Abstract
Purpose
To develop a method that can separate and quantify the fast (>1 kHz) and slow exchange transfer and magnetization transfer components in Z-spectra.
Methods
Z-spectra were recorded as a function of mixing time using a train of selective pulses providing variable delay multi-pulse (VDMP) build-up curves. Fast and slow transfer components in the Z-spectra were separated and quantified on a voxel-by-voxel basis by fitting the mixing time dependent CEST signal using a three-pool model.
Results
Phantom studies of glutamate (Glu) solution, bovine serum albumin (BSA) solution, and hair conditioner showed the capability of the proposed method to separate fast and slow transfer components. In vivo mouse brain studies showed a strong contrast between white matter and gray matter in the slow-transferring map, corresponding to an asymmetric component of the conventional semi-solid magnetization transfer contrast (MTC). In addition, a fast-transferring proton map was found that was homogeneous across the brain and attributed to the total contributions of the fast-exchanging protons from proteins, metabolites and a symmetric MTC component.
Conclusions
This new method provides a simple way to extract fast and slow transfer components from the Z-spectrum leading to novel MRI contrasts and providing insight into the different MTC contributions.
Keywords: Chemical Exchange Saturation Transfer (CEST), variable delay multi-pulse (VDMP), Magnetization Transfer Contrast (MTC), Fast-exchanging Protons, Slow Magnetization Transfer, Fast Magnetization Transfer
Introduction
CEST MRI enhances the detection sensitivity of dilute metabolites using their exchangeable protons (1-5). It has been applied to detect amide protons (APT-CEST) from proteins and peptides (6, 7), as well as endogenous metabolites (8-15). Although the signal is enhanced, a challenge for CEST is specificity, namely to separate exchanging protons from different molecules. Many exchanging protons, e.g. amine and hydroxyl groups, are broadened due to their rapid chemical exchange, causing their signals in Z-spectra to coalesce with the water resonance. Conventionally, the saturation power (B1) and length are optimized to achieve some selectivity to exchange rate (8, 15-18). However, contamination from both the exchange transfer and other magnetization transfer components from lipids, proteins, semi-solid macromolecules and other metabolites generally complicates this simplified approach. A recent strategy is to acquire a full Z-spectrum with low saturation power, and to fit it by assuming a Lorentzian line-shape for each exchanging pool, including water, amide, guanidinium and relayed nuclear Overhauser effect (rNOE) peaks (14, 19-21). Although this approach can improve specificity, it cannot separate the many overlapping signals such as those from amine and hydroxyl groups or from non-exchangeable aromatic protons that transfer magnetization to water through a rNOE process (2, 20). When applying high B1, the Z-spectrum becomes even more complicated because of stronger contamination by direct water saturation (DS), the increased signal from semi-solid macromolecules, and the overlapping coalesced signals of fast exchanging protons (4).
The development of pulsed-CEST provides a tool for Z-spectral editing in terms of separating different types of exchanging protons by their relaxation time, line-shape or exchange rate (12, 22-35). For instance, frequency-labeled exchange transfer (FLEX) MRI can selectively detect amide protons and aliphatic protons (26) in the brain, and chemical exchange rotation transfer (CERT) can extract the creatine signal (12, 31, 36). The off-resonance variable delay multi-pulse CEST (VDMP-CEST) (33, 35) is a pulsed CEST sequence in which the mixing time between pulses is varied to exploit the notion that slow-exchanging protons and slow relayed NOEs (rNOEs) require extra time to fully exchange with water after RF labeling. This is similar to the nuclear Overhauser spectroscopy (NOESY) and exchange spectroscopy (EXSY) experiments in high-resolution NMR (37). The off-resonance VDMP approach was originally used to detect slow-transferring magnetization of amide and aliphatic protons, by applying weak saturation pulses at one particular frequency. This low saturation power minimized contamination from fast-exchanging protons. Due to its sensitivity to exchange rates in the slow- to intermediate-range, VDMP-CEST can also be used as an transfer rate filter to remove the MTC contribution by performing difference editing as a function of mixing time, allowing selective detection of amide and aliphatic protons in human brain (33, 35). When monitoring fast-exchanging protons using VDMP MRI at high B1, the VDMP build curves (i.e., the CEST signal as a function of mixing time) become complicated due to the combined effects of fast and slow exchange transfer and conventional magnetization transfer effects at the same offset. Under such conditions, the CEST signals from slow- or fast-exchanging solute protons cannot be obtained by simple subtraction between two images. Recently, the CEST signals from fast-exchanging protons could be selected in tissues using the on-resonance VDMP technique (i.e. pulse offset at the water resonance frequency) with high-power binomial pulses to achieve their efficient labeling (34). This on-resonance VDMP (onVDMP) approach treats all fast-exchanging protons as one single pool, called the ‘total fast-exchanging proton’ (TFP) pool (38). As such, the onVDMP experiment closely resembles the T1ρ experiment (39-42), but with the added ability to distinguish slow- and fast-exchanging components based on their unique VDMP buildup patterns. However, similar to T1ρ dispersion, onVDMP lacks the chemical shift information needed for specificity. Here, we extend the idea of separating fast- and slow-transferring protons to the off-resonance VDMP sequence. In addition, we are more careful and use the general terminology “transfer”, which applies to both proton exchange and conventional magnetization transfer components, because the semisolid tissue components also have fast (due to exchangeable protons) and slow (due to rNOEs) transfer components. Thus, from now on we will use “fast-transferring” proton (FP) and “slow-transferring” proton pool nomenclature. The technique was verified on a glutamate (Glu) solution, a bovine serum albumin (BSA) solution, and an MTC phantom (hair conditioner). We then performed in vivo mapping of the signal contributions from the slow and fast exchange transfer and magnetization transfer components in healthy mouse brain.
Methods
MRI Pulse Sequence
The VDMP pulse sequence (27, 35) is shown in Fig. 1a. The width of the Gaussian pulse has to be long enough to minimize the DS, but cannot be too long in order to avoid suppressing the VDMP buildup curves (35). In practice, a pulse width of 10 ms was found to be a good balance as demonstrated in the supporting information and the simulations in the supporting figure S1. The magnetization transfer behavior for exchangeable protons during the VDMP sequence is very different for slow and fast transfer components (33-35). In tissue, both fast and slow transfer components are abundant (4, 43). Therefore, the VDMP build-up curves in vivo have to be fitted by at least a three-pool model, e.g. the water pool and slow- and fast-transferring proton pools, to account for the observed curves (34). The detailed theory can be found in the supporting information. The γ̄slowxslow and γ̄fastxfast maps, corresponding to the signals from the slow- and fast- transferring components generated by one single labeling pulse, respectively, will be determined using the VDMP buildup curves. Here, γ is the labeling fraction and x the transferring proton fraction relative to water. Notice that the signal transferred (ΔS/S0) due to the combined CEST and magnetization transfer effects will be the total effect of all pulses, including the enhancement factor that corrects for the number of pulses and the effect of T1 relaxation over the time spread of all pulses over the total preparation time (30). In current study, with a short preparation time, the total enhancement therefore is about 16 times the γ̄slowxslow or γ(x000304)fastxfast values.
Figure 1.
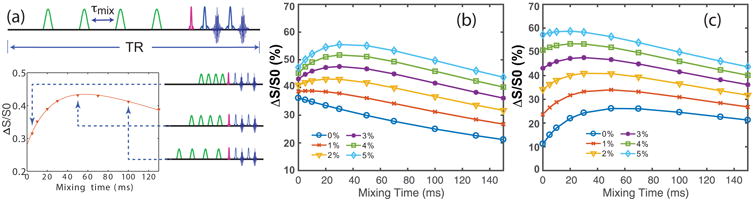
(a) The timing diagram of the off-resonance VDMP sequence. A train of Gaussian selective pulses is applied for saturation. The acquisition of VDMP buildup curves at each offset is illustrated below the sequence. It was performed by fixing the pulse number and increasing the inter-pulse delay, i.e., the mixing time tmix. (b) The simulated VDMP buildup curves by setting the fast-transferring proton contribution (γ̄fastxfast) as a constant (3% of the water proton magnetization), while the slow-transferring proton contribution (γ̄slowxslow) was varied from 0% to 5% of the water proton magnetization with an increment of 1%. (c) Simulated VDMP buildup curves using the same parameters as those in Fig. b except γ̄slowxslow was set to be constant (3% of the water proton magnetization), while γ̄fastxfast was varied from 0% to 5% of the water proton magnetization. Exchange rates were 2 kHz and 30 Hz for fast- and slow-transferring protons, respectively, T1w was 1.9 s, the pulse width was 10 ms, and the number of pulses was 16.
Simulations
In order to demonstrate the dependency on the concentrations of the fast- and slow-transferring components, the VDMP buildup curves were simulated using Eqs. S1-S2 in the supporting information with two sets of situations. In the first set, the fast-transferring proton fraction was assumed to be constant (3% of the water proton magnetization), while the slow-transferring proton fraction was varied (0% to 5% of the water proton magnetization). In the second set, the slow-transferring proton fraction was set at 3% of the water proton magnetization and the fast-transferring proton fraction was varied (0% to 5% of the water proton magnetization).
Phantoms
Glutamate (Glu, 50 mM, approximately 0.1% proton fraction), bovine serum albumin (BSA, 10%w/v, approximately 0.79% proton fraction for amides, 0.11% proton fraction for hydroxyls and 0.46% proton fraction for amines), and hair conditioner (Suave) were selected to represent a metabolite, a mobile protein and MTC pools found in tissue. BSA contains a large amount of both amine and amide protons, allowing demonstration of the capability of separating fast- (amine) and slow-exchanging (amide) protons by the current method. Except for the hair conditioner, phantoms were prepared in phosphate buffered saline (PBS), titrated to pH 7.3. All samples were studied in 5 mm NMR tubes.
Animals
The institutional animal care and use committee approved this study. Three adult female SCID mice (NCI, Frederick, MD), approximately 6-8 weeks old, were used. All animals were anesthetized using 2% isoflurane in airflow, followed by 1% to 1.5% isoflurane during the MRI scan.
MR Scanning
All MRI experiments were performed on a horizontal bore 11.7 T Bruker Biospec system (Bruker, Ettlingen, Germany). Phantoms were studied at room temperature. Images were acquired using a RARE sequence with TE = 4 ms, TR=5 s, slice thickness = 2 mm, and a matrix size of 32 × 32. The VDMP Z-spectra were collected from -3000 to 3000 Hz in increments of 200 Hz. For each offset, eight mixing times (5, 10, 20, 30, 50, 70, 100, and 125 ms) were collected with 16 Gaussian pulses of a 10 ms pulse width and three different maximum B1 values (2.9 μT, 5.9 μT and 11.8 μT). The matrix size is 64× 64 (with a FOV of 1.6 × 1.6 cm2) for high-resolution VDMP imaging on mouse brain. Two maximum B1 values (5.9 μT and 11.8 μT) were used on animal studies. T1 values on both mouse brain and phantoms were obtained using a variable-TR RARE sequence with variable TR (TR = 0.5, 1, 1.5, 2, 3.5, 5, 8 s).
Data Analysis
Data were analyzed with custom-written MATLAB scripts (34). When fitting the 3-pool VDMP build-up curves at each offset, three parameters (γ̄slowxslow, kslow, γ̄fastxfast) were varied. A chi-square (χ2) goodness-of-fit analysis was performed for each offset. An average transfer rate of 2 kHz was assumed for fast-transferring protons as the VDMP build-up curve is nearly independent of the transfer rate higher than 1 kHz (34). Then, we obtained three maps for each saturation offset: a slow-transferring proton map (γ̄slowxslow), the corresponding rate map (kslow), and a fast-transferring proton map (γ̄fastxfast). The measured T1w values obtained using the variable-TR RARE sequence were used in the fitting.
Results
Simulations
The simulated VDMP buildup curves shown in Figs. 1b, c demonstrate that the shape of the buildup curves is extremely sensitive to the ratio of slow- and fast-transferring protons: the addition of fast-transferring protons flattens the curves, while the addition of slow-exchanging protons shows increased saturation buildup. Therefore, the VDMP buildup curves have the potential to provide insight into the relative contributions of the two pools due to their different VDMP buildup patterns.
Phantoms
The VDMP Z-spectra of hair conditioner, Glu, and BSA (at two B1 levels) are plotted in Figs. 2a-d, together with the spectra for the slow ((γ̄slowxslow) and fast ((γ̄fastxfast) components obtained by fitting the VDMP build-up curves at each offset (Figs. 2e-h). The Z-spectra of hair conditioner (Figs. 2a,e) show mainly a slow-transferring component with an asymmetric profile with respect to the water resonance that has its frequency centered in the aliphatic proton range. On the contrary, only a fast-exchanging component is present in the transfer spectra of the Glu solution (Figs. 2b,f). The measured fast-transferring component at 2 ppm is around 1%, which is a factor of ten higher than the amine proton fraction (0.11%), which is due to the CEST effect enhancing the sensitivity by the single 10 ms pulse. The separating results of the BSA sample shows both fast- and slow-transferring components (Figs. 2g,h). A fast-transferring component is visible on the positive frequency side compared to water, showing a coalesced line-shape with water. Slow-transferring components are found on both sides, tentatively assigned to amide protons (3.6 ppm), and relayed NOEs (rNOEs) of aromatic protons at positive frequency (1-2.5 ppm) and of aliphatic protons at negative frequency (multiple resonances with apparent maximum at -3.6 ppm), respectively. The fast component shows a pattern similar to that of the Glu solution, and is tentatively assigned to the amine protons (20, 43). The fitting of the buildup curve is excellent (χ2 <2 × 10−3, Figs. 2i-l) in the frequency ranges where strong CEST signal is present. On the contrary, the fitting can be worse when the CEST signal is low compared to the noise background. The amide (3.6 ppm), rNOE (-3.6 ppm), and amine CEST (2 ppm) signals for the BSA solution are plotted as a function of saturation power in Fig. 2m. While the signal intensity of the slow exchanging component increases only slightly with B1, that of the fast-exchanging component increases about ten-fold (44, 45). The exchange rates of hair conditioner were determined to be around 36±2 Hz and 48±2 Hz at -3.6 ppm and 3.6 ppm, respectively, while the exchange rates were 23±3 Hz and 12±2 Hz for the BSA at the offsets of 3.6 ppm (amide proton) and -3.6 ppm (aliphatic protons), respectively. The measured slow-transferring component (0.25-0.4%) is smaller than the proton fraction (0.79%) in BSA due to two factors: the labeling fraction γ is lower than one and some of the amide protons have a very slow exchange rate with water. Similar to the CEST experiments on the Glu phantom, the measured fast-transferring component for amine protons in BSA with high labeling power (1.4% at 11.8 μT) are also higher than the amine proton fraction in BSA (0.46%).
Figure 2.
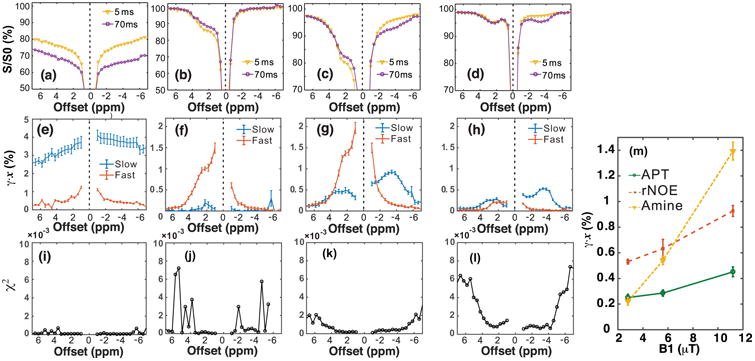
VDMP-CEST Z-spectra at mixing times of 5 ms and 70 ms for hair conditioner (a, e), glutamate (Glu) with peak saturation power 11.8 μT (b, f), BSA solution with peak saturation power 11.8 μT (c, g) and BSA solution with peak saturation power 2.9 μT (d, h). (e-h) The transfer spectra of the corresponding slow-(γ̄slowxslow) and fast-transferring (γ̄fastxfast) components obtained by fitting the VDMP build-up curves at different offsets for the phantoms in (a-d). (i-l) The chi-square goodness-of-fit test for each offset are plotted. (m) The APT, rNOE, and amine CEST signal of BSA solution obtained by fitting the VDMP build-up curves using a three-pool model as a function of peak saturation power. The error estimations were carried out by fitting the data pixel-by-pixel, and then calculating its average value and standard variation. The VDMP sequence consisted of 16 pulses of 10 ms length.
In vivo MRI
Figure 3 shows the VDMP Z-spectra at two mixing times (Figs. 3a,b), as well as the fitted transfer spectra for fast- and slow-transferring components (Figs. 3c,d) in the mouse brain recorded with saturation powers of 5.9 μT (Figs. 3a, c) and 11.8 μT (Figs. 3b,d). The chi-square goodness-of-fit is plotted in Figs. 3e,f and indicated excellent fitting for each offset (χ;2< 5 × 10−4) due to the strong MTC/CEST signal in vivo. The VDMP build-up curves, together with the fitting results from the three-pool model for the frequencies at ±3.6 ppm are also plotted for the low and high B1 values (Figs. 3g,h, respectively). The slow-transferring component spectra at both B1 levels strongly resemble those in the hair conditioner phantom and are assigned to arise mainly from the MTC component. In the current study, high saturation powers were applied, which caused the amide and aliphatic peaks to be obscured by the strong MTC components (35). In order to distinguish the MTC component with slow transfer rate from other chemical exchanging pools, such as the APT-CEST and the rNOE-CEST, the component is called slow-MTC. The slow-MTC shows a complicated line shape with a maximum signal around -3.6 ppm, and is not symmetric with respect to the water frequency. This is consistent with previous studies indicating that the slow-MTC pool arises mainly from the aliphatic chains of the immobilized macromolecules (46, 47). With a saturation power of 11.8 μT, the transfer rates (kslow) for the slow-MTC were 25±3 Hz and 35±2 Hz for the offsets at -3.6 ppm and 3.6 ppm, respectively. The fast-transferring component spectrum shows a constant background in the region studied, i.e., based on the signal from -7 to -4 ppm and to 4 to 7 ppm, which is attributed to a symmetric fast-MTC component that increases with B1 (∼0.5% at B1 = 5.9 μT and 1% at 11.8 μT). In addition to this MTC part there were larger fast components closer to water, showing a clear asymmetry with respect to the water resonance. These components are assigned mainly to the fast-exchanging protons (FP) from metabolite and mobile protein OH and NH2 groups and of course the symmetric fast-MTC baseline, most likely of OH protons in the semisolid. The assignments of slow-MTC, fast-MTC and FP pools are indicated in Fig. 3c. In current study, fast exchanging proton signals over a small frequency range are detected, compared to the total of all fast-transferring components, i.e. TFP, detected in onVDMP method (38). Therefore, the name FP is used to show the difference. Notice that the FP pool may have a partial contribution from the fast-MTC pool.
Figure 3.
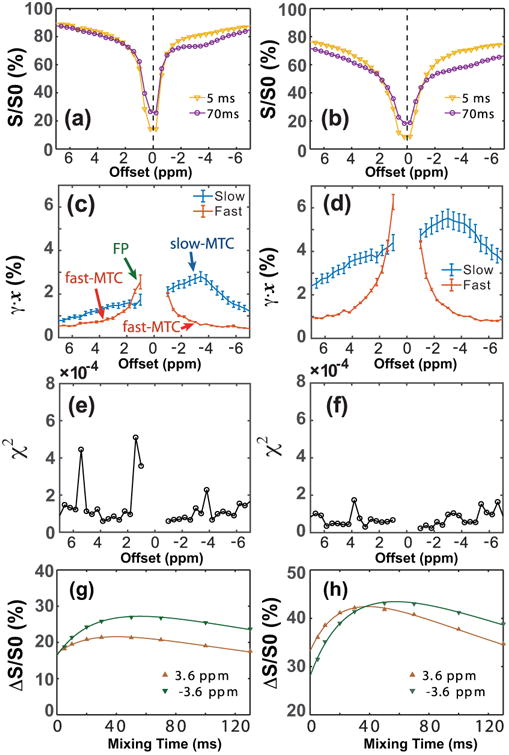
In vivo Z-spectra of the mouse brain at mixing times of 5 ms and 70 ms for the B1 values of (a) 5.9 μT and (b) 11.8 μT. The transfer spectra corresponding slow- (γ̄slowxslow) and fast-transferring (γ̄fastxfast) components are in (c,d). The corresponding chi-square goodness-of-fit for each offset are plotted (e,f). Experiments were performed using 16 Gaussian pulses (10 ms pulse width). (g,h) The VDMP build-up curves, as well as the fitting results using the three-pool model are plotted for the peak powers of 5.9 μT (g) and 11.8 μT (h), respectively. The curves of slow-MTC, fast-MTC and FP pools are indicated in (c). Six different regions-of-interest were chosen for the error estimations.
Slow-MTC, fast-MTC, and FP maps at ±3.6 ppm and 2 ppm are plotted in Fig. 4. The slow-MTC maps at the three frequency offsets all show a similar clear contrast between GM and WM, with strong saturation in white matter, confirming our assignment to an MTC origin. The fast-MTC and FP map show homogeneous intensity across the brain at the three offsets. This observation is consistent with previously reported slow-MTC and total fast proton (TFP) map measured using the on-resonance VDMP technique (34).
Figure 4.
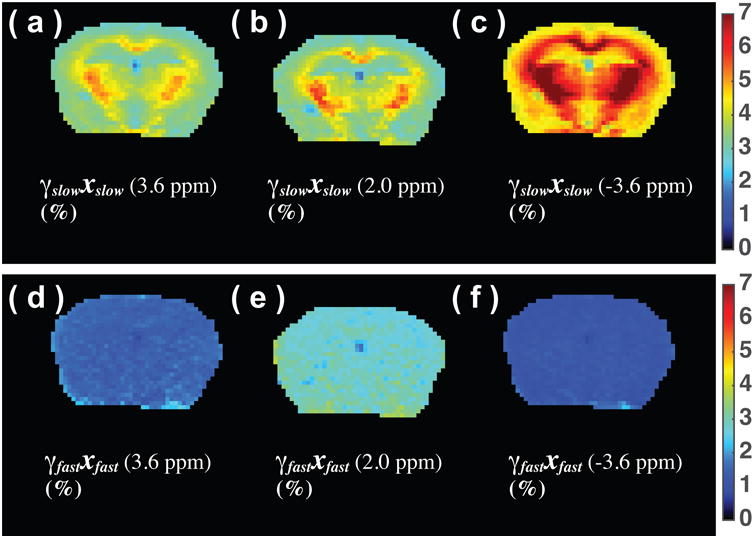
Slow-transfer component map (γ̄slowxslow) of a mouse brain at 3.6 ppm (a), 2 ppm (b) and -3.6 ppm offsets (c), as well as the fast-transfer proton (FP) images (γ̄fastxfast) at frequency offsets of 3.6 ppm (d), 2 ppm (e), and -3.6 ppm (f). The images were recorded using 16 Gaussian pulses (10 ms pulse width) with peak saturation power 11.8 μT.
Discussion
We demonstrated that off-resonance VDMP can be used as an transfer rate filter to distinguish and quantify slow- and fast-transferring components in Z-spectra by varying the mixing times. The method revealed several pools in tissues based on their different transfer rates that would be difficult to access using conventional MTC/CEST techniques.
The Z-spectrum of BSA (Figs.2c,d) at around 2-3 ppm is dominated by the amine protons particularly at high saturation power (Fig. 2c). The separation of amide and amine protons is extremely difficult with the commonly used Lorentzian line-shape fitting technique due to the serious overlap between the two proton groups and the coalesced shape of the amine resonance, while the off-resonance VDMP method could accomplish this (Figs. 2g&h). The amide protons, as well as the aromatic protons in BSA between 1-2.5 ppm, are clearly visible in the slow-exchanging Z-spectra.
Recently, a transient MTC technique, which resembles the VDMP approach, was used to study the MTC parameters in human brain at 7T MRI, which resembles the VDMP approach, and also provided information about the transfer rates and the line-shape of the macromolecular components (47, 48). The offVDMP scheme has the additional capability of separating the fast- and slow-transferring components that exist in Z-spectra. In the mouse brain, at the high saturation power applied in the current study, the amide and aliphatic peaks overlap with the broad slow-MTC line-shape. Therefore, the exchange rates at each offset become a proportionally averaged value between slow-MTC and the mobile-protein based amide/aliphatic proton transfer rates, and show a measurable difference at the aliphatic (-3.6 ppm, 25±3 Hz) and amide (3.6 ppm, 35±3 Hz) frequency offsets. The current study presents direct evidence that MTC contains fast-exchanging components, which is often neglected but in line with considerations put forward by previous study (49). The contributions to the fast components in the transfer spectra in the region between -4 ppm to 4 ppm are more difficult to assign. Besides some small effect remaining from mixing time dependence of the direct water saturation, the fast-transferring protons from mobile proteins and metabolites, i.e. the FP pool, and bound water and semi-solid OH groups may be the major sources. The line-shape of the FP pool closely resembles that of the fast-exchanging protons in vivo detected by other techniques (50, 51), in which the protein was also indicated as the major source for the FP pool. The complicated line-shapes and presence of multiple MTC pools suggest that caution is required when fitting the Z-spectra using simple Lorentzian line-shape analysis.
In the current mouse study, we used high B1 levels to focus on FP in addition to MTC contributions. Similar to conventional MTC, the slow-MTC also showed high contrast between the WM and GM in the mouse brain at all frequency offsets measured, which indicates that a large amount of slow-MTC probably arises from myelin lipids. The fast-MTC pool shows uniform signal across the brain, similar to the TFP map (34). Further studies are needed to confirm the contributions to the fast-MTC and FP. In the current study, the off-VDMP was performed at a wide range of saturation offsets to investigate the contributions of the fast- and slow-transferring protons in tissues. For the potential clinical application of the technique, the number of saturation offsets can be significantly reduced to a few frequencies of interest to reduce the experimental time.
Conclusion
It was shown that the offVDMP technique can extract fast- and slow-transferring components from the Z-spectra of phantoms and mouse brains in vivo. The broad slow-transferring component under high B1 situation shows asymmetric with respect to water was attributed mainly to slow-MTC. At large offsets, a symmetric MTC component (fast-MTC) was found. The results thus provide insight not only into all transferring proton pools in the brain, but especially offers a new technique that can obtain slow-MTC (asymmetric), fast-MTC (symmetric) and fast-exchanging proton components in tissue.
Supplementary Material
Figure S1: The simulated excitation profile of a train of Gaussian pulses (1, 4 and 16 pules) on water signal for the peak B1 values of 5.9 μT (180 degree) (a) and 11.8 μT (360 degree) (b). In the simulation, the water T1 and T2 were set to 2.0 s and 40 ms, respectively. The pulse width was 10 ms.
Acknowledgments
This work was supported by R01EB015032, P41EB015909 and R01EB019934. Lin Chen thanks the China Scholarship Council (201506310130) for financial support.
Grant support from NIH: R01EB015032, P41EB015909 and R01EB019934.
References
- 1.Ward KM, Aletras AH, Balaban RS. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST) J Magn Reson. 2000;143(1):79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 2.van Zijl PCM, Yadav NN. Chemical exchange saturation transfer (CEST): What is in a name and what isn't? Magn Reson Med. 2011;65(4):927–48. doi: 10.1002/mrm.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu G, Song X, Chan KW, et al. Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed. 2013;26(7):810–28. doi: 10.1002/nbm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Zijl PCM, Lam WW, Xu J, et al. Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.04.045. 10.1016/j.neuroimage.2017.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones KM, Pollard AC, Pagel MD. Clinical applications of chemical exchange saturation transfer (CEST) MRI. J Magn Reson Imaging. 2017 doi: 10.1002/jmri.25838. 10.1002/jmri.25838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Payen JF, Wilson DA, et al. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085–90. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Tryggestad E, Wen Z, et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med. 2011;17(1):130–4. doi: 10.1038/nm.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai K, Haris M, Singh A, et al. Magnetic resonance imaging of glutamate. Nat Med. 2012;18(2):302–6. doi: 10.1038/nm.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis KA, Nanga RP, Das S, et al. Glutamate imaging (GluCEST) lateralizes epileptic foci in nonlesional temporal lobe epilepsy. Sci Transl Med. 2015;7(309):309ra161. doi: 10.1126/scitranslmed.aaa7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling W, Regatte RR, Navon G, et al. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proc Natl Acad Sci U S A. 2008;105(7):2266–70. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Zijl PC, Jones CK, Ren J, et al. MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST) Proc Natl Acad Sci U S A. 2007;104(11):4359–64. doi: 10.1073/pnas.0700281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zu Z, Louie EA, Lin EC, et al. Chemical exchange rotation transfer imaging of intermediate-exchanging amines at 2 ppm. NMR Biomed. 2017;30(10) doi: 10.1002/nbm.3756. 10.1002/nbm.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang XY, Xie J, Wang F, et al. Assignment of the molecular origins of CEST signals at 2 ppm in rat brain. Magn Reson Med. 2017 doi: 10.1002/mrm.26802. 10.1002/mrm.26802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai K, Singh A, Poptani H, et al. CEST signal at 2ppm (CEST@2ppm) from Z-spectral fitting correlates with creatine distribution in brain tumor. NMR Biomed. 2015;28(1):1–8. doi: 10.1002/nbm.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haris M, Singh A, Cai K, et al. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nat Med. 2014;20(2):209–14. doi: 10.1038/nm.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon MT, Gilad AA, Zhou J, et al. Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): Ph calibration for poly-L-lysine and a starburst dendrimer. Magn Reson Med. 2006;55(4):836–47. doi: 10.1002/mrm.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin T, Autio J, Obata T, et al. Spin-locking versus chemical exchange saturation transfer MRI for investigating chemical exchange process between water and labile metabolite protons. Magn Reson Med. 2011;65(5):1448–60. doi: 10.1002/mrm.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JS, Xia D, Jerschow A, et al. In vitro study of endogenous CEST agents at 3 T and 7 T. Contrast Media Mol Imaging. 2016;11(1):4–14. doi: 10.1002/cmmi.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desmond KL, Moosvi F, Stanisz GJ. Mapping of amide, amine, and aliphatic peaks in the CEST spectra of murine xenografts at 7 T. Magn Reson Med. 2014;71(5):1841–53. doi: 10.1002/mrm.24822. [DOI] [PubMed] [Google Scholar]
- 20.Zaiss M, Windschuh J, Goerke S, et al. Downfield-NOE-suppressed amide-CEST-MRI at 7 Tesla provides a unique contrast in human glioblastoma. Magn Reson Med. 2017;77(1):196–208. doi: 10.1002/mrm.26100. [DOI] [PubMed] [Google Scholar]
- 21.Zhou IY, Wang E, Cheung JS, et al. Quantitative chemical exchange saturation transfer (CEST) MRI of glioma using Image Downsampling Expedited Adaptive Least-squares (IDEAL) fitting. Sci Rep. 2017;7(1):84. doi: 10.1038/s41598-017-00167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Zijl PCM, Sehgal AA. Proton Chemical Exchange Saturation Transfer (CEST) MRS and MRI. eMagRes. 2016;5(2) doi: 10.1002/9780470034590.emrstm1482. [DOI] [Google Scholar]
- 23.Song X, Gilad AA, Joel S, et al. CEST phase mapping using a length and offset varied saturation (LOVARS) scheme. Magn Reson Med. 2012;68(4):1074–86. doi: 10.1002/mrm.23312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheidegger R, Vinogradov E, Alsop DC. Amide proton transfer imaging with improved robustness to magnetic field inhomogeneity and magnetization transfer asymmetry using saturation with frequency alternating RF irradiation. Magn Reson Med. 2011;66(5):1275–85. doi: 10.1002/mrm.22912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinogradov E, Zhang S, Lubag A, et al. On-resonance low B1 pulses for imaging of the effects of PARACEST agents. J Magn Reson. 2005;176(1):54–63. doi: 10.1016/j.jmr.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Yadav NN, Jones CK, Hua J, et al. Imaging of endogenous exchangeable proton signals in the human brain using frequency labeled exchange transfer imaging. Magn Reson Med. 2013;69(4):966–73. doi: 10.1002/mrm.24655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones CK, Huang A, Xu J, et al. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. NeuroImage. 2013;77C:114–24. doi: 10.1016/j.neuroimage.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav NN, Jones CK, Xu J, et al. Detection of rapidly exchanging compounds using on-resonance frequency labeled exchange (FLEX) transfer. Magn Reson Med. 2012;68:1048. doi: 10.1002/mrm.24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones CK, Polders D, Hua J, et al. In vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T. Magn Reson Med. 2012;67(6):1579–89. doi: 10.1002/mrm.23141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman JI, McMahon MT, Stivers JT, et al. Indirect detection of labile solute proton spectra via the water signal using frequency-labeled exchange (FLEX) transfer. Journal of the American Chemical Society. 2010;132(6):1813–5. doi: 10.1021/ja909001q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zu Z, Janve VA, Xu J, et al. A new method for detecting exchanging amide protons using chemical exchange rotation transfer. Magn Reson Med. 2013;69(3):637–47. doi: 10.1002/mrm.24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng H, Xu J, Yadav NN, et al. (15)N Heteronuclear Chemical Exchange Saturation Transfer MRI. J Am Chem Soc. 2016;138(35):11136–9. doi: 10.1021/jacs.6b06421. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Yadav NN, Zeng HF, et al. Magnetization Transfer Contrast-Suppressed Imaging of Amide Proton Transfer and Relayed Nuclear Overhauser Enhancement Chemical Exchange Saturation Transfer Effects in the Human Brain at 7T. Magn Reson Med. 2016;75(1):88–96. doi: 10.1002/mrm.25990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Chan KW, Xu X, et al. On-resonance variable delay multipulse scheme for imaging of fast-exchanging protons and semisolid macromolecules. Magn Reson Med. 2017;77(2):730–9. doi: 10.1002/mrm.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Yadav NN, Bar-Shir A, et al. Variable delay multi-pulse train for fast chemical exchange saturation transfer and relayed-nuclear overhauser enhancement MRI. Magn Reson Med. 2014;71(5):1798–812. doi: 10.1002/mrm.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zu Z, Janve VA, Li K, et al. Multi-angle ratiometric approach to measure chemical exchange in amide proton transfer imaging. Magn Reson Med. 2012;68(3):711–9. doi: 10.1002/mrm.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavanagh J, Fairbrother WJ, Palmer AG, et al. Protein NMR Spectroscopy Principles and Practice. Academic Press; 2006. [Google Scholar]
- 38.Xu J, Schunke K, Chen L, et al., editors. ISMRM. Honolulu, HI, USA: Proceedings of the 25h Annual Meeting of ISMRM; 2017. Highly sensitive pH mapping during ischemia using Total Fast-exchanging Protons (TFP) imaging. [Google Scholar]
- 39.Jin T, Wang P, Zong X, et al. Magnetic resonance imaging of the Amine–Proton EXchange (APEX) dependent contrast. NeuroImage. 2012;59(2):1218–27. doi: 10.1016/j.neuroimage.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michaeli S, Sorce DJ, Springer CS, Jr, et al. T1rho MRI contrast in the human brain: modulation of the longitudinal rotating frame relaxation shutter-speed during an adiabatic RF pulse. J Magn Reson. 2006;181(1):135–47. doi: 10.1016/j.jmr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Grohn OH, Lukkarinen JA, Silvennoinen MJ, et al. Quantitative magnetic resonance imaging assessment of cerebral ischemia in rat using on-resonance T(1) in the rotating frame. Magn Reson Med. 1999;42(2):268–76. doi: 10.1002/(sici)1522-2594(199908)42:2<268::aid-mrm8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 42.Grohn OHJ, Kettunen MI, Makela HI, et al. Early detection of irreversible cerebral ischemia in the rat using dispersion of the magnetic resonance imaging relaxation time, T1rho. J Cereb Blood Flow Metab. 2000;20(10):1457–66. doi: 10.1097/00004647-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 43.van Zijl PCM, Zhou J, Mori N, et al. Mechanism of magnetization transfer during on-resonance water saturation. A new approach to detect mobile proteins, peptides, and lipids. Magn Reson Med. 2003;49(3):440–9. doi: 10.1002/mrm.10398. [DOI] [PubMed] [Google Scholar]
- 44.Meissner JE, Goerke S, Rerich E, et al. Quantitative pulsed CEST-MRI using Omega-plots. NMR Biomed. 2015 doi: 10.1002/nbm.3362. [DOI] [PubMed] [Google Scholar]
- 45.Wu R, Xiao G, Zhou IY, et al. Quantitative chemical exchange saturation transfer (qCEST) MRI - omega plot analysis of RF-spillover-corrected inverse CEST ratio asymmetry for simultaneous determination of labile proton ratio and exchange rate. NMR Biomed. 2015;28(3):376–83. doi: 10.1002/nbm.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hua J, Jones CK, Blakeley J, et al. Quantitative description of the asymmetry in magnetization transfer effects around the water resonance in the human brain. Magn Reson Med. 2007;58(4):786–93. doi: 10.1002/mrm.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang X, van Gelderen P, Duyn JH. Spectral characteristics of semisolid protons in human brain white matter at 7 T. Magn Reson Med. 2017;78(5):1950–8. doi: 10.1002/mrm.26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Gelderen P, Jiang X, Duyn JH. Rapid measurement of brain macromolecular proton fraction with transient saturation transfer MRI. Magn Reson Med. 2017;77(6):2174–85. doi: 10.1002/mrm.26304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liepinsh E, Otting G. Proton exchange rates from amino acid side chains--implications for image contrast. Magn Reson Med. 1996;35(1):30–42. doi: 10.1002/mrm.1910350106. [DOI] [PubMed] [Google Scholar]
- 50.Zhang XY, Wang F, Li H, et al. Accuracy in the quantification of chemical exchange saturation transfer (CEST) and relayed nuclear Overhauser enhancement (rNOE) saturation transfer effects. NMR Biomed. 2017;30(7) doi: 10.1002/nbm.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang XY, Wang F, Li H, et al. CEST imaging of fast exchanging amine pools with corrections for competing effects at 9.4 T. NMR Biomed. 2017;30(7) doi: 10.1002/nbm.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malyarenko DI, Zimmermann EM, Adler J, et al. Magnetization transfer in lamellar liquid crystals. Magn Reson Med. 2014;72(5):1427–34. doi: 10.1002/mrm.25034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: The simulated excitation profile of a train of Gaussian pulses (1, 4 and 16 pules) on water signal for the peak B1 values of 5.9 μT (180 degree) (a) and 11.8 μT (360 degree) (b). In the simulation, the water T1 and T2 were set to 2.0 s and 40 ms, respectively. The pulse width was 10 ms.


