Abstract
In the context of high adult mortality and an immense impact on the health burden of Zambia, a decomposition analysis of age- and cause-specific mortality in age group 15–59 was performed to determine the contributions to the gap in life expectancy at birth between males and females. Previous studies on decomposition have examined income groups, ethnicity, and regional differences’ contributions to gaps in life expectancy, but not the adult mortality age group 15–59. These studies focus on developed countries and few on developing countries. Arriaga’s decomposition method was applied to 2010 census and 2010–2012 sample vital registration with verbal autopsy survey (SAVVY) data to decompose contributions of age- and cause-specific adult mortality to the gap in life expectancy at birth between males and females. The decomposition analysis revealed that mortality was higher among males than females and concentrated in age groups 20–49. Age- and cause-specific adult mortality contributed positively, 50% of the years to the gap in life expectancy at birth between males and females. Major cause-specific mortality contributors to the gap in life expectancy were infectious and parasitic diseases (1.17 years, 26.3%), accidents and injuries (0.54 years, 12.2%), suicide and violence (0.30 years, 6.8%). Female HIV mortality offset male mortality. Neoplasms deaths among females contributed negatively to the gap in life expectancy (-0.22 years, -5.4%). Accidents, injuries, suicide, and violence are emerging major causes of death in age group 20–49 in Zambia which health policy and programmes should target.
Keywords: Decomposition-analysis, Age-cause-specific mortality, Verbal autopsy, Life expectancy, Adult mortality, Zambia
Highlights
-
•
Adult mortality contributed half of the years to the gender gap in life expectancy at birth.
-
•
Male mortality in age group 20–49 years contributed most to the gender difference in life expectancy at birth.
-
•
Accidents and injuries, and suicide and violence among males were major positive contributors to the gender gap in life expectancy at birth.
-
•
Female HIV mortality offset male mortality and contributed negatively to the gender life expectancy gap.
Introduction
There is growing interest in decomposing changes in life expectancy in the fields of demography and epidemiology. This is because life expectancy at birth is an important ingredient in international development composite indicators, such as the Human Development Index (HDI), as it summarises mortality conditions which are a reflection of the population’s health status (Silcocks et al., 2001, United Nations, 2016). Changes in life expectancy reflect either improvements or declines in mortality and living conditions of the population (Mondal and Shitan, 2014, Seale, 2000, Tarkiainen et al., 2012). Decomposition analysis determines age- and cause-specific mortality contributions that impact on changes in the life expectancy (Arriaga, 1984, Das Gupta, 1978). It unmasks information pertaining to inequalities in socioeconomic and health conditions that manifest themselves in widening gaps in life expectancy in the population (Hosseinpoor et al., 2012, Khang et al., 2010, Yang et al., 2012). Variations in life expectancy by region and socioeconomic status reflect differences in access to public health care in the population (Mondal and Shitan, 2014, Seale, 2000, Silber, 1992). Therefore, changes in life expectancy reflect effects of mortality in the age groups as well as due to cause-specific mortality. The effects of mortality changes comprise of two parts: the rate effects which are changes in the age-cause-specific mortality rates, and the compositional effects which are changes in age-specific mortality rates (Arriaga, 1984, Vaupel and Romo, 2002). A decomposition of age- and cause-specific mortality contributions to life expectancy changes provides relevant information for health policy programmes and interventions for targeting specific age groups and causes of death to improve the health status of the population in line with the national and sustainable development goals (SDGs).
Previous studies have decomposed changes in life expectancy mainly in North-American, European, Asian and Middle-East countries such as the United States of America (USA) (Trovato & Heyen, 2006), Canada (Auger et al., 2012, Trovato and Odynak, 2011), Japan (Trovato & Heyen, 2006), South Korea (Khang et al., 2010, Yang et al., 2012), China (Le, Ren, Shen, Li, & Zhang, 2015), Taiwan (Chen, Kwok, & Yip, 2012), Finland (Martikainen et al., 2001, Tarkiainen et al., 2012), France (Trovato & Heyen, 2006), Australia (Trovato & Lalu, 1997), Kuwaiti (Al-Ramadhan, 2008), and England and Wales (Trovato & Heyen, 2006). These studies decomposed life expectancy differences by examining age- and cause-specific mortality contributions by applying Arriaga’s (1984) decomposition method. Their findings have been consistent and mixed in some countries.
Few studies, however, have decomposed the contributions of age- and cause-specific mortality to differences in life expectancy between males and females in sub-Saharan African countries. This is largely so because of the lack of official statistics on causes of death in most of the sub-Saharan African countries, except for South Africa and Mauritius that routinely collect and publish causes of death data (Bah, 1998, Mberu et al., 2015, Rao et al., 2006).
Adult mortality in Zambia is among the highest in sub-Saharan Africa and is an issue of major concern as it poses a health burden on households, communities, health system and national economy (Ainsworth et al., 2005, Mutangadura and Webb, 1998). About 62 per cent of deaths reported by the 2010–2012 sample vital registration with verbal autopsy survey (SAVVY) were of adults (Central Statistical Office (CSO). 2014). The gap in life expectancy at birth between males and females varied and widened from 2 years in 1980 to 4.2 years in 2010 (Central Statistical Office (CSO) (CSO). 2012). The magnitude and direction of the changes in the life expectancy attributed to contributions of the adult mortality in age group 15–59 remain largely unknown. In the context of high adult mortality in Zambia, there is need to assess the extent to which age- and cause-specific adult mortality changes have contributed to differences in the life expectancy between males and females at birth through a decomposition analysis. Zambia is a landlocked country in southern Africa. The 2010 census estimated the population at 13.1 million with 51 per cent of population being female and 49 per cent male. The adult mortality age group 15 to 59 years comprises about half of the total population (Central Statistical Office [Zambia], 2012). About 40 per cent of the population live in urban areas. Zambia is one of the countries in southern Africa that has experienced a generalized HIV/AIDS epidemic with HIV prevalence rate of 13.3 per cent in age group 15–49 years (Central Statistical Office (CSO) [Zambia] et al., 2014). Nearly 61 per cent of the population live below a dollar a day (Central Statistical Office [Zambia], 2016).
The study seeks to answer the research question: what is the contribution of age- and cause-specific adult mortality rates to the gap in life expectancy at birth between males and females in Zambia? Answering this question generates useful information for national health policy relevant in understanding and focusing programmes on specific interventions needed to address widening gaps in life expectancy attributable to adult mortality conditions.
Literature review
Previous studies show that the contributions of age- and cause-specific mortality to changes in life expectancy vary from country to country after performing a decomposition analysis. For example, in Canada, USA, England and Wales, and France cancer mortality narrowed the life expectancy gap between males and females, whereas in Germany, Italy and Japan the gap widened (Trovato & Heyen, 2006). Cause-specific mortality attributed to accidents, violence and suicide in Japan contributed to widening the gap in life expectancy between males and females (Trovato & Heyen, 2006). In South Korea, age groups 20–44 and ages 50 years and above as well as liver disease, cardiovascular diseases, hypertension-related diseases, transport accidents, and suicide significantly contributed to differences in life expectance between males and females (Khang et al., 2010, Yang et al., 2012). In Quebec and Canada, lung cancer at early ages in Quebec and Cardiovascular diseases at older ages in Canada lowered life expectancy at birth (Auger et al., 2014). The Anglophone had narrower gaps in life expectancy than the Francophone. Tobacco-related causes of mortality contributed largely to the differences in life expectancy (Auger et al., 2012).
In Finland, alcohol-related diseases, cancers, ischaemic heart disease and smoking mortality among adults aged 25 years and above contributed the most to widening the gap in life expectancy (Martikainen et al., 2014, Tarkiainen et al., 2012). A decrease in ischaemic heart disease mortality among men aged 55–74, and in cardiovascular diseases mortality among women aged 65–84, significantly contributed to an increase in life expectancy (Martikainen et al., 2001). In Kuwaiti, gains in life expectancy were attributed to a reduction in mortality due to neoplams, diseases of the circulatory system, and accidents. Age groups 15–64 contributed the most to gains in life expectancy in both males and females (Al-Ramadhan, 2008). In China, gender differentials in the gap in life expectancy were attributed to age group 60–79 as well as cancers, circulatory diseases, respiratory diseases, traffic accidents and suicide (Le et al., 2015). In Australia, the age group 35–74 and heart disease, breast cancer, lung cancer, accidents and violence narrowed the gap. Whereas, prostate cancer and suicide contributed to widening the gap (Trovato & Lalu, 1997).
In sub-Saharan Africa, Bah (1998) in Mauritius found that mortality attributed to infectious and parasitic diseases was higher among males than females and played a major role in mortality transition. Age group 0–1 contributed the most to widening the gap in life expectancy between males and females. In high HIV/AIDS prevalence populations such as South Africa, a decomposition analysis of age-and cause-specific mortality contributions to the total difference in life expectancy if HIV was eliminated revealed that the age group 30–49 contributed to an increase in life expectancy following the introduction of antiretroviral therapy (Muhwava, Herbst, & Newell, 2013).
Reviewed studies show that many of them have decomposed age- and cause-specific mortality contributions to changes in life expectancy, however, few of them have considered the contributions of the adult mortality age group of 15–59, which is the aim of this study.
Methods
Data
The study used cross-sectional data from the 2010 Zambia census of population and housing (10 per cent sample) and the 2010–2012 SAVVY. The 2010 census collected information on household deaths in the last 12 months country-wide. Information on the age, sex and cause of death of deceased persons was collected. The census questionnaire had precoded categories for the question on causes of death. There is a limitation in this as the responses on causes of death were dependent on the respondent as no standard medical procedure was followed to establish the cause of death. The SAVVY is a nationally representative survey that used verbal autopsy questionnaires to collect more detailed information on causes of death for deceased persons aged 15 years and older in households that experienced a death in the last 12 months. A baseline census was conducted during the SAVVY and the population was adjusted to the national level using national census figures. Trained medical personnel coded and classified the causes of death using the International Classification of Diseases and Related Health Problems, 10th revision (ICD-10). The SAVVY used a World Health Organization methodology which is elaborated elsewhere (Central Statistical Office (CSO), 2014). Household deaths are faced with data quality issues such as under-reporting of deaths, age misreporting, and reference period errors. In addition, in households that have dissolved deaths are rarely captured. The reported deaths were evaluated for completeness using the Brass Growth Balance method (Brass, 1975). A detailed elaboration of the method can be found elsewhere (Brass, 1975, Moultrie et al., 2013). The Brass Growth Balance spreadsheet developed by Moultrie et al. (2013) and available on the website of the International Union for the Scientific Study of Population (IUSSP) was used to evaluate the completeness of death reporting.
Statistical analysis
Life tables for males and females were constructed using household deaths by age and sex from the census and survey. They were constructed separately for the 2010 census and 2010–2012 SAVVY data. Standard life table techniques were applied to estimate the life expectancies at birth and each age (Preston, Heuveline, & Guillot, 2001). Household deaths by age and sex were computed into age specific mortality rates () by dividing with the respective populations for each age and sex. The age-specific mortality rates () were then converted into life table age-specific probabilities of dying () using the method by Greville (1943). It was assumed that the observed age specific mortality rates () were equivalent to the life table age specific mortality rates () when converting to age-specific probabilities of dying using the method , where is the average number of person-years lived in the interval by those dying in the age interval. The values were estimated using the age distribution of deaths in the life table as recommended by Keyfitz (1966) using the expression: , where are life table deaths in the age interval. The values for the under-five ages, that is, (age 0) and (ages 1–4) were computed as recommended by Preston et al. (2001, 48). The age specific mortality rate at age 0 () was less than 0.107 (< 0.107) (Central Statistical Office (CSO) et al., 2014). The values for the open interval, that is, were adapted from the north model level 14 of the Coale and Demney (1983) family of model life tables. The choice of the north model of life table is based on previous studies that have used it on Zambia (Chisumpa & Dorrington, 2011). Furthermore, the open interval is not affected by AIDS mortality. A probability of dying of 1 was assigned to the open-ended interval age group (). The estimates of and were stabilized by performing three iterations (Preston et al., 2001, 45). The other life table functions (,, , and ) were defined and computed using the standard life table expressions (Preston et al., 2001). The constructed life tables for males and females served as inputs for the decomposition of age- and cause-specific mortality rates contributions to the gender gap in life expectancy at birth.
Decomposition of contributions of age- and cause-specific mortality rates to the gap in life expectancy was performed by applying the Arriaga (1984) method based on reviewed previous studies. The method measures change in life expectancy and decomposes the change into two components to show the contribution of each age group and causes of death to the total change. The two components of change are: direct and indirect effects with interaction effects which add up to the total effects and equate to the total change in life expectancy. The total effects represent the overall contributions in magnitude attributable to either age-specific mortality or cause-specific mortality rates responsible for changes in the life expectancy (Arriaga, 1984). Therefore, through decomposition, components that contributed the most in magnitude to the change in overall life expectancy can be determined. The direct effect is a change in the number of life-years in life expectancy resulting from mortality changes within each age group whereas the indirect effect is a change in the life expectancy attributed to the number of life-years due to persons alive at the end of the age interval of an age group resulting from mortality changes within the interval (Arriaga, 1984, 87). Additionally, there is an interaction effect which results from overall mortality changes affecting the life expectancy but cannot be explained or assigned to particular age groups (Arriaga, 1984, 88).
The direct effect (DE) is computed as: , where and are standard life table functions, x is the initial age of the age interval i, a is the age at which life expectancy is calculated; is the temporary life expectancy for the age interval, and t is the initial year of observation for the period of n years. For the indirect effect (IE), it is computed as: , where the life table functions are as defined above.
The Arriaga decomposition method was applied in a two-step process, first, computation of the age-specific mortality contributions to direct and indirect effects to obtain the total effects on the change in life expectancy. The age-specific change in mortality difference () between males and females within ages x and x+n is expressed as: , where the life table functions are as defined above. The first component of the expression represents direct effects while indirect and interaction effects are represented by the second component (Auger et al., 2014). Second, the age-cause-specific mortality contributions to changes () in life expectancy within ages x and x+n were estimated as: , where, is the proportion of deaths between ages x and x+n due to cause i, and is all-cause mortality between ages x and x+n. It is assumed that contributions in each age group to the overall are partitioned by the cause of death and that the contribution of each cause to the change in life expectancy for each age group is proportional to the contribution of the difference in mortality between males and females (Beltran-Sanchez, Preston, & Canudas-Romo, 2008).
The study quantified the magnitude of contributions of the age-and cause-specific mortality changes in the adult mortality age group 15–59 years to changes in life expectancy at birth. Pollard’s (1988) decomposition method was used to compare with the results produced by Arriaga’s decomposition method. Ponnapalli (2005) compared different decomposition methods and found that the Arriaga and Pollard life expectancy decomposition results are not sensitive to the decomposition methods. The comparison of Arriaga and Pollard life expectancy decomposition results was done to check for consistency. Stata version 14.2 (StataCorp, 2015) and Microsoft Office Excel 2010 (Microsoft Corporation, 2010) were used as statistical tools of analysis. Stata was used to extract household deaths and causes of death from the census and survey which were later exported to Excel spreadsheets for decomposition analysis.
Ethical consideration
The 2010 census and 2010–2012 SAVVY datasets were obtained from the Central Statistical Office in Zambia. Permission was sought and granted to use these datasets. The datasets were stripped off personal identifiers before they were made available for public use. The Central Statistical Office in Zambia adhered to all ethical requirements when undertaking the census and SAVVY.
Results
Life expectancy and causes of death
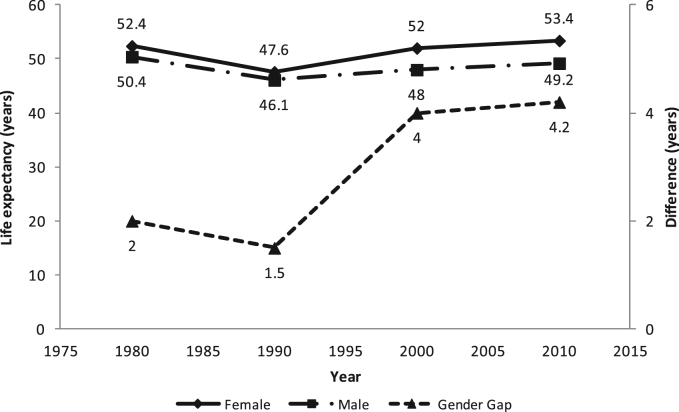
The life expectancy at birth for males and females varied in the 30-year period from 1980 to 2010 (Fig. 1). The figure also shows the widening gap in life expectancy at birth between males and females. The gender gap in life expectancy also entails that mortality remained higher for males than females during the 30-year period in Zambia.
Fig. 1.
Trend and difference in life expectancy at birth by sex, Zambia 1980–2010 Census.
Source: Central Statistical Office, (2012)
Table 1 shows the estimated life expectancy at birth of 49.8 years for males and 54.2 years for females derived from the constructed life tables using the 2010 census. This is slightly higher than the published estimates presented in Fig. 1 but are within acceptable margins. The 2010–2012 SAVVY estimated life expectancy at birth for males and females are 45.4 years and 49.5 years, respectively (Table 1). The SAVVY estimates are lower than those for the census. The gap in life expectancy between males and females is 4.4 for the census and 4.1 for the SAVVY, which is not far from the published gap of 4.2 years.
Table 1.
Life expectancy at birth and Percentage distribution of adult deaths by age, sex and cause of death, 2010 Census and 2010-2012 SAVVY.
| 2010 Census |
2010–2012 SAVVY |
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Life expectancy at birth | 49.8 | 54.2 | 45.4 | 49.5 |
| Age group | ||||
| 15–19 | 7.5 | 10.2 | 5.3 | 7.4 |
| 20–24 | 9.6 | 12.1 | 7.9 | 12.4 |
| 25–29 | 14.5 | 17.2 | 14.6 | 15.8 |
| 30–34 | 16.9 | 15.8 | 15.7 | 15.6 |
| 35–39 | 16.8 | 14.9 | 21.3 | 14.2 |
| 40–44 | 11.8 | 10.1 | 10.2 | 11.9 |
| 45–49 | 10.2 | 7.7 | 9.3 | 9.9 |
| 50–54 | 7.4 | 7.2 | 10.2 | 6.9 |
| 55–59 | 5.3 | 4.9 | 5.6 | 5.9 |
| Cause of death | ||||
| Accidents and injuries | 6.8 | 2.7 | 15.3 | 6.3 |
| Suicide and violence | 4.2 | 2.1 | – | – |
| Infectious and parasitic diseases | 76.3 | 82.1 | – | – |
| HIV disease | – | – | 37.9 | 44.1 |
| Diseases of circulatory system | – | – | 4.6 | 6.5 |
| Tuberculosis | – | – | 8.3 | 7.5 |
| Malaria | – | – | 6.6 | 6.5 |
| Neoplasms | – | – | 1.9 | 4.6 |
| Pneumonia/ARI | – | – | 2.9 | 1.7 |
| Diabetes mellitus | – | – | 1.9 | 1.6 |
| Diarrhoeal diseases | – | – | 1.3 | 1.5 |
| All other causes | 12.7 | 13.1 | 19.3 | 19.7 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 |
| Number (15–59) | 3632 | 3061 | 582 | 496 |
Source: Author’s computations using 2010 Zambia Census and 2010–2012 SAVVY data files
The percentage distributions of adult deaths by age group are comparable for the census and SAVVY for a number of age groups for both males and females, except for age group 15–19 as shown in Table 1. For the census, the highest proportion of deaths were in age groups 30–34 and 35–39 among males, whereas for the SAVVY, age group 35–39 had the highest proportion of deaths among males. The lowest proportions of deaths among males were in age group 55–59 for the census and 15–19 for the SAVVY. Among females, high proportions of deaths were concentrated in age groups 25–29, 30–34 and 35–39, and lowest in age group 55–59 for both the census and SAVVY.
The causes of death are not comparable between the 2010 census and 2010–2012 SAVVY for most causes, except for accidents and injuries as shown in Table 1. The census shows a higher proportion of females than males died from infectious and parasitic diseases. More males than females died from accidents and injuries. A higher proportion of males died from suicide and violence than females.
The SAVVY in Table 1 shows that the leading cause of death in age group 15–59 was HIV disease, with a higher proportion of female than male deaths. Accidents and injuries were second leading causes of death among males and were twice as high when compared to females. Tuberculosis was the second leading cause of death among females though a slightly higher proportion of males died from it. Nearly the same proportion of males and females died due to malaria. Slightly more females died from diseases of the circulatory system than males. Neoplasms claimed more female lives than male ones. Slightly more males died from pneumonia/ARI than females. There is a negligible difference in the proportion of males and females who died due to diabetes mellitus. All other causes of deaths claimed nearly the same proportions of male and female deaths.
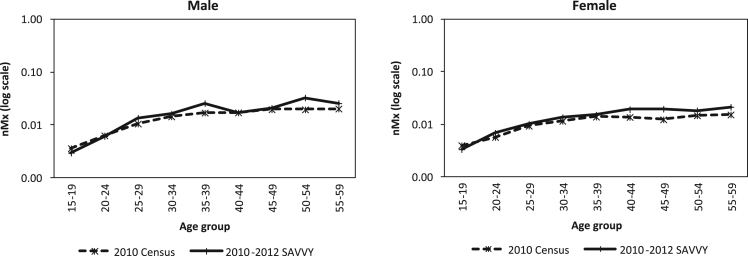
A comparison of census and SAVVY age specific mortality rates by sex for age group 15–59 shows that age specific mortality rates for both males and females follow the same pattern of increasing progressively with age (Fig. 2). However, the census mortality rates are smoother than those of the SAVVY. Male age specific mortality rates from the SAVVY show more irregularities than the female ones.
Fig. 2.
Adult Age Specific Mortality Rates by Sex, Zambia 2010 Census and 2010-2012 SAVVY.
Source: Author computations using 2010 Zambia Census and 2010–2012 SAVVY data files
Decomposition of contributions of age- and cause-specific mortality rates
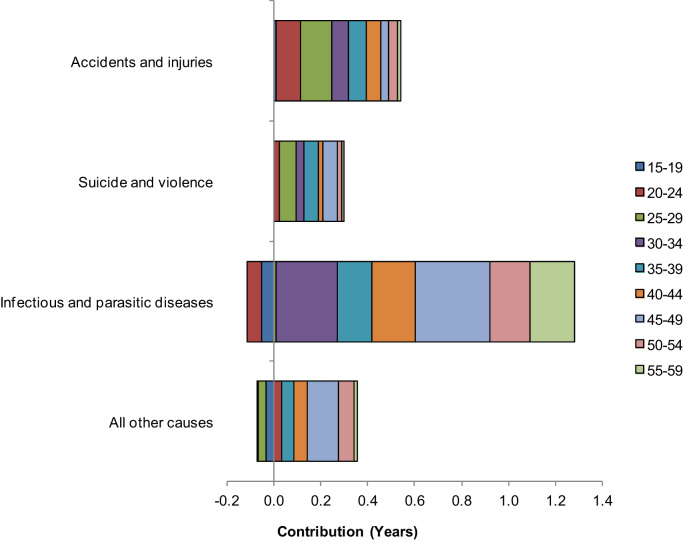
Contributions of age- and cause-specific adult mortality rates to the gap in life expectancy between males and females are presented in Fig. 3 represented by each bar whether for age group or cause of death for the 2010 census. A bar on the right side of the figure indicates a positive contribution to the gender gap in life expectancy whereas a bar on the left side shows a negative contribution to the gap.
Fig. 3.
Cause-specific mortality contributions to the gender life expectancy gap by age, Zambia, 2010 Census.
Source: Author computations using 2010 Zambia Census data files
A joint partitioning of the gap in life expectancy by age- and cause-specific mortality showed that infectious and parasitic diseases made larger contributions in age group 45–49 (0.31 years; 7.1 per cent) for the 2010 census. The pattern was more common among males than females (Fig. 3 and Table A1). Accidents and injuries had the most contributions in age group 25–29 (0.13 years; 3.0 per cent). Males succumbed more to accidents and injuries than females. Suicide and violence made larger contributions in age group 25–29 (0.07 years; 1.5 per cent). The pattern was more prevalent among males than females.
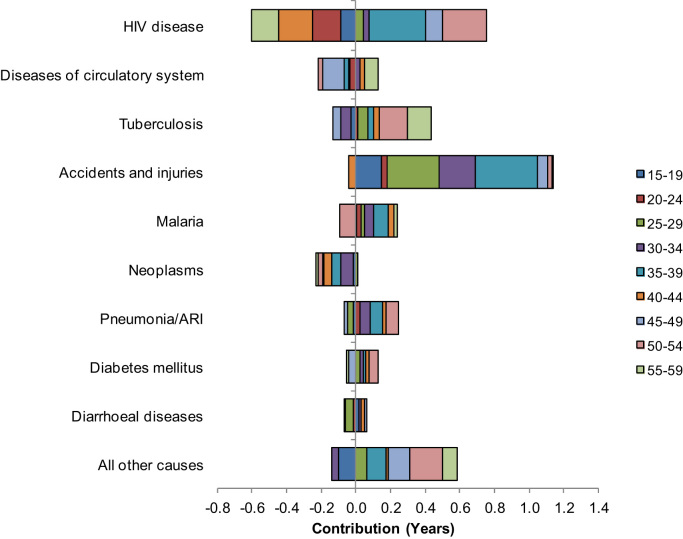
For the 2010-2012 SAVVY, a decomposition analysis of the gap in life expectancy by combining both the age- and cause-specific mortality revealed that the major cause of death contributor was accidents and injuries mostly in age group 35–39 (0.36 years; 8.9 per cent) (Fig. 4 and Table A2). Followed by age group 25–29 (0.30 years; 7.4 per cent). HIV disease had the most offsetting pattern in mortality between males and females. HIV disease made large contributions to widening of the gap in age groups 35–39 (0.32 years; 7.9 per cent); 50–54 (0.26 years; 6.3 per cent); and 45–49 (0.10 years; 2.5 per cent). However, these contributions were offset by age groups 15–19 (-0.09 years; -2.2 per cent); 20–24 (-0.16 years; -3.9 per cent); 40–44 (-0.20 years; -4.8 per cent); and 55–59 (-0.16 years; -3.8 per cent). This resulted in HIV disease contributing 0.16 years (3.9 per cent) to the life expectancy gender gap. Tuberculosis made its larger contributions in age groups 50–54 (0.16 years; 4.0 per cent) and 55–59 (0.14 years; 3.4 per cent). Pneumonia/ARI made its significant contributions through age groups 35–39 and 50–54 (0.07 years; 1.8 per cent) in either case. Malaria contributions were larger in age groups 35–39 (0.09 years) and 30–34 (0.05 years; 1.3 per cent). Neoplasms made the larger negative contributions to the gender gap in life expectancy at age groups 30–34 (-0.07 years; -1.7 per cent); 35–39 (-0.06 years; -1.4 per cent) and 40–44 (-0.04 years; -1.1 per cent). Diseases of the circulatory system also had a large contribution to narrowing the gap at age group 45–49 (-0.12 years; -3.0 per cent). Table A1, Table A2 in the Appendix provide additional information.
Fig. 4.
Cause-specific mortality contributions to the gender life expectancy gap by age, Zambia, 2010-2012 SAVVY.
Source: Author computations using 2010–2012 SAVVY data files
Discussion
The study examined contributions of age- and cause-specific mortality rates in age group 15–59 to determine changes in the gap in life expectancy at birth between males and females in Zambia using Arriaga’s decomposition method applied to 2010 census and 2010–2012 SAVVY data. Completeness of death reporting was evaluated using the Brass Growth Balance method and was found to be above the recommended threshold of 60 per cent completeness (male 99 per cent and female 82 per cent) to yield plausible estimates (Dorrington, 2013). The age- and cause-specific adult mortality contributed 50 per cent of the years to the gender gap in life expectancy at birth.
The cause-specific decomposition analysis, for the 2010 census, showed that infectious and parasitic diseases were the major positive contributor to the gender gap in life expectancy at birth, contributing 1.17 years (26.3 per cent). The broad categorisation of the causes of death makes it difficult to point out specifically which diseases made the most contribution. The infectious and parasitic diseases were concentrated in age group 45–49 years as the main contributor to the gender gap in life expectancy. The finding is consistent with previous studies (Rao et al., 2006).
Accidents and injuries were the second leading cause of death among males and they were concentrated in age group 20–39 years with age group 25–29 years (0.13 years) as the main contributor. Accidents and injuries contributed 0.54 years (12.2 per cent) to the gender gap in life expectancy. For the 2010–2012 SAVVY, accidents and injuries contributed 1.10 years (26.8 per cent) and were concentrated in age group 25–39 years, and the largest positive contributor was age group 35–39 years (0.36 years) to the gender life expectancy difference. This finding is consistent with previous studies conducted in other countries (Chen et al., 2012, Khang et al., 2010, Le et al., 2015, Yang et al., 2012). Accidents and injuries are emerging as a significant cause of death in Zambia due to the increased number of motor vehicles and accidents. The number of registered motor vehicles in Zambia was estimated at 337,513 and the number of road traffic accident fatalities was estimated at 1,388 per annum in 2010 (World Health Organization (WHO), 2013). The road-related fatality rate was estimated at 32.2 cases per 100,000 persons (World Health Organization (WHO), 2013). A study by the Ministry of Health (Zambia), on road traffic accidents found that the number of accidents increased by 45 per cent from 19,727 in 2008 to 29,118 in 2013 (Lusakatimes.com, 2015).
Many interventions have been devised by the government through the Road Transport and Safety Agency (RTSA), such as the public education campaign “Save lives Road Safety Awareness Campaign”, the seat belt campaign “Arrive Alive”, mounting of road blocks at check points, and inspection of motor vehicle fitness. However, despite all these interventions, road traffic accidents have continued to claim more lives in Zambia. In 2013, for example, 53 people were killed on the spot in what was termed as one of the worst road traffic accidents in Zambia, involving a passenger bus and a heavy duty truck (Lusakatimes.com, 2015). Some of the causes of road traffic accidents are over-speeding, misjudging of distance, cutting-in (lanes), drinking alcohol while driving, not observing traffic rules, overloading, poor state of vehicles and road infrastructure. These findings are consistent with what was found in Japan (Trovato & Heyen, 2006) and South Korea (Khang et al., 2010, Yang et al., 2012), where accidents and injuries contributed positively to the gap in life expectancy between males and females. Mortality due to accidents and injuries was higher among males than females because of risky behaviours that males engage in. It is estimated that 3 out of 4 road accident deaths are among males (World Health Organization (WHO), 2013). Waldon, McCloskey, and Earle (2005) attribute the high incidence of accident-related deaths among males to differences in biological and cultural or social factors that influence behavioural expectations by society of men and women.
Suicide and violence positively contributed 0.30 years (6.8 per cent) to the gender gap in life expectancy. Males in age group 25–49 contributed the most to the gap. The larger contributor was age group 25–29, positively contributing 0.07 years (1.5 per cent). Mortality due suicide and violence was more common among males than females. This is consistent with findings from Japan (Trovato & Heyen, 2006), Canada (Auger et al., 2012) and South Korea (Khang et al., 2010, Yang et al., 2012) where suicide and violence positively contributed to the gap in life expectancy (Auger et al., 2012, Trovato and Heyen, 2006, Yang et al., 2012). Suicide and violence has been attributed to several factors such as economic crisis, abuse of alcohol and drugs, violent environment, abuse, and poverty among others. Individuals, especially males resort to suicide and violence as a response to these problems. The suicide death rate in Zambia was estimated at 18.9 deaths per 100,000 in 2011 (World Health Organization (WHO), 2014). Waldon (1983) postulates that sex hormones and cultural influences contribute to differences in mortality between males and females. Male hormones contribute to higher violence-related deaths while female hormones are protective to the risk of ischematic heart disease. Societal cultural influences on men lead to hazardous behaviours such as alcohol consumption, cigarette smoking, drug abuse, and use of dangerous weapons which eventually result in higher mortality most among men (Waldon, 1983).
HIV disease was the leading cause of disease among adults, with higher mortality among females than males based on the SAVVY. This is consistent with previous findings (Mberu et al., 2015, Mudenda et al., 2011, Rao et al., 2006). HIV prevalence is higher among females than males in Zambia (Central Statistical Office (CSO) et al., 2014). Females have higher rates of HIV infection because of their vulnerability due to their low socioeconomic status. HIV disease positively contributed to the gender gap in life expectancy. It contributed 0.16 years (3.9 per cent). Male mortality was offset by female mortality. Male mortality was slightly higher than female mortality in age groups 35–39, 45–49 and 50–54 that contributed to the life expectancy gap. In contrast, female HIV mortality in age groups 15–19, 20–24, 40–44, and 55–59 negatively contributed to the life expectancy gap. However, the age group 35–39 positively contributed the most to the life expectancy gap, (0.32 years, 7.9 per cent). The gap in HIV deaths between males and females was small resulting in a marginal positive contribution to the gender gap in life expectancy.
Government health interventions on HIV/AIDS have over the years yielded positive results in reducing mortality following the introduction of antiretroviral therapy as well as behaviour change campaigns (Bendavid, Holmes, Bhattacharya, & Miller, 2012). For instance, by mid-2013, 503,420 adults were on antiretroviral therapy (ART) representing 92 per cent of those that needed ART. Furthermore, 81 per cent of those who initiated ART were still surviving 12 months after commencing treatment (National AIDS Council, 2014). Therefore, the marginal positive contribution to the gap in life expectancy by HIV disease may be attributed to the impact of ART. With a reduction in mortality there is a possibility of having negative contributions to the gender gap in life expectancy. In South Africa, an increase in life expectancy was observed following the introduction of antiretroviral therapy (Muhwava et al., 2013).
Tuberculosis positively contributed 0.30 years (7.4 per cent) to the gap in life expectancy. The contributing age groups were 50–59 with age group 50–54 being the major positive contributor. Male mortality due to tuberculosis, after decomposition, was higher than female mortality. For females, tuberculosis mortality was higher in age groups 15–19, 30–34, and 45–49 years. In Zambia, the tuberculosis prevalence rate was 347 cases per 100,000 persons in 2012. Government interventions such as the integrated disease surveillance and response strategy maintained the tuberculosis cure rate at 83 per cent in 2012 (Ministry of Health [Zambia], 2014). Neoplasms negatively contributed, -0.22 years (-5.4 per cent) to the gender gap in life expectancy. Neoplasms mortality was higher among females and concentrated in age groups 30–44 with age group 30–34 contributing, -0.07 years (-1.7 per cent) being the largest. The finding is consistent with studies conducted in Canada (Auger et al., 2014, Trovato and Heyen, 2006) and USA (Trovato & Heyen, 2006).
The results from the 2010 census and 2010–2012 SAVVY complement each other especially where there are similarities in the estimates and all point in the same direction, thus helping to understand the gender mortality differences in Zambia. The decomposition analysis has enhanced the demographic understanding of the contributions of age-and cause-specific adult mortality to the gap in life expectancy at birth between males and females in Zambia. The study findings are relevant for health policy and programmes in developing target specific interventions for age groups and causes of death in line with the national development goals and SDGs.
The study has several limitations. First, the lack of death data by age-sex and cause of death for previous censuses, that is, 1980, 1990, and 2000, meant that trend analysis could not be performed. Second, the Arriaga decomposition method may underestimate the contributions of cause-specific mortality at old ages (Auger et al., 2012, Yang et al., 2012). However, the impact of this is minimal since the study focused on age group 15–59. Third, the accuracy and reliability of cause of death data from the census and SAVVY was dependent on the respondent. Though, quality control measures were put in place by both the census and SAVVY, the cause of deaths reported by the census were precoded on the questionnaires and not verified by trained medical personnel. On the other hand, the causes of death reported by the SAVVY are taken to be more accurate and reliable as these were established and coded by trained and experienced medical personnel based on the verbal autopsy illness history of deceased persons. Therefore, the study findings on causes of death are more plausible for the SAVVY.
Conclusions
Age- and cause-specific adult mortality positively contributed, 50 per cent of the years, to the gender gap in life expectancy at birth. The major cause-specific mortality positive contributors to the gender gap in life expectancy were infectious and parasitic diseases and accidents and injuries in age group 15–59. The age-specific mortality positive contribution to the gender life expectancy gap was by age group 20–49 concentrated in males. Therefore, health policy and programme interventions should target emerging leading causes of death, that is, accidents and injuries; in addition to HIV/AIDS, tuberculosis, pneumonia, neoplasms among females, suicide and violence among males. The interventions should target age groups 20–49 for both males and females.
Acknowledgements
Central Statistical Office -Zambia for providing the datasets. Parts of this manuscript were extracted from the doctoral thesis by the first author at University of the Witwatersrand, Demography and Population Studies Programme, Johannesburg, South Africa. The authors are grateful to the anonymous external reviewers for their valuable and expert comments that assisted in improving the quality of the manuscript.
Acknowledgments
Availability of data and material
The 2010 Census dataset is available from IPUMS and the Central Statistical Office, Zambia. The 2010–2012 SAVVY dataset is available with written permission from the Central Statistical Office, Zambia.
Competing interests
The authors declare that they have no competing interests.
Funding
Authors received no funding.
Appendix
Table A1.
Cause-specific mortality contributions by age group, Zambia 2010 Census.
| Age |
Total Effect |
Accidents and injuries |
Suicide and violence |
Infectious and parasitic diseases |
All other causes |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | % | Year | % | Year | % | Year | % | Year | % | |
| 15–19 | -0.07 | -1.57 | 0.01 | 0.21 | 0.00 | 0.03 | -0.05 | -1.13 | -0.03 | -0.69 |
| 20–24 | 0.10 | 2.26 | 0.10 | 2.35 | 0.02 | 0.56 | -0.06 | -1.45 | 0.04 | 0.80 |
| 25–29 | 0.18 | 4.01 | 0.13 | 2.97 | 0.07 | 1.54 | 0.01 | 0.30 | -0.04 | -0.80 |
| 30–34 | 0.37 | 8.31 | 0.07 | 1.69 | 0.04 | 0.80 | 0.26 | 5.82 | 0.00 | 0.00 |
| 35–39 | 0.34 | 7.66 | 0.08 | 1.73 | 0.06 | 1.41 | 0.15 | 3.34 | 0.05 | 1.18 |
| 40–44 | 0.32 | 7.10 | 0.06 | 1.35 | 0.02 | 0.37 | 0.19 | 4.18 | 0.05 | 1.20 |
| 45–49 | 0.55 | 12.33 | 0.03 | 0.73 | 0.06 | 1.44 | 0.31 | 7.07 | 0.14 | 3.08 |
| 50–54 | 0.29 | 6.54 | 0.04 | 0.83 | 0.02 | 0.42 | 0.17 | 3.87 | 0.06 | 1.43 |
| 55–59 | 0.23 | 5.25 | 0.01 | 0.33 | 0.01 | 0.22 | 0.19 | 4.33 | 0.02 | 0.36 |
| Total | 2.30 | 51.89 | 0.54 | 12.18 | 0.30 | 6.80 | 1.17 | 26.32 | 0.29 | 6.58 |
Source: Author computations using 2010 Zambia Census data files
Table A2.
Cause-specific mortality contributions by age group, Zambia 2010–2012 SAVVY.
| Age |
Total Effect |
HIV disease |
Diseases of circulatory system |
Tuberculosis |
Accidents and injuries |
Malaria |
Neoplasms |
Pneumonia/ARI |
Diabetes mellitus |
Diarrhoeal diseases |
All other causes |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | % | Years | % | Years | % | Years | % | Years | % | Years | % | Years | % | Years | % | Years | % | Years | % | Years | % | |
| 15–19 | -0.06 | -1.46 | -0.09 | -2.17 | 0.00 | 0.02 | -0.03 | -0.62 | 0.15 | 3.61 | 0.00 | 0.11 | -0.01 | -0.34 | -0.01 | -0.34 | 0.00 | 0.00 | 0.02 | 0.37 | -0.10 | -2.43 |
| 20–24 | -0.11 | -2.80 | -0.16 | -3.92 | -0.03 | -0.78 | 0.01 | 0.26 | 0.03 | 0.80 | 0.03 | 0.71 | 0.00 | 0.05 | 0.02 | 0.59 | 0.00 | 0.00 | -0.02 | -0.38 | 0.00 | -0.08 |
| 25–29 | 0.44 | 10.70 | 0.04 | 1.07 | -0.01 | -0.16 | 0.06 | 1.41 | 0.30 | 7.41 | 0.02 | 0.39 | 0.01 | 0.18 | -0.03 | -0.74 | 0.03 | 0.68 | -0.05 | -1.13 | 0.07 | 1.60 |
| 30–34 | 0.26 | 6.32 | 0.04 | 0.89 | 0.02 | 0.52 | -0.06 | -1.51 | 0.21 | 5.04 | 0.05 | 1.33 | -0.07 | -1.72 | 0.06 | 1.44 | 0.02 | 0.44 | 0.02 | 0.42 | -0.04 | -0.90 |
| 35–39 | 0.91 | 22.35 | 0.32 | 7.86 | -0.03 | -0.75 | 0.03 | 0.84 | 0.36 | 8.86 | 0.09 | 2.13 | -0.06 | -1.38 | 0.07 | 1.80 | 0.01 | 0.34 | 0.00 | 0.00 | 0.11 | 2.68 |
| 40–44 | -0.15 | -3.57 | -0.20 | -4.79 | 0.03 | 0.72 | 0.03 | 0.76 | -0.04 | -0.91 | 0.03 | 0.69 | -0.04 | -1.08 | 0.02 | 0.42 | 0.02 | 0.48 | 0.02 | 0.39 | 0.01 | 0.33 |
| 45–49 | 0.07 | 1.61 | 0.10 | 2.48 | -0.12 | -3.03 | -0.05 | -1.14 | 0.06 | 1.40 | 0.00 | -0.06 | -0.01 | -0.13 | -0.02 | -0.48 | -0.04 | -0.95 | 0.02 | 0.46 | 0.12 | 3.06 |
| 50–54 | 0.61 | 15.04 | 0.26 | 6.28 | -0.03 | -0.62 | 0.16 | 4.01 | 0.03 | 0.68 | -0.09 | -2.28 | -0.02 | -0.60 | 0.07 | 1.79 | 0.05 | 1.15 | 0.00 | 0.00 | 0.19 | 4.64 |
| 55–59 | 0.13 | 3.25 | -0.16 | -3.83 | 0.08 | 1.98 | 0.14 | 3.40 | 0.00 | 0.04 | 0.02 | 0.47 | -0.02 | -0.41 | 0.00 | 0.00 | -0.02 | -0.42 | 0.00 | 0.00 | 0.08 | 2.02 |
| Total | 2.10 | 51.29 | 0.16 | 3.85 | -0.09 | -2.10 | 0.30 | 7.38 | 1.10 | 26.85 | 0.14 | 3.48 | -0.22 | -5.42 | 0.18 | 4.47 | 0.07 | 1.72 | 0.01 | 0.13 | 0.45 | 10.88 |
Source: Author computations using 2010–2012 SAVVY data files
References
- Ainsworth M., Beegle K., Koda G. The impact of adult mortality and parental deaths on primary schooling in north-western Tanzania. Journal of Development Studies. 2005;41:412–439. [Google Scholar]
- Al-Ramadhan M.A. Contributions of age and cause-specific mortality to gains in life expectancies among the Kuwait population. Genus. 2008;64:155–171. [Google Scholar]
- Arriaga E.E. Measuring and explaining the change in life expectancies. Demography. 1984;21:83–96. [PubMed] [Google Scholar]
- Auger N., Feuillet P., Martel S., Lo E., Barry A.D., Harper S. Mortality inequality in populations with equal life expectancy: Arriaga’s decomposition method in SAS, Stata, and Excel. Annals of Epidemiology. 2014;24:575–580. doi: 10.1016/j.annepidem.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Auger N., Harper S., Barry A.D., Trempe N., Daniel M. Life expectancy gap between the Francophone majority and Anglophone minority of a Canadian population. European Journal of Epidemiology. 2012;27:27–38. doi: 10.1007/s10654-011-9644-8. [DOI] [PubMed] [Google Scholar]
- Bah S.M. Assessing the contribution of age-sex differentials in causes of death due to infectious and parasitic diseases to the trends in age-sex differentials in life expectancy in Mauritius. Social Biology. 1998;45:260–272. doi: 10.1080/19485565.1998.9988977. [DOI] [PubMed] [Google Scholar]
- Beltran-Sanchez H., Preston S.H., Canudas-Romo V. An integrated approach to cause-of-death analysis: Cause-deleted life tables and decompositions of life expectancy. Demographic Research. 2008;19:1323–1347. doi: 10.4054/DemRes.2008.19.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendavid E., Holmes C.B., Bhattacharya J., Miller G. HIV development assistance and adult mortality in Africa. Journal of American Medical Association. 2012;307:2060–2067. doi: 10.1001/jama.2012.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass, W. (1975). Methods for estimating fertility and mortality from limited and defective data: Based on seminars held 16-24 September,1971 at the Centro Latinoamericano de Demografia (CELADE) San Jose, Costa Rica (p. 159). Chapel Hill, North Carolina: University of North Carolina, International Program of Laboratories for Population Statistics.
- Central Statistical Office (CSO), [Zambia], M.o.H., & ICF International . Central Statistical Office, Ministry of Health and ICF International; Rockville, Maryland: 2014. Zambia demographic and health survey 2013–2014. [Google Scholar]
- Central Statistical Office (CSO) Central Statistical Office; Analytical Report. Lusaka: 2012. Population and housing census-2010. [Google Scholar]
- Central Statistical Office (CSO) Central Statistical Office; Lusaka: 2014. Sample vital registration with verbal autopsy report: 2010–2012. [Google Scholar]
- Central Statistical Office [Zambia] Central Statistical Office; Analytical Report. Lusaka: 2012. Population and housing census-2010. [Google Scholar]
- Central Statistical Office [Zambia] Central Statistical Office; Lusaka: 2016. 2015 Living conditions monitoring survey (LCMS) report. [Google Scholar]
- Chen Y.-Y., Kwok R.C.L., Yip P.S.F. Decomposing the widening suicide gender gap: An experience in Taipei city, Taiwan. Journal of Affective Disorders. 2012;136:868–874. doi: 10.1016/j.jad.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Chisumpa V.H., Dorrington R. Estimating adult mortality in Zambia using information on survival of parents from surveys. African Population Studies. 2011;25(Supplement):113–126. [Google Scholar]
- Das Gupta P. A general method of decomposing a difference between two rates into several components. Demography. 1978;15:99–112. [PubMed] [Google Scholar]
- Dorrington R. The brass growth balance method. In: Moultrie T., Dorrington R., Hill A., Hill K., Timaeus I.M., Zaba B., editors. Tools for demographic estimation. International Union for the Scientific Study of Population; Paris: 2013. pp. 196–208. (demographicestimation.iussp.org) [Google Scholar]
- Greville T.N.E. Short methods of constructing life tables. Record from the American Institute of Actuaries. 1943;32:29–42. [Google Scholar]
- Hosseinpoor A.R., Lee J.H., Lynch J., Mathers C., Abou-Zahr C. International shortfall inequality in life expectancy in women and men, 1950–2010. Bulletin of World Health Organisation. 2012:90. doi: 10.2471/BLT.11.097378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyfitz N. A life table that agrees with the data. Journal of the American Statistical Association. 1966;61:305–312. [Google Scholar]
- Khang Y.-H., Yang S., Cho H.-J., Choi-Jung K., Yun S.-C. Decomposition of socioeconomic differences in life expectancy at birth by age and cause of death among 4 million South Korean public servants and their dependents. International Journal of Epidemiology. 2010;39:1656–1666. doi: 10.1093/ije/dyq117. [DOI] [PubMed] [Google Scholar]
- Le Y., Ren J., Shen J., Li T., Zhang C.-F. The changing gender differences in life expectancy in Chinese cities 2005–2010. PLoS ONE. 2015;10:e0123320. doi: 10.1371/journal.pone.0123320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusakatimes.com, 2015. Over 50 people injured in Chibombo in a bus accident. Lusakatimes: 〈http://www.lusakatimes.com/2015/04/14/over-50-people-injured-in-chibombo-in-a-bus-accident/〉.
- Martikainen P., Makela P., Peltonen R., Myrskyla M. Income differences in life expectancy: The changing contribution of harmful consumption of alcohol and smoking. Epidemiology. 2014;25:182–190. doi: 10.1097/EDE.0000000000000064. [DOI] [PubMed] [Google Scholar]
- Martikainen P., Valkonen T., Martelin T. Change in male and female life expectancy by social class: Decomposition by age and cause of death in Finland 1971-95. Journal of Epidemiology Community Health. 2001;55:494–499. doi: 10.1136/jech.55.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mberu B., Wamukoya M., Oti S., Kyobutungi C. Trends in causes of adult deaths among the urban poor: Evidence from Nairobi Urban Health and Demographic Surveillance System, 2003–2012. Journal of Urban Health: Bulletin of the New York Academy of Medicine. 2015;92:422–445. doi: 10.1007/s11524-015-9943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Microsoft Corporation . Microsoft Corporation; Excel: 2010. Microsoft office 2010. [Google Scholar]
- Ministry of Health [Zambia] Ministry of Health, Monitoring and Evaluation Unit; Lusaka: 2014. Annual health statistics bulletin 2012. [Google Scholar]
- Mondal N.I., Shitan M. Relative importance of demographic, socioeconomic and health factors on life expectancy in low- and lower-middle-income countries. Journal of Epidemiology. 2014;24:117–124. doi: 10.2188/jea.JE20130059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moultrie T., Dorrington R., Hill A., Hill K., Timaeus I., Zaba B., editors. Tools for demographic estimation. International Union for the Scientific Study of Population; Paris: 2013. (demographicestimation.iussp.org) [Google Scholar]
- Mudenda S.S., Kamocha S., Mswia R., Conkling M., Sikanyiti P., Potter D. Feasibility of using a World Health Organization standard methodology for sample vital registration with verbal autopsy to report leading causes of death in Zambia: Results of a pilot in four provinces, 2010. Population Health Metrics. 2011:9. doi: 10.1186/1478-7954-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhwava W., Herbst K., Newell M.-L. The Impact of HIV-related mortality on life expectancy: Evidence from the Africa Centre Demographic Surveillance Area. Southern African Journal of Demography. 2013;12:5–28. [Google Scholar]
- Mutangadura G., Webb D. The Socioeconomic impact of adult mortality and morbidity on households in Zambia. SAfAIDS News. 1998;6:14–15. [PubMed] [Google Scholar]
- National AIDS Council . Government of The Republic of Zambia-National AIDS Council; Lusaka: 2014. Zambia Country Report: Monitoring the Declaration of Comminttement to HIV and AIDS and the universal access report submitted to the United Nations General assembly special session on HIV and AIDS. [Google Scholar]
- Pollard J.H. On the decomposition of changes in expectation of life and differentials in life expectancy. Demography. 1988;25:265–276. [PubMed] [Google Scholar]
- Ponnapalli M.K. A comparison of different methods for decomposition of changes in expectation of life at birth and differentials in life expectancy at birth. Demographic Research. 2005;12:141–172. [Google Scholar]
- Preston S.H., Heuveline P., Guillot M. Blackwell Publishers; Oxford: 2001. Demography: Measuring and modeling population processes. [Google Scholar]
- Rao C., Lopez A.D., Hemed Y. Causes of death. In: Jamison D.T., Feachem R.G.A., Makgoba M.W., Bos E.R., Baingana F.K., Hofman K.J., editors. Disease and mortality in Sub-Saharan Africa. The International Bank of Reconstruction and Development/The World Bank; Washington, D.C., USA: 2006. pp. 43–58. [PubMed] [Google Scholar]
- Seale C. Changing patterns of death and dying. Social Science and Medicine. 2000;51:917–930. doi: 10.1016/s0277-9536(00)00071-x. [DOI] [PubMed] [Google Scholar]
- Silber J. Inequality in mortality: Measuring the contributions of various causes of death. Genus. 1992;48:93–107. [PubMed] [Google Scholar]
- Silcocks P.B., Jenner D.A., Reza R. LIfe expectancy as a summary of mortality in a population: Statistical considerations and suitability for use by health authorities. Journal of Epidemiology Community Health. 2001;55:38–43. doi: 10.1136/jech.55.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp . StataCorp; College Station, Texas: 2015. Stata 14.2 statistics/data analysis. [Google Scholar]
- Tarkiainen L., Martikainen P., Laaksonen M., Valkonen T. Trends in life expectancy by income from 1988 to 2007: Decomposition by age and cause of death. Journal of Epidemiology Community Health. 2012;66:573–578. doi: 10.1136/jech.2010.123182. [DOI] [PubMed] [Google Scholar]
- Trovato F., Heyen N.B. A varied pattern of change of sex differential in the G7 countries. Journal of Biosocial Science. 2006;38:391–401. doi: 10.1017/S0021932005007212. [DOI] [PubMed] [Google Scholar]
- Trovato F., Lalu N.M. Changing sex differences in life expectancy in Australia between 1970 and 1990. Journal of the Australian Population Association. 1997;14:187–200. doi: 10.1007/BF03029339. [DOI] [PubMed] [Google Scholar]
- Trovato F., Odynak D. Sex differences in life expectancy in Canada: Immigrant and native-born populations. Journal of Biosocial Science. 2011;43:353–368. doi: 10.1017/S0021932011000010. [DOI] [PubMed] [Google Scholar]
- United Nations . United Nations Development Programme; New York: 2016. Human development report 2016: Human development for everyone. [Google Scholar]
- Vaupel J.W., Romo V.C. Decomposing demographic change into direct vs. compositional components. Demographic Research. 2002;7:1–14. [Google Scholar]
- Waldon I. Sex differences in human mortality. Social Science Medicine. 1983;17:321–333. doi: 10.1016/0277-9536(83)90234-4. [DOI] [PubMed] [Google Scholar]
- Waldon I., McCloskey C., Earle I. Trends in gender differences in accidents mortality: Relationships to changing gender roles and other societal trends. Demographic Research. 2005;13:415–454. [Google Scholar]
- World Health Organization (WHO) World Health Organization; Geneva: 2013. Global status report on road safety.〈www.who.int/violence_injury_prevention/road_safety_status〉 (Accessed 12 August, 2017) [Google Scholar]
- World Health Organization (WHO) 2014. The World Health Statistics 2014. 〈www.who.int/gho/publications/world_health_statistics/en/〉. (Accessed 12 November 2017).
- Yang S., Khang Y.-H., Chun H., Harper S., Lynch J. The changing gender differences in life expectancy in Korea 1970–2005. Social Science Medicine. 2012;75:1280–1287. doi: 10.1016/j.socscimed.2012.04.026. [DOI] [PubMed] [Google Scholar]