Graphical abstract
Keywords: Urease, Urea, Ureolytic bacteria, Urease inhibitor, Urea metabolism
Abstract
Urea in diets of ruminants has been investigated to substitute expensive animal and vegetable protein sources for more than a century, and has been widely incorporated in diets of ruminants for many years. Urea is also recycled to the fermentative parts of the gastrointestinal (GI) tracts through saliva or direct secretory flux from blood depending upon the dietary situations. Within the GI tracts, urea is hydrolyzed to ammonia by urease enzymes produced by GI microorganisms and subsequent ammonia utilization serves the synthesis of microbial protein. In ruminants, excessive urease activity in the rumen may lead to urea/ammonia toxicity when high amounts of urea are fed to animals; and in non-ruminants, ammonia concentrations in the GI content and milieu may cause damage to the GI mucosa, resulting in impaired nutrient absorption, futile energy and protein spillage and decreased growth performance. Relatively little attention has been directed to this area by researchers. Therefore, the present review intends to discuss current knowledge in ureolytic bacterial populations, urease activities and factors affecting them, urea metabolism by microorganisms, and the application of inhibitors of urease activity in livestock animals. The information related to the ureolytic bacteria and urease activity could be useful for improving protein utilization efficiency in ruminants and for the reduction of the ammonia concentration in GI tracts of monogastric animals. Application of recent molecular methods can be expected to provide rationales for improved strategies to modulate urease and urea dynamics in the GI tract. This would lead to improved GI health, production performance and environmental compatibility of livestock production.
Introduction
Inspired by the discoveries that asparagine can substitute protein in yeast cultures, scientists started to consider non-protein nitrogen (NPN) amides as possible protein substitutes for ruminal microorganisms more than a century ago with initial focus on yeasts [1] that later switched to bacteria [2]. In the Germany of the early 20th century, several targeted trials explored the specific potential of urea as a replacement of feed protein in wethers [3], [4], [5], [6], goats [7], [8], [9], lactating dairy cows [10], [11], [12], and growing cattle [13]. While the concept of urea use in ruminant nutrition was well established in Germany by 1940 with almost 100 published studies [14], it was not widely accepted in the rest of the world [15]. The ruminant urea concept became accepted internationally only after publication of the reports of Bartlett and Cotton [16] and Hart et al. [17] on satisfactory growth of young cattle with dietary urea supplements. As reviewed by Reid [15], several subsequent studies confirmed that urea-nitrogen fed to ruminants was indeed converted to true protein. Subsequently, it was also established that composition of milk or blood including fatty acid profile and vitamins in milk and amino acid profile in blood of urea-fed lactating cows was similar to control cows [18]. Nowadays, urea has been accepted universally as an inexpensive ingredient to replace expensive animal and vegetable protein sources in various diets of ruminants.
The comprehensive research on the feeding and microbial conversion of urea from ruminant diets also created an interest to isolate urea-hydrolyzing microbes and examine urease activities for better understandings of urea metabolism in the rumen [19], [20], [21]. It soon became evident that excessive urease activity is present in the rumen [19], which may lead to ammonia toxicity if urea is fed in too high amounts, and proper feeding management is not followed [22]. Also in mono-gastric animals, high ammonia concentration in the vicinity of the intestinal mucosa may lead to pathological changes and increased turnover of the epithelial cells, resulting in futile energy and protein depletion, decreased nutrient absorption and impaired GI barrier functions [23], [24], [25]. Ureolytic bacteria and urease are key control factors for proper utilization of urea and reduction of ammonia toxicity in the GI tract; however, a systematic analysis of this perspective is not readily available in the current literature. Therefore, the present review delineates urease and ureolytic bacteria in the GI tract and their implication in urea metabolism of ruminant and mono-gastric animals.
Urease enzyme
Urease activity is widespread among the prokaryotes. Ureases from urea hydrolyzing bacteria are generally made up of two or three subunits (ureA, ureB, and ureC) and involve many accessory proteins (e.g., ureD, ureE, ureF, ureG, ureH, and ureI) for their activation [26], [27]. The major gene encoding for a urease functional subunit is ureC that has several highly conserved regions. Molecular characteristics (genetic as well as structural) of bacterial ureases have been described in details elsewhere [27] and shall not be repeated here. Instead, we will focus on activities and distribution of ureases in different segments of the GI tract of ruminant and monogastric animals.
Bacterial urease enzymes in ruminants
The intraruminal hydrolysis of urea to ammonia and CO2 was demonstrated by Lenkeit and Becker [28] who estimated that 10–20% of the ammonia produced in the rumen can be used for the synthesis of bacterial protein. They further proposed that the remaining ammonia is absorbed from the rumen and transported to the liver for partial recycling to the rumen as salivary urea. The ruminal urease activity was first characterized by Pearson and Smith [19] who found pH and temperature optima at pH 7–9 and 49 °C, respectively. The same authors postulated that urease activity of the ruminal digesta (∼0.2 mg ammonia-N/g/h) is so great that all urea ever likely to be fed would be readily converted to ammonia within 1 h. It was later confirmed that ureases produced by ruminal and other microorganisms rapidly hydrolyze urea to ammonia within 30 min to 2 h upon entering into the rumen either through feeds or recycled from blood via saliva and GI mucosa [29]. In a Rusitec fermenter system [30], the ureolytic activity was generally greater in microorganisms loosely adhered with the solid feed particles (in compartment 2) than in microorganisms present only in rumen fluid (strained rumen content; compartment 1) or in microorganisms tightly bound with solid feed particles (compartment 3). Specific urease activity was substantially greater in compartment 1 than in compartment 2, which reduced markedly with the depth of the compartment [30]. In the living animals, urease activity is mainly present in bacteria associated with the ruminal wall epithelium and in the rumen fluid [31]. The urease activity of bacteria associated with the mucosa of the rumen has been suggested to regulate the passage of urea from the blood into the rumen [32], [33]. The high ureolytic activity of bacteria attached to the ruminal wall when feeding a low protein diet is assumed to be one of the adaptive mechanisms to increase the entry of blood urea into the rumen across the ruminal wall. Theoretically, this may support microbial protein synthesis by enforcing urea reutilization in the rumen-liver nitrogen cycle. However, the distribution of the urease activity in different compartments of the microbial populations is variable to some extent. Ruminal wall urease activity by attached microorganisms was found to be altered depending upon the concentrations of dietary protein. When sheep were fed on a low-protein diet (23 g protein/day), the greatest urease activity was found in the bacteria adhering to the ruminal wall, followed by the ruminal fluid bacteria and lowest in the bacteria attached to solid feed matrix in the rumen [34]. However, bacteria associated with the ruminal wall and ruminal fluid had similar urease activity when sheep were fed with a high-protein diet (137 g protein/day), but both had significantly lower urease activity than in sheep with a low protein intake; the lowest urease activity being observed again in bacteria associated with ruminal feed particles [34]. Marini et al. [35] noted that the urease activity in the rumen wall of lambs was lowered by approximately 70% with a high-protein diet (253 g/kg DM) compared with a low-protein diet (98 g/kg DM). Although the ruminal urease activities were low in lambs fed high-protein diets, it was sufficient to hydrolyze about 10-times the urea recycled to the total GI tract of these lambs [35]. Thus, it has been argued that urease activity is unlikely a main regulating factor of the blood urea transfer into the GI tract [35], [36].
Bacterial urease enzyme in monogastric animals
In non-ruminants, the urease activity is present in the jejunum, ilium, cecum and colon; however, it is generally low (usually below 1.0 mg ammonia-N/g/h) compared with ruminant animals. Among the parts of the digestive tracts, highest urease activity was observed in the cecum of chickens (0.34 mg ammonia-N/g/h) and cecum and colon of pigs (0.84–1.24 mg ammonia-N/g/h) [37]. Urease activity is not present in the wall of the GI tracts of non-ruminants [37]. Karasawa et al. [38] reported that intestinal contents exhibited about 88% of the total urease activity, of which 95% was contributed by cecal contents and 5% by colo-rectal contents with no activity in the small intestinal contents. Of the total urease activity, intestinal tissues (cecum included), liver and kidney contributed 3, 6 and 2%, respectively. Due to low urease activity and use of ammonia by bacteria in the digestive tracts, poultry and pigs are less capable in utilizing urea when supplemented in the diets. Succinivibrionaceae WG-1 present in the foregut of tammar wallaby produced urease [39].
Unlike other monogastric animals, urease activity in the GI tract in rabbits would provide distinct advantages because of the synthesis of microbial protein in the large intestine and its reutilization for their coprophagy habits. In European hares, urease activity was detected to some extent in the stomach arising probably from cecotrophs, followed by no urease activity in the duodenum, and again detectable urease activity in the jejunum [40]. Expectedly, the large intestine (cecum and colon) contained highest urease activity with peak values of 4.2 mg ammonia-N/g/h) in the cecum [40]. Several studies showed that strong urease activity is present in the cecum of the rabbits [41], [42]. The urease activity was different between fundus and antral content of stomach and between cecum and soft feces [42]. Moreover, urease enzyme patterns were different between cecal content and soft feces of rabbits as zymograms showed two different bands. Similarly, Marounek et al. [43] reported that most of the total urease activity was present in the cecum of rabbits, followed by the colon with little activity in the duodenum, but no activity in the stomach. High level of urease activity in the cecum of hare and rabbit may imply intensive urea recycling as an adaptive mechanism to reduce the requirement of dietary protein.
Ureolytic bacteria
Ureolytic bacteria in ruminants
Following widespread research on urea utilization as a replacement of vegetable and animal protein sources in the ruminant diets, an interest emerged to isolate and identify urea-hydrolyzing microorganisms for a greater understanding of urea metabolism in the rumen (Table 1). Using culture-dependent methods, earlier workers isolated a few facultative ureolytic anaerobic bacteria predominantly related to staphylococci or micrococci [20], [21]. Gibbons and Doetsch [44] isolated a urea hydrolyzing bacterium from the rumen of normally fed cattle and assigned it to the species Bifidobacterium (Lactobacillus) bifidum. Later, few presumptively ureolytic bacteria related to Bacteroides sp., Ruminococcus sp., Propionibacterium sp., Streptococcus bovis and an anaerobic Lactobacillus sp. from cattle fed on semi-synthetic purified diets were isolated; however, the urease activities of the bacteria were not measured [45]. An ureolytic strain of Selenomonas ruminantium was isolated by John et al. [46] from the rumen of a steer. Cook [47] screened over 1000 rumen bacterial isolates from the rumen of sheep on different media and reported that urease activity was usually limited to Staphylococcus sp., Streptococcus sp., Klebsiella aerogenes and Lactobacillus casei var. casei. The ureolytic isolate of Streptococcus faecium expressed greater urease activity compared with the other bacteria, and was present in larger numbers in the rumen and accounted for the majority of the urease activity in the rumen of sheep fed forage-based diets [47]. Van Wyk and Steyn [48] reported that all bacterial isolates with urease activity were Gram-positive, facultative anaerobic and catalase-positive cocci. Among ten isolates, nine isolates were assigned to Staphylococcus saprophyticus and one isolate as Micrococcus varians. The Gram-positive facultative anaerobic cocci possibly accounted for a major proportion of the ruminal urease activity.
Table 1.
Bacteria from gastrointestinal tract of farm animals showing ureolytic or urease activity.
| Ureolytic bacteria | Niche | Reference |
|---|---|---|
| Bifidobacterium (Lactobacillus) bifidum | Rumen of cattle | Gibbons and Doetsch [44] |
| Bacteroides sp., Propionibacterium sp., Ruminococcus sp., Streptococcus bovis and Lactobacillus sp. | Rumen of cattle | Slyter et al. [45] |
| Selenomonas ruminantium | Rumen of a steer | John et al. [46] |
| Staphylococcus sp., Streptococcus sp., Klebsiella aerogenes and Lactobacillus casei var. casei | Rumen of sheep | Cook [47] |
| Staphylococcus saprophyticus and Micrococcus varians | Rumen of sheep | Van Wyk and Steyn [48] |
| Staphylococcus sp., Selenomonas ruminantium, Enterococcus faecium, Enterococcus faecalis and Lactobacillus sp. | Rumen of domesticated and wild ruminants | Lauková and Koniarová [49] |
| Ruminococcus bromii, Bifidobacterium sp., Succinivibrio dextrinosolvens, Treponema sp., Butyrivibrio sp., Peptostreptococcus productus and Prevotella ruminicola | Rumen of cattle | Wozny et al. [50] |
|
Clostridiaceae, Methylophilaceae Paenibacillaceae, Methylococcaceae, and Helicobacteraceae familiesa Marinobacter and Methylophilus generaa |
Rumen of dairy cows | Jin et al. [51]. |
| Clostridium coccoides, Clostridium innocuum, Peptostreptococcus productus, Peptostreptococcus micros, Fusobacterium russii, Peptococcus magnus and Fusobacterium sp. | Cecum of rabbits | Crociani et al. [41] |
| Eubacterium limosus, Staphylococcus spp., Selenomonas ruminantium, and Mitsuokella (previously Bacteroides) multiacidus | Feces of pigs | Varel et al. [52] |
| Succinivibrionaceae WG-1 | Foregut of tammar wallaby | Pope et al. [39] |
| Selenomonas ruminantium | Rumen | Smith et al. [53] |
| Ruminococcus albus 8 | Rumen of ruminants | Kim et al. [54] |
| Bacillus, unclassified Succinivibrionaceae, Pseudomonas, Haemophilus, Neisseria, Streptococcus and Actinomyces | Rusitec fermenter | Jin et al. [55] |
| Fibrobacter (previously Bacteroides) succinogenes S85, Prevotella (previously Bacteroides) ruminicola 23, Butyrivibrio fibrisolvens D1, Butyrivibrio sp. C3, Megasphaera elsdenii B159 and Selenomonas ruminantium GA192 | – | Chan and Jones [56] |
Since taxonomic assignments of Methylophilaceae, Methylococcaceae, and Helicobacteraceae families or Marinobacter and Methylophilus genera are based on sequencing of functional ureC gene rather than conventional cultivation- or 16S rRNA gene-based approaches, there is uncertainty if these are representative of true rumen bacteria.
Different bacterial strains exhibited varying urease activity. For example, Lauková and Koniarová [49] tested urease activity in many bacterial isolates, including Staphylococcus sp., Selenomonas ruminantium, Enterococcus sp. and Lactobacillus sp. isolated from the rumen of domesticated and wild ruminants. They reported that 56.7% of Selenomonas ruminantium isolates and 18.5% of lactobacilli isolates expressed medium urease activity, while 62.2% of the Enterococcus faecium isolates and all of Enterococcus faecalis isolates showed low urease activity. All the staphylococci isolates were ureolytic with medium or low urease activity. Streptococcus uberis and Streptococcus bovis did not express any urease activity.
Several strains of non-selectively isolated species from the rumen also showed urease activity, which included Ruminococcus bromii, Succinivibrio dextrinosolvens, Bifidobacterium sp., Treponema sp., Butyrivibrio sp., Peptostreptococcus productus and Prevotella ruminicola (previously known as Bacteroides ruminicola) [50]. Urease activity was expressed in most Peptostreptococcus productus isolates, while it was not tested in other bacterial isolates. Veillonella and Megasphaera and Propionibacterium did not exhibit urease activity.
Earlier culture-dependent methods did not detect most of the urease-producing bacteria in the rumen. Recent studies using molecular techniques indicate that the majority of the ureolytic bacteria in the rumen have not been isolated and identified. Using Illumina next-generation sequencing, Jin et al. [51] studied the urease ureC gene for analysis of abundances of predominant ureolytic bacteria in the rumen of dairy cows fed diets with urea (180 g/day) or without urea. The taxonomic classification of the ruminal ureC genes in dairy cows indicated that the majority of urease producing bacteria has yet to be identified [51]. The wall-associated bacteria (WAB) had ureolytic bacterial populations distinct from the bacteria associated with solid particles (SAB) and bacteria present in rumen fluid (LAB). Moreover, over 55% of the ureC gene sequences were not affiliated with any identified taxonomically assigned urease genes. Diversity of the ureC genes was lower for the rumen WAB than for the SAB and LAB. The ureC genes affiliated with Clostridiaceae, Paenibacillaceae, Methylococcaceae, Methylophilaceae and Helicobacteraceae families were highly abundant. The relative abundances of Marinobacter and Methylophilu genera were greater in the WAB than in the LAB and SAB [51].
Ureolytic bacteria in non-ruminants
Relatively little attention has been given to the ureolytic bacterial populations in monogastric animals including pigs and poultry, which is quite reasonable due to the insignificance of dietary urea in monogastric animals. However, endogenously produced urea that enters into the GI tract may have some significance in these animals depending upon species. In poultry, anaerobic uric acid hydrolytic bacteria have been found in the ceca of chickens, ducks, turkeys, guinea-fowl and pheasants at numbers between 5.4 × 108 and 1.8 × 1010/g of fresh cecal content [57]. Forty urea-degrading bacterial strains were isolated from the soft feces and cecal content of rabbits, which belonged to Clostridium coccoides, Clostridium innocuum, Peptostreptococcus productus, Peptostreptococcus micros, Fusobacterium russii, Peptococcus magnus and Fusobacterium sp., and showed substantial urease activity [41]. Varel et al. [52] noted widespread ureolytic bacterial numbers (25% of the total bacterial counts) in the feces of pigs fed a normal diet and urease activity of 0.48 mg ammonia/min/g dry feces. Out of 166 bacterial isolates from pigs, 55 isolates were ureolytic and most of them belonged to Streptococcus spp. (41 isolates) along with other genera, i.e., Eubacterium limosus (5 isolates), Staphylococcus spp. (2 isolates), Selenomonas ruminantium (2 isolates), Mitsuokella (Bacteroides) multiacidus (2 isolates) and others (3 isolates) [52]. To our knowledge, molecular techniques have not been employed to delineate the detailed ureolytic microbiota of the monogastric livestock animals but open a very promising future perspective.
Factors affecting urease activity and ureolytic bacteria
Urease synthesis is constitutive in some microorganisms [51], [58], [59], [60], [61]. In most ureolytic bacteria, however, urease synthesis is regulated by many factors, including the concentrations of urea, ammonia and dietary nitrogen, and the pH of the medium [26], [50], [51], [61], [62] (Table 2). Urease activity of Selenomonas ruminantium was reduced by high concentration of its reaction product (i.e., ammonia) [53]. In the Rusitec system, urease activity was enhanced with increased rate of urea infusion from 10 to 170 mg/day for a forage-based diet and 40 to 170 mg/day for a concentrate-based diet [30]. However, high ammonia concentrations and complex organic nitrogen sources in the ruminal fluid may suppress urease activity, but there are strain differences in the urease activity due to these factors [50]. Wozny et al. [50] noted that ammonia production from urea (an indicator of activity of the urease) was not detected (11 strains), or suppressed (7 strains) or unaffected (5 strains) when N concentration was increased in the medium. In a pure culture study with Ruminococcus albus 8 and different sources of nitrogen (i.e., urea, ammonia and peptides), growth of Ruminococcus albus 8 on urea and ammonia was similar, but increased urease transcript abundance and enzyme activity were noted in urea-grown cultures [54]. There is evidence that glutamine synthetase in Selenomonas ruminantium can regulate the synthesis of urease [53], [63] and the activities of both urease and glutamine synthetase enhanced several-folds when this bacterium was grown in an ammonia limiting condition. In a recent in vivo study, urease activity in the ruminal fluid of both cattle and yak increased linearly with increasing concentrations (64–235 g/kg diet) of dietary protein. At the same time, it seemed that ruminal ammonia concentrations (5.1–105 mg/L) were not increased enough to suppress urease activity of the rumen microbiota [64].
Table 2.
Factor affecting urease and ureolytic bacteria in the gastrointestinal tract of livestock animals.
| Factor | Response | Reference |
|---|---|---|
| Ni, urea | Urea (10 g/kg) increased urease activity in the rumen of sheep; Ni further increased urease activity when the diet contained 5 mg/kg of nickel | Spears et al. [65] |
| Mn, Mg, Ca, Sr, Ba, Co | Purified ruminal urease activity was decreased by the bivalent metals (5 and 10 mM) | Mahadevan et al. [66] |
| Ba, Ni, Mn | Stimulated urease activity at 2 and 20 mM metal ion concentrations in vitro with the fluid from the rumen of sheep | Spears et al. [67] |
| Cu, Zn, Cd | Inhibited urease activity at 2 and 20 mM in vitro with the fluid from the rumen of sheep | Spears et al. [67] |
| Sr, Ca, Co | Inhibited at 20 mM concentration, but not at 2 mM concentration in vitro with the fluid from the rumen of sheep | Spears et al. [67] |
| Mn, Mg, Ca, Sr, Ba | Stimulated urease activity in whole cell preparation of rumen bacteria | Jones et al. [68] |
| Na, K, Co | Inhibited urease activity in whole cell preparation of rumen bacteria | Jones et al. [68] |
| Ni | Sheep fed diets containing Ni at 5.32 mg/kg (5 mg/kg of Ni added) and urea at 10 g/kg had greater urease activity (2.5 vs. 12.7 µM ammonia nitrogen/min/mL) and ammonia concentration (66 vs. 88 mg/L) in the rumen | Spears et al. [67] |
| Monensin | Monensin at 33 mg/kg diet inhibited urease activity (5.80 vs. 1.97 7 µM ammonia/min/mL) in the rumen of steers | Starnes et al. [69] |
| Lasalocid | Lasalocid at 33 mg/kg diet inhibited urease activity (5.80 vs. 4.18 7 µM ammonia/min/mL) in the rumen of steers | Starnes et al. [69] |
| pH | Urease activity was optimum at pH 6.8–7.6. On both sides of this range, activity decreased linearly with pH | Muck [70] |
| Urea | Urea infusion in Rusitec increased urease activity | Czerkawski and Breckenridge [30] |
| Urea | Increased ureolytic bacterial population in rusitec fermenter | Jin et al. [55] |
| Urea | With isonitrogenous diets fed to cattle, ureolytic bacterial population was not affected or below 0.1% level | Zhou et al. [71] |
| Urea | Urea (160 g/day) addition to the basal diet (CP content of 167 g/kg) of cows did not alter the diversity and composition of the ureolytic bacteria and urease activity | Jin et al. [51] |
| Ammonia | High concentration reduces urease activity | Smith et al. [53] |
| Protein | With 23 g protein intake, high urease activity in ruminal wall associated bacteria, followed by ruminal fluid bacteria and lowest in solid feed associated bacteria. With 123 g protein intake, lower urease activity in sheep compared with a low protein diet; the lowest urease activity in bacteria associated with ruminal feed particles | Javorský et al. [34] |
| Protein | Urease activity in the rumen wall of lambs was lowered with a high-protein diet (253 g/kg DM) compared with a low-protein diet (98 g/kg DM) | Marini et al. [35] |
| Protein | Urease activity in ruminal fluid of both cattle and yak increased with increasing concentrations (64–235 g/kg diet) of dietary protein | Zhou et al. [64] |
| Nitrogen sources | In a pure culture study with Ruminococcus albus 8 and different sources of nitrogen (i.e., urea, ammonia and peptides), increased urease activity in urea-grown cultures | Kim et al. [54] |
The purified urease enzyme was inhibited by several divalent cations (Mn2+, Cu2+, Zn2+, Cd2+, Ni2+, Mg2+, Ba2+, Hg2+ and Co2+) [66]. At variance, Spears and Hatfield [67] reported that urease activity of incubated ruminal fluid was stimulated by a number of inorganic ions including Mn2+, Ni2+, and Ba2+, but was inhibited by Cu2+, Zn2+ and Cd2+. In the Rusitec fermenter system, urea supplementation (5 g/kg diet) significantly enhanced the proportion of ureolytic bacteria [55]. In this study, supplementation of urea only resulted in the greatest proportion of Actinobacteria and Proteobacteria, and the lowest proportion of Bacteroidetes. Bacillus was present in greater abundance in the urea-supplemented fermenters. The unclassified Succinivibrionaceae was also present at a greater relative abundance in the urea-treated fermenters. The abundances of both Streptococcus and Pseudomonas were comparatively high in the fermenters added with urea only. Urea supplementation increased the relative abundances of Neisseria, Actinomyces and Haemophilus genera. The changes of the relative abundances of these bacteria suggest that they are more responsive to urea. These bacteria contain urease genes and have urease activity [55]. However, these ureolytic genera were not detected or below 0.1% of total bacteria in the rumen of finishing bulls fed diets containing 0.8–2% urea compared with the control diet when all diets were isonitrogenous; nonetheless, urea supplementation changed some other bacterial populations such as Butyrivibrio, Coprococcus and a methanogenic Methanobrevibacter archaea [71]. In another study, supplementation with urea (160 g/day) to the basal diet (CP content of 167 g/kg) of cows did not significantly alter the diversity and composition of the ureolytic bacteria as noted from the analysis of the ureC genes [51]. In their study, the ammonia concentrations increased in the ruminal fluid of the urea-supplemented animals compared with those in the control animals, but the total urease activities of all ruminal content fractions were similar between the two groups. It was suggested that urease activity and ureolytic bacterial populations may be induced by endogenous urea on the basal high-protein diet, which did not further change despite supplementation of urea (160 g/day) in the basal diet [51]. This might also explain the discrepancies in the urease and ureolytic bacterial populations observed among the in vitro and in vivo system studies above, where the pure culture or mixed culture studies in vitro generally reported increased urease activity and ureolytic bacteria upon urea addition, which was not observed in the studies supplementing urea in vivo.
Implication of urease in urea metabolism in the rumen
Several reviews have been published on urea metabolism, ammonia absorption from the rumen, factors affecting urea utilization, urea toxicity and its signs and treatments [22], [72], [73], [74]. In this section, the main focus will be on the role of urease in urea metabolism and utilization. A schematic diagram is presented depicting the urea pool in the rumen, the urea hydrolysis by ruminal microorganism to ammonia by urease, the utilization of ammonia by the ruminal microbiota, and the excretion of urea and ammonia (Fig. 1). The urea pool in the rumen is fed from the diet and endogenous urea that recycles via the ruminal wall and salivary secretion.
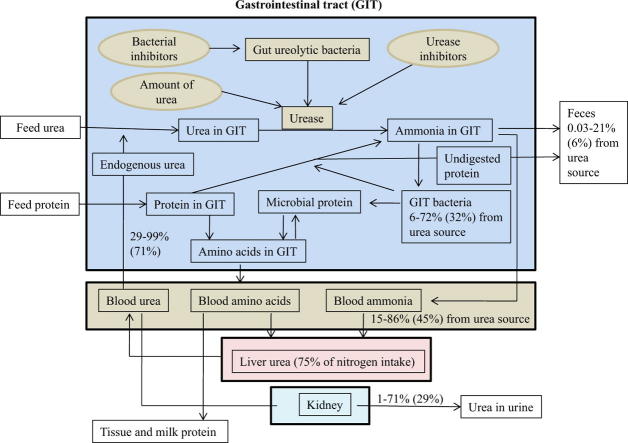
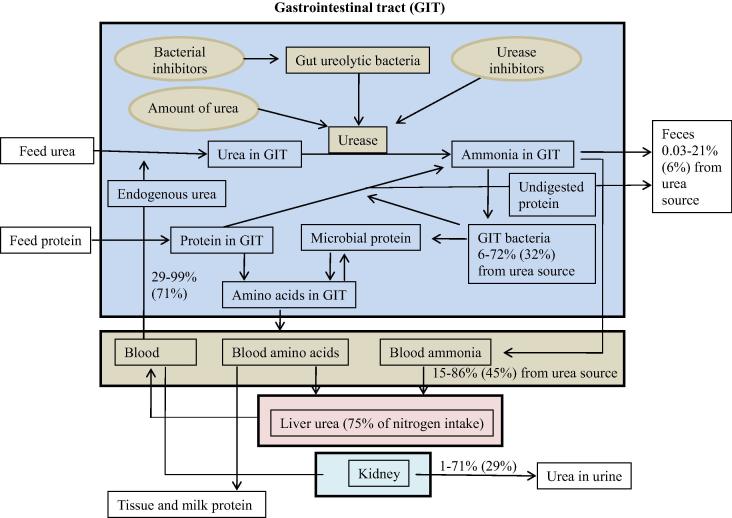
Fig. 1.
A schematic presentation of the role of urease and ureolytic bacteria in urea metabolism. Urea in gastrointestinal tracts (GIT) is hydrolyzed to ammonia by urease enzymes produced by ureolytic bacteria residing in the GIT. Urease activity in the GIT, especially in the rumen, is highly expressed; their suppression may aid to decrease ammonia toxicity and to improve utilization of protein in ruminants, and to lower ammonia concentration in GIT content in non-ruminants for improved GI health and production performance.
Urea kinetics in the GIT and body are quite variable depending upon diet composition, especially the amount and type of nitrogen intake, the relation of nitrogen intake relative to its requirement, and the amount and type of carbohydrate fermented in the rumen [73]. In ruminants, urea production in the liver (endogenous urea) may account for 27–143% (mean 75%) of nitrogen intake [75]. A range of 29–99% (mean 71%) of endogenous urea may recycle to the GIT and 1–71% (mean 29%) is eliminated through urine [75]. Endogenous urea recycled into GIT via saliva may represent 15–94% of total endogenous urea entry [73]. Approximately 25–90% of urea may be degraded in the post-ruminal digestive tract [73]. Of the urea entry into the GIT, 15–86% (mean 45%) may be absorbed as ammonia and enter the liver, 0.03–21% (mean 6%) are eliminated through feces, and 6–72% (mean 32%) are utilized for microbial protein synthesis [75]. Usually, 11–75% (mean 48%) of the GIT urea can be used for anabolism purposes in the body, most of which is contributed from microbial amino acids absorbed from the intestine [75].
The ruminal influx of urea is affected by a number of dietary factors, including amount of protein intake [35], [76], [77], [78], total dry matter intake [79], feeding frequency [80], dietary degradable protein concentration [81], [82], [83], [84], [85], organic matter digestibility and ruminal fermentable carbohydrate intake [85] and by ruminant animal species [76]. The concentrations of ammonia and short chain fatty acids (SCFA), ruminal CO2, ruminal pH, and plasma urea concentration are the resulting physicochemical signals of this dietary regulation [83], [85]. Of these, ammonia concentration in the ruminal fluid is negatively associated with urea transport to the rumen [85], while greater butyrate and CO2 concentrations enhance urea movement through the ruminal wall [73], [86].
The mechanisms behind this regulation have become much clearer in the last few years. The urea transport across the ruminal epithelium is mediated primarily through urea transporters (UT) located in the luminal and basolateral membrane of the ruminal epithelium [74], [87] with an additional role of aquaporins [88]. Urea transporters are expressed differentially depending on dietary protein concentration [89] with ruminal ammonia concentration being the major negative regulator of ruminal UT-B mRNA and protein expression [90]. On the other hand, SCFA upregulate UT-B expression at moderately low pH [90]. Similar to this long-term transcriptional regulation, SCFA and moderately low pH also upregulate urea influx capacity acutely while ammonia has the opposite effect [87]. This reciprocal regulation by SCFA and ammonia ensures that ammonia provision to ruminal microbes via the ruminal urea influx is adjusted to both the ammonia consumption capacity and the overall metabolic activity of the ruminal microbiota. Based on this coordinated short-term functional and long-term transcriptional regulation, the amount of urea recycled to the ruminant GI tract (as a proportion of total hepatic urea output) can vary from 29 to 99%; and nitrogen transfer across the GI tract may be considerably higher than nitrogen intake [91]. Interestingly, this pattern of regulated urea influx may be specific for the ruminant forestomach because we could not detect any acute regulation of cecal urea flux by SCFA and ammonium ions. As regards long-term adaptation, the highest cecal flux rates of urea were observed when fermentable protein was high in a low-fiber diet [92].
Given the proven role of ammonia in the dietary regulation of urea transport, it has also been suggested that urease at the ruminal wall may influence in the transfer of blood urea across the ruminal wall. The urease activity associated with the bacteria residing on the epithelium could help maintaining a localized concentration gradient of urea across the ruminal wall and hence augment greater rate of urea diffusion into the rumen [32]. In support of this concept, the expression of urease activity by the ruminal wall-adherent bacteria was shown to be regulated by the ammonia concentration in the rumen with high ammonia concentration retarding urease activity [32]. However, a very low ruminal urease activity in sheep fed high-protein diets (resulting in high ammonia concentration in the rumen) was sufficient to hydrolyze about 10 times the endogenous urea returned to the total GI tract [35]. As such, urease activity may not be a main factor controlling urea transfer into the GI tract [36], [89].
Nonetheless, the localization of urease in close proximity to the ruminal wall may have relevance beyond feeding the microbes with nitrogen. When urea is hydrolyzed into carbon dioxide and two ammonia molecules, the latter immediately associate with protons to form two ammonium ions. Consequently, the hydrolysis of each mole of urea buffers two moles of protons close to the surface of the ruminal epithelium. Although this buffering may be quantitatively minor for overall ruminal pH homeostasis [93], it may have relevance for the local pH regulation in the apical microclimate of the ruminal epithelial cells [87].
Finally, the sum of ammonia in the rumen is not only derived from hydrolysis of urea but also from the degradation of feed protein and deamination of amino acids by proteolytic bacteria and protozoa [94], [95]. The solubility and degradability of feed proteins differs greatly, and consequently the rate of hydrolysis of protein by ruminal proteolytic bacteria and protozoa varies substantially, which influences ammonia concentrations in the rumen [95], [96]. Ammonia produced from dietary protein or urea is used by the ruminal microorganisms for their growth, which is subsequently available to the host as microbial protein. Utilizations of ammonia by the microbiota is affected by different dietary factors, including availability of readily available energy and carbon source, amount of urea, amount and solubility of protein, adequate supply of phosphorus, sulfur and other minerals, and feeding management [22], [72]. The activities of urease and urea concentration in the rumen are the major determinants governing the extent of urea and protein utilization and urea/ammonia toxicity. The urease activity converting urea to the ruminal pool of ammonia is rapid and not rate-limiting. Therefore, concentration of ammonia in the rumen increases when large amounts of urea are fed to ruminants because the ability of the ruminal microorganisms to utilize ammonia for their growth cannot keep pace with the production of ammonia from urea and protein. Thus an increasing amount of ammonia is absorbed into the blood. As ammonia can be absorbed either diffusive as ammonia molecule (i.e., NH3) or via cation channels as ammonium ion (i.e., NH4+) through the ruminal mucosa [97], [98], its absorption depends upon several ruminal conditions, primarily pH [22], [97]. Especially at near neutral pH, it can reach quite sizable amounts [91]. Urea is not usually toxic; however ammonia is toxic to all mammals [22], [72], [99]. The inability of the liver to convert excessively absorbed ammonia from the rumen to non-toxic urea results in increased ammonia concentration in the blood, causing ammonia toxicity.
Urease inhibitors
Urease inhibitors in ruminants
Urea hydrolysis to ammonia in the rumen is very rapid, which can override its utilization by the ruminal microorganisms and lead to ammonia toxicity and wastage of nitrogen of feeds. Therefore, slowing down the urea hydrolysis may reduce ammonia loss and improve urea utilization. Coated urea or slow release urea products as protein supplements could constantly supply ammonia to ruminal microorganisms for their growth without the potential toxicity associated with feed-grade urea [72], [100], which may also improve nutrient utilization for low-quality forages and reduce plasma ammonia concentrations [101], [102].
Another strategy, which has been explored for many years to decrease the urease activity in the rumen, is the use of urease inhibitors (Table 3). A number of urease inhibitors such as acetohydroxamic acid (AHA), phosphoric phenyl ester diamide (PPD), N-(n-butyl) thiophosphoric triamide (NBPT), boric acid, bismuth compounds and hydroquinone decrease ureolytic activity [107], [124]. However, some of these compounds pose potential risks to animal and human health, thus precluding their use in production. These inhibitors usually work very well when tested in vitro. For example, hydroquinone at concentrations of 0.01, 0.1, 1 and 10 mg/L suppressed urease activity by 25, 34, 55 and 63% [107]. Supplementation of AHA decreased urease activity in vitro by 50%. However, the latter also reduced SCFA concentration and inhibited the growth of several bacterial species including Fibrobacter succinogenes (formerly known as Bacteroides succinogenes), Prevotella ruminicola, Butyrivibrio fibrisolvens, Butyrivibrio sp., Megasphaera (Peptostreptococcus) elsdenii, and Selenomonas ruminantium [56]. In a metagenomics approach, Jin et al. [55] identified that Bacillus, unclassified Succinivibrionaceae, Pseudomonas, Haemophilus, Neisseria, Streptococcus, and Actinomyces were the dominant ureC-containing bacterial genera that were induced by urea supplementation, of which the latter five were suppressed by AHA supplementation in the Rusitec fermenter. This leads to the conclusion that urease inhibitors have effects beyond urease inhibition; the growth of certain bacterial species is impaired, especially that of certain ureolytic species. Therefore, carbohydrate fermentation may also be compromised in parallel and the bacterial community may be required to reorganize itself.
Table 3.
Different urease inhibitors used to inhibit ureolytic bacteria and urease activity in the gastrointestinal tract of livestock animals.
| Urease inhibitor | System | Response | Reference |
|---|---|---|---|
| Hydroxyurea (25–125 mM) and Hydroxylamine (25–250 mM) | In vitro |
|
Mahadevan et al. [66] |
| Hydroxymate of different amino acids such as alanine, arginine, lysine, threonine, aspartic acid (0.01–1 mM) | In vitro |
|
Mahadevan et al. [66] |
| Phenylurea (12.5–62.5 mM) | In vitro |
|
Mahadevan et al. [66] |
| N-Ethylmaleimide (0.1–10 mM) | In vitro |
|
Mahadevan et al. [66] |
| Acetohydroxamic acid (0.001, 0.01 and 1 mM) | In vitro |
|
Makkar et al. [103] |
| Phenylphosphoryldiamidate (1 g/day) infusion into the rumen | Sheep |
|
Whitelaw et al. [104] |
| Phenylphosphoryldiamidate (1 g/day) infusion into the abomasum | Sheep |
|
Whitelaw et al. [104] |
| N (n-butyl) thiophosphoric triamide (0.125–4 g/day) | Sheep |
|
Ludden et al. [105] |
| N (n-butyl) thiophosphoric triamide (0.25 and 4 g/day) | Sheep fed 1.1 and 2% urea |
|
Ludden et al. [105] |
| Acetohydroxamic acid (90, 180 and 360 or 375 mg/kg body weight) | Sheep |
|
Streeter et al. [106] |
| Acetohydroxamic acid at 5 and 10 mM | In vitro |
|
Chan and Jones [56] |
| Hydroquinone at 0.01, 0.1, 1 and 10 mg/L | In vitro sheep rumen fluid |
|
Zhang et al. [107] |
| Phosphoric phenyl ester diamide (1 g/100 g N) | Dairy cows |
|
Voigt et al. [108] |
| Phosphoric phenyl ester diamide at 0.1, 0.5 and 1.0% of N | Cows |
|
Voigt et al. [109] |
| Phosphoric phenyl ester diamide at 1.0% of N | Cows fed 180 g urea/day |
|
Voigt et al. [110] |
| Vaccination, jack bean (Canavalia ensiformis L.) urease | Sheep |
|
Sidhu et al. [111] |
| Vaccination, jack bean urease | Sheep |
|
Glimp and Tillman [112] |
| Vaccination, jack bean urease | Buffalo fed with urea |
|
Sahota and Jethi [113] |
| Vaccination, jack bean urease | Calves |
|
Harbers et al. [114] |
| Vaccination, jack bean urease | Sheep |
|
Marini et al. [115] |
| Vaccination, UreC proteins of H. pylori | Cows |
|
Zhao et al. [116] |
| Penicillin (20 mg/kg) | Chickens |
|
Karasawa et al. [38] |
| Combination of chlortetracycline (110 mg/kg), sulfamethazine (110 mg/kg) and penicillin (55 mg/kg) | Pigs |
|
Varel et al. [52] |
| Chloroxytetracycline Yucca extract at 2 g/kg diet |
Chickens |
|
Yeo et al. [117] |
| Lactobacillus casei at 1.2 × 107 per kg diet | Chickens |
|
Yeo et al. [117] |
| Zinc oxide at 2.5 g/kg diet | Pigs |
|
Højberg et al. [118] |
| Copper sulfate at 175 mg/kg diet | Pigs |
|
Højberg et al. [118] |
| Copper sulfate at 125 mg/kg diet | Pigs |
|
Varel et al. [52] |
| Vaccination, jack bean urease | Pigs |
|
Glimp and Tillman [119] |
| Vaccination, jack bean urease | Pigs |
|
Kornegay et al. [120] |
| Vaccination, jack bean urease | Chickens |
|
Dang et al. [121] |
| Vaccination, jack bean urease | Guinea pigs |
|
Dang and Visek [122] |
| Vaccination, jack bean urease | Hens |
|
Pimentel and Cook [123] |
In vivo, Ludden et al. [105] investigated the effects of NBPT on ruminal protein metabolism and fermentation in three independent experiments on wethers lasting 14–15 days each. They identified linear dose effects of supplementing 0.125 to 4 g/day of NBPT with a feed containing 2% urea on decreases of ruminal urease activity, ruminal ammonia concentration and nitrogen retention, whereas ruminal urea concentration and urinary nitrogen excretion increased linearly. However, the inhibition of both urease activity and urea degradation diminished as the experiment progressed. The total SCFA concentration was diminished on day 2 of the experiment but also this effect was no longer present on day 15. Collectively, these experiments highlight a transient nature of the NBPT effect in vivo that could point to microbial adaptation [104], [105]. They also demonstrated that the most successful inhibition of urease activity in the early phase of supplementation was linked to decreased ruminal production of SCFA and decreased nitrogen retention, both of which are undesired effects.
A decreased fiber fermentation at the beginning of urease inhibitor supplementation was also observed for PPD by Voigt et al. [108] together with a longer lasting increase in the acetate:propionate ratio [109]. The activity of urease, the hydrolysis rate of urea and the ammonia concentration in the rumen were lower than control after 0.5–2 h of feeding. The effect of PPD on urea hydrolysis diminished with progressing time; however, it did not disappear with long-term supplementation for >160 days. 15N-tracing of the supplemented urea indicated that urea-N incorporation in chyme protein of the duodenum and milk protein was improved by applying PPD over 30 days [110]. Therefore, these authors concluded that urea utilization can be improved with long-term application of PPD [110].
Immunological inactivation of urease by immunization against jack bean (Canavalia ensiformis L.) urease also significantly reduced the urease activity and ammonia concentration in ruminal fluid [111]. It has been suggested that anti-urease antibodies enter into the GI tract through bile, mucus, saliva and other intestinal secretions [111], [122]. The urease activity decreased in the rumen, ileum and colon; and plasma ammonia concentration was lowered in the ruminal vein of immunized lambs [111], [112], [125]. A decreased ammonia concentration in the ruminal fluid was also observed in buffalo calves immunized against jack bean urease and fed a diet containing urea [113]. The immunization with jack bean urease also resulted in increased growth rate and feed efficiency in lambs [111], [112] and calves [114] fed urea-supplemented diets, which was perhaps due to reduced rate of urea hydrolysis in the rumen [111]. However, in a recent study, immunization with jack bean urease did not decrease ureolytic activity nor urea kinetics in sheep fed a high-protein (164 g/kg) diet [115]. The authors attributed this inability to a lack of immunological homology between jack bean urease and bacterial urease and to the inability of the antibodies to enter into GI content [115]. However, this controversy certainly needs further investigation.
A recent approach has attempted to use a component of urease protein for vaccination, which has similar homology for most of the bacterial urease types. The alpha subunit of urease (ureC) proteins in ruminal bacteria shares very analogous amino acid sequences, which are also greatly similar to that of Helicobacter pylori. Zhao et al. [116] used ureC proteins of H. pylori as a vaccine to produce anti-urease antibody titers in blood and the saliva of the immunized cows. After the fourth booster, the vaccinated cows had considerably decreased urease activity (by 17%) in the rumen than the control cows. The anti-urease antibodies also substantially lowered ureolysis and ammonia concentration in the ruminal fluid in vitro. Therefore, ureC of H. pylori appears to be an effective urease vaccine in ruminants because of its immunological homology with many rumen bacterial ureases. Nonetheless, a vaccine produced from a combination of different ureC clusters of rumen bacteria could be even more effective than ureC of H. pylori or ureC of single rumen bacteria [116].
Recently, several plant secondary metabolites, including tannins, saponins and essential oils, have been explored for their potential to improve rumen fermentation, to decreased methane emission and nitrogen excretion, and to enhance production performance and the health status of animals [96], [126], [127]. Studies are limited regarding the effects of these plant bioactive compounds on rumen urease activities and ureolytic microbiota. Tannins may inhibit the ureolytic bacteria in the rumen. For example, chestnut and quebracho tannins have shown to reduce the urease activity in feces of cows [128]. The reduction of urease activity in the rumen or feces may be attributed to the inhibition of ureolytic bacterial population by tannins or interaction between urease enzymes and tannins [127], [128]. A blend of tannins from chestnut (Castanea sativa; >78% hydrolysable tannins) and quebracho (Schonopsis lorentzii; >84% condensed tannins) at a 1:2 (w/w) ratio added in the diet at 2 g/kg feed decreased urease activity in the ruminal fluid of Holstein steers. This was accompanied by a reduction of some prominent ureolytic bacterial populations including Butyrivibrio and Treponema [129]. However, there is not much information available on the effect of plant bioactive compounds on the ureolytic bacterial populations. The regulation of urease activity in the rumen and ruminal bacteria is multifaceted and ureolytic bacteria present in the rumen are highly diverse in nature. Therefore, the factors regulating urease synthesis, as well as the impact of urea hydrolysis, dietary protein concentration and plant metabolites on the growth of the ureolytic bacteria, warrant further research in the complex rumen environment.
Urease inhibitors in non-ruminants
Excessive ammonia concentration in the GI tract may lead to retarded growth of monogastric animals, because ammonia produced from urea hydrolysis in the vicinity of intestinal mucosa can cause substantial damage to the epithelial cells. Consequently, an increase in turnover of the epithelial cells of the GI tract could occur, diverting available energy and protein from the growth and impairing the nutrient transport in the GI tract. For example, increased concentration of ammonia in the stomach of rats after urea instillation in the presence of urease caused a harmful effect on the gastric mucosa, including disruption of the surface epithelial cells, stasis of microcirculation, and necrosis of the mucosa [23]. In another study, urease caused gastritis induced by Helicobacter pylori; however a urease-negative strain of this bacterium did not exert gastritis symptoms in gnotobiotic piglets [24]. Therefore, decreasing urease activity and ammonia production in the GI tract may be implicated for improving growth performance and health of monogastric animals.
Feeding of a probiotic (2 g product per kg diet with 1.2 × 107 Lactobacillus casei) to young chickens reduced the urease activity in the small intestine (but not in the large intestine) at day 21 (no effect on day 42) of the trial [117]. The lowered urease activity was associated with increased body weight gain between 0 and 21 days of age without any effect on feed efficiency [117]. In this study, antibiotic (0.1% chloroxytetracycline) or yucca extract (2 g/kg diet) supplementation did not affect urease activity and ammonia concentration in the small and large intestine. Karasawa et al. [38] reported that dietary penicillin (20 mg/kg) decreased urease activity in cecal and colo-rectal contents. Penicillin reduced the urease activity in the cecal tissue to half of control activity but urease activities in other GI tissues were unaffected. Another antibiotic combination (AreoSP 250®) of chlortetracycline (110 mg/kg), sulfamethazine (110 mg/kg) and penicillin (55 mg/kg) in the diet significantly decreased the ureolytic bacterial population (27.2 versus 10.1% of total bacteria) in pigs [52]. However, urease activity and ammonia concentration were not affected by the antibiotic combination, which suggests that remaining ureolytic bacteria increased the synthesis of urease. Because the use of antibiotics in farm animal diets is discouraged or even prohibited in certain countries, alternative options are being explored for dietary supplements to improve production performance. In the study of Varel et al. [52], copper sulfate (125 mg/kg) decreased ureolytic bacterial number by 36% and also urease activity, but did not affect ammonia concentration in the feces. Højberg et al. [118] reported that dietary addition of zinc oxide at a high dose (2.5 g/kg) reduced or tended to reduce the urease activity in the porcine cecum and colon. The addition of copper sulfate (175 mg/kg feed) had no effect on the urease activity in this study. The authors did not measure ammonia concentrations in the digesta of the pigs.
Immunization against intestinal urease has also been attempted to suppress intestinal urease activity and ammonia concentration using jack bean urease in monogastric livestock, poultry and laboratory animals. After jack bean urease immunization, urease activity and ammonia concentration in the GI tract and its contents considerably decreased in pigs [119], [120], rats, mice, and guinea pigs [121], [122], [130]. The ureolytic activity of the GI contents of immunized animals was reduced by 40% compared with the control animals [120]. Immunity against urease increased growth rates in chickens, rats [122], and pigs [119], [120]. It has been postulated that the improved growth performance is related to a reduced rate of urea hydrolysis in the GI tract, reduced ammonia concentration in blood and consequently less energy expenditure to excrete ammonia as urea or uric acid. Even immunization of hens against jack bean urease increased fertility, hatchability and growth of chickens hatched from eggs laid by immunized hens [25], [123]. However, jack bean urease failed to produce antibodies against the urease of Helicobacter in vaccinated mice [131]. Despite the demonstrated effect of jack bean urease immunization on growth performance and reduction of urease in the GI tract, it has not been popular for practical application due to the short-lived nature of the antibody titers produced in response to non-adjuvant immunization of farm animals. Thus, intermittent immunization was required to maintain the required antibody titers [115]. However, the magnitude of the effects was comparable to using antibiotics in the feeds [132]. Overall, these studies imply that a better understanding of the urease-producing bacteria is needed for the practical application of urease inhibitors and urease immunization to obtain long-term benefits in animals.
Conclusions and future perspective
Urea feeding in ruminants as an inexpensive substitute for vegetable and animal proteins has been investigated for more than a century. A large extent of information related to the mechanisms of urea utilization by ruminal microorganisms has been generated. Urease activity and ureolytic microbiota in the rumen are fundamental in the utilization of urea in the rumen. They also largely influence the ammonia concentration in GI tract of monogastric animals with consequences for GI health and production performance. However, investigations on rumen urease and ureolytic bacteria are scarce, especially using culture-independent methods. Few urease inhibitors have been tried to decrease ammonia concentration, but their practical application in the field is not evident due to lacking or inconsistent experimental results and potential toxicity issues. Preparation of vaccines from a combination of different ureC clusters of rumen bacteria could be attempted to cover majority of the ruminal bacterial urease for an effective anti-urease immunization strategy. Some plant bioactive compounds could open new windows into the dietary modulation of urease and ureolytic bacteria; however, this potential is as yet largely unexplored. Finally, monitoring of the ureolytic bacterial population dynamics using recent molecular methods needs more attention to better understand and target urease activity in the GI tract of animals.
Acknowledgments
Conflict of interest
Authors declare that they have no conflicts of interest.
Compliance with Ethics Requirements
This is a review paper that does not contain any studies with human or animal subjects.
Acknowledgements
First author gratefully acknowledges the Alexander von Humboldt Foundation, Germany for awarding the Humboldt Research Fellowship.
Biographies

Amlan Kumar Patra, PhD, is employed as Assistant Professor, West Bengal University of Animal and Fishery Sciences in India since 2007, and currently works at Free University of Berlin, Germany as a Humboldt Research Fellow. Earlier, he worked at the American Institute for Goat Research of Langston University, USA as a post-doctoral Research Associate, and The Ohio State University, USA through a BOYSCAST fellowship from India. His research has focused on animal nutrition, rumen microbiology and gastrointestinal physiology. He has authored about 100 articles in journals, book chapters, and proceedings, and edited a Springer book. Currently, he serves as an Editor in Animal Feed Science and Technology and an Associate Editor in Frontiers in Veterinary Science.

Jörg Rudolf Aschenbach, Dr. med. vet., is Full Professor and Head of the Institute of Veterinary Physiology at the Freie Universität Berlin (Germany) since 2010. Before that, he was Full Professor at the University of Veterinary Medicine Vienna (Austria) and postdoctoral researcher at Leipzig University (Germany). The topics of his research have focused mainly on gastrointestinal and metabolism physiology in farm animal species. He has authored 130 research and review articles. He has served on Editor-in-chief and Associate Editor levels in the past. Currently, he is Editor of Leipziger Blaue Hefte and supporting five editorial boards.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Zuntz N. Bemerkungen über die Verdauung und den Nährwert der Cellulose. Observations on the digestion and nutritive value of cellulosePflugers Archiv Eur J Physiol. 1891;49:477–483. [Google Scholar]
- 2.Müller M. Untersuchungen über die bisher beobachtete eiweißsparende Wirkung des Asparagins bei der Ernährung. Investigations on the hitherto observed protein-sparing effect of asparagine in nutritionPflugers Arch. 1906;112:245–291. [Google Scholar]
- 3.Thaer W. Untersuchungen über den Eiweissersatz durch Amide. Investigations on the replacement of protein by amidesLandwirtsch Versuchsstat. 1909;70:413–444. [Google Scholar]
- 4.Friedlaender K. Zur Frage des Eiweissersatzes durch Amide. Regarding the question of protein replacement by amidesLandwirtsch Versuchsstat. 1917;67:283–312. [Google Scholar]
- 5.Morgen A., Schöler G., Windheuser K., Ohlmer E. Über den Ersatz von Eiweiß durch Harnstoff bei Hammeln und Milchtieren. Regarding the replacement of protein by urea in wethers and dairy animalsLandwirtsch Versuchsstat. 1921;99:1–26. [Google Scholar]
- 6.Morgen A., Windheuser C., Ohlmer E. Über den Ersatz von Eiweiss durch Harnstoff bei Hammeln und Milchtieren. Regarding the replacement of protein by urea in wethers and dairy animalsLandwirtsch Versuchsstat. 1922;99:1–26. [Google Scholar]
- 7.Lawrow B.A., Moltschanowa O.P., Ochotnikowa A.J. Zur Frage nach der Stickstoffausnutzung des zur Nahrung zugesetzten Harnstoffes bei einem jungen Wiederkäuer (Böcklein) Regarding the question of nitrogen utilization of diet-added urea in the young ruminant (young buck)Biochem Z. 1924;153:71–85. [Google Scholar]
- 8.Ungerer E. Harnstoff und Glykokoll als Eiweißersatz in Versuchen an Milchziegen. Ein Beitrag zur Frage über den Nährwert der Amidstoffe. Urea and glycocoll as protein substitutes in trials on dairy goats. A contribution to the question regarding the nutritional value of amide substancesBiochem Z. 1924;147:275–355. [Google Scholar]
- 9.Paasch E. Fütterungsversuch an Ziegen mit Ammoniumazetat, Harnstoff und Hornmehl als Eiweißersatz. Feeding trial in goats using ammonium acetate, urea and horn meal as protein substitutesBiochem Z. 1925;160:333–385. [Google Scholar]
- 10.Richardsen M. Milchviehfütterungsversuche mit Harnstoff [Dairy cow feeding trials using urea] Fühling’s Landwirtsch Z 1921;71:325–7.
- 11.Völtz W., Dietrich W., Jantzon H. Die Verwertung des Harnstoffs für die Milchleistung nach Versuchen an Kühen. Utilization of urea for milk performance according to trials on cowsBiochem Z. 1921;130:323–328. [Google Scholar]
- 12.Morgen A., Windheuser C., Ohlmer E. Über den Ersatz von Eiweiss durch Harnstoff bei Milchtieren. Regarding the replacement of protein by urea in dairy animalsLandwirtsch Versuchsstat. 1922;99:359–366. [Google Scholar]
- 13.Fingerling G. Ersatz des Nahrungseiweisses durch Harnstoff beim wachsenden Rinde. Replacement of dietary protein by urea in the growing cattleLandwirtsch Versuchsstat. 1937;128:235–246. [Google Scholar]
- 14.Spark A. Entwicklung der Ernährungsforschung bei Wiederkäuern 1900–1950. [Development of nutritional research in ruminants 1900–1950] Tierärztl Hochsch Hannover, Thesis 2006.
- 15.Reid J.T. Urea as a protein replacement for ruminants: a review. J Dairy Sci. 1953;36:955–996. [Google Scholar]
- 16.Bartlett S., Cotton A.G. Urea as a protein substitute in the diet of young cattle. J Dairy Res. 1938;9:263–272. [Google Scholar]
- 17.Hart E.B., Bohstedt G., Deobald H.J., Wegner M.I. The utilization of simple nitrogenous compounds such as urea and ammonium bicarbonate by growing calves. J Dairy Sci. 1939;22:785–798. [Google Scholar]
- 18.Virtanen A.I. Milk production of cows on protein-free feed. Science. 1966;153:1603–1614. doi: 10.1126/science.153.3744.1603. [DOI] [PubMed] [Google Scholar]
- 19.Pearson R.M., Smith J.A.B. The utilization of urea in the bovine rumen. 2. The conversion of urea to ammonia. Biochem J. 1943;37:148–153. doi: 10.1042/bj0370148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appleby J.C. The isolation and classification of proteolytic bacteria from the rumen of the sheep. J Gen Microbiol. 1995;12:526–533. doi: 10.1099/00221287-12-3-526. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn T.H., Hobson P.N. Further studies on the isolation of proteolytic bacteria from the sheep rumen. J Gen Microbiol. 1962;29:69–81. doi: 10.1099/00221287-29-1-69. [DOI] [PubMed] [Google Scholar]
- 22.Patra A.K. Urea/ammonia metabolism in the rumen and toxicity in ruminants. In: Puniya A.K., Singh R., Kamra D.N., editors. Rumen microbiology: from evolution to revolution. Springer; India: 2015. pp. 329–341. [Google Scholar]
- 23.Murakami M., Yoo J.K., Teramura S., Yamamoto K., Saita H., Matuo K. Generation of ammonia and mucosal lesion formation following hydrolysis of urea by urease in the rat stomach. J Clin Gastroenterol. 1990;12:S104–S109. doi: 10.1097/00004836-199001001-00018. [DOI] [PubMed] [Google Scholar]
- 24.Eaton K.A., Brooks C.L., Morgan D.R., Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pimentel J.L., Cook M.E., Jonsson J.M. Research note: increased growth of chicks and poults obtained from hens injected with jackbean urease. Poult Sci. 1991;70:1842–1844. doi: 10.3382/ps.0701842. [DOI] [PubMed] [Google Scholar]
- 26.Mobley H., Island M.D., Hausinger R.P. Molecular biology of microbial ureases. Microbiol Rev. 1995;59:451–480. doi: 10.1128/mr.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konieczna I., Żarnowiec P., Kwinkowski M., Kolesińska B., Frączyk J., Kamiński Z. Bacterial urease and its role in long-lasting human diseases. Curr Protein Pept Sci. 2012;13:789–806. doi: 10.2174/138920312804871094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenkeit W., Becker M. Das Schicksal des Harnstoffs der Amidflocken im Pansen. The fate of urea of the amide flakes in the rumenZeitschrift für Tierernährung und Futtermittelkunde. 1938;1:97–101. [Google Scholar]
- 29.Rekib A., Sadhu D.P. Effect of feeding higher doses of urea on the rumen metabolism in goat. Indian Vet J. 1986;45:735–739. [PubMed] [Google Scholar]
- 30.Czerkawski J.W., Breckenridge W. Distribution and changes in urease (EC 3.5.1.5) activity in Rumen Simulation Technique (Rusitec) Br J Nutr. 1982;47:331–348. doi: 10.1079/bjn19820042. [DOI] [PubMed] [Google Scholar]
- 31.Rybosová E., Javorský P., Havassy I., Horský K. Urease activity of adherent bacteria in the sheep rumen. Physiol Bohemoslov. 1984;33:411–416. [PubMed] [Google Scholar]
- 32.Cheng K.J., Wallace R.J. The mechanism of passage of endogenous urea through the rumen wall and the role of ureolytic epithelial bacteria in the urea flux. Br J Nutr. 1979;42:553–557. doi: 10.1079/bjn19790147. [DOI] [PubMed] [Google Scholar]
- 33.Wallace R.J., Cheng K.J., Dinsdale D., Orskov E.R. An independent microbial flora of the epithelium and its role in the ecomicrobiology of the rumen. Nature. 1979;279:424–426. doi: 10.1038/279424a0. [DOI] [PubMed] [Google Scholar]
- 34.Javorský P., Rybosová E., Havassy I., Horský K., Kmet V. Urease activity of adherent bacteria and rumen fluid bacteria. Physiol Bohemoslov. 1987;36:75–81. [PubMed] [Google Scholar]
- 35.Marini J.C., Klein J.M., Sands J.M., Van Amburgh M.E. Effect of nitrogen intake on nitrogen recycling and urea transporter abundance in lambs. J Anim Sci. 2004;82:1157–1164. doi: 10.2527/2004.8241157x. [DOI] [PubMed] [Google Scholar]
- 36.Norton B.W., Janes A.N., Armstrong D.G. The effects of intraruminal infusions of sodium bicarbonate, ammonium chloride and sodium butyrate on urea metabolism in sheep. Br J Nutr. 1982;48:265–274. doi: 10.1079/bjn19820112. [DOI] [PubMed] [Google Scholar]
- 37.Michnová E., Boda K., Tomás J., Havassy I. Urease activity in the contents and tissues of the sheep, pig and chicken gastrointestinal apparatus. Physiol Bohemoslov. 1979;28:545–550. [PubMed] [Google Scholar]
- 38.Karasawa Y., Ono T., Koh K. Inhibitory effect of penicillin on caecal urease activity in chickens fed on a low protein diet plus urea. Br Poult Sci. 1994;35:157–160. doi: 10.1080/00071669408417681. [DOI] [PubMed] [Google Scholar]
- 39.Pope P., Smith W., Denman S., Tringe S., Barry K., Hugenholtz P. Isolation of Succinivibrionaceae implicated in low methane emissions from Tammar wallabies. Science. 2011;333:646–648. doi: 10.1126/science.1205760. [DOI] [PubMed] [Google Scholar]
- 40.Stepan’kov A.A., Kuznetsova T.A., Vecherskii M.V. Urease activity in the gastrointestinal tract of the European hare (Lepus europaeus) Biol Bull. 2017;44:224–227. [Google Scholar]
- 41.Crociani F., Biavati B., Castagnoli P., Matteuzzi D. Anaerobic ureolytic bacteria from caecal content and soft faeces of rabbit. J Appl Bacteriol. 1984;57:83–88. doi: 10.1111/j.1365-2672.1984.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 42.Crociani F., Matteuzzi D., Minardi A., Brigidi P., Gioffré F. Urease activity in gastrointestinal tract of rabbit and electrophoretic behaviour of urease. Ann Inst Pasteur Microbiol. 1986;137A:287–294. doi: 10.1016/s0769-2609(86)80035-7. [DOI] [PubMed] [Google Scholar]
- 43.Marounek M.S., Vovk J., Skřivanová V. Distribution of activity of hydrolytic enzymes in the digestive tract of rabbits. Br J Nutr. 1995;1995(73):463–469. doi: 10.1079/bjn19950048. [DOI] [PubMed] [Google Scholar]
- 44.Gibbons R.J., Doetsch R.N. Physiological study of an obligately anaerobic ureolytic bacterium. J Bacteriol. 1959;77:417–428. doi: 10.1128/jb.77.4.417-428.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slyter L.L., Oltgen R.R., Kern D.L., Weaver J.M. Microbial species including ureolytic bacteria from the rumen of cattle fed purified diets. J Nutr. 1968;94:185–192. doi: 10.1093/jn/94.2.185. [DOI] [PubMed] [Google Scholar]
- 46.John A., Isaacson H.R., Bryant M.P. Isolation and characteristics of a ureolytic strain of Selenomonas ruminantium. J Dairy Sci. 1974;57:1003–1014. doi: 10.3168/jds.s0022-0302(74)85001-0. [DOI] [PubMed] [Google Scholar]
- 47.Cook A.R. The elimination of urease activity in Streptococcus faecium as evidence for plasmid coded urease. J Gen Microbiol. 1976;9:49–58. doi: 10.1099/00221287-92-1-49. [DOI] [PubMed] [Google Scholar]
- 48.Van Wyk L., Steyn P.L. Ureolytic bacteria in sheep rumen. J Gen Microbiol. 1975;91:225–232. doi: 10.1099/00221287-91-2-225. [DOI] [PubMed] [Google Scholar]
- 49.Lauková A., Koniarová I. Survey of urease activity in ruminal bacteria isolated from domestic and wild ruminants. Microbios. 1995;84:7–11. [PubMed] [Google Scholar]
- 50.Wozny M.A., Bryant M.P., Holdeman L.V., Moore W.E.C. Urease assay and urease-producing species of anaerobes in the bovine rumen and human feces. Appl Microbiol. 1977;33:1097–1104. doi: 10.1128/aem.33.5.1097-1104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin D., Zhao S., Zheng N., Bu D., Beckers Y., Denman S.E. Differences in ureolytic bacterial composition between the rumen digesta and rumen wall based on ureC gene classification. Front Microbiol. 2017;8:385. doi: 10.3389/fmicb.2017.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varel V.H., Robinson I.M., Pond W.G. Effect of dietary copper sulfate, Aureo SP250, or clinoptilolite on ureolytic bacteria found in the pig large intestine. Appl Environ Microbiol. 1987;53:2009–2012. doi: 10.1128/aem.53.9.2009-2012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith C.J., Hespell R.B., Bryant M.P. Regulation of urease and ammonia assimilatory enzymes in Selenomonas ruminantium. Appl Environ Microbiol. 1981;42:89–96. doi: 10.1128/aem.42.1.89-96.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J.N., Henriksen E.D., Cann I.K.O., Mackie R.I. Nitrogen utilization and metabolism in Ruminococcus albus 8. Appl. Environ Microbiol. 2014;80:3095–3102. doi: 10.1128/AEM.00029-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin D., Zhao S., Wang P., Zheng N., Bu D., Beckers Y. Insights into abundant rumen ureolytic bacterial community using rumen simulation system. Front Microbiol. 2016;7:1006. doi: 10.3389/fmicb.2016.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan C.C., Jones G.A. Effect of acetohydroxamic acid on growth and volatile fatty acid production by rumen bacteria. Can J Microbiol. 1973;19:27–33. doi: 10.1139/m73-004. [DOI] [PubMed] [Google Scholar]
- 57.Barnes E.M., Impey C.S. The occurence and properties of uric acid decomposing anaerobic bacteria in the avian caecum. J Appl Bacteriol. 1974;37:393–409. doi: 10.1111/j.1365-2672.1974.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 58.Burbank M.B., Weaver T.J., Williams B.C., Crawford R.L. Urease activity of ureolytic bacteria isolated from six soils in which calcite was precipitated by indigenous bacteria. Geomicrobiol J. 2012;29:389–395. [Google Scholar]
- 59.Carter E.L., Flugga N., Boer J.L., Mulrooney S.B., Hausinger R.P. Interplay of metal ions and urease. Metallomics. 2009;1:207–221. doi: 10.1039/b903311d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zotta T., Ricciardi A., Rossano R., Parente E. Urease production by Streptococcus thermophilus. Food Microbiol. 2008;25:113–119. doi: 10.1016/j.fm.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Collins C.M., D’Orazio S.E. Bacterial ureases: structure, regulation of expression and role in pathogenesis. Mol Microbiol. 1993;9:907–913. doi: 10.1111/j.1365-2958.1993.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 62.Weeks D.L., Sachs G. Sites of pH regulation of the urea channel of Helicobacter pylori. Mol Microbiol. 2001;40:1249–1259. doi: 10.1046/j.1365-2958.2001.02466.x. [DOI] [PubMed] [Google Scholar]
- 63.Smith C.J., Bryant M.P. Introduction to metabolic activities of intestinal bacteria. Am J Clin Nutr. 1979;32:149–157. doi: 10.1093/ajcn/32.1.149. [DOI] [PubMed] [Google Scholar]
- 64.Zhou J.W., Liu H., Zhong C.L., Degen A.A., Yang G., Zhang Y. Apparent digestibility, rumen fermentation, digestive enzymes and urinary purine derivatives in yaks and Qaidam cattle offered forage-concentrate diets differing in nitrogen concentration. Livst Sci. 2018;208:14–21. [Google Scholar]
- 65.Spears J.W., Smith C.J., Hatfield E.E. Rumen bacterial urease requirement for nickel. J Dairy Sci. 1977;60:1073–1076. doi: 10.3168/jds.S0022-0302(77)83990-8. [DOI] [PubMed] [Google Scholar]
- 66.Mahadevan S., Sauer F., Erfle J.D. Studies on bovine rumen bacterial urease. J Anim Sci. 1976;42:745–753. doi: 10.2527/jas1976.423745x. [DOI] [PubMed] [Google Scholar]
- 67.Spears J.W., Hatfield E.E. Nickel for ruminants I. Influence of dietary nickel on ruminal urease activity. J Anim Sci. 1978;47:1345–1350. doi: 10.2527/jas1978.4761345x. [DOI] [PubMed] [Google Scholar]
- 68.Jones G.A., MacLeod R.A., Blackwood A.C. Ureolytic rumen bacteria: II. Effect of inorganic ions on urease activity. Can J Microbiol. 1964;10:379–387. doi: 10.1139/m64-051. [DOI] [PubMed] [Google Scholar]
- 69.Starnes S.R., Spears J.W., Froetschel M.A., Croom W.J., Jr. Influence of monensin and lasalocid on mineral metabolism and ruminal urease activity in steers. J Nutr. 1984;114:518–525. doi: 10.1093/jn/114.3.518. [DOI] [PubMed] [Google Scholar]
- 70.Muck R.E. Urease activity in bovine feces. J Dairy Sci. 1982;65:2157–2163. [Google Scholar]
- 71.Zhou Z., Meng Q., Li S., Jiang L., Wu H. Effect of urea-supplemented diets on the ruminal bacterial and archaeal community composition of finishing bulls. Appl Microbiol Biotechnol. 2017;101:6205–6216. doi: 10.1007/s00253-017-8323-4. [DOI] [PubMed] [Google Scholar]
- 72.Kertz A.F. Review: urea feeding to dairy cattle: a historical perspective and review. Prof Anim Sci. 2010;26:257–272. [Google Scholar]
- 73.Huntington G.B., Archibeque S.L. Practical aspects of urea and ammonia metabolism in ruminants. J Anim Sci. 2000;77(suppl E):1–11. [Google Scholar]
- 74.Abdoun K., Stumpff F., Martens H. Ammonia and urea transport across the rumen epithelium: a review. Anim Health Res Rev. 2006;7:43–59. doi: 10.1017/S1466252307001156. [DOI] [PubMed] [Google Scholar]
- 75.Batista E.D., Detmann E., Valadares Filho S.C., Titgemeyer E.C., Valadares R.F.D. The effect of CP concentration in the diet on urea kinetics and microbial usage of recycled urea in cattle: a meta-analysis. Animal. 2017;11:1303–1311. doi: 10.1017/S1751731116002822. [DOI] [PubMed] [Google Scholar]
- 76.Zhou J.W., Mi J.D., Titgemeyer E.C., Guo X.S., Ding L.M., Wang H.C. A comparison of nitrogen utilization and urea metabolism between Tibetan and fine-wool sheep. J Anim Sci. 2015;93:3006–3017. doi: 10.2527/jas.2014-8865. [DOI] [PubMed] [Google Scholar]
- 77.Mutsvangwa T., Davies K.L., McKinnon J.J., Christensen D.A. Effects of dietary crude protein and rumen-degradable protein concentrations on urea recycling, nitrogen balance, omasal nutrient flow, and milk production in dairy cows. J Dairy Sci. 2016;99:6298–6310. doi: 10.3168/jds.2016-10917. [DOI] [PubMed] [Google Scholar]
- 78.Prates L.L., Valadares R.F.D., Valadares Filho S.C., Detmann E., Ouellet D.R., Batista E.D. Investigating the effects of sex of growing Nellore cattle and crude protein intake on the utilization of recycled N for microbial protein synthesis in the rumen by using intravenous 15N-urea infusion. Anim Feed Sci Technol. 2017;231:119–130. [Google Scholar]
- 79.Gao W., Gao X., Chen A., Zhang F., Chen D., Liu C. Effect of dietary dry matter intake on endogenous nitrogen flows in growing lambs. J Anim Physiol Anim Nutr. 2017;101:e383–e393. doi: 10.1111/jpn.12618. [DOI] [PubMed] [Google Scholar]
- 80.Sarraseca A., Milne E., Metcalf M.J., Lobley G.E. Urea recycling in sheep: effects of intake. Br J Nutr. 1998;79:79–88. doi: 10.1079/bjn19980011. [DOI] [PubMed] [Google Scholar]
- 81.Wickersham T.A., Titgemeyer E.C., Cochran R.C., Wickersham E.E., Gnad D.P. Effect of rumen degradable intake protein supplementation on urea kinetics and microbial use of recycled urea in steers consuming low-quality forage. J Anim Sci. 2008;86:3079–3088. doi: 10.2527/jas.2007-0325. [DOI] [PubMed] [Google Scholar]
- 82.Rémond D., Bernard L., Savary-Auzeloux I., Noziere P. Partitioning of nutrient net fluxes across the portal-drained viscera in sheep fed twice daily: effect of dietary protein degradability. Br J Nutr. 2009;102:370–381. doi: 10.1017/S0007114508199470. [DOI] [PubMed] [Google Scholar]
- 83.Kiran D., Mutsvangwa T. Effects of partial ruminal defaunation on urea-nitrogen recycling, nitrogen metabolism, and microbial nitrogen supply in growing lambs fed low or high dietary crude protein concentrations. J Anim Sci. 2010;88:1034–1047. doi: 10.2527/jas.2009-2218. [DOI] [PubMed] [Google Scholar]
- 84.Batista E.D., Detmann E., Titgemeyer E.C., Valadares Filho S.C., Valadares R.F., Prates L.L. Effects of varying ruminally undegradable protein supplementation on forage digestion, nitrogen metabolism, and urea kinetics in Nellore cattle fed low-quality tropical forage. J Anim Sci. 2016;94:201–216. doi: 10.2527/jas.2015-9493. [DOI] [PubMed] [Google Scholar]
- 85.Kennedy P.M., Milligan L.P. The degradation and utilization of endogenous urea in the gastrointestinal tract of ruminants: a review. Can J Anim Sci. 1980;60:205–221. [Google Scholar]
- 86.Abdoun K., Stumpff F., Rabbani I., Martens H. Modulation of urea transport across sheep rumen epithelium in vitro by SCFA and CO2. Am J Physiol Gastrointest Liver Physiol. 2010;298:G190–G202. doi: 10.1152/ajpgi.00216.2009. [DOI] [PubMed] [Google Scholar]
- 87.Lu Z., Stumpff F., Deiner C., Rosendahl J., Braun H., Abdoun K. Modulation of sheep ruminal urea transport by ammonia and pH. Am J Physiol Regul Integr Comp Physiol. 2014;307:R558–R570. doi: 10.1152/ajpregu.00107.2014. [DOI] [PubMed] [Google Scholar]
- 88.Walpole M.E., Schurmann B.L., Górka P., Penner G.B., Loewen M.E., Mutsvangwa T. Serosal-to-mucosal urea flux across the isolated ruminal epithelium is mediated via urea transporter-B and aquaporins when Holstein calves are abruptly changed to a moderately fermentable diet. J Dairy Sci. 2015;98:1204–1213. doi: 10.3168/jds.2014-8757. [DOI] [PubMed] [Google Scholar]
- 89.Marini J.C., Van Amburgh M.E. Nitrogen metabolism and recycling in Holstein heifers. J Anim Sci. 2003;81:545–552. doi: 10.2527/2003.812545x. [DOI] [PubMed] [Google Scholar]
- 90.Lu Z., Gui H., Yao L., Yan L., Martens H., Aschenbach J.R. Short-chain fatty acids and acidic pH upregulate UT-B, GPR41, and GPR4 in rumen epithelial cells of goats. Am J Physiol Regul Integr Comp Physiol. 2015;308:R283–R293. doi: 10.1152/ajpregu.00323.2014. [DOI] [PubMed] [Google Scholar]
- 91.Kiran D., Mutsvangwa T. Effects of barley grain processing and dietary ruminally degradable protein on urea nitrogen recycling and nitrogen metabolism in growing lambs. J Anim Sci. 2007;85:3391–3399. doi: 10.2527/jas.2007-0081. [DOI] [PubMed] [Google Scholar]
- 92.Stumpff F., Lodemann U., Van Kessel A.G., Pieper R., Klingspor S., Wolf K. Effects of dietary fibre and protein on urea transport across the cecal mucosa of piglets. J Comp Physiol B. 2013;183:1053–1063. doi: 10.1007/s00360-013-0771-2. [DOI] [PubMed] [Google Scholar]
- 93.Aschenbach J.R., Penner G.B., Stumpff F., Gäbel G. Ruminant nutrition symposium: role of fermentation acid absorption in the regulation of ruminal pH. J Anim Sci. 2011;89:1092–1107. doi: 10.2527/jas.2010-3301. [DOI] [PubMed] [Google Scholar]
- 94.Wallace R.J. Ruminal microbial metabolism of peptides and amino acids. J Nutr. 1996;126:1326S–1334S. doi: 10.1093/jn/126.suppl_4.1326S. [DOI] [PubMed] [Google Scholar]
- 95.Patra A.K., Yu Z. Effects of vanillin, quillaja saponin, and essential oils on in vitro fermentation and protein-degrading microorganisms of the rumen. Appl Microbiol Biotechnol. 2014;98:897–905. doi: 10.1007/s00253-013-4930-x. [DOI] [PubMed] [Google Scholar]
- 96.Patra A.K., Saxena J. The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr Res Rev. 2009;22:204–219. doi: 10.1017/S0954422409990163. [DOI] [PubMed] [Google Scholar]
- 97.Leonhard-Marek S., Stumpff F., Martens H. Transport of cations and anions across forestomach epithelia: conclusions from in vitro studies. Animal. 2010;4:1037–1056. doi: 10.1017/S1751731110000261. [DOI] [PubMed] [Google Scholar]
- 98.Rosendahl J., Braun H.S., Schrapers K.T., Martens H., Stumpff F. Evidence for the functional involvement of members of the TRP channel family in the uptake of Na+ and NH4+ by the ruminal epithelium. Pflugers Arch. 2016;468:1333–1352. doi: 10.1007/s00424-016-1835-4. [DOI] [PubMed] [Google Scholar]
- 99.Lewis D. Ammonia toxicity in the ruminant. J Agric Sci. 1960;55:111–117. [Google Scholar]
- 100.Taylor-Edwards C.C., Hibbard G., Kitts S.E., McLeod K.R., Axe D.E., Vanzant E.S. Effects of slow-release urea on ruminal digesta characteristics and growth performance in beef steers. J Anim Sci. 2009;87:200–208. doi: 10.2527/jas.2008-0912. [DOI] [PubMed] [Google Scholar]
- 101.Ribeiro S.S., Vasconcelos J.T., Morais M.G., Ítavo C.B.C.F., Franco G.L. Effects of ruminal infusion of a slow-release polymer-coated urea or conventional urea on apparent nutrient digestibility, in situ degradability, and rumen parameters in cattle fed low-quality hay. Anim Feed Sci Technol. 2011;164:53–61. [Google Scholar]
- 102.Huntington G.B., Magee K., Matthews A., Poore M., Burns J. Urea metabolism in beef steers fed tall fescue, orchardgrass, or gamagrass hays. J Anim Sci. 2009;87:1346–1353. doi: 10.2527/jas.2008-1444. [DOI] [PubMed] [Google Scholar]
- 103.Makkar H.P.S., Sharma O.P., Dawra R.K., Negi S.S. Effect of acetohydroxamic acid on rumen urease activity in vitro. J Dairy Sci. 1981;64:643–648. doi: 10.3168/jds.S0022-0302(81)82624-0. [DOI] [PubMed] [Google Scholar]
- 104.Whitelaw F.G., Milnde J.S., Wright S.A. Urease (EC 3.5.1.5) inhibition in the sheep rumen and its effect on urea and nitrogen metabolism. Br J Nutr. 1991;66:209–225. doi: 10.1079/bjn19910026. [DOI] [PubMed] [Google Scholar]
- 105.Ludden P.A., Harmon D.L., Huntington G.B., Larson B.T., Axe D.E. Influence of the novel urease inhibitor N-(n-butyl) thiophosphoric triamide on ruminant nitrogen metabolism: II. Ruminal nitrogen metabolism, diet digestibility, and nitrogen balance in lambs. J Anim Sci. 2000;78:188–198. doi: 10.2527/2000.781188x. [DOI] [PubMed] [Google Scholar]
- 106.Streeter C.L., Oltjen R.R., Slyter L.L., Fishbein W.N. Urea utilization in wethers receiving the urease inhibitor, acetohydroxamic acid. J Anim Sci. 1969;29:88–93. doi: 10.2527/jas1969.29188x. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y.G., Shan A.S., Bao J. Effect of hydroquinone on ruminal urease in the sheep and its inhibition kinetics in vitro. Asian-Australas J Anim Sci. 2001;14:1216–1220. [Google Scholar]
- 108.Voigt J., Piatkowski B., Bock J. Studies on the effect of phosphoric phenyl ester diamide as inhibitor of rumen urease in dairy cows. 3. Digestibility of the nutrients and bacterial protein synthesis. Arch Tierernahr. 1980;30:835–840. doi: 10.1080/17450398009425097. [DOI] [PubMed] [Google Scholar]
- 109.Voigt J., Piatkowski B., Bock J. Studies on the effect of phosphoric phenyl ester diamide as inhibitor of the rumen urease of dairy cows. 1. Influence on urea hydrolysis, ammonia release and fermentation in the rumen. Arch Tierernahr. 1980;30:811–823. doi: 10.1080/17450398009425094. [DOI] [PubMed] [Google Scholar]
- 110.Voigt J., Piatkowski B., Bock J. Studies on the effect of phosphoric phenyl ester diamide as inhibitor of the rumen urease of dairy cows. 2. The metabolism of 15N-urea. Arch Tierernahr. 1980;30:825–834. doi: 10.1080/17450398009425096. [DOI] [PubMed] [Google Scholar]
- 111.Sidhu K.S., Jones L.E.W., Tillman A.D. Effect of urease immunity on growth, digestion and nitrogen metabolism in ruminant animals. J Anim Sci. 1968;27:1703–1708. doi: 10.2527/jas1968.2761703x. [DOI] [PubMed] [Google Scholar]
- 112.Glimp H.A., Tillman A.D. Effect of jackbean urease injections on performance, anti-urease production and plasma ammonia and urea levels in sheep. J Anim Sci. 1965;24:105–112. doi: 10.2527/jas1965.241105x. [DOI] [PubMed] [Google Scholar]
- 113.Sahota R.S., Jethi R.K. Immunological control of endogenous urease activity in buffalo calves. Zentralbl Veterinarmed A. 1981;28:247–251. doi: 10.1111/j.1439-0442.1981.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 114.Harbers L.H., Tillman A.D., Visek W.J., Glimp H.A. Some effects of jackbean urease immunity in young calves. J Anim Sci. 1965;24:102–104. doi: 10.2527/jas1965.241102x. [DOI] [PubMed] [Google Scholar]
- 115.Marini J.C., Simpson K.W., Gerold A., Van Amburgh M.E. The effect of immunization with jackbean urease on antibody response and nitrogen recycling in mature sheep. Livest Prod Sci. 2003;81:283–292. [Google Scholar]
- 116.Zhao S., Wang J., Zheng N., Bu D., Sun P., Yu Z. Reducing microbial ureolytic activity in the rumen by immunization against urease therein. BMC Vet Res. 2015;11:94. doi: 10.1186/s12917-015-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yeo J., Kim K. Effect of feeding diets containing an antibiotic, a probiotic, or yucca extract on growth and intestinal urease activity in broiler chicks. Poult Sci. 1997;76:381–385. doi: 10.1093/ps/76.2.381. [DOI] [PubMed] [Google Scholar]
- 118.Hojberg O., Canibe N., Poulsen H.D., Hedemann M.S., Jensen B.B. Influence of dietary zinc oxide and copper sulfate on the gastrointestinal ecosystem in newly weaned piglets. Appl Environ Microbiol. 2005;71:2267–2277. doi: 10.1128/AEM.71.5.2267-2277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Glimp H.A., Tillman A.D. Effect of jackbean urease injections and chlortetracycline on rate of gain and feed efficiency in swine. J Anim Sci. 1964;23:963–966. [Google Scholar]
- 120.Kornegay E.T., Miller E.R., Hoefer J.A. Urease toxicity in growing pigs. J Anim Sci. 1965;24:51–56. doi: 10.2527/jas1965.24151x. [DOI] [PubMed] [Google Scholar]
- 121.Dang H.C., Visek W.J., Dubois K.P. Effect of urease injection on body weight of growing rats and chicks. Proc Soc Exp Biol Med. 1960;105:164–167. doi: 10.3181/00379727-105-26046. [DOI] [PubMed] [Google Scholar]
- 122.Dang H.C., Visek W.J. Some effects of urease administration on laboratory animals. Am J Physiol. 1964;206:731–737. doi: 10.1152/ajplegacy.1964.206.4.731. [DOI] [PubMed] [Google Scholar]
- 123.Pimentel J.L., Cook M.E. Improved growth in the progeny of hens immunized with jackbean urease. Poult Sci. 1988;67:434–439. doi: 10.3382/ps.0670434. [DOI] [PubMed] [Google Scholar]
- 124.Krajewska B., Ureases I. Functional, catalytic and kinetic properties: a review. J Mol Catal B Enzym. 2009;59:9–21. [Google Scholar]
- 125.Sidhu K.S., Hall L.T., Easley L.R., Jones E.W., Tillman A.D. Effect of urease immunity on urease and antiurease activities in ruminants. J Nutr. 1969;99:16–22. doi: 10.1093/jn/99.1.16. [DOI] [PubMed] [Google Scholar]
- 126.Patra A.K., Saxena J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry. 2010;71:1198–1222. doi: 10.1016/j.phytochem.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 127.Patra A.K., Min B.R., Saxena J. Dietary tannins on microbial ecology of the gastrointestinal tract in ruminants. In: Patra A.K., editor. Dietary phytochemicals and microbes. Springer; The Netherlands: 2012. pp. 237–262. [Google Scholar]
- 128.Powell J.M., Aguerre M.J., Wattiaux M.A. Tannin extracts abate ammonia emissions from simulated dairy barn floors. J Environ Qual. 2011;40:907–914. doi: 10.2134/jeq2010.0492. [DOI] [PubMed] [Google Scholar]
- 129.Diaz Carrasco J.M., Cabral C., Redondo L.M., Pin Viso N.D., Colombatto D., Farber M.D. Impact of chestnut and quebracho tannins on rumen microbiota of bovines. BioMed Res Int. 2017;2017 doi: 10.1155/2017/9610810. ID 9610810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Visek W.J. Studies on urea hydrolysis in birds and mammals. Am J Vet Res. 1962;23:569–574. [PubMed] [Google Scholar]
- 131.Chen M., Lee A., Hazell S.L., Hu P., Li Y. Lack of protection against gastric helicobacter infection following immunisation with jack bean urease: the rejection of a novel hypothesis. FEMS Microbiol Lett. 1994;116:245–250. doi: 10.1111/j.1574-6968.1994.tb06710.x. [DOI] [PubMed] [Google Scholar]
- 132.Visek V.J. The mode of growth promotion by antibiotics. J Anim Sci. 1978;46:1447–1469. [Google Scholar]