Graphical abstract
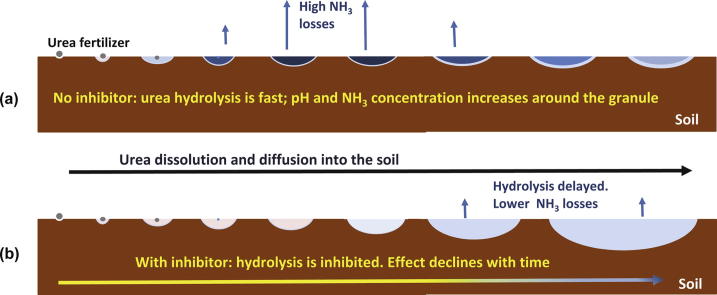
Schematic diagram of urea dissolution, diffusion and hydrolysis in the soil. (a) Without an inhibitor, hydrolysis is fast (dark blue color) causing NH3/NH4+ accumulation and increasing the pH close to the soil surface around the fertilizer granule, driving NH3 volatilization. As the ammonia species are less mobile in soil, diffusion is limited. (b) The inhibitor maintains urea unhydrolyzed for some time. Urea has no electrical charges and diffuses easily into the soil solution. When the effect of the inhibitor phases down and urea starts to hydrolyze, both the pH and the NH3/NH4+ concentrations are lower (light blue color) as a result of dilution. Part of the urea is incorporated into the soil before hydrolysis; the NH3 produced inside the soil is retained by the negative charges of colloidal material and losses are reduced even if no rain or irrigation incorporates urea into the soil.
Keywords: NBPT, NPPT, Ammonia volatilization, Soil urease, Nutrient use efficiency, Urea, Nitrogen fertilizer
Abstract
Urea is the most widely used nitrogen (N) fertilizer, with a projected increase in annual demand of 1.5% in the coming years. After its application to soil, urea undergoes hydrolysis via the urease enzyme, causing increases in the soil pH in the surrounding area of the granules and resulting in NH3 losses that average 16% of N applied worldwide and can reach 40% or more in hot and humid conditions. The use of urease inhibitors is an effective way to reduce NH3 losses. Several compounds act as urease inhibitors, but only N-(n-butyl) thiophosphoric triamide (NBPT) has been used worldwide, being the most successful in a market that has grown 16% per year in the past 10 years. Only in the past three years other compounds are being commercially launched. In comparison to urea, NBPT-treated urea reduces NH3 loss by around 53%. Yield gain by NBPT usage is of the order of 6.0% and varies from −0.8 to 10.2% depending on crop species. Nitrification inhibitors usually increase NH3 volatilization and mixing them with urease inhibitors partially offsets the benefits of the latter in reducing NH3 loss. The efficacy of NBPT to reduce NH3 loss is well documented, but there is a need for further improvement to increase the period of inhibition and the shelf life of NBPT-treated urea.
Introduction
Compared to the use of other nutrients, the use of nitrogen (N) fertilizers in agriculture in highest: in 2018/2019 over 107 Mt of N will be produced by the fertilizer industry worldwide and approximately 55% of the N fertilizers are urea [1]. Urea demand is forecast to increase by 1.5% per year and new urea plants are being commissioned to operate in the near future [2]. Urea has advantages for industry such as high N concentration (45–46% N) and lower production costs compared to other N sources. However, urea applied to soils undergoes fast hydrolysis, producing ammonia (NH3), which can be lost to the atmosphere. Ammonia losses can be both an economic problem (because less nutrient is left for plants to take up, affecting yields), and an environmental issue. Losses of NH3 in agriculture and livestock systems worldwide are estimated to be 37 Mt of N [3], [4], [5].
The amounts of N lost as NH3 vary with soil and environmental conditions: they are higher when urea is surface-applied to light soils (i.e., low cation exchange capacity), with high temperatures and moisture content [6], [7] and high N rates [8]. Band application usually results in higher losses than broadcasting fertilizer because of the high N rate effect. The global average losses of NH3 from urea fertilizers are estimated to be close to 14% (range of 10–19%) [9], but they can reach up to 40% of applied urea-N in tropical soils because of high temperatures [8], [10].
Incorporation of urea into the soil is an effective way of reducing or even preventing NH3 volatilization losses. This can be done by mechanical operations or by rain or irrigation. Holcomb et al. [11] observed that the application of approximately 15 mm of water soon after urea fertilization was sufficient to incorporate the fertilizer into the soil and reduce NH3 losses by 90%, which is in the range of 10–20 mm of rain or irrigation that is reported to significantly reduce NH3 volatilization [7]. Depending on the soil properties, even a shallow mechanical incorporation (i.e., 3 cm) can reduce losses, but Rochette et al. [12] found negligible NH3 volatilization only when urea was incorporated at depths greater than 7.5 cm.
Urea incorporation is, therefore, part of the so-called best management practices for increasing nutrient use efficiency. However, incorporation is not always possible or feasible, as in perennial crops, where it can cause mechanical damage to the roots, or in crops with a thick mulch of crop residues, such as sugarcane [13] and, sometimes, no-till areas. Mechanical incorporation requires higher-power tractors and is time-consuming, which restricts its use in large farms. Therefore, surface-application of urea is the predominant practice in many situations despite the risk of high N losses. Alternatives to overcome this risk include N sources other than urea and urea fertilizer formulations, such as slow or controlled-release fertilizers and urea amended with additives to reduce losses by temporarily blocking soil ureases and preventing urea hydrolysis for some time [14], [15]. Nitrogen sources such as ammonium sulfate and ammonium nitrate are not subject to NH3 volatilization losses in acid soils but are more expensive per unit N. Moreover, ammonium nitrate faces increasing restrictions because of its use as explosive material.
Neem oil and neem cake (extracted from Azadirachta indica (A. Juss)) have been used as urease inhibitor, in (primarily) India. Neem also exhibits other properties of agronomic interest such as nitrification inhibition and pesticide effect [16]. The effect of neem coated urea to reduce urease activity has been demonstrated [17] but some studies noted that neem, or one of its active ingredients – azadirachtin – seems to stimulate urease activity [18], [19]. Neem has more potential as a nitrification inhibitor [18], [20] and for this reason all urea used in India since 2015 is mandatorily coated with neem [21]. Other non-inhibiting products such as zeolites have shown mixed results in decreasing NH3 volatilization from urea application [22] or have no effect [23]. However, in this text, only the urease inhibitors will be covered.
There is long held interest in compounds that inhibit ureases in soils. Metals such as Ag, Hg, Cu, Cd, Co, Zn, and others were long known to inhibit urea hydrolysis and were tested as fertilizer additives [24], [25]. Boric acid can also decrease urea hydrolysis [26]. Bock and Kissel [6] reviewed early works with organic compounds tested as soil urease inhibitors. Kiss and Simihaian [27] reported that over 14,000 compounds or mixtures of compounds have been tested for their effects on soil urease activity, and many of them were patented for that purpose. Hydroquinone and some benzoquinones were known to inhibit urease activity, but the best results were obtained with structural analogues of urea [15], [27]. There are many compounds in the latter family that showed inhibitory effects, but the one that stood out and most successfully reached the market is N-(n-butyl) thiophosphoric triamide (NBPT), traded as Agrotain, in the USA starting in the mid-1990s. Today, different brands of NBPT are sold as additives to urea in many countries worldwide [28].
Following the commercial success of NBPT and the large potential market for urea additives, there is renewed interest in new molecules or formulations of urease inhibitors. There is room for improvement, as NBPT has a relatively short period of effective urease inhibition in soils, especially under high temperatures [14]. Several urea analogues were shown to more effectively inhibit urea hydrolysis in vitro than NBPT [29] but none of the compounds tested by these authors is commercial so far. A formulation containing NBPT and NPPT (N-(n-propyl) thiophosphoric triamide) has been successfully tested [30], [31] and has reached the market in the past two years under the brand name Limus. A new urease inhibitor, N-(2-nitrophenyl) phosphoric triamide (2-NPT), was developed in Germany in the early 2000s, has been tested under field conditions [32] and is also reaching the market, which is, so far, amply dominated by NBPT. They are all urea analogues.
Compounds such as phenolic aldehydes and benzoylthioureas are being developed as novel urease inhibitors [33], [34] and are the subject of another chapter of this special issue.
NBPT has already a solid position in the market of non-commodity fertilizers. It is estimated that 14 Mt of specialty fertilizers, including controlled-release, slow-release, sulfur-coated urea, and urease- and nitrification inhibitor-treated fertilizers were produced worldwide in 2016: urea containing NBPT accounted for 7.4 Mt, or 53% [28]. Sales are estimated to have increased at a rate of 16% per year in the past 10 years. The demand for urease inhibitor is expected to continue to grow at a pace of 10–12% per year in the next 10 years. Environmental regulations may help to increase the demand for such products. For instance, Germany has passed legislation requiring that by 2020 all urea fertilizer used in that country either be incorporated into the soil or amended with urease inhibitors [28].
Urease in soils
The urease enzyme is common in nature and is present in animals, plants and microorganisms. In soil, most of the urease enzyme comes from syntheses realized by microorganism and plant materials [35], [36]. Paulson and Kurtz [37] estimated that 79–89% of urease activity in soils is derived from extracellular enzymes adsorbed to soil colloids. The activity of urease enzyme is higher in plant materials than in soil, and thus areas with crop residues, such as no-till, tend to show higher enzyme activity. Barreto and Westerman [38] observed a threefold increase in urease activity in no-till system compared with that in the soil of a conventional tillage area.
Urease activity depends on soil moisture. In dry soil conditions the rate of urease hydrolysis is low [39]; however, it increases gradually as the water content of soil increases until it reaches 20% [35]. Above that level, the hydrolysis is largely unaffected by changes in soil moisture. Therefore, urea hydrolysis – and the consequent NH3 formation – tends to be high in moist soils, especially under high temperature; conversely, urea applied to dry soils has slow hydrolysis, allowing more time for reducing volatilization losses by soil incorporation with mechanical means, rain or irrigation [7], [10].
Urea hydrolysis, catalyzed by urease enzymes, is a fast process in soils, and involves proton consumption, increasing the soil pH in the surrounding area of fertilizer granules [7], [40]. Overrein and Moe [41] showed increase in soil pH from 6.5 to 8.8 after three days of urea application. The urea hydrolysis results in ammonium and CO2 production, according to the following simplified equation [10]:
As urea hydrolysis consumes protons (H+), the soil pH increases driving the equilibrium between NH4+ and NH3 towards the formation of the gaseous form.
Mechanism of action of urease inhibitors
NBPT strongly blocks three active sites of the urease enzyme, forming a bond of tridentate nature, with two nickel centers and one oxygen from the carbamate bridge linking both metals, reducing the probability of urea to reach the nickel atom [42]. Other phosphoramide derivatives, similar to NBPT, show the same mechanisms of action [6], [27], [29]
NBPT is not the direct inhibitor of urease; it must be converted into N-(n-butyl) phosphoric triamide (NBPTO). The factors influencing this conversion are not clear, but the reaction is faster in soils with aerobic conditions (occurring in minutes or hours) and can take days under anaerobic conditions [43]. NBPT has shown higher efficiency in delaying urea hydrolysis than the direct application of NBPTO, which is degraded faster [44].
Before the advent of organic molecules as urease inhibitor in agriculture, metals were widely tested [24], [25]. Shaw [25] evaluated the action of metals and showed this sequence of urease inhibition power: Ag+ ∼ Hg2+ > Cu2+ > Cd2+ > Co2+ > Ni2+ > Zn2+ = Sn2+ = Mn2+ = Pb2+. The metals inhibit urease by creating a chemical bond with one or more sulfhydryl group active sites to produce insoluble sulfites; consequently, the metal with higher affinity to the enzyme that forms the more insoluble sulfite will be the stronger inhibitor [25]. Usually, the inhibitory effect of metals is less than that of phosphoramides [6]. Moreover, the application of heavy metals in soils may cause environmental problems.
Boric acid can also inhibit urea hydrolysis because boric acid acts as an analogous substrate [26]. The urease enzyme has in its active site two atoms of nickel bonded with one hydroxyl. The mechanism of the action of boric acid on urease is that it symmetrically fits between both nickel centers and shows a geometric similarity to the urea molecule [26].
Pesticides may affect soil enzymes acting as alternative substrate. However, azadirachtin – the active ingredient of neem – showed opposite results, increasing urease activity because it acted as a source of energy for microorganisms instead of a urease inhibitor [19], although neem has been recommended for addition to urea in India [21].
Method of application of urease inhibitors
In the first studies to test urease inhibitors, the potential inhibitors were added directly to the soil [6], [35]; however, given the ubiquitous nature of the enzyme in soil and the fact that urea, even when broadcast over the soil, usually is confined to a limited portion of the soil, efficient reduction of NH3 losses could be achieved with small amounts of inhibitors added directly to the fertilizer.
The urease inhibitors, such as NBPT, are applied mainly as liquid formulation coating urea fertilizer granules, which guarantees a homogeneous cover and efficacy [14]. NBPT can also be added to the urea melt before granulation. There is little or no difference in the performance of NBPT to reduce NH3 losses when coated or incorporated into the urea granule [14], [45]. However, NBPT applied in the melt prolonged the storage time significantly compared to coated applications [45].
In Brazil, a fertilizer based on urea containing copper and boric acid is commercially available with the purposes of reducing NH3 loss and supplying B and Cu. Stafanato et al. [46] compared urea amended with Cu (Cu sulfate) and B (boric acid), as pellets (Cu and B mixed with urea before pelleting) or coated with the salts. Both methods were equally effective with reduction of NH3 volatilization losses of 30%, on average, compared to urea; however, their efficacy was still lower than the 85% reduction obtained by NBPT. Among the micronutrient treatments, the greater NH3 loss reduction was obtained by increasing the B concentration in the formulation, equivalent to an application of 10 kg ha−1B [46], much above the usual field recommendations for this nutrient. However, the benefits of boric acid and Cu added to urea to reduce NH3 losses are not consistent: some studies have shown a reduction in losses [46], [47], [48], [49], whereas others reported no effect [23], [27], [50], [51], [52].
Effect on plant germination and metabolism
Urease inhibitors delay urea hydrolysis in soil and, in this way, decrease the intensity that the soil pH and NH3/NH4+ concentration is increased in the surrounding area of the fertilizer granule, thus reducing the toxic effect of high ammonia concentration on seed germination [53], [54], [55]. Grant and Bailey [53] reported a decrease in seed damage due to the addition of NBPT in urea compared with untreated urea, which increased the stand density and promoted a higher yield of barley. In a study with rice, Qi et al. [56] showed that the addition of NBPT to urea reduced the damage to seed germination and increased root growth. Urea treated with NBPT decreased the damage to canola seedlings, which resulted in higher grain yield [54].
NBPT can be absorbed by plants and change some metabolic pathways reducing urease activity and glutamine synthetase activity, which are associated with N assimilation [57], [58]. Therefore, NBPT can cause transient yellowing of leaf tips caused by urea toxicity soon after application. However, plants usually recover quickly and no effects on growth have been reported [14], [57].
Stability, longevity, and efficacy of urease inhibitors
NBPT degrades over time when applied to urea, which may limit the shelf life of treated fertilizer [45], [59]. The addition of organic amendments, such as peat, decreases the shelf life of NBPT treated urea [59]. Watson et al. [45] found that the storage half-life of NBPT treated urea was 20 weeks at 25 °C, but NBPT degradation was much smaller when urea was stored at 4 °C. In this study, when the urease inhibitor was added to the urea melt before granulation, the stability was longer than when NBPT was coating the urea granules [45]. Soares [60] found that urea coated with NBPT could be safely stored for 12 weeks at 25 °C but only 4 weeks at 35 °C. Cantarella et al. [61] studied the effectiveness of urea coated with NBPT and stored in warehouses in two locations in Brazil. They found that urea stored in the southern site (mild temperature) still performed as well as the freshly treated urea after 9 months, whereas the urea stored in the north-central warehouse (hotter) was significantly less effective after 6 months, although it still reduced NH3 losses compared with the untreated urea.
The solvents and other additives in the NBPT solution used to impregnate urea fertilizer seem to play a role in the stability and longevity of the commercial products. NBPT manufacturers change the solvent’s composition to improve overall performance, including storability, so the results of previous studies must be observed with care. However, the stability of urea coated with NBPT is still a matter of concern, especially in conditions in which fertilizer must be stored for a long period before use.
NBPT also undergoes microbial degradation in the soil. The rate of degradation depends on soil temperature, microbial activity, and soil pH. NBPTO, the product of the oxidation of NBPT, is more susceptible to degradation than NBPT [62], which explains why the inhibitor is more stable in the stored fertilizer than when applied to the soil.
Several soil factors are responsible for controlling the magnitude of NH3 loss and, in some cases, it is not possible to accurately estimate the effect of a single soil characteristic in controlling NH3 loss. NBPT degrades faster in acidic than in alkaline soils, which affects the longevity of the inhibitor in the soil [62], [63]. In fact, Soares [60] observed that NBPT treated urea reduced NH3 volatilization by 52–53%, compared to urea, in soils with pH (in 0.01 M CaCl2 solution) 5.6 and 6.4, but the reduction was only 18% at soil pH 4.5. Accordingly, the efficacy of urease inhibitor was lower in very acidic soils than in neutral or alkaline soils in the meta-analysis of Silva et al. [64].
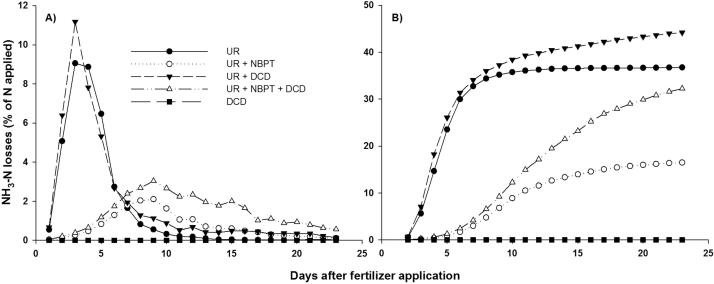
The time-period in which NBPT remains active will determine its effectiveness to reduce NH3 losses. In hot soils, degradation may begin after two to four days [65] but can take up to 10 or 15 days in low-temperature soils, such as those in temperate climate regions [45]. A typical curve of NH3 volatilization in a Brazilian Oxisol at 25 °C is shown in Fig. 1. The peak of NH3 losses of urea occurred only 2–4 days after fertilization in this warm and moist soil whereas the peak with urea coated with NBPT occurred on the 7th day. Not only was the peak delayed, but its size was also reduced. Ammonia volatilization started 4 days after fertilization with NBPT, indicating that the inhibitor was already starting to degrade. The inhibitor persistence in the soil directly affects its effectiveness in controlling NH3 losses. Changing the composition of the solvent and/or of the inhibitors used in the formulation is a strategy that may help to increase the longevity of the inhibitor both in stored urea and in the soil [66].
Fig. 1.
Daily (A) and cumulative (B) ammonia volatilization losses after urea application with urease (NBPT) and nitrification (DCD) inhibitors. Reprinted from Soares et al. [65], with permission from Elsevier.
Usually the efficacy of urease inhibitors is calculated considering the basal NH3 loss of a urea-based fertilizer [22], [64], [67], [68]. By this approach, the efficacy of urease inhibitor in lowering NH3 loss will be at its maximum when the conditions for NH3 loss from urea are extreme. Incorporation of urea into the soil and irrigation are management practices that effectively reduce NH3 loss from urea [11], [12], [22].
Split urea application can either decrease or have no effect in lowering NH3 loss [22]. Such inconsistencies are associated with the weather and soil conditions in the time of fertilizer application. Therefore, the efficacy of urease inhibitor in decreasing NH3 loss under split application depends on the conditions of fertilizer application and magnitude of NH3 loss. On the other hand, increasing N rates [22], [69], the application of urea over crop residues or to soils with high moisture and temperature, usually cause enhanced NH3 loss [13], [22] and hence, makes the use of urease inhibitors more attractive as a tool to increase N use efficiency. Conversely, low temperature or dry conditions may limit urea hydrolysis and, thus NH3 losses [13], [32].
As NH3 losses are usually concentrated in the first 2–5 days after urea application and NBPT shows a relatively short time protection (Fig. 1), the ideal situation for the urease inhibitors’ performance is for mechanical incorporation, rain or irrigation to occur up to 5–7 days after fertilization with urea containing inhibitors, while the inhibitory potential is still high, depending on soil temperature or moisture. Indeed, the results of field studies showed reductions in NH3 volatilization higher than 85% due to NBPT when rain occurred within 5 days after urea application to maize and pasture. NH3 losses of untreated urea were high: 37 and 18% of the applied N to maize and pasture, respectively, whereas the corresponding NH3 losses of urea + NBPT were 5% and 3% [15]. This was a situation in which the use of urease inhibitors clearly paid off. However, even when the urease inhibitors degrade before rain occurs, a significant NH3 loss reduction is usually observed. This is, for instance, the case in the data shown in Fig. 1, in which NH3 volatilization was measured under controlled conditions in which the fertilized soil remained in a chamber for more than 20 days without any water addition. The treatment with urea lost 36% of the N as NH3 whereas the treatment with urea + NBPT lost only 16% [65].
Even if relatively short-lived, the effect of urease inhibitor allows urea hydrolysis to slow down, permitting urea to diffuse into the soil, thus diluting urea and NH3 concentration on the soil surface; in addition, when urea diffuses and hydrolyses a few millimeters inside the soil, the NH3 produced may react with soil acidity or be bound to soil’s negative charges, decreasing volatilization losses (See Graphical Abstract). The effect of such urea incorporation will be higher in soils with higher buffering capacity and acidity.
The concentration of urease inhibitors in urea fertilizer may affect their efficacy. Usually, the concentrations of NBPT in commercial fertilizers vary from 500 to 1200 mg NBPT per kilogram of urea. In the meta-analysis of Silva et al. [64] a slight decrease in volatilization loss was observed when NBPT rates increased from 530 to >1060 mg kg−1. Similar findings were observed by Mira et al. [70], who found a reduction in NH3 losses when NBPT rates were increased up to 1000 mg kg−1. However, apparently there is no yield gain in increasing NBPT rates >1060 mg kg−1 [64]. Despite the potential of higher NBPT rates in reducing NH3 loss, the lack of yield gain and the increase in cost for farmers limit the recommendation to increase NBPT rates in the short term. In addition, as recent evidences showed that NBPT can be absorbed by plant roots, high NBPT rates may also affect the internal N metabolism of plants even if the effect is transitory [57], [71].
Interaction between urease and nitrification inhibitors
The main purpose of using nitrification inhibitors is to reduce the conversion of ammonium (NH4+) to nitrate (NO3−), reducing the potential of nitrate leaching [72], which is one important pathway of N loss in agriculture. Nitrification inhibitors also reduce nitrous oxide (N2O) emissions from fertilizers, [73], [74], which has a positive environmental impact as N2O is a potent greenhouse gas. However, the avoided N2O emission is of little significance for plant nutrition, because the denitrification process usually causes low amounts of N loss.
The association of urease and nitrification inhibitors has, therefore, the potential to increase N use efficiency by addressing two important N loss mechanisms. However, the combination of both inhibitors in urea may not give the expected beneficial effect. In fact, several studies have shown that nitrification inhibitors added to urea increase NH3 loss. Treating urea with nitrification inhibitors showed potential to increase NH3 loss by 38% in a study by Pan et al. [22], and this may decrease the benefits of the urease inhibitor [65], [75], [76], [77]. The magnitude of the interaction between the two inhibitors depends on the soil properties. In most studies, the mixture of both inhibitors still reduced NH3 loss when compared with untreated urea, i.e., the urease inhibitor was still effective, but the results are less than those obtained with the urease inhibitor alone [75]. However, in an Oxisol study with low cation exchange capacity, Soares et al. [65] observed that the nitrification inhibitor could offset the effect of the urease inhibitor in reducing NH3 volatilization losses (Fig. 1). Nevertheless, these authors showed that there was no direct effect of the nitrification inhibitor on the urease inhibitor; the nitrification inhibitor, by blocking nitrification, caused the soil NH3/NH4+ concentration to remain high for a longer period, allowing volatilization losses to continue [65]. In addition, the nitrification inhibitor, by blocking nitrification, decreases soil acidification, which also helps to reduce NH3 loss.
Effect of urease inhibitors on NH3 volatilization losses and crop yield
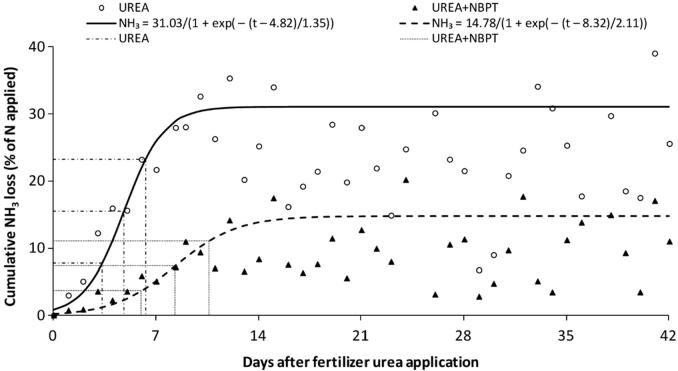
Urea treated with urease inhibitor shows a decrease in daily volatilization loss (Fig. 1) which results in a reduction in NH3 loss (Fig. 2). The meta-analysis by Silva et al. [64] revealed an accumulated NH3 loss of 31% for urea and 15% for urea + NBPT in a wide range of soil, weather, and management conditions. The reduction in urea hydrolysis by the urease inhibitor slows the NH3 loss in the days after fertilization; the data of Fig. 2, for example, shows that 50% of the total NH3 loss occurred 4.8 or 8.3 days after fertilization for urea and urea + NBPT, respectively.
Fig. 2.
Cumulative NH3 loss during 42 d after application of urea and urea treated with N-(n-butyl) thiophosphoric triamide (NBPT). The points of the curves correspond to the arithmetic average of daily cumulative NH3 loss compiled from 35 studies. The dash-dotted lines (urea) and dotted lines (urea + NBPT) represent (X axis) the number of days that elapsed for 20, 50, and 75% of the total NH3 losses to occur. The R2 value was 0.99 for both the urea and the urea + NBPT models. Reprinted from Silva et al. [64] with permission from The American Society of Agronomy.
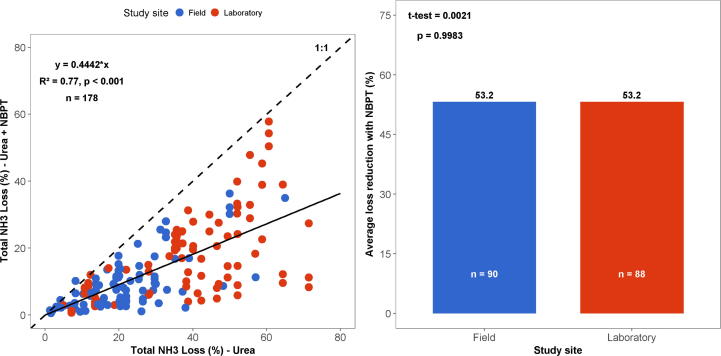
Pooling together data of NH3 loss of urea and NBPT-treated urea from several studies presented in Table 1 of Supplementary Material, it is clear that, in the vast majority of the studies, the NH3 loss of NBPT-treated urea is lower than that of urea (Fig. 3). Most of the points are located below the ratio 1:1, demonstrating that NBPT significantly lowered NH3 loss in comparison with urea (P < 0.001). More importantly, there was no difference between data obtained under field or laboratory conditions (p = 0.9983), indicating a reduction of 53% in NH3 loss in both conditions (Fig. 3). Such a result demonstrates that both field and laboratory studies agree regarding the effectiveness of NBPT in lowering NH3 loss from urea (Fig. 3).
Fig. 3.
Total NH3 loss of urea and urea + NBPT (left) and the average reduction in NH3 loss by treating urea with NBPT in comparison to untreated urea (right) considering the selected studies presented in Table 1-S of the Supplementary Material.
Four independent meta-analyses have been published recently on the effect of enhanced efficiency fertilizers (EEF) such as urease inhibitors, nitrification inhibitors or controlled release fertilizers in reducing volatilization loss or increasing N use efficiency/crop yield (Table 1). In summary, treating urea with a urease inhibitor reduced the NH3 loss from 52 to 54% when compared to untreated urea, which is very close to the result reported in Fig. 3.
Table 1.
Compilation of data of four meta-analyses recently published regarding the effect of urease inhibitor (UI), controlled release fertilizer (CRF) and nitrification inhibitor (NI) in NH3 loss, crop yield and N use efficiency (NUE) compared to urea-based fertilizer.
| Meta-analyses papers | NH3 loss |
Crop yield |
NUE |
||||||
|---|---|---|---|---|---|---|---|---|---|
| UI | CRF | NI | UI | CRF | NI | UI | CRF | NI | |
| Reduction or increase (%) in comparison to urea-based fertilizer | |||||||||
| [68]a | – | – | – | +5.0 | +6.5 | +6.0 | +5.0 | +2.0 | +13.0 |
| [67] | – | – | – | +10.0 | – | +5.0 | +12.0 | – | +5.0 |
| [22] | −54.0 | –68.0 | +38.0 | – | – | – | – | – | – |
| [64] | −52.0 | – | – | +5.2 | – | – | – | – | – |
The study of Linquist et al. [68] present data of a single crop (rice). In the same study, NUE is originally presented as N uptake.
Despite the high potential of urease inhibitors to reduce NH3 loss [13], [22], [64], [65], [70], the effect on crop yield and N use efficiency (NUE) is much more limited and ranges from a yield increase of 5–12% in most studies (Table 1). The relatively small yield gains reported in the literature also help to determine the rates of inhibitor in the fertilizer.
The reason is that, in many cases, most of the N taken up by crops comes from the soil; N from the fertilizer, although important to determine yields, is a complement. In this way, the N saved by the urease inhibitor may not translate into yield increases [64]. However, the N preserved in the soil-plant system, as a consequence of the use of urease inhibitors reducing NH3 losses, contributes to building up soil N reserves. Moreover, less NH3 volatilization also brings environmental benefits. Ammonia may be deposited in the vicinity or be transported over long distances when NH3 reacts with acids to form ammonium aerosols such as (NH4)2SO4 or NH4HSO4 [78]. Nitrogen deposited elsewhere may cause undesirable effects, including indirect emissions of greenhouse gases, soil acidification, and biodiversity loss [4], [79].
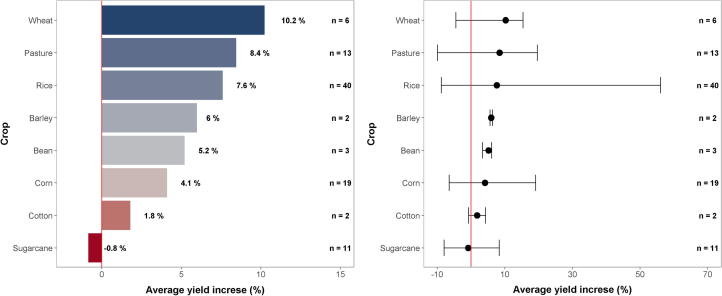
In the average of 96 observations presented in Table 2-S of the Supplementary Material, the use of urease inhibitor promoted yield gain of 6.0% for several crops in comparison to the untreated urea. This value is similar to the 5.2% and 7.5% yield gain of several crops reported by Silva et al. [64], 10% by Abalos et al. [67], 5.7% by Linquist et al. [68] for rice, and 5–14% for maize [15]. Table 2-S shows that per crop, the yield gain ranged from -0.8% in sugarcane to +10.2% in wheat (Fig. 4). Despite the absence of statistical treatment of the data, such differences in yield gain promoted by a urease inhibitor are dependent on the crop growth cycle, which indirectly affects responsiveness to N. Cereal crops and pasture showed the highest yield gains due to urease inhibitor addition (Fig. 4). Cereal crops have short growth cycle which increases the chance of N response, and pasture biomass yields are largely improved by N fertilization [67]. On the other hand, the relatively limited effect of urease inhibitor in increasing the yield of cotton and sugarcane (Fig. 4) can be related to particularities of the N nutrition for both crops. In cotton, excessive N fertilization stimulates vegetative growth and delays maturity, with the potential to compromise yield and nutrient use efficiency [80], [81]. Reduced N responsiveness of sugarcane is associated with a long crop growth cycle that increases uptake of N mineralized from soil organic matter and reduces the dependency of N from fertilizers [82].
Fig. 4.
Average yield increase per crop by treating urea with NBPT in comparison to the untreated urea, considering the selected studies presented in Table 2-S of the Supplementary Material. Error bars (right) represent the maximum and minimum values found in the original studies.
Conclusions and future perspectives
Urease inhibitors have been on the market for 20 years, with growing acceptance by farmers. Much is already known about this class of products given the large volume of scientific literature available. Their efficacy in reducing NH3 volatilization is well documented. However, some limitations, such as the short period of effective inhibition and the limited shelf life, should stimulate much-needed research and development efforts. Moreover, it should be acknowledged that, although urease inhibitors greatly reduce NH3 volatilization losses, they do not eliminate them. In situations where potential losses are high, the use of surface-applied urea, even with urease inhibitors, may result in sizeable N losses. Therefore, the challenges remain to further improve present urease inhibitors, to develop new molecules or mixture of molecules, to improve formulations, and integrate them with agronomic practices capable of reducing losses and increasing NUE.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
The following research grants are acknowledged: HC: FAPESP 2015/50305-8; FAPESP/NWO 2013/50365-5; CNPq 311.197/2013-2; RO: FAPESP 2014/05591-0; CNPq 308.007/2016-6; JRS: FAPESP 2016/08741-8 and FAPESP 2014/26767-9.
Biographies

Heitor Cantarella is a soil scientist and currently is the head of the Soils and Environmental Resources Center at the Agronomic Institute of Campinas, in Brazil. He is one of the coordinators of the FAPESP’s Bioenergy Program (BIOEN), and of the Nutrient for Life in Brazil. He holds a Degree in Agronomy (FCA-UNESP, Brazil, 1974), a Master and Ph.D. degrees in Soil Fertility, both from Iowa State University, USA (1981 and 1983). His expertise is in soil fertility and plant nutrition, fertilizer recommendation, nitrogen, fertilizer use efficiency, and environmental issues related with fertilizer use in agriculture. He has extensively studied NH3 and N2O losses from fertilizers and mitigating strategies including the use of urease and nitrification inhibitors. Among recent professional recognitions are the International Plant Nutrition Institute Award on Plant Nutrition (Brazil, 2016) and the IFA Norman Borlaug Award (Zurich, Switzerland, 2017).

Rafael Otto is a soil scientist, with degrees in Agronomy (2005), Master (2008) and PhD (2012) degree from Luiz de Queiroz College of Agriculgure (ESALQ), at the University of São Paulo, in Piracicaba, Brazil. In 2013 he spent one year as a Pos-Doc researcher at the Center for Nuclear Energy in Agriculture in Piracicaba. Later that year he joined the Soil Science Department at ESALQ-USP where he is Professor of Soil Fertility and Fertilizers. His research interest includes nitrogen management for biofuel production, development of new fertilizers and use of nanomaterials to supply plant nutrients.

Johnny R. Soares holds a B.S. degree in Agronomy by the University of São Paulo, Piracicaba, Brazil, MSc. and pH.D. in Tropical and Subtropical Agriculture by the Agronomic Institute, in Campinas, Brazil. Currently, he is a Post-Doc researcher at the School of Agricultural Engineering at the University of Campinas, Campinas, Brazil, where he works on the Global Sustainable Bioenergy initiative, conducting geospatial and environmental analysis of pasture intensification for bioenergy. His area of interest is biogeochemical cycles, greenhouse gases, agronomic efficiency of nitrogen fertilizers and nitrous oxide emissions.

Aijânio G. B. Silva earned his BSc degree in Agronomy in 2010 at the Federal Rural University of Rio de Janeiro, RJ, Brazil. He received his MSc and PhD degree in Science (Soil and Plant Nutrition) in 2013 and 2017, respectively, Luiz de Queiroz College of Agriculgure (ESALQ), at the University of São Paulo, in Piracicaba, Brazil. He is currently postdoctoral scientist at the same institution. His current research interests is on the effect of sugarcane straw removal on soil attributes, sugarcane plant growth and greenhouse gases emissions. Other research topics includes plant nutrition, soil fertility and fertilizers.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jare.2018.05.008.
Appendix A. Supplementary material
References
- 1.IFA International Fertilizer Association. Fertilizer outlook 2017–2021. IFA annual conference – 22–24 May 2017 Marrakech (Marocco). Paris: IFA International Fertilizer Association, Services PITaA; 2017 June 2017.
- 2.IFA International Fertilizer Association. Short-term fertilizer outlook 2017–2018 report. Paris: IFA International Fertilizer Association, Services PITaA; 2017 November 2017.
- 3.Bittman S., Dedina M., Howard C.M., Oenema O., Sutton M.A. Centre for Ecology and Hydrology (CEH); Edinburgh: 2014. Options for ammonia mitigation: guidance from the UNECE Task Force on Reactive Nitrogen. [Google Scholar]
- 4.Sutton M.A., Bleeker A., Howard C.M., Bekunda M., Grizzetti B., de Vries W. Centre for Ecology and Hydrology; Edinburgh: 2013. Our Nutrient World: the challenge to produce more food and energy with less pollution. [Google Scholar]
- 5.Sutton M.A., Erisman J.W., Dentener F., Möller D. Ammonia in the environment: from ancient times to the present. Environ Pollut. 2008;156:583–604. doi: 10.1016/j.envpol.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Bock B.R., Kissel D.E. National Fertilizer Development Center – NFDC; Muscle Shoals, AL, USA: 1988. Ammonia volatilization from urea fertilizers. [Google Scholar]
- 7.Terman G.L. Volatilization losses of nitrogen as ammonia from surface-applied fertilizers, organic amendments, and crop residues. Adv Agron. 1979;31:189–223. [Google Scholar]
- 8.Cantarella H., Mattos D., Quaggio J.A., Rigolin A.T. Fruit yield of Valencia sweet orange fertilized with different N sources and the loss of applied N. Nutr Cycl Agroecosys. 2003;67(3):215–223. [Google Scholar]
- 9.Bouwman A.F., Boumans L.J.M., Batjes N.H. Estimation of global NH3 volatilization loss from synthetic fertilizers and animal manure applied to arable lands and grasslands. Global Biogeochem Cycl. 2002;16(2) [Google Scholar]
- 10.Nitrogênio Cantarella H. In: Fertilidade do Solo. Novaes R.F., Hugo A.V.V., Barros N.F., Cantarutti R.B., Neves J.C.L., editors. Sociedade Brasileira de Ciência do Solo; Viçosa: 2007. pp. 375–470. [Google Scholar]
- 11.Holcomb J.C., Sullivan D.M., Horneck D.A., Clough G.H. Effect of irrigation rate on ammonia volatilization. Soil Sci Soc Am J. 2011;75(6):2341–2347. [Google Scholar]
- 12.Rochette P., Angers D.A., Chantigny M.H., Gasser M.-O., MacDonald J.D., Pelster D.E. Ammonia volatilization and nitrogen retention: how deep to incorporate urea? J Environ Qual. 2013;42(6):1635–1642. doi: 10.2134/jeq2013.05.0192. [DOI] [PubMed] [Google Scholar]
- 13.Cantarella H., Trivelin P.C.O., Contin T.L.M., Dias F.L.F., Rossetto R., Marcelino R. Ammonia volatilisation from urease inhibitor-treated urea applied to sugarcane trash blankets. Sci Agric. 2008;65(4):397–401. [Google Scholar]
- 14.Trenkel M.E. 2nd ed. IFA – Intl. Fertilizer Industry Association; Paris: 2010. Slow- and controlled-release and stabilized fertilizers: an option for enhancing nutrient use efficiency in agriculture. [Google Scholar]
- 15.Chien S.H., Prochnow L.I., Cantarella H. Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv Agron. 2009;102:267–322. [Google Scholar]
- 16.Mondal E., Chakraborty K. Azadirachta indica – a tree with multifaceted applications: an overview. J Pharm Sci Res. 2016;8(5):299–306. [Google Scholar]
- 17.Mohanty S., Patra A.K., Chhonkar P.K. Neem (Azadirachta indica) seed kernel powder retards urease and nitrification activities in different soils at contrasting moisture and temperature regimes. Bioresour Technol. 2008;99(4):894–899. doi: 10.1016/j.biortech.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Sridharan G.V., Hazarika D., Bhat M.R., Suraksha R.S., Singh S. Nitrification inhibition studies of neem coating on urea prills. Asian J Chem. 2017;29(1):196–198. [Google Scholar]
- 19.Kizilkaya R., Samofalova I., Mudrykh N., Mikailsoy F., Akca I., Sushkova S. Assessing the impact of azadirachtin application to soil on urease activity and its kinetic parameters. Turkish J Agric For. 2015;39(6):976–983. [Google Scholar]
- 20.Kashiri H.O., Kumar D. Coating of essential oils onto prilled urea retards its nitrification in soil. Arch Agron Soil Sci. 2017;63(1):96–105. [Google Scholar]
- 21.Singh B. Agronomic benefits of neem coated urea – a review. International fertilizer association review papers. Paris: International Fertilizer Association; 2016.
- 22.Pan B., Lam S.K., Mosier A., Luo Y., Chen D. Ammonia volatilization from synthetic fertilizers and its mitigation strategies: a global synthesis. Agric Ecosyst Environ. 2016;232:283–289. [Google Scholar]
- 23.Faria L.A., Nascimento C.A.C., Vitti G.C., Luz P.H.C., Guedes E.M.S. Loss of ammonia from nitrogen fertilizers applied to maize and soybean straw. R Bras Ci Solo. 2013;37:969–975. [Google Scholar]
- 24.Tabatabai M.A. Effects of trace elements on urease activity in soils. Soil Biol Biochem. 1977;9(1):9–13. [Google Scholar]
- 25.Shaw W.H.R. The inhibition of urease by various metal ions. J Am Chem Soc. 1954;7:2160–2163. [Google Scholar]
- 26.Benini S., Rypniewski W.R., Wilson K.S., Mangani S., Ciurli S. Molecular details of urease inhibition by boric acid: insights into the catalytic mechanism. J Am Chem Soc. 2004;126:3714–3715. doi: 10.1021/ja049618p. [DOI] [PubMed] [Google Scholar]
- 27.Kiss S., Simihaian M. Kluwer Academic Publishers; Doordrech: 2002. Improving efficiency of urea fertilizers by inhibition of soil urease activity. [Google Scholar]
- 28.Ramspacher A. Assessment of the global market for slow and controlled release, stabilized and water-soluble fertilizers. Presentaton at IFA strategic forum 2017. Paris: IFA – International Fertilizer Association; 2017 November 2017.
- 29.Domínguez M.J., Sanmartín C., Font M., Palop J.A., San Francisco S., Urrutia O. Design, synthesis, and biological evaluation of phosphoramide derivates as urease inhibitors. J Agric Food Chem. 2008;56:3721–3731. doi: 10.1021/jf072901y. [DOI] [PubMed] [Google Scholar]
- 30.Li S., Li J., Lu J., Wang Z. Effect of mixed urease inhibitors on N losses from surface-applied urea. Int J Agric Sci Technol. 2015;3(1):23–27. [Google Scholar]
- 31.Li Q., Yang A., Wang Z., Roelcke M., Chen X., Zhang F.-S. Effect of a new urease inhibitor on ammonia volatilization and nitrogen utilization in wheat in north and northwest China. Field Crop Res. 2015;175:96–105. [Google Scholar]
- 32.Schraml M., Gutser R., Maier H., Schmidhalter U. Ammonia loss from urea in grassland and its mitigation by the new urease inhibitor 2-NPT. J Agric Sci. 2016;154(8):1453–1462. [Google Scholar]
- 33.Horta L.P., Mota Y.C.C., Barbosa G.M., Braga T.C., Marriel I.E., Fátima A. Urease inhibitors of agricultural interest inspired by structures of plant phenolic aldehydes. J Braz Chem Soc. 2016;27(8):1512–1519. [Google Scholar]
- 34.Brito T.O., Souza A.X., Mota Y.C.C., Morais V.S.S., de Souza L.T., Fatima A. Design, syntheses and evaluation of benzoylthioureas as urease inhibitors of agricultural interest. RSC Adv. 2015;5(55):44507–44515. [Google Scholar]
- 35.Bremner J.M., Mulvaney R.L. Urease activity in soils. In: Burns R.G., editor. Soil enzymes. Academic Press; London: 1978. pp. 149–196. [Google Scholar]
- 36.Frankenberger W.T., Tabatabai M.A. Amidase and urease activities in plants. Plant Soil. 1982;64:153–166. [Google Scholar]
- 37.Paulson K.N., Kurtz L.T. Locus of urease activity in soil. Soil Sci Soc Am J. 1969;33(6):897–901. [Google Scholar]
- 38.Barreto H.J., Westerman R.L. Soil urease activity in winter wheat residue management systems. Soil Sci Soc Am J. 1989;53:1455–1458. [Google Scholar]
- 39.Volk G.M. Efficiency of fertilizer urea as affected by method of application, soil moisture, and lime. Agron J. 1966;58(3):249–252. [Google Scholar]
- 40.Ernst J.W., Massey H.F. The effects of several factors on volatilization of ammonia formed from urea in the soil. Soil Sci Soc Am J. 1960;24(2):87–90. [Google Scholar]
- 41.Overrein L.N., Moe P.G. Factors affecting urea hydrolysis and ammonia volatilization in soil. Soil Sci Soc Am J. 1967;31(1):57–61. [Google Scholar]
- 42.Manunza B., Deiana S., Pintore M., Gessa C. The binding mechanism of urea, hydroxamic acid and N-(N-butyl)-phosphoric triamide to the urease active site. A comparative molecular dynamics study. Soil Biol Biochem. 1999;31(5):789–796. [Google Scholar]
- 43.Watson C.J., editor. Urease activity and inhibition – principles and practices. The International Fertilizer Society Meeting; London: 2000. [Google Scholar]
- 44.Hendrickson L.L., Douglass E.A. Metabolism of the urease inhibitor N-(n-butyl)thiophosphoric triamide (NBPT) in soils. Soil Biol Biochem. 1993;25(11):1613–1628. [Google Scholar]
- 45.Watson C.J., Akhonzada N.A., Hamilton J.T.G., Matthews D.I. Rate and mode of application of the urease inhibitor N-(n-butyl) thiophosphoric triamide on ammonia volatilization from surface-applied urea. Soil Use Manag. 2008;24:246–253. [Google Scholar]
- 46.Stafanato J.B., Goulart R.S., Zonta E., Lima E., Mazur N., Pereira C.G. Volatilização de amônia oriunda de ureia pastilhada com micronutrientes em ambiente controlado. R Bras Ci Solo. 2013;37:726–732. [Google Scholar]
- 47.Cancellier E.L., Silva D.R.G., Faquin V., Gonçalves B.d.A., Cancellier L.L., Spehar C.R. Ammonia volatilization from enhanced-efficiency urea on no-till maize in Brazilian cerrado with improved soil fertility. Cienc Agrotec. 2016;40:133–144. [Google Scholar]
- 48.Dominghetti A.W., Guelfi D.R., Guimarães R.J., Caputo A.L.C., Spehar C.R., Faquin V. Nitrogen loss by volatilization of nitrogen fertilizers applied to coffee orchard. Cienc Agrotec. 2016;40:173–183. [Google Scholar]
- 49.Nascimento C.A.C., Vitti G.C., Faria L.A., Luz P.H.C., Mendes F.L. Ammonia volatilization from coated urea forms. R Bras Ci Solo. 2013;37:1057–1063. [Google Scholar]
- 50.Bayrakli F. Ammonia volatilization losses from different fertilizers and effect of several urease inhibitors, CaCl2, and phosphogypsum on losses from urea. Fertil Res. 1990;23:147–150. [Google Scholar]
- 51.Chaperon S., Sauvé S. Toxicity interactions of cadmium, copper, and lead on soil urease and dehydrogenase activity in relation to chemical speciation. Ecotoxicol Environ Saf. 2008;70:1–9. doi: 10.1016/j.ecoenv.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 52.Faria L.A., Nascimento C.A.C., Ventura B.P., Florim G.P., Luz P.H.C., Vitti G.C. Hygroscopicity and ammonia volatilization losses from nitrogen sources in coated urea. R Bras Ci Solo. 2014;38:942–948. [Google Scholar]
- 53.Grant C.A., Bailey L.D. Effect of seed-placed urea fertilizer and N-(n-butyl)thiophosphoric triamide (NBPT) on emergence and grain yield of barley. Can J Plant Sci. 1999;79(4):491–496. [Google Scholar]
- 54.Grant C.A., Derksen D.A., McLaren D., Irvine R.B. Nitrogen fertilizer and urease inhibitor effects on canola seed quality in a one-pass seeding and fertilizing system. Field Crop Res. 2011;121:201–208. [Google Scholar]
- 55.Xiaobin W., Jingfeng X., Grant C.A., Bailey L.D. Effects of placement of urea with a urease inhibitor on seedling emergence, N uptake and dry matter yield of wheat. Can J Soil Sci. 1995;75:449–452. [Google Scholar]
- 56.Qi XWW, Shah F, Peng S, Huang J, Cui K, Liu H, et al. Ammonia volatilization from urea-application influenced germination and early seedling growth of dry direct-seeded rice. Sci World J 2012;2012(Article ID 857472. Doi:10.1100/2012/757472):7p. [DOI] [PMC free article] [PubMed]
- 57.Artola E., Cruchaga S., Ariz I., Moran J.F., Garnica M., Houdusse F. Effect of N-(n-butyl) thiophosphoric triamide on urea metabolism and the assimilation of ammonium by Triticum aestivum L. Plant Growth Regul. 2011;63(1):73–79. [Google Scholar]
- 58.Cruchaga S., Lasa B., Jauregui I., Gonzáles-Murua C., Aparicio-Tejo P.M., Ariz I. Inhibition of endogenous urease activity by NBPT application reveals differential N metabolism responses to ammonium or nitrate nutrition in pea plants: a physiological study. Plant Soil. 2013;373:813–827. [Google Scholar]
- 59.Gioacchini P., Giovannini C., Marzadori C., Antisari L.V., Simoni A., Gessa C. Effect of N-(n-butyl)thiophosphoric triamide added to peat and leather in urea-based fertilizers on urea hydrolysis and ammonia volatilization. Commun Soil Sci Plant Anal. 2000;31(19–20):3177–3191. [Google Scholar]
- 60.Soares J.R. Agronomic Institute of Campinas; Campinas: 2011. Efeito de inibidores de urease e de nitrificação na volatilização de NH3 pela aplicação superficial de ureia no solo (Effect of urease and nitrification inhibitors on NH3 volatilization with surface-application of urea to soil) [Google Scholar]
- 61.Cantarella H., Soares J.R., Sousa R.M., Otto R., Sequeira C.H. 2016 International nitrogen initiative conference: solutions to improve nitrogen use efficiency for the world; Melbourne, Australia: 2016. Stability of urease inhibitor added to urea. [Google Scholar]
- 62.Engel R.E., Towey B.D., Gravens E. Degradation of the urease inhibitor NBPT as affected by soil pH. Soil Sci Soc Am J. 2015;79(6):1674–1683. [Google Scholar]
- 63.Engel R.E., Williams E., Wallander R., Hilmer J. Apparent persistence of N-(n-butyl) thiophosphoric triamide is greater in alkaline soils. Soil Sci Soc Am J. 2013;77(4):1424–1429. [Google Scholar]
- 64.Silva A.G.B., Sequeira C.H., Sermarini R.A., Otto R. Urease inhibitor NBPT on ammonia volatilization and crop productivity: a meta-analysis. Agron J. 2017;109(1):1–13. [Google Scholar]
- 65.Soares J.R., Cantarella H., Menegale M.L.C. Ammonia volatilization losses from surface-applied urea with urease and nitrifications inhibitors. Soil Biol Biochem. 2012;52:82–89. [Google Scholar]
- 66.Pro D., Huguet S., Arkoun M., Nugier-Chauvin C., Garcia-Mina J.M., Ourry A. From algal polysaccharides to cyclodextrins to stabilize a urease inhibitor. Carbohydr Polym. 2014;112:145–151. doi: 10.1016/j.carbpol.2014.05.075. [DOI] [PubMed] [Google Scholar]
- 67.Abalos D., Jeffery S., Sanz-Cobena A., Guardia G., Vallejo A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric Ecosyst Environ. 2014;189:136–144. [Google Scholar]
- 68.Linquist B.A., Liu L., van Kessel C., van Groenigen K.J. Enhanced efficiency nitrogen fertilizers for rice systems: meta-analysis of yield and nitrogen uptake. Field Crop Res. 2013;154:246–254. [Google Scholar]
- 69.Turner D.A., Edis R.B., Chen D., Freney J.R., Denmead O.T., Christie R. Determination and mitigation of ammonia loss from urea applied to winter wheat with N-(n-butyl) thiophosphorictriamide. Agric Ecosyst Environ. 2010;137(3–4):261–266. [Google Scholar]
- 70.Mira A.B., Cantarella H., Souza-Netto G.J.M., Moreira L.A., Kamogawa M.Y., Otto R. Optimizing urease inhibitor usage to reduce ammonia emission following urea application over crop residues. Agric Ecosys Environ. 2017;248:105–112. [Google Scholar]
- 71.Zanin L, Tomasi N, Zamboni A, Veranini Z, Pinton R. The urease inhibitor NBPT negatively affects DUR3-mediated uptake and assimilation of urea in maize roots. Front Plant Sci 2015;6(Article 1007):12p. [DOI] [PMC free article] [PubMed]
- 72.Subbarao G.V., Ito O., Sahrawat K.L., Berry W.L., Nakahara K., Ishiwawa T. Scope and strategies for regulation of nitrification in agricultural systems – challenges and opportunities. Crit Rev Plant Sci. 2006;25(4):303–335. [Google Scholar]
- 73.Soares J.R., Cassman N.A., Kielak A.M., Pijl A., Carmo J.B., Lourenço K.S. Nitrous oxide emission related to ammonia-oxidizing bacteria and mitigation options from N fertilization in a tropical soil. Sci Rep. 2016;6:30349. doi: 10.1038/srep30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Snyder C.S., Davidson E.A., Smith P., Venterea R.T. Agriculture: sustainable crop and animal production to help mitigate nitrous oxide emissions. Cur Opin Environ Sustain. 2014;9–10:46–54. [Google Scholar]
- 75.Frame W. Ammonia volatilization from urea treated with NBPT and two nitrification inhibitors. Agr J. 2017;109(1):1–10. [Google Scholar]
- 76.Zaman M., Saggar S., Blennerhassett J.D., Singh J. Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biol Biochem. 2009;41:1270–1280. [Google Scholar]
- 77.Nastri A., Toderi G., Bernati E., Govi G. Ammonia volatilization and yield response from urea applied to wheat with urease (NBPT) and nitrification (DCD) inhibitors. Agrochim. 2000;44(5–6):231–239. [Google Scholar]
- 78.Galloway J.N., Dentener F.J., Capone D.G., Boyer E.W., Howarth R.W., Seitzinger S.P. Nitrogen cycles: past, present, and future. Biogeochem. 2004;70(2):153–226. [Google Scholar]
- 79.Behara S.N., Sharma M., Aneja V.P., Balasubramanian R. Ammonia in the atmosphere: a review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ Sci Pollut Res. 2013;20:8092–8131. doi: 10.1007/s11356-013-2051-9. [DOI] [PubMed] [Google Scholar]
- 80.Li P., Dong H., Zheng C., Sun M., Liu A., Wang G. Optimizing nitrogen application rate and plant density for improving cotton yield and nitrogen use efficiency in the North China Plain. PLoS ONE. 2017;12(10):e0185550. doi: 10.1371/journal.pone.0185550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yeates S.J., Constable G.A., McCumstie T. Irrigated cotton in the tropical dry season. I: Yield, its components and crop development. Field Crop Res. 2010;116(3):278–289. [Google Scholar]
- 82.Otto R., Castro S.A.Q., Mariano E., Castro S.G.Q., Franco H.C.J., Trivelin P.C.O. Nitrogen use efficiently for sugarcane-biofuel production: what is next. Bioenerg Res. 2016;9:1272–1289. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.