetes.
Acknowledgments
This research was supported by the National Institutes on Aging grants (R01AG036921, R01AG044007), and by the NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Number KL2TR001071.
Footnotes
Potential Conflicts of Interest:
Dr. Izzetoglu has a very minor share in the company that manufactures the fNIRS device used in this study. All other authors have no conflicts of interest to report in relation to the current article.
Contribution statement
Roee Holtzer: conception and design, acquisition of data, analysis and interpretation of data, drafting the article and final approval; Claudene J George: interpretation of data, revising the article and final approval; Meltem Izzetoglu: data processing, revising the article and final approval; Cuiling Wang: data analysis and interpretation, revising the article and final approval.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam U, Riley DR, Jugdey RS, Azmi S, Rajbhandari S, D’Aout K, Malik RA. Diabetic Neuropathy and Gait: A Review. Diabetes Ther. 2017;8(6):1253–1264. doi: 10.1007/s13300-017-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allet L, Armand S, Golay A, Monnin D, de Bie RA, de Bruin ED. Gait characteristics of diabetic patients: a systematic review. Diabetes Metab Res Rev. 2008;24(3):173–191. doi: 10.1002/dmrr.809. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and progression of rigidity and gait disturbance in older persons. Neurology. 2004;63(6):996–1001. doi: 10.1212/01.wnl.0000138432.16676.4b. [DOI] [PubMed] [Google Scholar]
- Ayaz H, Izzetoglu M, Platek SM, Bunce S, Izzetoglu K, Pourrezaei K, Onaral B. Registering fNIR data to brain surface image using MRI templates. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2671–2674. doi: 10.1109/IEMBS.2006.260835. [DOI] [PubMed] [Google Scholar]
- Ayers EI, Tow AC, Holtzer R, Verghese J. Walking while talking and falls in aging. Gerontology. 2014;60(2):108–113. doi: 10.1159/000355119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj P, Wijesekara N, Liyanapathirana M, Newsholme P, Ittner L, Fraser P, Verdile G. The Link between Type 2 Diabetes and Neurodegeneration: Roles for Amyloid-beta, Amylin, and Tau Proteins. J Alzheimers Dis. 2017;59(2):421–432. doi: 10.3233/jad-161192. [DOI] [PubMed] [Google Scholar]
- Brach JS, Talkowski JB, Strotmeyer ES, Newman AB. Diabetes mellitus and gait dysfunction: possible explanatory factors. Phys Ther. 2008;88(11):1365–1374. doi: 10.2522/ptj.20080016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundel M, van den Heuvel M, de Bresser J, Kappelle LJ, Biessels GJ. Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci. 2010;299(1–2):126–130. doi: 10.1016/j.jns.2010.08.048. [DOI] [PubMed] [Google Scholar]
- CDC. National diabetes statistics report, 2014. CDC; 2014. National Diabetes Surveillance System. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. [Google Scholar]
- Chen M, Blumen HM, Izzetoglu M, Holtzer R. Spatial Coregistration of Functional Near-Infrared Spectroscopy to Brain MRI. J Neuroimaging. 2017;27(5):453–460. doi: 10.1111/jon.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RJ, Selb J, Gagnon L, Phillip D, Schytz HW, Iversen HK, … Boas DA. A systematic comparison of motion artifact correction techniques for functional near-infrared spectroscopy. Front Neurosci. 2012;6:147. doi: 10.3389/fnins.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bresser J, Tiehuis AM, van den Berg E, Reijmer YD, Jongen C, Kappelle LJ, … Biessels GJ. Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care. 2010;33(6):1309–1314. doi: 10.2337/dc09-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46(12):1604–1610. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]
- Duff K, Humphreys Clark JD, O’Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol. 2008;23(5):603–612. doi: 10.1016/j.acn.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Ivanoff J, Asplund CL, Marois R. Isolation of a central bottleneck of information processing with time-resolved FMRI. Neuron. 2006;52(6):1109–1120. doi: 10.1016/j.neuron.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan SB, Yucel MA, Akin A. Analysis of task-evoked systemic interference in fNIRS measurements: insights from fMRI. Neuroimage. 2014;87:490–504. doi: 10.1016/j.neuroimage.2013.10.024. [DOI] [PubMed] [Google Scholar]
- Filmer HL, Mattingley JB, Dux PE. Improved multitasking following prefrontal tDCS. Cortex. 2013;49(10):2845–2852. doi: 10.1016/j.cortex.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Filmer HL, Mattingley JB, Marois R, Dux PE. Disrupting prefrontal cortex prevents performance gains from sensory-motor training. J Neurosci. 2013;33(47):18654–18660. doi: 10.1523/jneurosci.2019-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramigna V, Pellegrino G, Cerasa A, Cutini S, Vasta R, Olivadese G, … Quattrone A. Near-Infrared Spectroscopy in Gait Disorders: Is It Time to Begin? Neurorehabil Neural Repair. 2017;31(5):402–412. doi: 10.1177/1545968317693304. [DOI] [PubMed] [Google Scholar]
- Harada T, Miyai I, Suzuki M, Kubota K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res. 2009;193(3):445–454. doi: 10.1007/s00221-008-1643-y. [DOI] [PubMed] [Google Scholar]
- Hernandez ME, Holtzer R, Chaparro G, Jean K, Balto JM, Sandroff BM, … Motl RW. Brain activation changes during locomotion in middle-aged to older adults with multiple sclerosis. J Neurol Sci. 2016;370:277–283. doi: 10.1016/j.jns.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69(11):1375–1388. doi: 10.1093/gerona/glu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Mahoney J, Verghese J. Intraindividual variability in executive functions but not speed of processing or conflict resolution predicts performance differences in gait speed in older adults. J Gerontol A Biol Sci Med Sci. 2014;69(8):980–986. doi: 10.1093/gerona/glt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J. Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage. 2015;112:152–159. doi: 10.1016/j.neuroimage.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Schoen C, Demetriou E, Mahoney JR, Izzetoglu M, Wang C, Verghese J. Stress and gender effects on prefrontal cortex oxygenation levels assessed during single and dual-task walking conditions. Eur J Neurosci. 2016 doi: 10.1111/ejn.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Allali G, Izzetoglu M, Wang C, Mahoney JR. Neurological Gait Abnormalities Moderate the Functional Brain Signature of the Posture First Hypothesis. Brain Topogr. 2016;29(2):334–343. doi: 10.1007/s10548-015-0465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across-neuropsychological test variability and incident dementia. JAMA. 2008;300(7):823–830. doi: 10.1001/jama.300.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20(2):215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Wang C, Verghese J. Performance variance on walking while talking tasks: theory, findings, and clinical implications. Age (Dordr) 2014;36(1):373–381. doi: 10.1007/s11357-013-9570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Yuan J, Verghese J, Mahoney JR, Izzetoglu M, Wang C. Interactions of Subjective and Objective Measures of Fatigue Defined in the Context of Brain Control of Locomotion. J Gerontol A Biol Sci Med Sci. 2016 doi: 10.1093/gerona/glw167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi Y. Hemodynamic signals in fNIRS. Prog Brain Res. 2016;225:153–179. doi: 10.1016/bs.pbr.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Izzetoglu M, Chitrapu P, Bunce S, Onaral B. Motion artifact cancellation in NIR spectroscopy using discrete Kalman filtering. Biomed Eng Online. 2010;9:16. doi: 10.1186/1475-925X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzetoglu M, Izzetoglu K, Bunce S, Ayaz H, Devaraj A, Onaral B, Pourrezaei K. Functional near-infrared neuroimaging. IEEE Trans Neural Syst Rehabil Eng. 2005;13(2):153–159. doi: 10.1109/TNSRE.2005.847377. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545302/5648/1181. [pii] [DOI] [PubMed] [Google Scholar]
- Kooistra M, Geerlings MI, Mali WP, Vincken KL, van der Graaf Y, Biessels GJ. Diabetes mellitus and progression of vascular brain lesions and brain atrophy in patients with symptomatic atherosclerotic disease. The SMART-MR study. J Neurol Sci. 2013;332(1–2):69–74. doi: 10.1016/j.jns.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: Increase in dual-task costs from young adulthood to old age. Psychology and Aging. 2000;15(3):417–436. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- Maidan I, Bernad-Elazari H, Giladi N, Hausdorff JM, Mirelman A. When is Higher Level Cognitive Control Needed for Locomotor Tasks Among Patients with Parkinson’s Disease? Brain Topogr. 2017;30(4):531–538. doi: 10.1007/s10548-017-0564-0. [DOI] [PubMed] [Google Scholar]
- Maidan I, Nieuwhof F, Bernad-Elazari H, Reelick MF, Bloem BR, Giladi N, … Mirelman A. The Role of the Frontal Lobe in Complex Walking Among Patients With Parkinson’s Disease and Healthy Older Adults: An fNIRS Study. Neurorehabil Neural Repair. 2016;30(10):963–971. doi: 10.1177/1545968316650426. [DOI] [PubMed] [Google Scholar]
- Maksimovic A, Hanewinckel R, Verlinden VJ, Ligthart S, Hofman A, Franco OH, … Ikram MA. Gait characteristics in older adults with diabetes and impaired fasting glucose: The Rotterdam Study. J Diabetes Complications. 2016;30(1):61–66. doi: 10.1016/j.jdiacomp.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Manschot SM, Biessels GJ, de Valk H, Algra A, Rutten GE, van der Grond J, Kappelle LJ. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia. 2007;50(11):2388–2397. doi: 10.1007/s00125-007-0792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman A, Maidan I, Bernad-Elazari H, Shustack S, Giladi N, Hausdorff JM. Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn. 2017;115:41–46. doi: 10.1016/j.bandc.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognit Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. S0010-0285(99)90734-X [pii] [DOI] [PubMed] [Google Scholar]
- Moheet A, Mangia S, Seaquist ER. Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci. 2015;1353:60–71. doi: 10.1111/nyas.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C, Beare R, Phan T, Starkstein S, Bruce D, Romina M, Srikanth V. Neuroimaging and its Relevance to Understanding Pathways Linking Diabetes and Cognitive Dysfunction. J Alzheimers Dis. 2017;59(2):405–419. doi: 10.3233/jad-161166. [DOI] [PubMed] [Google Scholar]
- Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, … Srikanth V. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013;36(12):4036–4042. doi: 10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378(9786):169–181. doi: 10.1016/s0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- Palta P, Carlson MC, Crum RM, Colantuoni E, Sharrett AR, Yasar S, … Golden SH. Diabetes and Cognitive Decline in Older Adults: The Ginkgo Evaluation of Memory Study. J Gerontol A Biol Sci Med Sci. 2017 doi: 10.1093/gerona/glx076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves ND, Brown SJ, Petrovic M, Boulton AJM, Vileikyte L. How Does Self-Perceived Unsteadiness Influence Balance and Gait in People With Diabetes? Preliminary Observations. Diabetes Care. 2017;40(5):e51–e52. doi: 10.2337/dc16-2183. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12(1):174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, Bennett DA, … Rosano C. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68(11):1379–1386. doi: 10.1093/gerona/glt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92(3):454–462. doi: 10.2522/ptj.20100397. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D’Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14(5):721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Sadanand S, Balachandar R, Bharath S. Memory and executive functions in persons with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2016;32(2):132–142. doi: 10.1002/dmrr.2664. [DOI] [PubMed] [Google Scholar]
- Sawacha Z, Gabriella G, Cristoferi G, Guiotto A, Avogaro A, Cobelli C. Diabetic gait and posture abnormalities: a biomechanical investigation through three dimensional gait analysis. Clin Biomech (Bristol, Avon) 2009;24(9):722–728. doi: 10.1016/j.clinbiomech.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Schwenk M, Zieschang T, Oster P, Hauer K. Dual-task performances can be improved in patients with dementia: a randomized controlled trial. Neurology. 2010;74(24):1961–1968. doi: 10.1212/WNL.0b013e3181e39696. WNL.0b013e3181e39696 [pii] [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, … Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. 305/1/50 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elderen SG, de Roos A, de Craen AJ, Westendorp RG, Blauw GJ, Jukema JW, … van der Grond J. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology. 2010;75(11):997–1002. doi: 10.1212/WNL.0b013e3181f25f06. [DOI] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Lipton RB, Wang C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc. 2012;60(10):1901–1905. doi: 10.1111/j.1532-5415.2012.04145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Mahoney J, Ambrose AF, Wang C, Holtzer R. Effect of cognitive remediation on gait in sedentary seniors. J Gerontol A Biol Sci Med Sci. 2010;65(12):1338–1343. doi: 10.1093/gerona/glq127. [DOI] [PubMed] [Google Scholar]
- Verghese J, Wang C, Ayers E, Izzetoglu M, Holtzer R. Brain activation in high- functioning older adults and falls: Prospective cohort study. Neurology. 2016 doi: 10.1212/wnl.0000000000003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Age-related changes in brain activation during a delayed item recognition task. Neurobiology of Aging. 2007;28(5):784–798. doi: 10.1016/j.neurobiolaging.2006.03.002. [DOI] [PubMed] [Google Scholar]